ABSTRACT
Chemicals are used extensively in modern societies. Evidence-based, updated safety information is necessary for using chemicals correctly and for protecting the health of humans and environment. This paper describes safety information life cycle of chemicals and highlights why it is important that individuals need to acquire critical toxicological information literacy skills to identify reliable information. Six competence standards of toxicological information literacy are defined according to the principles of the Association of College and Research Libraries. We also discuss the reliability of open access information sources.
Introduction
All humans should have access to reliable information about chemicals, either as individual substances or their mixtures (). Many kinds of chemicals are an essential part of our everyday life (ECHA Citation2021a). The public also views chemicals as manufactured substances. But natural substances are also chemicals; in fact, it does not make any difference whether the substance is man-made or originates from nature to a compound’s mechanisms and effects (Reeser Citation2013). Regulatory authorities issue permission before chemicals can be marketed. Information requirements about chemicals are defined in legislation, which is an essential tool for authorities when they regulate the presence, use, transport, and handling of chemicals on the market and ultimately their disposal.
Table 1. A list of 14 types of important regulated chemicals in societies. Their use in the national and global market is regulated according to the appropriate legislation at the national or worldwide level.
Three general information requirements issued by regulatory organizations must be fulfilled to obtain marketing permission; these are based on legislations about chemicals. The use of chemicals needs to be safe for humans, and they are not allowed to harm the environment. Secondly, the use of substances needs to have a justified purpose, i.e., the substances need to be effective. The third requirement is that the products should be of high quality. The required information of chemicals is through a collaboration by experts in many disciplines; it needs to be based on a systematic and comprehensive evaluation and it should be transparent so that the public can understand the process and discuss its validity to ensure that chemicals are used wisely and safely.
Chemical safety information is the sphere of expertise of toxicologists. The objective of toxicology is to increase safety in the world and to make the world a better place in which to live (Tuomisto Citation2006). Thus, reliable information is needed, and this extends to the ability to recognize non-reliable information. It is evident that people view what they think of as reliable information of chemicals will depend on their prejudices, level of education, and the culture in which they live, and this will be associated largely on their information literacy skills. In other words, they need to be able to recognize sources of reliable information and avoid non-reliable sites.
The aim of this paper is to describe, how safety information of chemicals is produced, and how primary data of safety studies with substances is transferred from experts to the public. In addition, it evaluates challenges to disseminate toxicological information. One challenge is recognizing reliable information when it is sourced from an open and non-controlled information network like today’s internet. Information literacy skills in toxicology are defined in our paper.
Safety information life cycle of substances
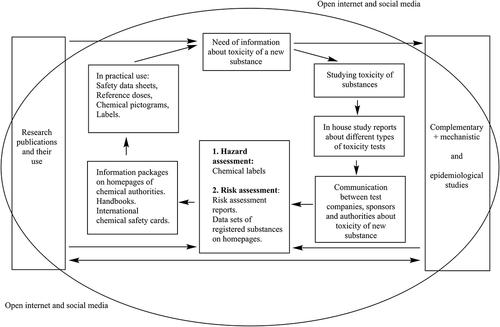
Traditional toxicology (science of poisons) has developed into a discipline concerned with the safety of substances either as individual compounds or their mixtures (Wexler and Hayes Citation2019). The type of safety information available differs on whether the chemical substances are new or are already on the market. A new chemical substance requires marketing authorization, and its toxic properties need to be systematically evaluated in a series of toxicity tests; these tests are stipulated in both national and global legislation. These include harmonized guidelines (OECD Citation2021a), following good laboratory practice (OECD Citation2021b) and good manufacturing practice (EMA Citation2021) in test laboratories according to the study plans of the substance’s sponsors. In addition, safety information is obtained from target-specific mechanistic and epidemiological studies. Systematic testing of old substances – from the present perspective – has been sometimes inadequate and complementary toxicity testing is required also for these materials. The nature of information emerging from toxicity tests is mostly descriptive, but it is useful for assessment a chemical’s toxicity or safety. With respect to new chemicals, the toxicity/safety testing programs vary according to the intended use of chemicals – are they medicines, pesticides, food additives, industrial chemicals, cosmetics, etc.?
The aim of safety information is to identify hazards and to undertake a risk assessment to ensure the safe use of chemicals and to implement risk management programs, if needed. An updated safety information protocol may generate the need for additional information about old chemical substances (). In the case of a new substance, its toxicity must be evaluated in a wide range of toxicity tests, which reveal different kinds of toxicities such as acute toxicity, skin and eye irritation and corrosion, sensitization, organ toxicity, reproductive toxicity, developmental toxicity, genotoxicity, carcinogenicity, or aspiration toxicity (ECHA Citation2021b; UNECE Citation2021). Usually, the manufacturing company itself does not conduct all of these tests in-house, instead some contract research organization (CRO) will perform the studies for the sponsoring company with the test details being transferred to the sponsor company. Reports of all toxicity tests are combined and assessed in processes of hazard or risk assessment. The outcomes of hazard assessment are not only hazard descriptions but can also be delivered as simple pictograms, hazard and precautionary statements in labeling. The outcome of risk assessment is a risk assessment report; this contains a description of the risks associated with the substance and reference dose for its safe use. This information is available on the homepages of authorities and delivered as safety data sheets.
All this information is necessary to obtain marketing authorization of a chemical; the information packages in which the data is stored is called a dossier. For example, chemical dossiers are used in the REACH process for evaluating chemical in the EU. This information is open and is used to construct data sets of registered substances, information packages, safety data sheets, and international chemical safety cards on homepages of chemical authorities and other interested parties or it may be appearing in handbooks and databanks. This information is provided to workers, customers, and other people in a variety of forms, e.g., as chemical pictograms and labels, safety data sheets in product packages, which make safe use possible, if the information is understood and the product is correctly used. There are other important outcomes of the risk assessment procedure, e.g., reference doses such as the calculation of allowable daily intake of food additives, derived no-effect level, occupational exposure limits, and dosing protocols of drugs. These are intended to protect users from adverse health effects of substances and to ensure that exposures are kept at a safe level ().
Information obtained in the standardized toxicity tests is largely descriptive; if one wishes to understand better a substance’s overall effects, then complementary studies are needed (). These may be related to clarifying its mechanism of action, or they may involve epidemiology. These are especially important for certain types of chemicals such as pharmaceuticals, biocides, and pesticides, which act via target molecules. Typically, these types of tests are conducted in universities and other research organizations undertaking basic research. Scientists present their results in conferences and write original research publications and review articles, which are peer-reviewed and published in the scientific forums. They are beneficial in clarifying and understanding the descriptive information emerging from toxicity tests and for also assessing the hazard and particularly the risks associated with the compound. This basic research information forms the supplementary cycle of safety information of chemicals and is intended to supplement the descriptive information from toxicity tests to the authorities and users of chemicals. Safety information life cycle of chemicals is an application of the original model of Subramanyam describing the evolution of scientific information (Subramanyam Citation1981) and the modified model of an information life cycle prepared by Curl (Curl Citation2001).
The safety information of many chemicals has become more accessible than earlier because it is freely and easily available on the internet. Although today there may be a vigorous debate about certain chemicals in social media channels, one must remember that lay-persons are not involved in producing the primary safety information of chemicals. Nonetheless, there is a dynamic interaction between social media and the other layers of the chemical’s scientific assessment, which is presented as the surrounding circle in . When evaluating information about some compound on social media, one needs to be able to judge whether the information is reliable or non-reliable. There are three types of non-reliable information, i.e., disinformation, misinformation, and malinformation (Pennycook and Rand Citation2021). Disinformation is information that is false or inaccurate and was created with a deliberate intention to mislead people. Quackery (pseudo medicines) (Cassileth and Yarett Citation2012; Leino et al. Citation2021) denying adverse health effects of smoking by the tobacco industry (Michaels Citation2008) and anti-corona vaccination campaigns are examples of disinformation. Misinformation is information that is false, inaccurate, or misleading. Unlike disinformation, misinformation has not necessarily been created deliberately to mislead. An incorrect interpretation of original data or results of safety studies by a trustworthy person may spread misinformation. Malinformation refers sensationalism and to some kind of exaggeration and embellishment, startling images, explicit content (e.g., uncommon adverse health effects publicized as the main property of a substance, exaggerating rare adverse effects), and other violations of accepted norms. Reliable information is particularly important if exposure to a health endangering chemical takes place leading to intoxications.
The value of information
The rapid evolvement of the digital realm of information, in other words open information to all who can use the internet, has changed the use and dissemination of health-related toxicity information (Benigeri and Pluye Citation2003; Jadad and Gagliardi Citation1998). The professional gatekeepers have lost their monopoly to use and interpret the scientific information for the everyday use of each citizen. The rapid growth of information – both factual and fanciful – have amplified this situation so that even toxicology professionals are faced with challenges of keeping up to date.
We have all become aware of the huge expansion of false information – some of it disseminated deliberately or others based on false information and/or expertise. Some authorities claim that this may be the most challenging, even life-threatening threat in the daily use of health information (Wu and McCormick Citation2018). The challenge here seems to be that expertise is needed in conducting, interpreting, and applying scientific results – that is always demanding and needs a long specialist education. The interpretation should always be based on a critical evaluation of the situation and how that information will be used or abused. One reason is that a lot of safety information about a chemical is obtained from animal and in vitro models and then applied to protect human beings.
Functioning in this type of operational environment needs first and foremost critically educated information literate citizens (Shanbhag Citation2006). Furthermore, it also demands an active participation from the researchers and other academic and professional bodies – including academic libraries – to disseminate science-based information on the internet. Information is a commodity that is utilized on a daily basis; human beings apply it in a classical sense when making plans or decisions and in this respect, they try to make sense also about what certain scientific results mean for their everyday lives (Wiebe Citation2015).
The traditional division in primary, secondary, tertiary information works fine in the academic and research type of environment, but one must bear in mind that humans always seek information that is easy to understand and apply. For example, scientific review articles strive to make a consensus summary of the present knowledge. This is because nobody can master all the knowledge produced even in a small field of expertise. One can even say that the internet has given a birth to quaternary type of information that may seem to be relevant but is based on falsifications, incorrect interpretations, or just personal opinions about how things should be in the natural world.
Information literacy in toxicology
An information literate person recognizes the need of information, finds it efficiently, can identify the difference between valuable and weak information, uses information appropriately, and is able to recognize the credentials of those who have produced the information. Toxicological information literacy can be defined more precisely by applying the former ALA information literacy standards (ALA ACRL Citation2000). In that sense, toxicologically literate persons have knowledge about toxicology and have learned to detect misinformation, disinformation, or malinformation of chemicals and combat the threat that they pose (). When they have mastered these information literacy skills, they can understand the behavior of people and interact creatively with individuals with opposing opinions and cope with difficult situations.
Table 2. Standards of toxicological information literacy. Six standards were modified according to the several discipline standards of ALA 2000 (ALA ACRL Citation2000).
These standards are useful tools in focusing the information management studies for both academics and students at the university level. They can even be used in the context of lay-person education on all levels where toxicological information literacy is needed to pinpoint the aspects to be studied in more detail.
Reliable open access information sources in toxicology
Conventional scientific publishing is a controlled process that starts at manuscript preparation and submission, continues to accepting (or rejecting) the manuscript and ends to database storage of the finished publication. Peer review has been universally employed in this process to ensure the scientific quality of publications (Azam Ali and Watson Citation2016).
Internet as a whole is not controlled publishing environment. There are no built-in quality assurance processes except some voluntary efforts like HONcode Certification (Health On the Net Citation2020). Therefore, it is essential that the information users critically evaluate the reliability of internet information sources such as websites before they are utilized. The evaluation criteria usually focus on the following aspects (Keselman et al. Citation2019; UC Berkeley Library Citation2021):
Authority
Purpose
Objectivity
Scientific argumentation
Vocabulary, rhetoric, and presentation
Relevance to user’s information need
Background sources used and cited
Being up-to-date
There is an abundance of reliable toxicological information sources available. The sources can be broadly categorized into books, scientific journals, databases, and websites of recognized operators (Wexler et al. Citation2020a, Citation2020b). A financial subscription is required to access many of the information sources, especially journals, which hinder access to potential users not affiliated to academia or other subscribing organizations.
Open access publishing is advantageous from many standpoints (Björk Citation2017; Elsabry Citation2017). One of the most important advantages is free access of publications for everyone. The open publishing model has gained increasing popularity. Today, there are more than 16,000 peer reviewed open access journals (DOAJ Citation2021). It is estimated that about 35% of all journal articles written in English are openly available (Fernanda Citation2020). There are also repositories that collect non-reviewed open materials such as manuscripts, preprints, and preliminary reports (Loan and Sheikh Citation2016). The disadvantage of open publishing is the appearance of so-called “predatory journals.” Their aim is mainly to defraud money from authors under the cover of the article processing charges. The quality of predatory journals is questionable (Wang, Pourang, and Burrall Citation2019). Recognized operators provide free and reliable information about all chemicals on their websites.
Conclusions
Reliable and comprehensible information about chemicals is the starting point for their safe use. Key characteristics of reliable information from open sources are authority, purpose, objectivity, scientific argumentation, relevance, and being up to date. Safety information life cycle of chemicals for regulatory purposes was provided in this paper. Toxicologists produce this information in collaboration with several disciplines, e.g., chemistry, biology, biochemistry, pharmacology, pathology, physiology, molecular biology, medicine, public health, and economics. Open information appears in many kinds of formats: databases, journals, books, websites of recognized operators and chemical-related information such as product labels and safety data sheets so that it can be utilized appropriately by a wide spectrum of users ranging from the public to experts and researchers. People should be aware of these reliable sources, avoid nonreliable information, and be assured that they have access to more reliable information than simply can be obtained by typing in some key word into a google-type search. Collaborations between toxicologists and library personnel during the education and later in career produce toxicologists who are updated information literate individuals. They benefit from the toxicological information literate skills by being competent and cooperative lifelong learners, finding information efficiently, appreciating the difference between valuable and weak information, and then using it ethically correctly in whatever task they are doing.
Acknowledgments
We thank Professor Markku Pasanen for valuable and constructive comments and Dr. Ewen MacDonald for the linguistic advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- ALA ACRL. 2000. Information literacy competency standards for higher education. Accessed May 26, 2021. http://hdl.handle.net/11213/7668.
- Azam Ali, P., and R. Watson. 2016. Peer review and the publication process. Nursing Open 3 (4):193–202. doi:10.1002/nop2.51.
- Benigeri, M., and P. Pluye. 2003. Shortcomings of health information on the internet. Health Promotion International 18 (4):381–86. doi:10.1093/heapro/dag409.
- Björk, B.-C. 2017. Open access to scientific articles: A review of benefits and challenges. Internal and Emergency Medicine 12 (2):247–53. doi:10.1007/s11739-017-1603-2.
- Cassileth, B. R., and I. R. Yarett. 2012. Cancer quackery: The persistent popularity of useless, irrational ‘alternative’ treatments. Oncology (Williston Park) 26 (8):754–58.
- Curl, S. R. 2001. Subramanyam revisited: Creating a new model for information literacy instruction. College and Research Libraries 62 (5):455–64. doi:10.5860/crl.62.5.455.
- DOAJ. 2021. DOAJ : The directory of open access journals. Accessed June 1, 2021. https://doaj.org/.
- ECHA. 2021a. Chemicals in our life. Accessed May 26, 2021. https://chemicalsinourlife.echa.europa.eu/.
- ECHA. 2021b. Understanding CLP. Accessed May 26, 2021. https://echa.europa.eu/regulations/clp/understanding-clp.
- Elsabry, E. 2017. Claims about benefits of open access to society (beyond academia). In Expanding Perspectives on Open Science: Communities, Cultures and Diversity in Concepts and Practices - Proceedings of the 21st International Conference on Electronic Publishing, 34–43. doi:10.3233/978-1-61499-769-6-34.
- EMA. 2021. Good manufacturing practice. Accessed May 26, 2021. https://www.ema.europa.eu/en/human-regulatory/research-development/compliance/good-manufacturing-practice.
- Fernanda, M. 2020. Is open access publication useful for all research fields? Presence of funding, collaboration and impact. Scientometrics 125 (1):689–716. doi:10.1007/s11192-020-03652-w.
- Health On the Net. 2020. HONcode certification. Accessed August 10, 2021. https://www.hon.ch/en/certification.html.
- Jadad, A. R., and A. Gagliardi. 1998. Rating health information on the internet: Navigating to knowledge or to babel? JAMA?: The Journal of the American Medical Association 279 (8):611–14. doi:10.1001/jama.279.8.611.
- Keselman, A., C. Arnott Smith, A. C. Murcko, and D. R. Kaufman. 2019. Evaluating the quality of health information in a changing digital ecosystem. Journal of Medical Internet Research 21 (2):e11129. doi:10.2196/11129.
- Leino, V., R. Airaksinen, M. Viluksela, and K. Vähäkangas. 2021. Toxicity of colloidal silver products and their marketing claims in Finland. Toxicology Reports 8:106–13. doi:10.1016/j.toxrep.2020.12.021.
- Loan, F. A., and S. Sheikh. 2016. Analytical study of open access health and medical repositories. The Electronic Library 34 (3):419–34. doi:10.1108/EL-01-2015-0012.
- Michaels, D. 2008. Doubt is their product : How industry’s assault on science threatens your health. New York: Oxford University Press.
- OECD. 2021a. OECD test guidelines for chemicals. Accessed May 7, 2021. https://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm.
- OECD. 2021b. Good laboratory practice (GLP). Accessed May 7, 2021. https://www.oecd.org/chemicalsafety/testing/good-laboratory-practiceglp.htm.
- Pennycook, G., and D. G. Rand. 2021. The psychology of fake news. Trends in Cognitive Sciences 25 (5):388–402. doi:10.1016/j.tics.2021.02.007.
- Reeser, D. 2013. Natural versus synthetic chemicals is a gray matter. Accessed May 26, 2021. https://blogs.scientificamerican.com/guest-blog/natural-vs-synthetic-chemicals-is-a-gray-matter/.
- Shanbhag, S. 2006. Alternative models of knowledge production: A step forward in information literacy as a liberal art. Library Philosophy and Practice, no. 2, University of Idaho Library. https://digitalcommons.unl.edu/libphilprac/71/.
- Subramanyam, K. 1981. Scientific and technical information resources. Books in library and information science . Vol. 33. New York, NY: Dekker.
- Tuomisto, J. 2006. Word to Eurotox (43th congress of the European societies of toxicology, Dubrovnik, September 20. Accessed May 26, 2021. https://www.mv.helsinki.fi/home/tammisol/Eurotox06.pdf.
- UC Berkeley Library. 2021. Evaluating resources. Accessed August 5, 2021. https://guides.lib.berkeley.edu/evaluating-resources.
- UNECE. 2021. About the GHS. Accessed May 26, 2021. https://unece.org/about-ghs.
- Wang, J. Z., A. Pourang, and B. Burrall. 2019. Open access medical journals: Benefits and challenges. Clinics in Dermatology 37 (1):52–55. doi:10.1016/j.clindermatol.2018.09.010.
- Wexler, P., and A. N. Hayes. 2019. The evolving journey of toxicology : A historical glimpse. In Casarett and Doull’s toxicology : The basic science of poisons, ed. C. D. Klaassen, 3–23. New York: McGraw-Hill Education.
- Wexler, P., S. Gilbert, A. Mohapatra, S. Bobst, A. Hayes, and S. Humes. 2020a. Information resources in toxicology : Volume 1: Background, resources, and tools. 5th ed. London: Academic Press.
- Wexler, P., S. Gilbert, A. Mohapatra, S. Bobst, A. Hayes, and S. T. Humes. 2020b. Information resources in toxicology : Volume 2: The global Arena. 5th ed. London: Academic Press.
- Wiebe, T. J. 2015. The information literacy imperative in higher education. Liberal Education 102 (1):52–57.
- Wu, J. T., and J. B. McCormick. 2018. Why health professionals should speak out against false beliefs on the internet. AMA Journal of Ethics 20 (11):1052–58. doi:10.1001/amajethics.2018.1052.