Abstract
Following its introduction into Australia, the rust fungus Puccinia xanthii var. parthenii-hysterophorae has played an important role in the integrated approach to manage Parthenium hysterophorus. The rust was imported from Australia and established within quarantine facilities in South Africa. Supplementary host-specificity testing of the rust on indigenous members from the tribe Heliantheae and 13 commercial sunflower cultivars was conducted. The rust was shown to be highly specific to parthenium, with only a single incident of symptom development on leaves of the native Spilanthes mauritiana. The symptoms on S. mauritiana were considered to be a laboratory artifact as no further symptoms were observed on this species during subsequent inoculations. Microscopic evaluations revealed that although basidiospores germinate on the surface of S. mauritiana leaves, they do not penetrate the epidermis. Spilanthes mauritiana is host to Puccinia africana, but cross-inoculation greenhouse studies showed that although P. africana is assumed to belong to the P. xanthii complex, it can be separated as a morphospecies from P. xanthii var. parthenii-hysterophorae as it is highly specific to its host. Based on these results it was concluded that P. xanthii var. parthenii-hysterophorae was suitable for release as a biological control agent on Pa. hysterophorus in South Africa.
Introduction
Parthenium hysterophorus L. (Asteraceae) originates from tropical America around the Gulf of Mexico (Haseler Citation1976) and occurs from the southern USA to central South America (Dale Citation1981). This highly invasive, noxious weed has become widespread in Africa, many South Pacific Islands, Asia and Australia (Navie et al. Citation1996, Evans Citation1997, McConnachie et al. Citation2010). In South Africa this weed was first observed in KwaZulu-Natal in the 1880s (Wood Citation1897), became prevalent in the 1980s in the aftermath of cyclone Demoina in 1984 (van der Laan Citation2006), and has since spread to the Mpumalanga and North West provinces (McConnachie et al. Citation2010).
Parthenium is an annual herb that can reach a height of 2 m, producing thousands of seeds that remain viable for several years (Navie et al. Citation1996). It invades disturbed areas and has a high incidence in areas that are regularly disturbed by floods (McFadyen Citation1992). Its allelopathic nature is well documented (Kanchan and Jayachandra Citation1980, Citation1981, van der Laan Citation2006), as well as its ability to reduce crop production and the carrying capacity of natural rangelands (McFadyen Citation1992, Navie et al. Citation1996, Evans Citation1997). Parthenium causes severe allergic responses in humans and livestock (Towers and Subba Rao Citation1992).
Existing management practices for parthenium weed in South Africa consist of mechanical and chemical approaches, which are often uneconomic and carry associated health implications from exposure to the plant. It is widely recognised that they should be supplemented with biological control, which is sustainable, cost effective and environmentally friendly (Evans Citation1997). Surveys conducted in the native range of parthenium weed revealed that there were numerous organisms that suppressed growth of the plant, suggesting that implementing a biological control programme was a good management option (Haseler Citation1976). Eleven agents were released in Australia against parthenium weed (McFadyen Citation1992), and evaluation studies revealed that biological control (using insect and fungal agents) significantly improved grass production in Australian rangelands (Dhileepan Citation2007).
The two most important pathogens associated with Pa. hysterophorus in its native range are two rust fungi, Puccinia abrupta Dietel & Holw. var. partheniicola (H.S.Jacks.) Parmalee and Puccinia xanthii Schwein. var. parthenii-hysterophorae Seier, H.C.Evans & Á.Romero (previously referred to as Puccinia melampodii Dietel & Holw.) (Evans Citation1987a, Citation1997, Parker Citation1989). Puccinia abrupta var. partheniicola was found to be already present in South Africa (Wood and Scholler Citation2002), presumably having been introduced along with its host plant. In Mexico, this rust was damaging to parthenium weed in semi-arid, high-altitude areas, but in humid, lowland situations infection was very light and scattered (Evans Citation1987b). The optimal temperature for germination of urediniospores and infection of plants is 15 °C (Fauzi et al. Citation1999). Therefore, this rust fungus will have little impact in the low-altitude, high-temperature areas in which the weed is currently spreading in South Africa. However, it may impact the weed in high-altitude, low-temperature areas. For this reason, the research focus was mainly on P. xanthii var. parthenii-hysterophorae, which was collected from the humid lowland areas in Mexico (Parker Citation1989) and since 2000 has been successfully used as a biological control agent against Pa. hysterophorus in Australia (Tomley Citation2000).
The life cycle, biology and host-specificity of P. xanthii var. parthenii-hysterophorae were extensively investigated in Mexico (Romero and Duran Citation2000) and by CABI-UK (Seier et al. Citation1997, Tomley Citation2000) before release of the rust fungus in Australia. Puccinia xanthii var. partheniihysterophorae is an autoecious, microcyclic rust fungus, producing both telia and basidiospores on one host (Parmelee Citation1967). The teliospores germinate over a wide temperature range (optimum being 25 °C) and produce basidiospores (optimum at 22 °C), which directly penetrate the host epidermis (Romero and Duran Citation2000, Tomley Citation2000, Kelaniyangoda and Ekanayake Citation2010). Symptoms of P. xanthii var. parthenii-hysterophorae first appear approximately 9 d after infection and can be observed as chlorotic spots on the leaves (Seier et al. Citation1997). Ten to 12 d after infection, telia erupt from the centre of these chlorotic spots, which gradually extend outwards (Tomley Citation2000). Under severe infection these chlorotic spots can coalesce, leading to leaf necrosis and die-back. Stem infections have also been observed (Seier et al. Citation1997, Tomley Citation2000).
Prior to the release of P. xanthii var. parthenii-hysterophorae in Australia, host-specificity testing was conducted on over 60 plant species within the family Asteraceae, as well as on 13 sunflower cultivars (M Seier, CABI, UK, pers. comm.). Limited infection was observed on a few plants, but in most cases the infection consisted of abnormal or abortive sporulation and the level of sporulation was much less than those on parthenium weed (Seier et al. Citation1997, Tomley Citation2000). A field experiment was conducted in Australia after the release of P. xanthii var. partheniihysterophorae, to confirm the host range under natural conditions (Tomley Citation2000). Although all plants on which symptoms developed under quarantine conditions were included, limited sporulation occurred on Calendula officinalis only (Tomley Citation2000). Since its release in Australia in 2000 there have been no reports of P. xanthii var. parthenii-hysterophorae infecting any plant other than Parthenium hysterophorus.
Puccinia xanthii is associated with various species within the tribe Heliantheae, namely Ambrosia spp. and Xanthium spp. (Bremer Citation1994). However, molecular and crossinoculation studies concluded that P. xanthii consists of a complex of morphospecies that can be separated by their hosts (Seier et al. Citation2009). In addition, Spilanthes mauritiana (Pers.) DC. is host to Puccinia africana Cooke in South Africa (Doidge Citation1950). This rust is morphologically indistinguishable from Puccinia xanthii (Henderson Citation1970) and is likely part of the P. xanthii complex.
The objective of this study was to evaluate the risk associated with introducing the Puccinia xanthii var. parthenii-hysterophorae strain originally collected in Mexico then released into Australia, into South Africa. This paper reports on the host-specificity testing conducted in South Africa on native species supplementary to those tested in Australia. In addition, cross-inoculation studies were performed using P. africana and P. xanthii var. partheniihysterophorae to determine whether they are specific to their known hosts.
Materials and methods
Plant material
Seeds of Pa. hysterophorus were collected from mature plants in the field and sown in seedling trays in a greenhouse maintained at 20–25 °C (day) and 18–20 °C (night). Seedlings were transplanted into 18 cm diameter plastic pots. Seeds were collected regularly for propagation. These plants were used to culture the rust fungus, P. xanthii var. parthenii-hysterophorae and for tests. Test plants were obtained either as seeds, seedlings or mature plants from the field or from local nurseries, and were maintained under the same conditions as parthenium plants.
Maintenance of rust culture and inoculation of plants
Parthenium leaves with air-dried teliospores of P. xanthii var. parthenii-hysterophorae strain W1496 were obtained from the Alan Fletcher Research Station, Brisbane, Australia, and imported into the plant pathogen quarantine facility of the ARC–PPRI Stellenbosch campus in 2004. Potted parthenium plants were inoculated using the inverted leaf-disk method of Morin et al. (Citation1993), in which leaf discs with rust pustules were placed on water agar (1.5%, w:v) in a Petri dish. The Petri dish was attached with an adhesive to the bottom of a 25 cm diameter pot. The surface of the plant and the inoculum were misted with water before the pot was inverted over a pot of similar size containing the test plant. The pots were sealed together to form an incubation chamber. The plants were incubated at 22 °C for 24 h, after which the inoculation chambers were dismantled and the plants were transferred to a glasshouse with 20-25 °C (day) and 18-20°C (night) temperatures.
Host-specificity testing
The tribe Heliantheae, to which both parthenium weed and sunflower belong, has its major diversity in the Americas. There are few members indigenous to South Africa, with six genera and nine species in total. None of the native Heliantheae required for testing were available commercially, but attempts were made to field-collect as many species as possible. Representative species from four indigenous genera of the Heliantheae were tested (), as well as 13 locally produced commercial sunflower cultivars.
Table 1 : Host-specificity testing of Puccinia xanthii var. partheniihysterophorae conducted in South Africa
Parthenium and test plants were inoculated as previously described. Inoculations were conducted using batches of plants (six pots per batch), each pot in its own incubation chamber. Parthenium plants were inoculated at the rosette stage and sunflower plants were inoculated at the six-leaf stage. Each pot contained four sunflower plants or a single plant of the other test species. The experiment was repeated three times, using six pots per species for each experiment. In each batch one parthenium plant was included as a control. The plants were monitored for presence or absence of symptoms, i.e. pustule development, 26 d after inoculation when the control plants were heavily infected with well-developed telia.
A subsample of inoculated leaves of the indigenous species were harvested and, after clearing in Carnoy's solution (75% alcohol, 25% acetic acid) for a period of at least 48 h, the leaves were removed and stained with 0.1% aniline blue in lactophenol for a period of 6–24 h, depending on the thickness of the leaf. They were mounted in lactic acid (50% aqueous solution) and examined microscopically.
Cross-inoculation
The rust pathogen Puccinia africana was collected from Spilanthes mauritiana plants in Umhlanga, KwaZulu-Natal (29°42′44.9″ S, 31°05′37.5″ E). This rust was established on S. mauritiana plants in the quarantine facilities at ARC-PPRI, Stellenbosch, and subsequently multiplied for inoculation studies. Cross-inoculation studies were conducted using nine Pa. hysterophorus and S. mauritiana plants each. In each experiment three S. mauritiana plants and three Pa. hysterophorus plants were inoculated with each respective rust fungus, using the incubation chamber technique, as previously described and the experiment was repeated three times. A subsample of the inoculated leaves was harvested from each plant and examined microscopically, using the technique described above.
Results and discussion
No symptoms or pustules developed on any of the 13 sunflower cultivars tested. During host-specificity testing in Australia, two Australian sunflower cultivars (not cultivated in South Africa) were infected under glasshouse conditions (Seier et al. Citation1997). However, teliospores from these infections were shown to be infertile and field testing undertaken in Australia demonstrated that P. xanthii var. parthenii-hysterophorae poses no risk to sunflower production. Another P. xanthii form that occurs on Xanthium strumarium has been shown to be able to infect a few sunflower cultivars under artificial conditions (Alcorn Citation1976, Morin et al. Citation1993, Pretorius et al. Citation2000) and field conditions (Alcorn and Kochman Citation1976, Gulya Citation2002). However, in Mexico, sunflower is cultivated extensively where P. xanthii var. parthenii-hysterophorae occurs naturally, and to date there has been no record of this rust affecting any sunflower cultivar (Farr and Rossman Citation2012). It was concluded therefore that P. xanthii var. partheniihysterophorae does not pose a risk to sunflower production in South Africa.
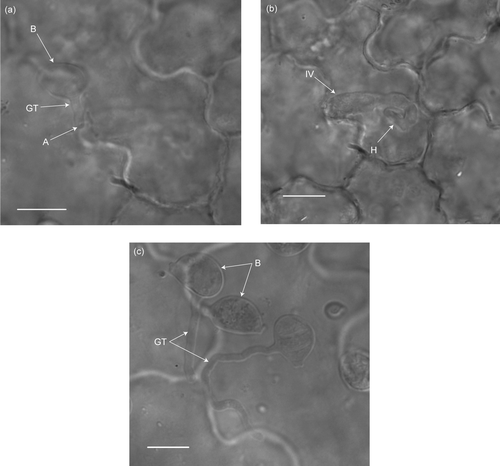
No sporulation was recorded on any of the other plant species tested, except for a very limited number of pustules on Spilanthes mauritiana. On one occasion only, two leaves, each on a different plant, bore a total of three pustules. Microscopic examination of S. mauritiana leaves revealed that although basidiospores of P. xanthii var. parthenii-hysterophorae germinated, they failed to penetrate the leaf epidermis (). It was concluded that the once-off infection of S. mauritiana by P. xanthii var. parthenii-hysterophorae was a false negative result because of artificial high inoculum loads characteristic of testing under glasshouse conditions (Evans et al. Citation2001), as found for some species tested in Australia (Seier et al. Citation1997).
Figure 1: Light micrographs (1000×) of the infection process of Puccinia xanthii var. parthenii-hysterophorae on Parthenium hysterophorus and Spilanthes mauritiana. (a) Puccinia xanthii var. parthenii-hysterophorae germinated basidiospore (B) with a short germ-tube (GT) that has formed a simple appressorium (A) on the host epidermis at 24 h after inoculation on Parthenium hysterophorus. (b) Puccinia xanthii var. parthenii-hysterophorae intra-epidermal vesicle (IV) with a primary hypha (H) 24 h after inoculation on Parthenium hysterophorus. (c) Puccinia xanthii var. parthenii-hysterophorae germinated basidiospore (B) producing a long germ-tube (GT), without penetration, 24 h after inoculation on Spilanthes mauritiana. Scale bars = 10 μm
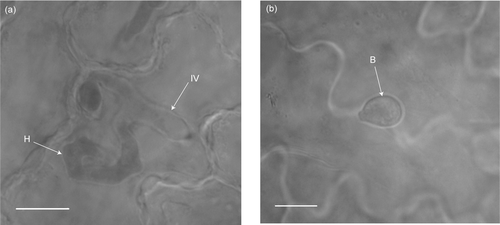
Spilanthes mauritiana was highly susceptible to Puccinia africana, but in contrast Pa. hysterophorus was not affected by this rust (). This indicates that although P. africana might form part of the Puccinia xanthii complex, it differs from the race occurring on parthenium and is considered to be a different form.
Figure 2: Light micrographs of the infection process of Puccinia africana on Spilanthes mauritiana and Parthenium hysterophorus. (a) Puccinia africana intra-epidermal vesicle (IV) with a branching primary hypha (H) 48 h after inoculation on Spilanthes mauritiana. (b) Puccinia africana basidiospore (B) not germinating on Parthenium hysterophorus 48 h after inoculation. Scale bars = μ10 m
Given the high level of host specificity demonstrated before release of P. xanthii var. parthenii-hysterophorae in Australia (M Seier, pers. comm.), together with the additional testing reported here, it is considered that P.xanthii var. parthenii-hysterophorae is safe for introduction into South Africa for the biological control of Pa. hysterophorus. Furthermore, this rust fungus has been demonstrated to be an effective agent under favourable climatic conditions. In Mexico P. xanthii var. parthenii-hysterophorae appeared to be well adapted to dry conditions as it was found to persist in areas where rain was absent for four months, but was most aggressive during the wet season (Tomley Citation2000). In Australia, P. xanthii var. parthenii-hysterophorae established rapidly and over a wide range in northern Queensland (Dhileepan et al. Citation2006). The performance of the rust, however, varied between years depending on rainfall, with the highest impact during a higher-rainfall year (Dhileepan Citation2007). It is expected that P. xanthii var. parthenii-hysterophorae will contribute greatly to the management of parthenium weed infestations in the warm, lower-altitude regions of South Africa, where there are currently no biological control agents implemented against this weed.
Acknowledgements
We thank Gwen Samuels, Lea Orien and Christopher Xhegwana of ARC-PPRI for assistance with plant and fungal culture maintenance. We also gratefully acknowledge financial support from the Department of Water and Environmental Affairs ‘Working for Water Programme’.
References
- Alcorn , JL. 1976 . Host range of Puccinia xanthii . Transactions of the British Mycological Society , 66 : 365 – 367 .
- Alcorn , JL and Kochman , JK. 1976 . A field record of Puccinia xanthii on sunflower . Australian Plant Pathology Society Newsletter , 5 : 33
- Bremer , K. 1994 . Asteraceae: cladistics and classification , Portland : Timber Press .
- Dale , IJ. 1981 . Parthenium weed in the Americas . Australian Weeds , 1 : 8 – 14 .
- Dhileepan K , Florentine SK , Lockett CJ. 2006 . Establishment, initial impact and persistence of parthenium summer rust Puccinia melampodii in north Queensland . In: Preston C , Watts JH , Crossman ND , Proceedings of the 15th Australian Weeds Conference , 24–28 September 2006 , Adelaide , Australia . Adelaide : Weed Management Society of South Australia . pp 577 – 580 .
- Dhileepan , K. 2007 . Biological control of Parthenium (Parthenium hysterophorus) in Australian rangelands translates to improved grass production . Weed Science , 55 : 97 – 501 .
- Doidge , EM. 1950 . The South African fungi and lichens to the end of 1945 . Bothalia , 5 : 1 – 1094 .
- Evans , HC. 1987a . Fungal pathogens of some subtropical and tropical weeds and the possibilities for biological control . Biocontrol News and Information , 8 ( 1 ) : 7 – 30 .
- Evans , HC. 1987b . Life-cycle of Puccinia abrupta var. partheniicola, a potential biological control agent of Parthenium hysterophorus . Transactions of the British Mycological Society , 88 : 105 – 111 .
- Evans , HC. 1997 . Parthenium hysterophorus: a review of its weed status and the possibilities for biological control . Biocontrol News and Information , 18 : 89N – 98N .
- Evans , HC , Fröhlich , J and Shamoun , SF. 2001 . “ Biological control of weeds ” . In Bio-exploitation of filamentous fungi , Edited by: Pointing , SB and Hyde , KD . 349 – 401 . Hong Kong : Fungal Diversity Press .
- Farr DF , Rossman AY. 2012 . Fungal Databases . Systematic Mycology and Microbiology Laboratory, Agricultural Research Service, US Department of Agriculture . Available at http://nt.ars-grin.gov/fungaldatabases/ [accessed 21 June 2012] .
- Fauzi , MT , Tomley , AJ , Dart , PJ , Ogle , HJ and Adkins , SW. 1999 . The rust Puccinia abrupta var. partheniicola, a potential biocontrol agent of parthenium weed: environmental requirements for disease progress . Biological Control , 14 : 141 – 145 .
- Gulya , TJ. 2002 . First report of Puccinia xanthii on sunflower in North America . Plant Disease , 86 : 564
- Haseler , WH. 1976 . Parthenium hysterophorus L. in Australia . Pest Articles and News Summaries , 22 : 515 – 517 .
- Henderson , DM. 1970 . Rust fungi from East Africa . Notes of the Royal Botanic Garden Edinburgh , 30 : 395 – 407 .
- Kanchan SD , Jayachandra . 1980 . Allelopathic effects of Parthenium hysterophorus L. exudation of inhibitors through roots , Plant and Soil 53 : 27 – 35 .
- Kanchan SD , Jayachandra . 1981 . Effects of Parthenium hysterophorus on nitrogen-fixing and nitrifying bacteria , Canadian Journal of Botany 59 : 199 – 202 .
- Kelaniyangoda , DB and Ekanayake , HMRK. 2010 . Puccinia melampodii Diet. and Holow. as a biological control agent of Parthenium hysterophorus . Journal of Food and Agriculture , 1 : 13 – 19 .
- McConnachie , AJ , Strathie , LW , Mersie , W , Gebrehiwot , L , Zewdie , K , Abdurehim , A , Abrha , B , Araya , T , Asaregew , F Assefa , F . 2010 . Current and potential geographical distribution of the invasive plant Parthenium hysterophorus (Asteraceae) in eastern and southern Africa . Weed Research , 51 : 71 – 84 .
- McFadyen , RE. 1992 . Biological control against parthenium weed in Australia . Crop Protection , 11 : 400 – 407 .
- Morin , L , Auld , BA and Brown , JF. 1993 . Host range of Puccinia xanthii and post-penetration development on Xanthium occidentale . Canadian Journal of Botany , 71 : 959 – 965 .
- Navie , SC , McFadyen , RE , Panetta , FD and Adkins , SW. 1996 . The biology of Australian weeds 27. Parthenium hysterophorus L . Plant Protection Quarterly , 11 : 76 – 88 .
- Parker A. 1989 . Biological control of parthenium weed using two rust fungi . In: Delfosse ES , Proceedings of the 7th International Symposium on Biological Control of Weeds , 6-11 March , Rome , Italy . Rome : Ministero d'Agricoltura e delle Foreste; Melbourne: CSIRO . pp 27 – 36 .
- Parmelee , JA. 1967 . The autoecious species of Puccinia on Heliantheae in North America . Canadian Journal of Botany , 45 : 2267 – 2327 .
- Pretorius , ZA , van Wyk , PS and Kriel , WM. 2000 . Occurrence of Puccinia xanthii on sunflower in South Africa . Plant Disease , 84 : 924
- Romero A , Duran Z. 2000 . Germination and the infection process of the rust fungus Puccinia melampodii on the weed Parthenium hysterophorus . Fitopatologia 35 : 155 – 162 (in Spanish, with English abstract) .
- Seier MK , Harvey JL , Romero A , Kinnersley RP. 1997 . Safety testing of the rust Puccinia melampodii as a potential biocontrol agent of Parthenium hysterophorus L . In: Mahadeveppa M , Patil VC , First international conference on parthenium management , 6–8 October 1997 , Dharwad , India . Dharwad : University of Agricultural Sciences . pp 63 – 69 .
- Seier , MK , Morin , L , van der Merwe , M , Evans , HC and Romero , A. 2009 . Are the microcyclic species Puccinia melampodii and Puccinia xanthii conspecific? . Mycological Research , 113 : 1271 – 1282 .
- Tomley AJ. 2000 . Puccinia melampodii (summer rust), a new biocontrol agent for parthenium weed . In: Swarbrick JT , Proceedings of the 6th Queensland Weed Symposium , 10–13 July 2000 , Queensland , Australia . Brisbane : Weed Science Society of Queensland . pp 126 – 129 .
- Towers GHN , Subba Rao PV. 1992 . Impact of the pan-tropical weed, Parthenium hysterophorus L. on human affairs . In: Richardson RG , Proceedings of the 1st International Weed Control Congress , 17–21 February 1992 , Melbourne , Australia . Melbourne : Weed Science Society of Victoria . pp 134 – 138 .
- van der Laan M. 2006 . Allelopathic interference potential of the alien invader plant Parthenium hysterophorus . MSc thesis , University of Pretoria , South Africa .
- Wood JM. 1897 . Report on Natal Botanic Gardens for the year 1896 . Durban : Durban Botanic Society .
- Wood , AR and Scholler , M. 2002 . Puccinia abrupta var. partheniicola on Parthenium hysteophorus in southern Africa . Plant Disease , 86 : 327