Abstract
Aims: To create a standardized home exercise therapy program that could be implemented by international sites to provide a consistent level of therapeutic intervention for pediatric patients participating in an ongoing Phase-III, randomized, controlled trial of repeat abobotulinumtoxinA injections for pediatric upper limb spasticity (NCT02106351). Methods: Physical therapists, occupational therapists, and medical doctors worked collaboratively to design an exercise therapy program to be implemented in the home setting. In this article, we describe the development process and the finalized program that is currently being used in the Phase-III trial. Results: The final program is presented as a “toolbox” for therapists, and includes a standardized step-wise process for choosing the most appropriate exercises and functional activities to achieve the agreed treatment goals of each abobotulinumtoxinA injection. The core toolbox includes: a clear protocol for clinicians, information sheets, signature of commitment forms, exercise score charts, and the library of exercises and functional activities that therapists choose from to aid the patient in achieving their treatment goals. Conclusions: Implementation of this home therapy program provides a standardized background of good practice against which to test the efficacy of abobotulinumtoxinA. Preliminary data show that the program is readily accepted by patients and their families.
Although cerebral palsy (CP) is considered “non-progressive” in terms of the original brain lesion, the underlying upper motor neuron syndrome causes progressive musculoskeletal pathology (Kerr Graham & Selber, Citation2003), ultimately resulting in reduced functional use of the limbs, deformity, and, in some children, a learned non-use of the affected limb (Hoare & Imms, Citation2004). Therefore, a key aim of therapy is to improve motor function, support motor development, and enable participation in age-appropriate activities (Strobl et al., Citation2015). With this aim in mind, it is generally well-accepted that occupational and/or physical therapy should form the foundation of long-term treatment plans for CP, because such treatment focuses on the development of skills necessary for the performance of activities for daily living and independence (Steultjens et al., Citation2004).
It has been estimated that between 50% and 70% of children with CP will have upper limb spasticity, with higher prevalence rates in children with hemiparesis (Colver, Fairhurst, & Pharoah, Citation2014; Santos et al., Citation2015). Botulinum toxin type-A (BoNT-A) is a commonly used pharmacologic option to reduce upper limb spasticity (Hoare, Citation2014; Strobl et al., Citation2015), and accumulating evidence now suggests that long-term BoNT-A treatment is more effective when combined with a physical/occupational therapy intervention, compared to BoNT-A therapy alone (Ferrari et al., Citation2014; Hoare et al., Citation2010; Karaca, Unlu, Kose, Gonen, & Cakci, Citation2016; Lin et al., Citation2015; Wallen, O’Flaherty, & Waugh, Citation2007). Based on data from 10 randomized controlled trials, the most recent Cochrane review concluded that “BoNT-A should not be used in isolation but should be accompanied by planned occupational therapy (Hoare et al., Citation2010).” However, while the utility of BoNT-A in managing upper limb spasticity due to CP is well established through clinical use, the current literature remains largely limited to small clinical trials of short duration, often without an adequate comparator (Delgado et al., Citation2010; Reeuwijk, van Schie, Becher, & Kwakkel, Citation2006). Few trials have provided adequate information on the rehabilitation strategies (if any) employed.
To address such evidence gaps, an international multicenter Phase-III clinical trial of abobotulinumtoxinA (Dysport®; Ipsen, Wrexham, UK) for upper limb spasticity due to CP (NCT02106351) was initiated in 2014 with the objective of demonstrating the efficacy and safety of abobotulinumtoxinA across repeat injections. Like its associated trial in pediatric patients with lower-limb spasticity (Delgado et al., Citation2016), this study of pediatric upper limb spasticity (PULS) has been designed to include modern standards of evaluation such as the assessment of efficacy across the different International Classification of Functioning, Disability and Health (ICF) domains of body function and structure, activity and participation. Considering the necessity of combining BoNT-A with occupational and/or physical therapy, and since not every child has access to the same standards of care, it was decided to include a standardized home therapy program to be followed in conjunction with abobotulinumtoxinA injections into the upper arm (for all patients, in both the control and active treatment arms).
Our objective was to create a home therapy program that can be implemented internationally for all patients participating in the trial. We describe here the development of the program and the processes required for its standardized implementation in the Phase-III PULS trial. Although the trial is ongoing, a subset of children completed their treatment cycles and a preliminary feasibility report is also presented.
Methods
An international working group formed by nine occupational therapists, physical therapists, and medical doctors was invited by the principle investigator of the PULS Phase-III trial to design the Home Exercises Therapy Program (HETP) over a series of face-to-face meetings and telephone calls. Members of the working group were all specialists in their respective fields and drawn from the international study sites involved in the Phase III study. Study sites were recruited based on their extensive experience in the use of BoNT-A treatment for pediatric CP spasticity management and ability to participate in clinical trials. The development process began in May 2013 and was completed in March 2014. Final HETP documentation was reviewed and approved by the working group before submitting the program for peer-review by the steering committee of the Phase III trial, consisting of specialist pediatric neurologists and neurorehabilitation doctors, and then for Institutional Review Board (IRB) approval as part of the overall appraisal of the Phase-III PULS trial protocol. No significant changes were required to the proposed materials at either stage.
Throughout the development process, the working group considered the needs of the target patient population for the PULS trial (NCT02106351), which recruited male and female children (2–17 years old) with a diagnosis of CP (GMFCS level I-IV and MACS I-V). Patients were required to have increased muscle tone/spasticity in at least one upper limb, with a Modified Ashworth Scale (MAS) (Bohannon & Smith, Citation1987) score ≥2 in the primary targeted muscle group (PTMG) of the study limb. The PTMG could be the elbow or wrist flexors, and additional muscles of the upper limb could be injected based on the child’s goals and clinical presentation.
Literature Search
First, to investigate whether similar programs had been developed previously, and to inform the development of the HETP, a literature search was performed in June 2013 using a combination of Medical Subject Headings terms and key words. Key words of relevance to protocol development were: cerebral palsy and home program. Language (English only) and date limits (January 2000–June 2013) were also applied. The search was performed using the PubMed and Medline databases. Bibliographic reference lists of papers identified during the database search were reviewed manually by two reviewers to identify any relevant studies not identified through the electronic database searches. There were no disagreements between the reviewers.
Overall, nine articles were identified and all were reviewed in detail (Campbell et al., Citation2012; Daichman, Johnston, Evans, & Tecklin, Citation2003; Harbourne, Willett, Kyvelidou, Deffeyes, & Stergiou, Citation2010; Kim et al., Citation2012; Novak, Citation2011; Novak & Cusick, Citation2006; Novak, Cusick, & Lannin, Citation2009; Novak, Cusick, & Lowe, Citation2007; Piggot, Hocking, & Paterson, Citation2003; Seifart, Unger, & Burger, Citation2010). Taken collectively, the literature strongly supported the use of home programs for children with CP. Of the nine articles, the working group agreed that the series of three studies by Novak et al. provided the most robust and repeatable source of evidence, clearly outlining the process for creating home programs (Novak & Cusick, Citation2006; Novak et al., Citation2009; Novak et al., Citation2007). The information from these identified articles was then used to inform the working group during their development of the final program.
Development of the Home Therapy Program
Novak et al. defined five key phases for development of a home therapy program: (1) establishing a collaborative relationship with the child’s parent/caregiver; (2) collaborative goal setting; (3) constructing the home therapy program; (4) supporting the program implementation; and (5) evaluating the outcomes (Novak & Cusick, Citation2006). To implement the five key phases of HETP development the working group determined that practical guidelines for selecting and teaching the home program exercises/activities needed to be defined. Using the approach outlined by Novak et al., the working group met in person to define what components (i.e. documents and training) the HETP should include and the process for development (i.e. task list to develop each component, ).
Table 1. Task List
The working group decided that core components of the HETP would include a library of therapeutic exercises/activities, advice on goal setting and goal attainment scaling (GAS, which is used as an efficacy outcome measure in the Phase-III study), and supporting information materials for the patients and their families. Thus, three separate subgroups:
Developed the library of exercises and functional activities that could be expected to be beneficial following an injection of abobotulinumtoxinA. Exercises were proposed and chosen based on the working group’s own experience. The subgroup also arranged the photographs of the exercises being demonstrated for use to ensure the manual was self-explanatory.
Reviewed the literature for GAS in spasticity management (Steenbeek, Ketelaar, Galama, & Gorter, Citation2007; Turner-Stokes, Citation2009) and examples of suitable treatment goals and developed a manual to train clinicians on developing GAS goals and incorporating the goals in the patient’s HETP. Emphasis was placed on building a collaborative relationship based on family preferences and setting SMART (specific, measurable, achievable, relevant, and timed) goals (Bovend'Eerdt, Botell, & Wade, Citation2009). All therapists received detailed training on the implementation of GAS, including goal setting, negotiation, and scoring.
Developed supporting materials and documentation required for standardized implementation of the HETP, including introduction of the program to the participating families, parent/child information sheets, commitment forms, and an incentive chart.
All HETP documents were translated (and back-translated as a quality control check) from English into Arabic, Czech, Hebrew, French, Polish, Spanish, and Turkish.
Implementation and Evaluation
Therapists at all sites received detailed face-to-face training on the implementation of the HETP, including how to introduce and explain the program to the patients and their families, use the manual to choose relevant exercises/activities, as well as goal setting, negotiation, and GAS scoring. Success of the HETP was evaluated by family acceptance to commit to the program and to use it throughout the Phase-III PULS trial. At each trial visit (Week 2, Week 4, Week 6, Week 12, and Week 16), the patient/parent/caregiver was asked to report on the frequency of home exercises by filling in a score chart or a paper diary.
Results
The finalized HETP was presented as a “toolbox” for clinicians at each trial site (in their respective language), and included patient and family materials, a library of exercises and functional activities, and a detailed protocol for clinicians. The toolbox is provided in the supplemental appendix to this article.
Patient and Family Materials
A set of six documents was developed for use by patients and their families.
Separate patient (if applicable) and parental guardian information sheets were written in the first person to engage the patient and their families, and were designed as templates that could be easily personalized. Both types of information sheet were structured to explain how the patient’s muscles are different due to spasticity, the purpose of the HETP, and provide tips on how to make the exercises more fun. The parental guardian information sheet also included information on the general principles of the program and any precautions.
Separate patient (if able) and parental guardian signature of commitment forms explaining the patient’s diagnosis, the reason for receiving BoNT-A injections, and the reason the patient should participate in the HETP.
Exercise score charts to record the exercises performed each day. Exercises were scored as “good”, “incomplete”, or “not done.”
Library of exercises and functional activities (hard copy folder).
Library of Exercises and Functional Activities
The final library of therapeutic exercises/activities was presented on a CD-ROM and in a hard copy binder. Exercises were organized by primary target joint. Passive range of motion (PROM) exercises was developed for the shoulder adductor and internal rotator muscles, elbow flexor muscles, forearm pronator muscles, and wrist/finger flexor muscles. Patients and families were instructed that the PROM exercises needed to be sustained stretches lasting 20–60 seconds per repetition, with at least five repetitions per day. Strengthening exercises were developed for the shoulder abductor and external rotator muscles, elbow extensor muscles, forearm supinator muscles, and wrist/finger extensor muscles. The minimum expectation was that the patient would engage in therapeutic strengthening exercises and functional activities at least five times per week for at least 15 minutes per session (based on the recommendation of at least one hour per week as decribed by Novak et al., Citation2009).
If active GAS goals were identified, the patients were also required to practice the goals. Based on the child’s individual presentation, the therapist decided whether these active exercises/functional activities should be performed independently or with assistance, and whether they should be performed against gravity or resistance.
Protocol for Clinicians
Clinicians were provided a detailed protocol for goal setting and implementation of the HETP, including:
Information on how to set goals using GAS methodology
A detailed description of the stepwise process for choosing the most appropriate exercises/activities to achieve the agreed goals of treatment
A description of how to discuss the HETP with the patient and their parental guardians.
The process for goal setting and goal assessment was based on published GAS methodology, and we used available GAS materials for our training (Turner-Stokes, Citation2009). In the first step, the research team along with the patient and family agreed to between one and three goals. GAS goals were individualized to the patient, and for the purposes of the Phase-III study, the goals were categorized into four sub-domains: active function, passive function, pain, and other. Examples of goals could include increased independence with self-care or improvements in leisure activities (active function), improved passive range for the ease of hygiene and prevention of skin breakdown (passive function), reduction of pain in the upper limb (pain), or another goal that is meaningful to the patient but does not meet the criteria of the first three sub-domains (other). Next, the research team established the muscles to be injected based on the patient’s goals and clinical presentation. A standardized flow chart was also developed to provide guidance on which exercises/activities to choose () based on the patient’s independence level to perform the task or movement targeted. As part of the protocol, all therapists were required to provide the patient and their families with:
At least one exercise for improving PROM, usually restricted by the increased tone of the muscles injected. If the patient did not have full PROM following abobotulinumtoxinA injection, the protocol expected that PROM exercises were completed for a minimum of 5 days per week to stretch the injected muscles.
A list of age appropriate exercises or functional activities to promote active range of motion/strengthening for the muscle groups opposing those that were injected.
Advice to practice the agreed functional tasks.
Figure 1. Standardized processes for (a) exercise selection based on goals (b) engaging patients and families.
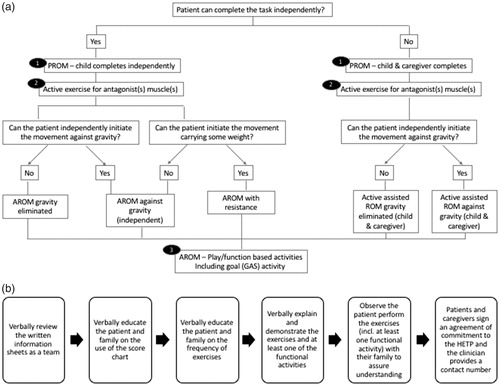
The HETP was introduced to the family using six steps designed to ensure understanding and commitment from all involved (). The final protocol accepted that flexibility in the chosen goals and exercises is required, and allowed therapists to tailor exercises based on family feedback. As an example, one patient wished to “perform better” when playing volleyball and the goal achievement levels were defined such that the expected outcome (score 0) at 6-weeks post-injection was to “hit a beach ball (i.e. larger ball) overhead with two hands at least 80% of the time,” the better than expected outcome (score +1) was to “hit a volleyball overhead with two hands at least 80% of the time,” and the much better than expected outcome (score +2) was to “hit a volleyball overhead with two hands and symmetrical movement of left and right side.” The exercises chosen to support this goal included PROM elbow extension as well as tricep dips and wall push-ups for strengthening. The functional activity was to practice hitting a beach ball overhead working on the use of the left upper arm.
Study personnel at each of the 31 sites were trained on implementation of the HETP at a series of training the trainer meetings. At least one clinician from each country in the trial received advanced training so that they could provide suitable training and support to clinicians from other sites in that country. Refresher courses were provided every 9 to 12 months to ensure a high quality of training was maintained across all trial sites.
Evaluation of the Program
The Phase-III PULS trial is ongoing at 31 sites across eight countries. As of January 2017, 65 children from 20 international sites had completed their injection cycles, providing interim information on the success of the HETP. Thus far, all families agreed to follow the HETP (as evidenced by 100% agreement in the parent/caregiver commitment forms). Overall, 61 children (94%) began the HETP immediately following injection of abobotulinumtoxinA and two families began with a delay of a week and two others after a delay of 1–4 months (unknown reasons). Two-thirds of families opted to complete the prescribed exercises five times per week, and one-third of families opted to complete the prescribed exercises once daily (i.e. 7 times per week).
Of the 65 children who had completed the Phase-III PULS trial by December 2016; seven received one abobotulinumtoxinA treatment (i.e. completed one treatment cycle), 21 received two treatments, 25 received three treatments, and 12 received four treatments. All but two of the 65 families (97%) maintained the frequency of the HETP throughout their participation in the trial and across all relevant treatment cycles (as verified by score charts). Data is missing for one family and the other discontinued the home therapy program six weeks after receiving the second injection.
Discussion
To the best of our knowledge, this is the first standardized HETP developed for use in a Phase-III trial of BoNT-A therapy. The anecdotal feedback from therapists has been positive, with therapists emphasizing how the HETP helps with patient engagement and in getting patients and families to think about how to incorporate exercises in their daily lives. From the authors’ experience, the standard HETP format appears to be acceptable across the diverse range of countries and cultures included in the Phase III study. In addition, the high proportion of families reporting continued use of the HETP throughout the trial confirms family acceptance and continued adherence to the standards of therapy outlined in the program.
The finalized HETP demonstrates that the process for developing a home therapy program as first outlined by Novak and Cusick (Citation2006) is useful and can be implemented across countries and cultures. As a team of clinicians from very different countries and cultures, we found that the development of written information sheets and commitment forms provided a consistent way to build trust, educate, and engage with children and their parents/caregivers. A collaborative goal setting environment was achieved through implementation of GAS which was found to provide a structured process that all therapists could readily follow. As described in several published papers, the setting and negotiation of SMART goals is helpful in engaging and motivating the patient because the resultant goals are worded in a way that has direct meaning to the lives of patients and their families (Bovend'Eerdt et al., Citation2009; Steenbeek et al., Citation2007; Tilton et al., Citation2016; Turner-Stokes, Fheodoroff, Jacinto, Maisonobe, & Zakine, Citation2013; Turner-Stokes, Williams, & Johnson, Citation2009).
Our main aim was to build a comprehensive library of exercises/activities to provide a standardized therapy program that could easily be personalized to the specific needs of each patient, and tailored to their treatment goals. Our program was purposefully designed to support the effectiveness of BoNT-A therapy with the idea of providing a standardized biomechanical therapy approach for an interventional trial. It was therefore necessary to focus on muscle-specific exercises and activities that promote attainment of patient-centered goals. Exercises were chosen by the working group based on their own clinical experience, and the manual was intended to help close a gap in the modern literature of appropriate exercises for children following an injection of BoNT-A into the upper limb. Function was not specifically addressed in the manual because it is emphasized throughout the process of goal setting and goal attainment scaling. For patients that selected active goals, therapists could embed AROM and strengthening into activities based on the child’s interests and GAS goals. Asking the patients and families to complete five sessions of 15 minutes per week allowed for one missed session per week, while still maintaining the frequency of practice needed for clinically significant change (Novak et al., Citation2009).
Limitations of our development process include the lack of a formalized process for cross-cultural adaptation and lack of child/family feedback on the HETP design. Nevertheless, all active exercises were designed to be functionally useful and as fun as possible. The working group aimed to develop exercises that could be performed at home with readily available equipment (e.g. cups, balls, sand, and water). While the manual was designed with a regulatory focus for use in a Phase III trial (e.g. assessing “compliance”), it is important to note that therapists were given the flexibility to support individualized modification of home therapy at each trial visit. In line with Step 4 of the process recommended by Novak et al., parents were continually asked for feedback on the use of the program and therapists had full capability to adapt the exercises as needed.
The high acceptance of the home therapy program (thus far) supports its feasibility and clinical application. This trial will not provide information on the impact of the HETP on functional attainment. It will, however, provide a standardized background of good practice against which to test the efficacy of abobotulinumtoxinA. Recent experience in a Phase-III trial of abobotulinumtoxinA for pediatric lower limb spasticity has shown the relevance of robust training and standardization of outcome measurement in providing a robust dataset that can show statistically significant and clinically relevant differences between treatment groups – both in measures of spasticity and hypertonia and in functional measures including the GAS (Delgado et al., Citation2016; Tilton et al., Citation2016). We also believe the HETP can be implemented to improve standards of care for children with CP across countries and cultures, particularly where resources are limited.
Conclusions
It is widely acknowledged that children with neurologic disabilities require opportunities for repeated practice of new tasks (Hubbard, Parsons, Neilson, & Carey, Citation2009), and the home therapy approach recognizes that families are better positioned than health professionals to perform these exercises with the frequency required for functional impact. It should be noted that our evidence for family acceptance and continued adherence of the program is based on interim analyses from the first 65 children who completed the trial. The final analysis will be published separately and will provide further information about the acceptability of the program. We hope that the full and transparent description of the HETP provided here will not only help with the design of future interventional trials, but also provide guidance for the development of a “tried and tested” program that has been shown to be readily accepted by patients and their families.
Acknowledgments
The authors wish to acknowledge the feedback from the trial investigators and their teams as well as the patients and their families. We also thank Anita Chadha-Patel, PhD (ACP Clinical Communications Ltd, funded by Ipsen Pharma) for editorial support in the development of this manuscript.
Disclosure statement
The authors received payment to train clinicians on intervention protocols related to the study. All authors (external from Ipsen) have been investigators in Ipsen-sponsored clinical trials (including the presented PULS study), and they or their institutions have received payment for participation. CV is employed by Ipsen Pharma. In addition, ACJ was employed directly by Ipsen as expert consultant towards the development of this manual.
Additional information
Notes on contributors
Angela Shierk
Angela Shierk, PhD, OTR, is an Occupational Therapist, Department of Clinical Research, Texas Scottish Rite Hospital for Children, Dallas, TX, USA, and Adjunct Faculty, Texas Woman’s University, Denton, TX, USA.
A. Cecilia Jiménez-Moreno
Cecilia Jiménez-Moreno, PhD is a Research Physiotherapist currently at the University of Newcastle, Institute of Genetic Medicine, and previously at Hospital San José Celaya, Celaya, Guanajuato, Mexico.
Heather Roberts
Heather Roberts, PhD, OTR, is an Occupational Therapist, Department of Clinical Research, Texas Scottish Rite Hospital for Children, Dallas, TX, USA, and Assistant Professor, Texas Woman’s University, Denton, TX, USA.
Shirley Ackerman-Laufer
Shirley Ackerman-Laufer, B.P.T, M.Sc. is a Senior Physiotherapist at the Edmond & Lilly Safra Hospital for Children, Sheba Medical Center, Tel Hashomer, Israel.
Gretchen Backer
Gretchen Backer, BS, PT is currently the Director of Program Review at the Michigan Department of Health and Human Services and was at William Beaumont Hospital, Royal Oak, Michigan, USA at the time of this study.
Rachel Bard-Pondarre
Rachel Bard-Pondarre is a Lead Physiotherapist at the Centre Médico-Chirurgical de Réadaptation des Massues – Croix-Rouge Française, Lyon, France.
Cigdem Cekmece
Cigdem Cekmece is an Assistant Professor in the Yahya Kaptan Vocational School, Department of Occupational Therapy, at Kocaeli University, Izmit, Turkey.
Weronika Pyrzanowska
Weronika Pyrzanowska is a Senior Physiotherapist at the Non-public Healthcare Unit Mazovian Neurorehabilitation and Psychiatry Center in Zagorze, Wiazowna, Poland.
Claire Vilain
Claire Vilain is a Medical Development Director (Neurology) at Ipsen Innovation, Les Ulis, France.
Mauricio R. Delgado
Mauricio R. Delgado, M.D., is a Pediatric Neurologist, Texas Scottish Rite Hospital for Children, Dallas, TX, USA and Professor, Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, TX, USA.
References
- Bohannon, R. W., & Smith, M. B. (1987). Interrater reliability of a modified ashworth scale of muscle spasticity. Physical Therapy, 67, 206–207.
- Bovend'Eerdt, T. J., Botell, R. E., & Wade, D. T. (2009). Writing smart rehabilitation goals and achieving goal attainment scaling: A practical guide. Clinical Rehabilitation, 23, 352–361. doi:10.1177/0269215508101741
- Campbell, S. K., Gaebler-Spira, D., Zawacki, L., Clark, A., Boynewicz, K., deRegnier, R. A.,… Zhou, X. J. (2012) Preterm, infants with periventricular brain injury: A pilot study. The Journal of Pediatric Rehabilitation Medicine, 27, 403–427. doi:10.3233/PRM-2011-0185
- Colver, A., Fairhurst, C., & Pharoah, P. O. (2014). Cerebral palsy. Lancet, 383, 1240–1249. doi:10.1016/S0140-6736(13)61835-8
- Daichman, J., Johnston, T. E., Evans, K., & Tecklin, J. S. (2003). The effects of a neuromuscular electrical stimulation home program on impairments and functional skills of a child with spastic diplegic cerebral palsy: A case report. Pediatric Physical Therapy, 15, 153–158. doi:10.1097/01.PEP.0000083121.26982.1D
- Delgado, M. R., Hirtz, D., Aisen, M., Ashwal, S., Fehlings, D. L., McLaughlin, J., … Vargus-Adams, J. (2010). Practice parameter: Pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): Report of the quality standards subcommittee of the American Academy of Neurology and the practice committee of the child neurology society. Neurology, 74, 336–343. doi:74/4/336 [pii]
- Delgado, M. R., Tilton, A., Russman, B., Benavides, O., Bonikowski, M., Carranza, J., … Picaut, P. (2016). Abobotulinumtoxina for equinus foot deformity in cerebral palsy: A randomized controlled trial. Pediatrics, 137, e20152830–e20152839. doi:10.1542/peds.2015-2830
- Ferrari, A., Maoret, A. R., Muzzini, S., Alboresi, S., Lombardi, F., Sgandurra, G., … Cioni, G. (2014). A randomized trial of upper limb botulimun toxin versus placebo injection, combined with physiotherapy, in children with hemiplegia. Research in Developmental Disabilities, 35, 2505–2513. doi:10.1016/j.ridd.2014.06.001
- Harbourne, R. T., Willett, S., Kyvelidou, A., Deffeyes, J., & Stergiou, N. (2010). A comparison of interventions for children with cerebral palsy to improve sitting postural control: A clinical trial. Physical Therapy, 90, 1881–1898. doi:10.2522/ptj.2010132
- Hoare, B. (2014). Rationale for using botulinum toxin a as an adjunct to upper limb rehabilitation in children with cerebral palsy. Journal of Child Neurology, 29, 1066–1076. doi:10.1177/0883073814533196
- Hoare, B. J., & Imms, C. (2004). Upper-limb injections of botulinum toxin-a in children with cerebral palsy: A critical review of the literature and clinical implications for occupational therapists. The American Journal of Occupational Therapy (AJOT), 58, 389–397.
- Hoare, B. J., Wallen, M. A., Imms, C., Villanueva, E., Rawicki, H. B., & Carey, L. (2010). Botulinum toxin a as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (update). The Cochrane Database of Systematic Reviews, 1, CD003469. doi:10.1002/14651858.CD003469.pub4
- Hubbard, I. J., Parsons, M. W., Neilson, C., & Carey, L. M. (2009). Task-specific training: Evidence for and translation to clinical practice. Occupational Therapy International, 16, 175–189. doi:10.1002/oti.275
- Karaca, B., Unlu, E., Kose, G., Gonen, E., & Cakci, A. (2016). Outcomes of botulinum toxin type a injection followed by rehabilitation in cases of cerebral palsy with upper extremity involvement. Journal of Child Neurology, 31, 357–363. doi:10.1177/0883073815596609
- Kerr Graham, H., & Selber, P. (2003). Musculoskeletal aspects of cerebral palsy. The Journal of Bone and Joint Surgery. British Volume, 85, 157–166.
- Kim, D.-A., Lee, J.-A., Hwang, P.-W., Lee, M.-J., Kim, H.-K., Park, J.-J., … Lee, N.-G. (2012). The effect of comprehensive hand repetitive intensive strength training (CHRIST) using motion analysis in children with cerebral palsy. Annals of Rehabilitation Medicine, 36, 39–46. doi:10.5535/arm.2012.36.1.39
- Lin, Y. C., Huang, C. Y., Lin, I. L., Shieh, J. Y., Chung, Y. T., & Chen, K. L. (2015). Evaluating functional outcomes of botulinum toxin type a injection combined with occupational therapy in the upper limbs of children with cerebral palsy: A 9-month follow-up from the perspectives of both child and caregiver. PLoS One, 10, e0142769. doi:10.1371/journal.pone.0142769
- Novak, I. (2011). Parent experience of implementing effective home programs. Physical & Occupational Therapy in Pediatrics, 31, 198–213. doi:10.3109/01942638.2010.533746
- Novak, I., & Cusick, A. (2006). Home programmes in paediatric occupational therapy for children with cerebral palsy: Where to start? Australian Occupational Therapy Journal, 53, 251–264. doi:10.1111/j.1440-1630.2006.00577.x
- Novak, I., Cusick, A., & Lannin, N. (2009). Occupational therapy home programs for cerebral palsy: Double-blind, randomized, controlled trial. Pediatrics, 124, e606–e614. doi:10.1542/peds.2009-0288
- Novak, I., Cusick, A., & Lowe, K. (2007). A pilot study on the impact of occupational therapy home programming for young children with cerebral palsy. The American Journal of Occupational Therapy : Official Publication of the American Occupational Therapy Association, 61, 463–468.
- Piggot, J., Hocking, C., & Paterson, J. (2003). Parental adjustment to having a child with cerebral palsy and participation in home therapy programs. Physical & Occupational Therapy in Pediatrics, 23, 5–29.
- Reeuwijk, A., van Schie, P. E., Becher, J. G., & Kwakkel, G. (2006). Effects of botulinum toxin type A on upper limb function in children with cerebral palsy: A systematic review. Clinical Rehabilitation, 20, 375–387.
- Santos, C. A., Franco de Moura, R. C., Lazzari, R. D., Dumont, A. J., Braun, L. A., & Oliveira, C. S. (2015). Upper limb function evaluation scales for individuals with cerebral palsy: A systematic review. Journal of Physical Therapy Science, 27, 1617–1620. doi:10.1589/jpts.27.1617
- Seifart, A., Unger, M., & Burger, M. (2010). Functional electrical stimulation to lower limb muscles after botox in children with cerebral palsy. Pediatric Physical Therapy, 22, 199–206. doi:10.1097/PEP.0b013e3181dbd806
- Steenbeek, D., Ketelaar, M., Galama, K., & Gorter, J. W. (2007). Goal attainment scaling in paediatric rehabilitation: A critical review of the literature. Developmental Medicine & Child Neurology, 49, 550–556. doi:10.1111/j.1469-8749.2007.00550.x
- Steultjens, E. M., Dekker, J., Bouter, L. M., van de Nes, J. C., Lambregts, B. L., & van den Ende, C. H. (2004). Occupational therapy for children with cerebral palsy: A systematic review. Clinical Rehabilitation, 18, 1–14.
- Strobl, W., Theologis, T., Brunner, R., Kocer, S., Viehweger, E., Pascual-Pascual, I., & Placzek, R. (2015). Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins (Basel), 7, 1629–1648. doi:10.3390/toxins7051629
- Tilton, A., Russman, B., Aydin, R., Dincer, U., Escobar, R., Kutlay, S., & Delgado, M., R. (2016). Abobotulinumtoxina (Dysport) improves function according to goal attainment in children with dynamic equinus due to cerebral palsy. Journal of Child Neurology, 32, 482–487.
- Turner-Stokes, L. (2009). Goal attainment scaling (gas) in rehabilitation: A practical guide. Clinical Rehabilitation, 23, 362–370. doi:10.1177/0269215508101742
- Turner-Stokes, L., Fheodoroff, K., Jacinto, J., Maisonobe, P., & Zakine, B. (2013). Upper limb international spasticity study: Rationale and protocol for a large, international, multicentre prospective cohort study investigating management and goal attainment following treatment with botulinum toxin a in real-life clinical practice. BMJ Open, 3, e002230. doi:10.1136/bmjopen-2012-002230
- Turner-Stokes, L., Williams, H., & Johnson, J. (2009). Goal attainment scaling: Does it provide added value as a person-centred measure for evaluation of outcome in neurorehabilitation following acquired brain injury? Journal of Rehabilitation Medicine, 41, 528–535. doi:10.2340/16501977-0383
- Wallen, M., O’Flaherty, S. J., & Waugh, M.-C. A. (2007). Functional outcomes of intramuscular botulinum toxin type a and occupational therapy in the upper limbs of children with cerebral palsy: A randomized controlled trial. The Archives of Physical Medicine and Rehabilitation, 88, 1–10. doi:10.1016/j.apmr.2006.10.017
