Abstract
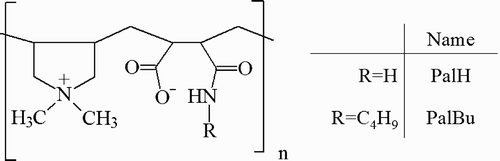
A series of hydrophobically modified polyampholytes has been synthesized by the copolymerization of the cationic monomer (N,N′‐diallyl‐N,N′‐dimethylammonium chloride) and the anionic monomers maleamic acid or butylmaleamic acid, and their influence on the inverse micellar region of the quaternary system sodium dodecylsulfate/toluene‐pentanol (1:1)/water has been investigated. The polymers increase the water solubilization capacity of the microemulsion at a polymer concentration ≥5%. However, the polyampholyte‐modified inverse microemulsions show a temperature dependent behavior, by the formation at 40°C of a narrow bicontinuous channel between the L2 (w/o) and the L1 (o/w) microemulsion phases. In this case, hydrophobic interactions between the surfactant alkyl tails and the hydrophobic side chains of the copolymer play an important role, too. The reverse microemulsion droplets were used as a template for the synthesis of BaSO4 nanoparticles. The polymers, which are involved in the redispersion process, influence the size and the stability of the nanoparticles formed by preventing their aggregation. Finally, monodisperse BaSO4 nanoparticles with an average size of 5 nm, thus, can be recovered and characterized by dynamic light scattering, zeta potential measurements, and transmission electron microscopy.
Introduction
Microemulsions are transparent, isotropic, thermodynamically stable dispersions of two immiscible fluids, commonly water and oil, stabilized by surfactant molecules.Citation1–3 Four component systems consisting of surfactant, cosurfactantCitation4 (generally a short chain linear alcohol), oil and water have many important features and can form normal micellar oil‐in‐water [o/w], as well as reverse micellar water‐in‐oil [w/o] or bicontinuous phases. One important feature of water‐in‐oil microemulsions is their abilility to solubilize guest molecules such as polyelectrolytes or inorganic precursor salts.
In recent years, the use of microemulsions for the synthesis of nanoparticles has received considerable attention:Citation5–9 the water droplets can indeed act as microreactors for the nucleation and growth of the particles, and the size of the aqueous core should control the final size of the nanoparticles. The extremely small size of these particles opens new fields of application such as catalysts,Citation10 Citation11 semiconductors,Citation12 Citation13 electrooptical devices,Citation14 or advanced ceramics.Citation15 However, in most cases the stability of the surfactant film is not strong enough to avoid the continuous growth of the particles out of the droplets. Another problem is the recovery of the nanoparticles from the reverse micelles avoiding a particle aggregation.
A topic that has thus found an increasing interest is the incorporation of non‐charged or charged polymers into microemulsionsCitation16–20 and their influence on the control of particle size and stability.Citation21–24 It is well established that the added polymer can influence the droplet size,Citation25 the droplet‐droplet interactions,Citation26 as well as the stability of the surfactant filmCitation27 by the formation of polymer‐surfactant complexes. One further advantage of the polymer‐modified microemulsions is the possibility to stabilize the primary formed nanoparticles via a polymer adsorption (steric or electrostatic stabilization).Citation28 Citation29 Finally, the polymer‐modified‐nanoparticles can be redispersed without flocculation after solvent evaporation according to the procedure described in Koetz et al.Citation30
The aim of the present article was to examine the influence of hydrophobic side chains of new polyampholytes on the phase behavior as well as the properties of the polyampholyte‐modified microemulsions. For that reason we synthesized copolymers of a cationic monomer (N,N′‐diallyl‐N,N′‐dimethylammonium chloride) with maleamic acid and butylmaleamic acid respectively. Finally, the polyampholyte‐modified microemulsions were used as new templates for the BaSO4 nanoparticles formation. After solvent evaporation the polyelectrolyte‐stabilized particles were examined by dynamic and electrophoretic light scattering in combination with transmission electron microscopy.
Experimental
Materials
Pentanol (99+%), toluene (99+%), sodium dodecylsulfate (SDS), maleic anhydride, butylamine, and sodium hydroxyde were obtained from Fluka and were used without further purification. Barium chloride (BaCl2) and sodium sulfate (Na2SO4) were purchased from Merck. N,N′‐diallyl‐N,N′‐dimethylammonium chloride and maleamic acid were supplied by Sigma‐Aldrich and used as received.
Water was purified with the Modulab PureOne water purification system (Continental).
Monomer Synthesis
N‐Butylmaleamic Acid Citation31
One mole of maleic anhydride is dissolved in 100 ml toluene in a three‐neck flask fitted with a condenser, and a magnetic stirrer and heated at 80°C. One mole 4‐butylamine in 70 ml toluene is added dropwise to the solution under stirring and the reaction was heated at 80°C for 3 hours. After cooling down the reaction mixture, a white precipitate is observed. The product is then filtered off, washed with toluene, and twice recrystallized in a methanol‐water (1:1) mixture (50 g/100 mL). The yield of the reaction is 55%. The composition of the monomer is verified by 300 MHz 1H NMR (Bruker AMX‐300) in DMSO‐d6, δ (ppm): 0.9 (t, 3H); 1.3 (sextet, 2H); 1.45 (quintet, 2H); 3.15 (quart, 2H); 6.25 (d, 1H); 6.4 (d, 1H); 9.1(s, 1H). The purity is checked by elemental analysis. Calculated (C8H13NO3): C 56.13%; H 7.65%; N 8.18%. Found: C 55.81%; H 8.21%; N 8.18%.
Polymer Synthesis
Poly‐(N,N′‐dially‐N,N′‐dimethylammonium‐alt‐maleamic carboxylate), PalH
The preparation of the polymer PalH (), is similar to a procedure described earlier in the literature.Citation32 Citation33 A free radical polymerization is carried out with the aqueous solutions of the monomers, at pH 8.5 adjusted by using sodium hydroxyde and at 60°C for a period of 16 hours. The reaction is realized in a nitrogen atmosphere and with the 4,4′‐azobis‐(4‐cyano‐pentanoic acid) (V501, Wako) as an initiator (concentration 0.02 mol/L). PalH obtained is purified by ultrafiltration and freeze‐dried. The yield of the reaction is approximately 38%.
1H NMR (300 MHz, D2O, δ in ppm): 1.0–;2.9 (8H, ‐CH‐, ‐CH2‐); 3.0–3.5 (6H, N‐CH3‐); 3.6–3.9 (4H, N‐CH2‐). Elemental analysis (in %): Calculated (C12H20N2O3): C 59.98; H 8.39; N 11.66. Found: C 55.75; H 9.55; N 10.23.
The molecular weight of PalH is determined from viscosity data by using the molecular weight dependency of the intrinsic viscosity, according to the Mark‐Houwink equation.Citation33 Citation34 It has to be noted that this is a relative method resulting in an apparent value of Mv≈22,000 g/mol for the polyampholyte PalH.
Poly‐(N,N′‐dially‐N,N′‐dimethylammonium‐alt‐N‐butyl‐maleamic carboxylate), PalBu
The procedure for the synthesis and purification of the polymer PalBu () is the same as for PalH. The yield of the reaction is about 40%.
1H NMR (300 MHz, D2O, δ in ppm): 0.8–4.0 (27H, ‐CH‐, ‐CH2‐, ‐CH3). Elemental analysis (in %): Calculated (C16H28N2O3): C 64.84; H 9.52; N 9.45. Found: C 60.20; H 10.62; N 8.71.
The molecular weight of PalBu is Mv≈22,000 g/mol.
Methods
Phase Diagrams
The isotropic phase was determined optically by titrating the oil‐alcohol/surfactant mixture with water or the corresponding aqueous polyelectrolyte solution at different temperatures (22°C, 30°C, and 40°C) by using a thermostated bath. At first, a mixture of the surfactant, oil, alcohol, and water (or the aqueous polymer solution) was shaken or treated in an ultrasonic bath until the system became optically clear. The area of the isotropic phase is determined by the dropwise addition of water (or the aqueous polymer solution) to the system.
Conductivity Measurements
The conductivity was measured with a microprocessor conductivity meter (LF 2000, WTW) at 22°C by using a Haake B3 thermostat.
Rheology
The rheological properties of the mixtures were investigated with a low‐stress rheometer (LS 100, Paar Physica) by using a Couette geometry. The samples were maintained at a constant temperature (±0.5°C) using a Haake B3 thermostat. Shear viscosities as a function of the shear rate were measured in the shear rate range between 1–1000 s−1.
Nanoparticle Formation
Two separately prepared microemulsions containing 10 mmol/L of BaCl2 and 10 mmol/L of Na2SO4 respectively, were mixed together. After shaking, BaSO4 nanoparticles were formed spontaneously in the inverse microemulsion droplets. Afterward, the mixture was dried in a vacuum oven at a temperature of 30°C for 48 hours to remove the solvents (water, toluene, and pentanol). The remaining powder was redispersed in water (0.5 wt%) with an ultrasound finger and characterized by means of dynamic and electrophoretic light scattering.
Dynamic Light Scattering
To determine the particle size and particle size distribution, dynamic light scattering measurements were carried out at 25°C at a fixed angle of 173° by using a Nano Zetasizer 3600 (Malvern) equipped with a He‐Ne laser (4 mW) and a digital autocorrelator.
Zeta Potential Measurements
Electrophoretic light scattering was used for measuring the surface charge of the nanoparticles. This technique allows the determination of the zeta potential of the nanoparticles at the effective shear plane between the moveable and non‐moveable part of the double layer. Measurements were carried out with a Zetasizer 4 (Malvern) at a fixed angle of 90°.
Transmission Electron Microscopy (TEM)
The morphology and size of the BaSO4 particles were determined by transmission electron microscopy (EM 902, Zeiss). Samples were prepared by dropping a small amount of the redispersed BaSO4‐solutions on copper grids, dried, and examined in the transmission electron microscope at an acceleration voltage of 90 kV.
Ultra‐High Resolution Scanning Electron Microscopy (Cryo‐SEM)
The structure of the microemulsions were examined by Ultra‐high resolution scanning electron microscopy (S‐4800, Hitachi). Samples were frozen in liquid nitrogen. Afterward, the sample were freeze‐fractured at −97°C using standard procedures and transferred into the cryo‐SEM.
Results
Polyelectrolyte‐Modified Systems
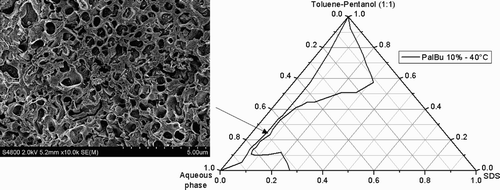
The oil component of the system is a toluene‐pentanol mixture 1:1 (volume ratio). The partial phase diagram of the quaternary system SDS/toluene‐pentanol (1:1)/water shows at 22°C an optically clear area in the oil corner, which can be related to the formation of a water in oil microemulsion (L2 phase or inverse swollen micelles), as well as in the water corner, which can be related to an oil in water microemulsion (L1 phase) (see ). The L2 phase was examined by Cryo‐SEM, and the micrograph in reveals individual droplets of water as expected.
Fig. 2. Partial phase diagrams at 22°C of the quasi‐ternary system SDS/toluene‐pentanol (1:1)/water in presence of different PalH concentrations. Cryo‐SEM micrograph of the L2 phase without polymer.
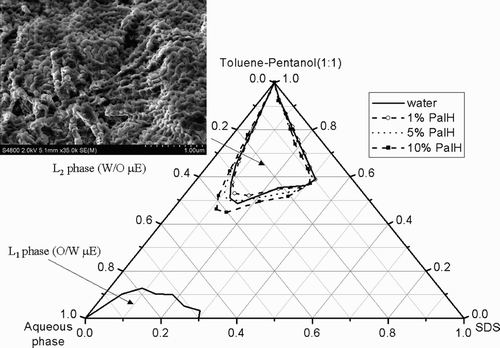
When water is substituted by a 1% wt PalH‐solution, a slight reduction of the initial inverse microemulsion area is observed (). However at a polymer concentration of 5%, the L2 phase is slightly increased. For 10% polymer, the L2 phase is significantly enlarged and the maximum of water solubilization is shifted from 36.3% to 42.2% at a constant surfactant concentration.
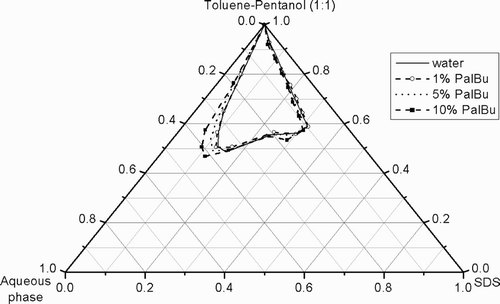
In comparison to that, the partial phase diagrams were examined by replacement of the water with aqueous PalBu‐solutions (). At a polymer concentration ≥5%, an extension of the L2 phase is observed. At 10% polymer solution, the maximum of water solubilization in the oil continuous microemulsion is shifted to 41.5% at a constant surfactant concentration.
Fig. 3. Partial phase diagrams at 22°C of the quasi‐ternary system SDS/toluene‐pentanol (1:1)/water in presence of different PalBu concentrations.
For both polymer‐modified microemulsions, the increase of the water solubilization capacity can be related to an increase of the bending elasticity of the surfactant film due to weak Coulombic interactions between the cationic functional groups of the polyampholyte and the anionic head groups of the surfactant.
Influence of the Temperature on the Phase Behavior
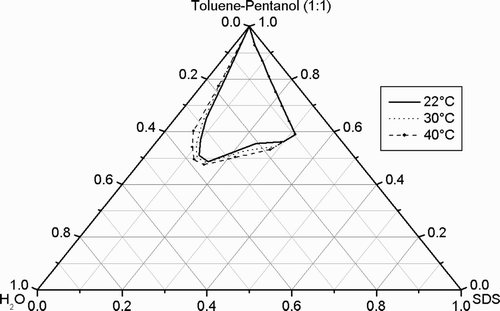
When the temperature of the unmodified system was increased from 22°C to 30°C, no significant change of the microemulsion area can be observed, but a further increase of the temperature to 40°C induces a slight widening of the L2 phase (). Broadly, when water was substituted by a 1% polymer solution no significant changes were observed.
Fig. 4. Partial phase diagram of the quasi‐ternary system SDS/toluene‐pentanol (1:1)/water at different temperatures.
With the incorporation of the polyelectrolyte PalH in the microemulsion, the effect of the temperature becomes more significant (). Indeed, for 5 wt% polymer, it is noticeable that the L2 phase is extended toward the water corner when the temperature increases (). Noteworthy, at 40°C and for a polymer concentration of 10% (), it becomes possible to incorporate in the microemulsion droplets until 46.1% of aqueous solution, content which represents an increase of 9.8% compared to the unmodified system.
Fig. 5. Partial phase diagrams of the quasi‐ternary system SDS/toluene‐pentanol (1:1)/water in presence of different PalH or PalBu concentrations, and at different temperatures.
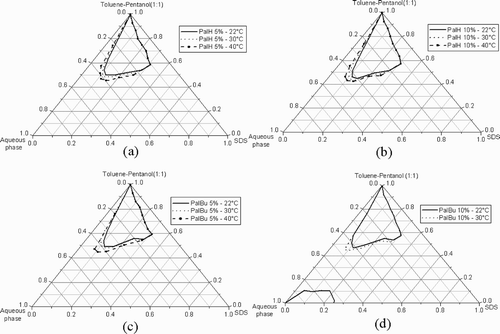
Concerning the PalBu‐modified microemulsion, the isotropic phase range also is affected by the increase of temperature. At 40°C with a polymer concentration of 5 wt%, the part of incorporated water is already at 44.6% ().
When the PalBu concentration is increased to 10%, the maximum of solubilization reaches 46.7% at 30°C (), that means 10.4% more than for the unmodified system.
Surprisingly, at 40°C and at a polymer concentration of 10%, the polymer PalBu induces the formation of a phase channel (). Starting from the L2 phase, the formation of the phase channel can be explained by an increase of the water solubilization capacity of the inverse swollen micelles, combined by a change of the spontaneous curvature of the surfactant film to zero. The Cryo‐SEM micrograph in reveals the bicontinuous structure of the studied microemulsion in the channel between the L1 and the L2 phases. The differences with the micrograph of the L2 phase in are notable. Similar results were observed by our group by adding poly(N‐vinyl‐2‐pyrrolidone)Citation35 or poly(ethylene glycol)Citation36 to the SDS/Xylene‐pentanol/water system, or by Bellocq by adding poly(ethylene glycol) into the AOT/isooctane/water system.Citation37
L2‐Phase Characterization
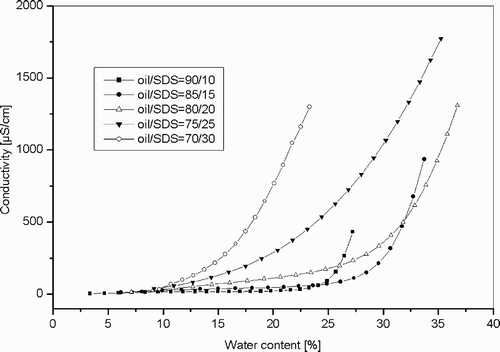
The L2 phase, as an oil‐continuous microemulsion with spherical water droplets, can be characterized by conductivity measurements because of the low conductivity of the oil phase and the high sensitivity with regard to structural changes, that is, the dynamics and coalescence of droplets.
As seen in , the conductivity of the non modified system remains first constant when water is added. A further increase of the water content was accompanied by a steep increase of the conductivity. This jump in the conductivity is characteristic for the percolation of charges through the droplets (dynamic processes of temporary cluster formation).
A boundary of percolation can thus be determined for the system SDS/toluene‐pentanol/water as a function of the mixture composition (). The shift of the percolation boundary was investigated in presence of the two copolymers PalH and PalBu, at 1% and 10% ().
Fig. 8. Boundary of percolation for the system SDS/toluene‐pentanol (1:1)/water depending on the polymer added and its concentration.
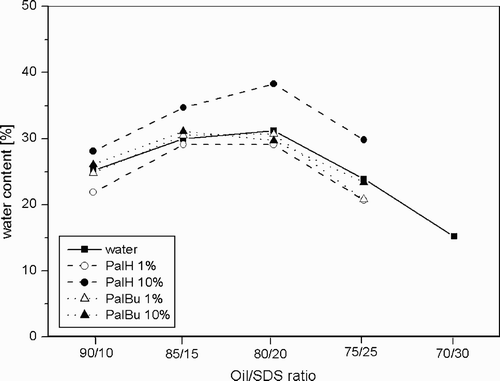
First of all, it has to be mentioned that the percolation still exists in presence of polyelectrolytes. Futhermore, the polymer PalBu does not affect the percolation phenomena between the microemulsion droplets and no change or shift can be (within the experimental error) noticed even if the concentration of PalBu is increased to 10%.
When the 1% PalH solution was incorporated into the system, no significant change can be observed. However, if the concentration is increased to 10%, the results show a shift of the percolation boundary to higher water contents. For the oil/SDS ratio 80/20, a maximum shift of 7% can be measured in comparison to the unmodified system.
For a more comprehensive discussion, the conductivity measurements were accompanied by rheological measurements. These could provide information about the interaction of the microemulsion surfactant film with the polyelectrolytes. Four isotropic mixtures, samples A, B, C, and D (see ) have been studied in much more detail by means of shear experiments.
Table 1 Composition for samples A–D
All systems investigated, that is, the nonmodified and polyelectrolyte‐modified microemulsions show a Newtonian flow behavior.
Our rheological data show for the unmodified system that the shear viscosity increases from B to D (). However, the weight ratio Rw (water/surfactant) is increased from B→D, and the surfactant concentration is constant. Therefore, the shear viscosity increase with increasing water content can be related to the increasing droplet size and/or droplet‐droplet interactions.
Fig. 9. Shear viscosities of the different polyelectrolyte modified microemulsions as a function of the water content.
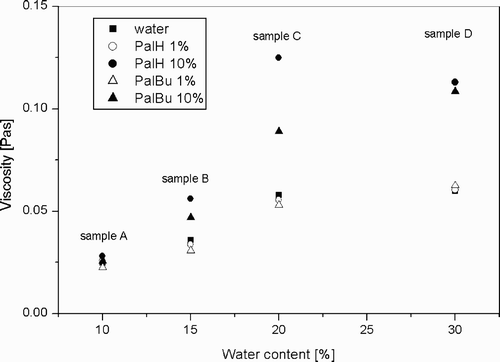
At the points A, B and C, Rw is constant (see ), but the content of the aqueous solution is increased in the order: A→B→C. Under these conditions, one can expect a constant droplet size but increasing droplet number. The experimental data () show for the unmodified system that the shear viscosity is twice as large at higher water contents. This can be explained by a simple concentration effect (i.e., increase of the number of microemulsion droplets with an approximately uniform size).
When 1 wt% of polymer is incorporated into the microemulsion, no change of the shear viscosity is observed, whatever the polymer added. But, at a polymer concentration of 10 wt% a significant increase of the shear viscosity has to be mentioned for samples B, C, and D. For instance sample C with 10% PalH shows a viscosity twice as high as the sample without polymer.
At higher polymer concentrations, one can assume that the polymers interact with the surfactant film, which leads to an increase of the droplet‐droplet interactions and, hence, to an increase of the shear viscosity.
Nanoparticle Formation
Polyelectrolyte‐modified microemulsions seem to be interesting template phases for the nanoparticle formation. Indeed, the incorporation of special polymers into the L2 phase can affect the nanoparticle formation process as follows:
increase of the film stability of the droplets due to polyelectrolyte‐surfactant interactions | |||||
control of the nanoparticle growth process due to polyelectrolyte‐nanoparticle interactions | |||||
stabilization of the nanoparticles formed due to electrosteric stabilization generated by the polyelectrolyte | |||||
Our interest was now focused on the effect of the zwitterionic polyelectrolytes PalH and PalBu, varying in the hydrophobicity, with regard to the formation of BaSO4 nanoparticles. For this process, two adequate microemulsions respectively containing BaCl2 and Na2SO4 were mixed together. A more detailed description of the BaSO4 particle formation process previously was described by our group in Koetz et al.Citation29 Due to the spontaneous collision of the microemulsions droplets, BaSO4 particles are formed and can be directly characterized in solution by dynamic light scattering and after solvent evaporation by transmission electron microscopy. The formation of nanoparticles was investigated for three different microemulsion compositions (points A, B, and C; see ) in the unmodified system as well as in the 10 wt% polyelectrolyte‐modified systems.
The dynamic light scattering experimental results listed in reveal that the nanoparticles formed have a very small average size, around 5 nm for points A and B, independently of the system used. For the point C, one can observe smaller BaSO4 particles, around 2 nm in presence of PalH and 3.2 nm in presence of PalBu, in comparison to 3.9 nm observed for the unmodified system.
Table 2 Results of dynamic light scattering experiments
In a second step, the microemulsions containing the BaSO4 nanoparticles were dried in a vacuum oven at 30°C to remove the solvents. The crystalline powders obtained were then redispersed in water and the dispersions were characterized again by dynamic light scattering () in combination with transmission electron microscopy.
From the unmodified microemulsion, much larger particle aggregates in the order of 100–350 nm are observed. When the samples are filtered with a 200 nm filter, smaller nanoparticles of about 3 nm size can be measured, in addition to bigger aggregates. These results show that the primary particles formed in the microemulsion tend to aggregate during the drying process and are not sufficiently stabilized.
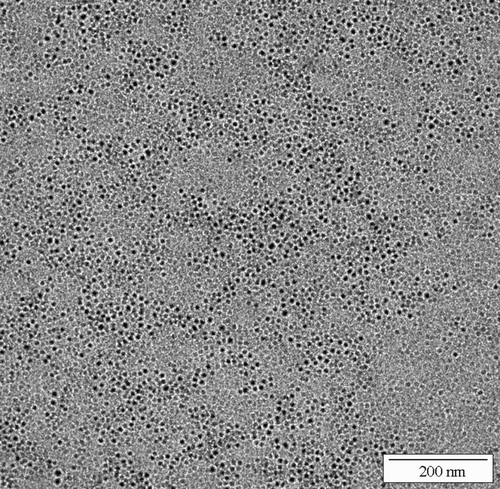
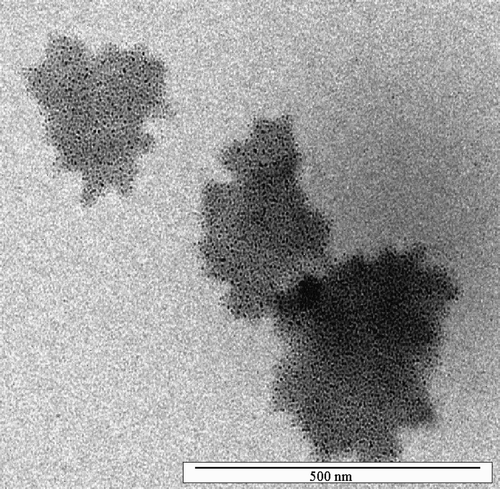
On the other hand, a significant decrease of the particle size can be induced by adding polyelectrolytes to the system. Indeed, in presence of PalH or PalBu, the primary particles do not aggregate during the drying process and small nanoparticles of about 4 nm can be redispersed (). In good agreement, the TEM micrographs show small BaSO4 particles with an average particle size of 5 nm, obtained in the PalH‐modified system () and in the PalBu‐modified system (). However, there is no significant difference between the two polymers studied here with regard to their properties as stabilizer.
In addition, the BaSO4 nanoparticles were studied by means of electrophoretic light scattering to measure the electrokinetic potential (zeta potential) at the particle surface. The particles obtained from the unmodified system reveal a negative zeta potential of about −43 mV. Hence, the surface charge of the particles is dominated by the adsorption layer of the anionic surfactant.
The results obtained for the PalH‐modified‐particles show a higher zeta potential of about −32 mV. In this case, one can assume that the polymer, adsorbed at the particle surface, tends to decrease the negative potential of the particles surface. Concerning the copolymer PalBu, the same conclusion can be made from the zeta potential of −37 mV.
This means, the nanoparticles produced are stabilized due to the adsorption of the polyampholyte.
Conclusion
Our investigations have revealed that an optically clear region of the phase diagram in the SDS/toluene‐pentanol (1:1)/water system still exists after incorporating the polyampholytes PalH and PalBu. In general, the copolymers induce an extension of the isotropic phase in direction of the water corner, enforced by increasing the concentration of the polymer. This effect can be explained by a change in the bending elasticity of the surface film induced by Coulombic interactions between the functional groups of the polyelectrolyte and the surfactant head groups.
The copolymers added have a quite similar effect on the L2 phase at room temperature (22°C), but a different behavior at higher temperature. The incorporation of small hydrophobic side chains (butyl group) along the copolymer backbone involves the formation of a bicontinuous channel when the temperature arises 40°C. Assumingly, PalBu, as hydrophobically modified copolymer, will tend to form microdomains at room temperature.Citation38 These microdomains disappear at higher temperature. In this case, in addition to the electrostatic interactions between the surfactant head group and the polymer, hydrophobic interactions between the surfactant tail and the hydrophobic side chains have to be taken into account. These additional hydrophobic‐hydrophobic interactions affect the surfactant film stability and increase the solubilization capacity of the microemulsion droplets, which is then combined with a change of the spontaneous curvature of the surfactant film to zero, followed by the formation of a bicontinuous phase channel.
One feature of the w/o microemulsion droplets (L2 phase) is their low viscosity and Newtonian flow behavior. The increase of the shear viscosity as a function of the polymer concentration confirms well the assumption of polyelectrolyte‐stuffed inverse microemulsion droplets. Moreover, the PalH induces an increase of the viscosity of the mixture in comparison to PalBu. This can be explained by the fact that the polymer PalBu, involved in the formation of microdomains at room temperature, does not interact so strongly with the surfactant film as PalH.
Another interesting feature of the SDS/toluene‐pentanol (1:1)/water inverse microemulsions is their high conductivity due to percolation phenomena. The percolation still exists in presence of polyelectrolytes. It also is notable that the polymer PalH at a concentration of 10% delays the percolation of the droplets. This can be explained by a modification of the interfacial film due to the presence of the polymer and by an increase of the repulsion between the droplets. However, the polymer PalBu does not affect the percolation, which can be explained again by the formation of microdomains.
Finally we show that the SDS‐based microemulsions can be successfully used as reactors for the BaSO4 particle formation. After solvents evaporation, the particles can be redispersed in water. In presence of polymers, aggregation phenomena, occurring during the drying‐process, are well prevented, and monodisperse BaSO4 nanoparticles with an average size of 5 nm can be obtained. The copolymers were indeed involved in the nanoparticles formation by increasing the film stability of the microemulsions droplets due to polyelectrolyte‐surfactant interactions, and by stabilizing the particles due to electrosteric stabilization, thus, preventing their aggregation. Moreover the zeta potential measurements confirmed the stabilization of the nanoparticles produced due to the adsorption of the polyelectrolyte layer.
Further experiments should be focused on the characterization of the bicontinuous channel formed with PalBu and its use as template phase. In addition, new polyampholytes with longer hydrophobic side chains will also be synthesized in order to study the influence of the copolymer structure on the behavior of the microemulsion and with regard to their use as a template phase.
The authors thank Prof. A. Laschewsky, University of Potsdam & Fraunhofer Institut für Angewandte Polymerforschung (Golm), for providing access to his synthesis laboratories and for fruitful discussions on polymer synthesis. Dr. B. Tiersch and Frau S. Rüstig,University of Potsdam, are acknowledged for the TEM and Cryo‐SEM micrographs.
References
- Langevin , D. 1988 . Microemulsions . Acc. Chem. Res. , 21 ( 7 ) : 255 – 260 .
- Prince , L. M. 1997 . Microemulsions Edited by: Prince , L. M. New York : Academic .
- Holmberg , K. 1998 . Micelles, Microemulsions and Monolayers Edited by: Shah , O. New York : Marcel Dekker .
- Stilbs , P. , Rapacki , K. and Lindman , B. 1983 . Effect of alcohol cosurfactant length on microemulsion structure . J. Colloid Interface Sci. , 95 : 585
- Barnickel , P. , Wokaun , A. , Sager , W. and Eicke , H. F. 1992 . Size tailoring of silver colloids by reduction in w/o microemulsions . J. Colloid Inter. Sci. , 148 ( 1 ) : 80
- Curri , M. L. , Agostiano , A. , Catalano , M. and Chiavarone , L. 2000 . Synthesis and characterization of CdS nanoclusters in a quaternary microemulsion: the role of the cosurfactant . J. Phys. Chem. B , 104 : 8391 – 8397 .
- Lin , J. , Zhou , W. and O'Connor , C. J. 2001 . Formation of ordered arrays of gold nanoparticles from CTAB reverse micelles . Mater. Lett. , 49 : 282 – 286 .
- Chen , F. , Xu , G. Q. and Hor , A. 2003 . Preparation and assembly of colloidal gold nanoparticles in CTAB‐stabilized reverse microemulsion . Mater. Lett. , 57 : 3282 – 3286 .
- Capek , I. 2004 . Preparation of metal nanoparticles in water‐in‐oil (w/o) microemulsions . Adv. Coll. Int. Sci. , 110 : 49 – 74 .
- Beck , D. D. and Siegel , R. W. 1992 . The dissociative adsorption of hydrogen sulfide over nanophase titanium dioxide . J. Matter. Res. , 7 : 2840
- Mayer , A. B.R. and Mark , J. E. 1997 . Transition metal nanoparticles protected by amphiphilic block copolymers as tailored catalyst systems . Colloid Polym. Sci , 275 : 333 – 340 .
- Moffitt , M. and Eisenberg , A. 1995 . Size control of nanoparticles in semiconductor‐polymer composites. 1. Control via multiplet aggregation numbers in styrene‐based random ionomers . Chem. Mater. , 7 : 1178 – 1184 .
- Holmes , J. D. , Bhargava , P. A. , Korgel , B. A. and Johnston , K. P. 1999 . Synthesis of cadmium sulfide Q particles in water‐in‐CO2 microemulsions . Langmuir , 15 : 6613
- Brus , L. 1991 . Quantum crystallites and nonlinear optics . Appl. Phys. A , 53 : 465
- Herrig , H. and Hempelmann , R. 1996 . A colloidal approach to nanometre‐sized mixed oxide ceramic powders . Mater. Lett. , 27 : 287
- Bagger–Jorgensen , H. , Olsson , U. , Iliopoulos , I. and Mortensen , K. 1997 . A nonionic microemulsion with adsorbing polyelectrolyte . Langmuir , 13 : 5820
- Lianos , P. 1996 . Comment on the effect of addition of water‐soluble polymers in water‐in‐oil microemulsions made with anionic and cationic surfactants . J. Phys. Chem. , 100 : 5155
- Plucinski , P. and Reitmeir , J. 1997 . The interactions between polyelectrolytes and AOT in an oil/water system . Coll. Surf. A , 122 : 75
- Beitz , T. , Koetz , J. and Friberg , S. E. 1998 . Polymer‐modified ionic microemulsions formed in the system SDS/water/xylene/pentanol . Progr. Colloid Polym. Sci. , 111 : 100 – 106 .
- Note , C. , Koetz , J. and Kosmella , S. 2006 . Influence of hydrophobically modified polyelectrolytes on CTAB‐based w/o microemulsions . Coll. Surf. A , 283 : 1334 – 1342 .
- Jacobs , B. , Sottmann , T. , Strey , R. , Allgaier , J. , Willner , L. and Richter , D. 1999 . Amphiphilic block copolymers as efficiency boosters for microemulsions . Langmuir , 15 : 6707 – 6711 .
- Beitz , T. , Koetz , J. , Wolf , G. , Kleinpeter , E. and Friberg , S. E. 2001 . Poly(N‐vinyl‐2‐pyrrolidone) and 1‐octyl‐2‐pyrrolidone modified ionic microemulsions . J. Coll. Interf. Sci. , 240 : 581 – 589 .
- Lang , J. 1996 . Reply to comment on the effect of addition of water‐soluble polymers in water‐in‐oil microemulsions made with anionic and cationic surfactants . J. Phys. Chem. , 100 : 5156
- Maugey , M. and Bellocq , A. M. 1999 . Effect of added salt and poly(ethylene glycol) on the phase behavior of a balanced AOT‐water‐oil system . Langmuir , 15 : 8602
- Suarez , M. J. , Levy , H. and Lang , J. 1993 . Effect of addition of polymer to water‐in‐oil microemulsions on droplet size and exchange of material between droplets . J. Phys. Chem. , 97 : 9808
- Gonzalez–Blanco , C. , Rodriguez , L. J. and Velazquez , M. M. 1997 . Effect of the addition of water‐soluble polymers on the structure of aerosol OT water‐in‐oil microemulsions: a Fourier transform infrared spectroscopy study . Langmuir , 13 : 1938
- Meier , W. 1996 . Poly(oxyethylene) adsorption in water‐in‐oil microemulsions: a conductivity study . Langmuir , 12 : 1188
- Koetz , J. , Saric , M. , Kosmella , S. and Tiersch , B. 2004 . Influence of polyelectrolytes on lecithin‐based w/o microemulsions and BaSO4‐nanoparticle formation . Progr. Colloid Polym. Sci. , 129 : 1 – 10 .
- Koetz , J. , Bahnemann , J. , Lucas , G. , Tiersch , B. and Kosmella , S. 2004 . Polyelectrolyte‐modified microemulsions as new templates for the formation of nanoparticles . Coll. Surf. A: Phys. Eng. Aspects , 250 : 423 – 430 .
- Koetz , J. , Bahnemann , J. , Kosmella , S. and Peter , M. 2004 . WO 2004/056928 A3
- Coleman , L. E. , Bork , J. F. and Dunn , H. 1959 . Reaction of primary aliphatic amines with maleic anhydride . J. Org. Chem. , 24 ( 1 ) : 135 – 136 .
- Hahn , M. , Jaeger , W. , Schmolke , R. and Behnisch , J. 1990 . Synthesis of regular polyampholytes by copolymerization of maleic acid with allyl and diallyl amine derivatives . Acta Polym. , 41 : 107 – 112 .
- Thünemann , A. F. , Sander , K. and Jaeger , W. 2002 . Polyampholyte‐dressed micelles of fluorinated and hydrogenated dodecanoic acid . Langmuir , 18 : 5099 – 5105 .
- Dautzenberg , H. , Jaeger , W. , Koetz , J. , Philipp , B. , Seidel , Ch. and Stscherbina , D. 1994 . “ Electrochemical and spectroscopic characterization of polyelectrolytes ” . In Polyelectrolytes: Formation, Characterization and Application Edited by: Dautzenberg , H. , Jaeger , W. , Koetz , J. , Philipp , B. , Seidel , Ch. and Stscherbina , D. Munich : Carl Hanser Verkag .
- Koetz , J. , Beitz , T. , Kosmella , S. and Tiersch , B. 2001 . Polymer-modified microemulsions . Proceedings CESIO 5th World Surfactant Congress Firenze (Italy) , 1 : 499 – 506 .
- Koetz , J. , Andres , S. , Kosmella , S. and Tiersch , B. 2005 . BaSO4 nanorods produced in polymer-modified bicontinuous microemulsions . Composite Interface , 3 ( 4–6 ) : 461 – 475 .
- Bellocq , A. M. 1998 . Phase equilibria of polymer‐containing microemulsions . Langmuir , 14 : 3730
- Strauss , U. P. and Chiao , Y. 1986 . Hydrophobic polyampholytes . Macromolecules , 19 : 355 – 358 .