Further studies by the authors on the work presented in “Oxidative Copolymerization of Aniline with o- and p-Nitroaniline by Ammonium per Sulfate: Kinetic and Pathway” by M. Rashid and Suhail Subhiar, which was published the Journal of Dispersion Science and Technology, volume 30, number 3, pages 297–304, did not produce matching UV and IR spectral data.
The authors have provided the following updated figures and additional reference to clarify the new-found data.
3.3. FTIR Spectral Studies
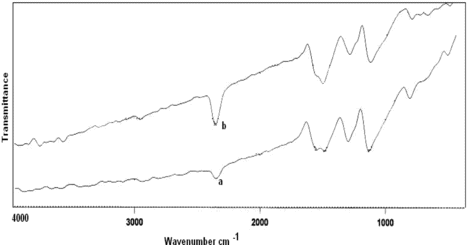
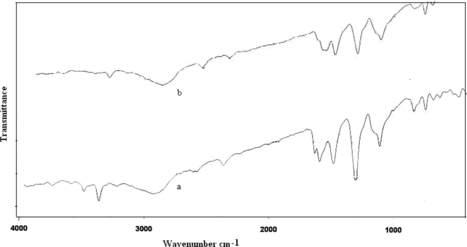
FTIR spectra of poly(A-co-ONA) 1:1 and 1:2 (curve a, b) is shown in Figure . It is clear that the distinctive peaks corresponding to C=C stretching vibrations of quinoid rings appears at 1556 cm−1 and for benzenoid rings appears at 1479 cm−1. The mode at 1295 cm−1 is assigned as partly to C–N stretching and partly to the ring stretching vibrations. The band at 808 cm−1 is due to the N–H out of plane bending absorption. The stretching peaks of N–H appeared in the range of 3360–34500 cm−1. The peaks corresponding to 1295 cm−1 in poly(A-co-ONA) 1:1 and at 1290 cm−1 in poly(A-co-ONA) 1:2 are attributed to NO stretching vibrations due to NO2 groups. Figure (curve a, b) shows IR spectra of poly(A-co-PNA) 1:1, 1:2, respectively. The benzenoid and quinoid stretching vibrations of C=C appeared at 1489 cm−1 and 1582 cm−1, respectively. The strong peaks appeared to 1300 cm−1 and 1305 cm−1 are attributed to N–O stretching vibrations of NO2 groups. The peak near 2915 cm−1 is due to C–H stretching absorption. The broad region of 2915–3365 cm−1 is ascribed to N–H stretching vibrations in poly(A-co-PNA).
3.4. Electronic Spectral Studies
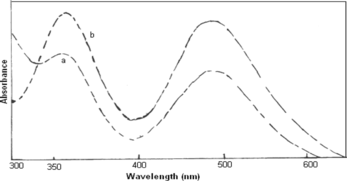
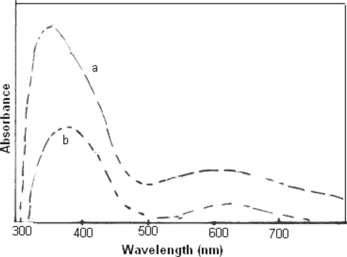
UV-vis absorption spectra of the protonated form of polyanilines dissolved in 1M HC1 (Figure , curve a) and deionized water (curve b) shows two main absorption, the benzenoid π−π* transition at 350 nm and the “exciton” transition at 490 nm.[21,22] The nitro group substitution on the benzene rings was responsible for the change in π electrons distribution in the phenyl rings. NO2 groups cause an increase in the band gap due to their strong electron-withdrawing tendency. Figure (curve a, b) shows UV-vis spectra of poly(A-co-ONA) 1:1, 1:2, dissolved in DMSO. The blue shift is observed in absorption band of π−π* of both the copolymers of poly(A-co-ONA) 1:1 and poly(A-co-ONA) 1:2. The blue shift in the absorption band of π−π* transition is due to the presence of NO2 substitution, which results in larger steric strain increasing the torsional angle between adjacent rings and also results to increase in the band gap.[22,23]
REFERENCE
- Ismail , Y.A. ( 2004 ) Studies on aniline based conducting polymers. Ph.D. Thesis, Aligarh Muslim University, Aligarh, India.