Abstract
The effects of three fertiliser solutions (20:20:20, 15:5:25 and 12:30:10 NPK) with electrical conductivity (EC) of 1, 1.5 or 2 mS cm−1 on growth and flowering of Cymbidium ‘Sleeping Nymph’ were investigated over three years. One-year-old tissue-cultured propagules of ‘Sleeping Nymph’ were planted singly in plastic pots in a polyhouse. Plant height, leaf length, pseudobulb length and girth, and number of pseudobulbs per clump were highest in treatment T5 (20:20:20 NPK at 1.5 mS cm−1). The EC of the fertiliser solution had a significant influence on the number of spikes per plant, number of florets per spike, spike length and rachis length. The number of florets per spike and spike length were highest with fertiliser solution with an EC of 1.0 mS cm−1. The number of spikes per plant, florets per spike, spike length and rachis length (1.75, 11.25, 46.3 cm and 22 cm, respectively) were highest in treatment T4 (12:30:10 NPK at 1 mS cm−1). Plant nutrient content was highest with fertiliser solution with an EC of 2.0 mS cm−1. An EC of 0.8–1.0 mS cm−1 for the pour-through leachate from the growing medium was optimal for highest-quality flowering of the Cymbidium hybrid.
Introduction
Cymbidium orchids are among the most commercially important cut flower crops because of the size, shape, colour and longevity of the blooms (Barman et al. Citation2008). The flowers are thick, waxy and bear five pointed petals per bloom. Cymbidium is one of the most popular and desirable orchids because of the beauty of the flowers, which are available in a rainbow of colours and are used in floral arrangements and floral corsages. The genus Cymbidium originated from the foothills of the Himalayas and is distributed in tropical and subtropical Asia and northern Australia. North-eastern India is endowed with a diverse and congenial environment for growing Cymbidium but is yet to embark on a significant cut flower trade, particularly of Cymbidium.
Orchids in the wild usually absorb nutrients dissolved in rain water, which contains up to 15 ppm dissolved nutrients. In their natural habitat, plants grow in accumulated humus and on naturally decomposed organic matter in tree joints (Poole and Sheehan Citation1973). In general, Cymbidium plants require a cool climate, porous medium, limited nutrition and judicious watering. A wide variety of materials can be used successfully for growing orchids, as reported by Sheehan (Citation1958). Moss (Citation1955) recommended peat moss for Cymbidium, because it produced superior growth, resisted decay, and remained in better physical condition for 2–3 years.
With the introduction of different growing media for orchid cultivation, the fertilisation programme and recommended nutritional dose has become controversial (Sheehan Citation1961). Poole and Seeley (Citation1978) noted deficiency symptoms in Cymbidium plants supplied with a low concentration of nitrogen (N). Lunt and Koranek (1961) stated that a high concentration of liquid N fertiliser promoted vegetative growth at the expense of flowering in Cymbidium. Phalaenopsis orchids are severely affected by omission of N, phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg) from the nutrient solution, which results in considerable reduction of the total leaf area and flower pedicel development was almost completely inhibited (Yoneda et al. Citation1997). The slow development of deficiency symptoms in orchids is related to their remarkable ability to remobilise minerals from older leaves and other storage organs, such as pseudobulbs, to meet the nutrient demands of new growth. This ‘efficient recycling’ phenomenon observed in most tropical orchids may be attributed to their epiphytic origin, where the supply of minerals is scanty and unpredictable. Tang et al. (Citation1999) reported that application of 0.3% NPK (15:30:15) fertiliser solution 1–2 times each week significantly enhanced the flowering of Dendrobium.
In Cymbidium hybrids, vegetative buds emerge in autumn, winter, spring and early summer depending on the type of hybrid (de Kreij and van den Berg Citation1990). During growth of the shoots, generative buds emerge in the axils of the leaves and the bud develops into spikes. Good vegetative growth is needed to obtain a large number of generative buds, but vegetative growth that is too lush can reduce generative bud formation. Application of a high N concentration during vegetative growth and omission of fertiliser during generative bud emergence resulted in the highest spike production in Cymbidium (Arnold Bik and van den Berg Citation1983). Orchids are less tolerant of increased electrical conductivity (EC) of the fertiliser solution than many terrestrial plant species (Miles Citation1982). A high EC of the fertiliser solution decreased yield of Dendrobium flowers (Imamura et al. Citation1986). De Kreij and van den Berg (Citation1990) reported that the flower number on Cymbidium spikes decreased with fertiliser solution with an EC exceeding 1.4 dS m−1. Recommended orchid fertilisation programmes are varied and often create confusion. Therefore, the present investigation was undertaken with the objective to determine the optimum EC of the fertiliser solution for improved growth and spike production in the Cymbidium hybrid ‘Sleeping Nymph’.
Material and methods
The experiment was carried out at the National Research Centre for Orchids, Pakyong, India, from March 2007 to February 2010. One-year-old tissue-cultured plants of Cymbidium ‘Sleeping Nymph’ were planted singly in plastic pots (30 cm diameter) and grown in a polyhouse. The growing medium comprised a mixture of leaf mould, cocochips and brick pieces (4:2:1, by volume). The total N, P and K contents of the leaf mould were 0.22%, 0.10% and 0.56%, respectively, and those of the cocochips were 0.17%, 0.03% and 0.75%, respectively. The brick pieces contained trace amounts of N, P and K.
The day temperature during winter (November to March) varied from 10 to 15 °C and during the wet season (April to October) it varied from 15 to 25 °C. The different graded doses of NPK fertiliser were prepared by mixing the appropriate quantities of ammonium nitrate, ammonium dihydrogen phosphate and potassium nitrate. The treatments comprised T1 (control), T2 (20:20:20 NPK at 1 mS cm−1), T3 (15:5:25 NPK at 1 mS cm−1), T4 (12:30:10 NPK at 1 mS cm−1), T5 (20:20:20 NPK at 1.5 mS cm−1), T6 (15:5:25 NPK at 1.5 mS cm−1), T7 (12:30:10 NPK at 1.5 mS cm−1), T8 (20:20:20 NPK at 2.0 mS cm−1), T9 (15:5:25 NPK at 2.0 mS cm−1), and T10 (12:30:10 NPK at 2.0 mS cm−1). The control treatment received only stream water (EC 0.5 mS cm−1; pH 6.2; water-soluble N [ and
] 84 mg kg−1; water-soluble P 34 mg kg−1; water-soluble K 60 mg kg−1; water-soluble Ca 10 mg kg−1; water-soluble Mg 5.5 mg kg−1; and water-soluble iron [Fe], manganese [Mn], copper [Cu] and zinc [Zn] of 0.6, 0.43, 0.08 and 0.12 mg kg−1, respectively) throughout the experimental period. During winter (November to February), application of fertiliser was restricted to once per month, whereas in other seasons fertiliser was applied at 15-day intervals. Plants were irrigated twice per week during March to October and once per week during November to February. Flowering was initiated in October 2009, i.e. two years after planting.
The flowering parameters such as number of spikes per plant, number of florets per spike, spike length and rachis length were recorded in 2009–2010. The EC of the leachate was measured at vegetative and reproductive stages. The leachate was collected using the pour-through technique: three representative pots were labeled for each replication and deionised water was applied to saturate the pot without leaching. The pots were placed on clean saucers and enough deionised water was poured over the surface of the medium in each pot to obtain approximately 70 ml leachate. Deionised water was applied to the pots slowly and evenly to prevent channeling of water through the mix or down the sides of the container and thus prevent dilution of the leachate. The leachate from three saucers was poured into a jar, swirled to make it evenly mixed, then subjected to routine analysis of EC (Wright Citation1986). The EC was measured with a conductivity meter (CM 180, ELICO, Hyderabad, India).
Leaf and flower spikes were collected from all treatments during flowering. All samples were dried in the shade and finally in a hot air oven at 65 °C, ground and passed through a 80-mesh sieve (180 µm). Dried samples (1.0 g) were digested with a diacid mixture (HNO3:HClO4 = 9:4; Jackson Citation1973). After digestion of the samples, the total N was determined with the micro-Kjeldahl method (Bremner Citation1965), total P was determined with the vanodomolybdophosphoric acid yellow-colour method (Jackson Citation1973), and total K was determined with the flame photometric method (Jackson Citation1973). The following methods were used to determine water-soluble nutrient contents: the method of Subbiah and Asija (Citation1956) for N, that of Watanabe and Olsen (Citation1965) for P, and the flame photometric method (Jackson Citation1973) for K. Water-soluble Ca and Mg were determined by the versanate method (Hesse Citation1971). Water-soluble Fe, Mn, Cu and Zn were measured with an atomic absorption spectrophotometer (AAnalyst 100, Perkin Elmer, Norwalk, CT, USA).
The experiment was set up in a completely randomised design with three replications per treatment and consisting of 10 plants per replication (i.e. 30 plants per treatment). Thus, a total of 300 plants were used for the 10 treatments in the study. All data were analysed by analysis of variance (ANOVA). Multiple comparisons were performed with Duncan's multiple range test using the MSTATC statistical computer programme (version 5; Michigan State University, East Lansing, MI, USA).
Results and discussion
Growth parameters
Electrical conductivity of the NPK fertiliser solution significantly (F 9,20 = 2.39, p ≤ 0.05) influenced the growth parameters of Cymbidium ‘Sleeping Nymph’ (). The highest plant height, leaf length, pseudobulb length, pseudobulb girth and pseudobulb number (80.9 cm, 60.6 cm, 6.57 cm, 3.78 cm and 7, respectively) were recorded in plants that received the T5 treatment (20:20:20 NPK at 1.5 mS cm−1). Plant height in the T5 treatment was 24% higher than that of the control and was similar to that recorded in treatments T8 and T10. An increase of 25.5% in leaf length over the control was recorded in treatment T5 and similar increases were recorded in treatments T2, T7, T8 and T10. The pseudobulb length and girth gradually increased with increasing concentrations of fertiliser solution with an EC of up to 1.5 mS cm−1 and thereafter showed a declining trend. The pseudobulb length and girth showed significant differences among the treatments and an increase of 44% and 40%, respectively, over that of the control was recorded in treatment T5.
Table 1 : Effect of electrical conductivity of the fertiliser solution on growth of Cymbidium ‘Sleeping Nymph’. Within a column, means followed by the same superscript letter are not significantly different at the 0.05 level of probability with Duncan's multiple range test
Consistent with our results, Barman et al. (Citation2008) found that application of 200 ppm each of N, P and K resulted in the highest pseudobulb diameter in Cymbidium ‘Soulhunt- 6’. The pseudobulb number per clump gradually increased with increasing concentrations of fertiliser solution with an EC of up to 2 mS cm−1; an increase of 55.5% over the control was recorded in treatment T8, which was statistically similar to treatments T5, T6, T9 and T10. These results confirm that the number of pseudobulbs per clump increased with elevation in EC of the fertiliser solution. However, growth parameters such as plant height, leaf length, pseudobulb length and pseuodbulb girth gradually increased with EC of the fertiliser solution up to 1.5 mS cm−1, irrespective of fertiliser dose (20:20:20, 15:05:25 or 12:30:10 NPK). The increase in growth parameters up to EC of 1.5 mS cm−1 might be because of the increasing concentration of N, P and K in the growing medium and resulted in improved nutrient uptake leading to optimal growth of the plant. However, at a higher EC, salts accumulated in the medium. This was evident from the pour-through leachate and might have severely affected the roots, leading to lower nutrient uptake and consequently reduced growth.
Further increase in EC of the 20:20:20, 15:05:25 and 12:30:10 NPK solutions above 1.5 mS cm−1 caused a gradual decrease in the growth parameters (). In a study by Wang (Citation1998), it was revealed that a nutrient solution EC of 1.4 dS m−1 resulted in increased root injury and stunted growth in Phalaenopsis hybrids. In the present study, the fertiliser dose of 20:20:20 NPK for all levels of EC (1–2 mS cm-1) was superior to doses of 15:05:25 and 12:30:10 NPK in terms of growth characteristics. The results of the present investigation are in agreement with the findings of Naik et al. (Citation2010), who observed that a 20:20:20 NPK ratio at 0.1% was suitable for growth of Cymbidium ‘Pine Clash Moon Venus’ at the intermediate growth stage (second-year growth). Plant growth parameters were increased in response to higher EC of the fertiliser solution, which agreed with the findings of Lee and Lin (Citation1987) and Wang and Gregg (Citation1994).
Flowering parameters
Application of different graded doses of fertiliser solution with different EC significantly (F 9,20 = 2.39, p ≤ 0.05) influenced flowering parameters such as number of spikes per plant, number of floret per spike, spike length and rachis length over those of the control (). However, a fertiliser solution with EC of 2 mS cm−1 (T8, T9 and T10) resulted in no flower initiation. It was observed that application of 12:30:10 NPK at 1 mS cm−1 (T4) resulted in the highest flowering parameters among all of the treatments. The highest number of spikes per plant (1.75) was recorded in treatment T4 and closely followed by treatment T6 (15:5:20 NPK at 1.5 mS cm−1). The highest number of florets per spike of 11.25 was recorded in treatment T4 followed by T3 (15:5:25 NPK at 1 mS cm−1) and T6 (15:5:20 NPK at 1.5 mS cm−1).
Figure 1: Effect of electrical conductivity of the fertiliser solution on flowering of Cymbidium ‘Sleeping Nymph’. Bars are the mean ± SE (n = 15). T1 = Control, T2 = 20:20:20 NPK at 1 mS cm−1, T3 = 15:5:25 NPK at 1 mS cm−1, T4 = 12:30:10 NPK at 1 mS cm−1, T5 = 20:20:20 NPK at 1.5 mS cm−1, T6 = 15:5:25 NPK at 1.5 mS cm−1, T7 = 12:30:10 NPK at 1.5 mS cm−1, T8 = 20:20:20 NPK at 2.0 mS cm−1, T9 = 15:5:25 NPK at 2.0 mS cm−1, T10 = 12:30:10 NPK at 2.0 mS cm−1
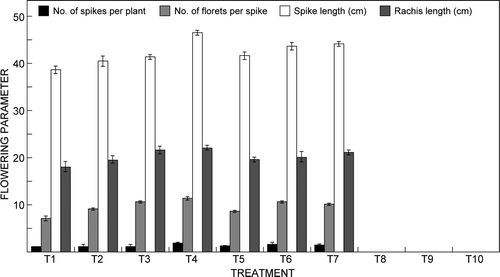
The highest spike length of 46.35 cm was registered in treatment T4 followed by T3. Similarly, rachis length was highest (22 cm) in treatment T4 followed by treatment T3. Wang (Citation1996) observed that Phalaenopsis plants fertigated with reverse osmosis water with EC of 0.03 mS cm−1 produced flowers of larger size compared with plants fertigated with municipal water with EC of 1.4 mS cm−1. Gordon (Citation1990) recommended that fertiliser high in P be used prior to development of the inflorescence in order to achieve the best flowering in Phalaenopsis. The number of spikes per plant gradually increased with increasing concentration of fertiliser solution up to EC of 1.5 mS cm−1. Further increase in EC of the fertiliser solution inhibited flowering. The NPK dose of 12:30:10 at EC of 1 or 1.5 mS cm−1 was superior to 20:20:20 and 15:5:25 NPK doses to increase the flower number of Cymbidium ‘Sleeping Nymph’.
The results of the present investigation support those of Wang and Gregg (Citation1994), who observed that increased application rate of 20:8.6:16.6 NPK fertiliser from 0.25 to 1.0 g l−1 resulted in increased flower number and stalk diameter of a white-flowered Phalaenopsis hybrid for two consecutive flowering seasons. High N application rates to Cymbidium reduces the inflorescence:shoot ratio because of poor inflorescence production and vigorous vegetative shoot formation (Arnold Bik and van den Berg Citation1983, de Kreij and van den Berg Citation1990, Lunt and Kofranek Citation1961, Powell et al. Citation1988). In Cymbidium Mary Pinchess ‘Del Rey’, high EC associated with high N concentration in the nutrient supply, compared with low EC, resulted in a higher number of inflorescences per unit greenhouse area (de Kreij and van den Berg Citation1990). However, this increase reflected the increased number of vegetative shoots that produced inflorescences, not the promotion of reproductive growth.
Nutrient content in the plant
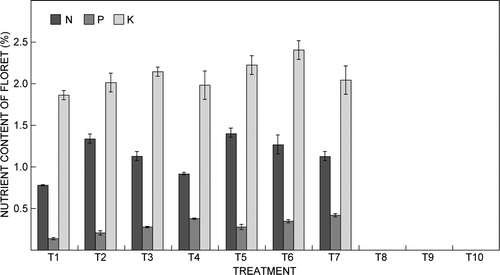
The N, P and K contents of leaves at harvest () showed a significant (F 9,20 = 2.39, p −1 ≤ 0.05) increase with increasing concentration in the fertiliser solution applied. The highest N, P and K contents of leaves were 2.21%, 0.66% and 1.98%, respectively, in the treatments T8 (20:20:20 NPK at 2.0 mS cm−1), T10 (12:30:10 NPK at 2.0 mS cm−1) and T9 (15:5:25 NPK at 2.0 mS cm−1). A higher EC of the fertiliser solution might have resulted in better maintenance of N, P and K contents in the growing medium, which enhances the nutrient content in the leaf (Wang Citation1998).
Table 2 : Effect of electrical conductivity of the fertiliser solution on the leaf nitrogen (N), phosphorus (P) and potassium (K) contents at flowering. Within a column, means followed by the same superscript letter are not significantly different at the 0.05 level of probability with Duncan's multiple range test
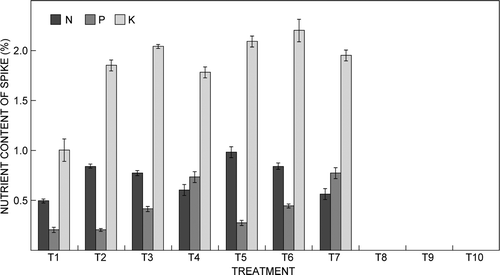
The N, P and K contents of florets gradually increased with application of NPK fertiliser solution with EC up to 1.5 mS cm−1 (). However, plants treated with fertiliser solution with EC of 2 mS cm−1 did not produce flowers. The highest N content of florets was 1.4% in treatment T5, which was 82% higher than that of the control. The P content of florets was highest at 0.41% in treatment T7 (12:30:10 NPK at 1.5 mS cm−1), which represented a 215% increase over that of the control. The highest K content of florets was 2.39% in treatment T6 (15:5:25 NPK at 1.5 mS cm−1), which was 29% higher than that of the control. The N, P and K contents of the spike () increased with increasing EC of fertiliser NPK up to 1.5 mS cm−1. The highest N, P and K contents of the spike were 0.98%, 0.77% and 2.2% in treatments T5, T7 and T6, respectively.
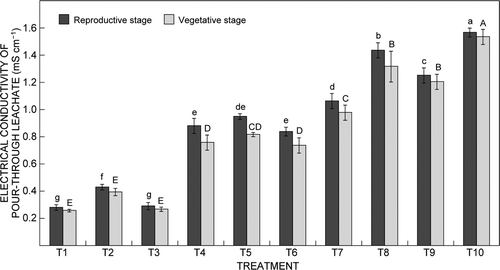
Leachate analysis
Application of different graded doses of NPK with different EC significantly (F 9,20 = 2.39, p ≤ 0.05) influenced the EC of the leachate, which in turn influenced the flowering parameters of Cymbidium ‘Sleeping Nymph’ (). The increasing EC of the pour-through leachate throughout the experiment might be due to the increased concentration of soluble salts in the leachate resulting from the application of different graded doses of NPK. This result supports findings of Wang (Citation1996), who reported that increasing fertiliser concentration caused increase in the EC of the leachate. Application of the 12:30:10 NPK dose resulted in the highest EC of the pour-through leachate from the growing medium followed by 20:20:20 NPK, and the lowest EC was recorded with the 15:5:25 NPK dose, at all levels of EC throughout the experiment. The optimal flowering parameters recorded in treatment T4 resulted in EC of the pour-through leachate of 0.876 mS cm−1. Further increase in EC of the pour-through leachate above 0.876 mS cm−1 resulted in a gradual decrease in the flowering parameters. The permissible limit of EC of the pour-through leachate for optimal flowering was 0.8–1.0 mS cm−1. However, the EC of the pour-through leachate did not affect the growth phase of Cymbidium ‘Sleeping Nymph’, even at a higher EC of the pour-through leachate of 1.5 mS cm−1 in treatment T10, and significant improvements in growth parameters were observed.
Figure 4: Effect of electrical conductivity (EC) of the fertiliser solution on changes in EC of the pour-through leachate at different growth stages of Cymbidium ‘Sleeping Nymph’. Bars are the mean ± SE (n = 15). Bars with the same letter are not significantly different at the 0.05 level of probability with Duncan's multiple range test
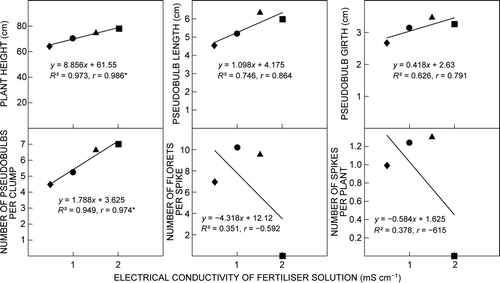
Correlation and regression analyses between the EC of the fertiliser solution and plant growth and flowering parameters are shown in . A linear negative correlation was observed between EC of the fertiliser solution with flowering parameters such as number of florets per spike and number of spikes per plant. However, the correlations between EC of the fertiliser solution and growth parameters such as plant height, pesudobulb length and girth, and number of pesudobulbs per clump were positive. The highest negative correlation, between EC of the fertiliser solution and number of spikes per plant, was −0.615. The highest significant positive correlation was 0.986 (p ≤ 0.05) between EC of the fertiliser solution and plant height. Linear regression analysis indicated that EC of the fertiliser solution accounted for 97.3%, 74.6%, 62.6%, 94.9%, 35.1% and 37.8% of the variation in plant height, pesudobulb length, pseudobulb girth, number of pseudo-bulbs per clump, number of florets per spike and number of spikes per plant, respectively.
Conclusions
The fertiliser solutions with EC of 1.5 mS cm−1 were optimal for growth of Cymbidium ‘Sleeping Nymph’. A higher EC of the fertiliser solution up to 2 mS cm−1 resulted in a higher number of pseudobulbs per clump and decreased the pseudobulb girth. A fertiliser solution with EC up to 1 mS cm−1 was optimal for flowering of Cymbidium ‘Sleeping Nymph’. An EC higher than 1 mS cm−1 had a severely detrimental effect on flowering. A pour-through leachate with EC of 0.8–1.0 mS cm−1 was adjudged to be the maximum permissible limit for flowering of Cymbidium ‘Sleeping Nymph’.
References
- Arnold Bik , R and van den Berg , ThJM. 1983 . Effect of substrate and nitrogen supply on yield and quality of mini-Cymbidium . Acta Horticulturae , 150 : 289 – 295 .
- Barman , D , Rajni , K , Naik , SK and Upadhyaya , RC. 2008 . Production of Cymbidium Soulhunt - 6 by manipulating cultural practices under partially modified greenhouse . Indian Journal of Horticulture , 65 : 69 – 72 .
- Bremner , JM. 1965 . “ Total nitrogen ” . In Methods of soil analysis, part 2: Chemical and microbiological properties , Edited by: Black , CA . 1149 – 1178 . Madison : American Society of Agronomy .
- De Kreij , C and van den Berg , ThJM. 1990 . Effect of electrical conductivity of the nutrient solution and fertilization regime on spike production and quality of Cymbidium . Scientia Horticulturae , 44 : 293 – 300 .
- Gordon , B. 1990 . Culture of the Phalaenopsis orchid , Rialto , California : Laid-Back Publications .
- Hesse , PR. 1971 . A textbook of soil chemical analysis , London : John Murray Publishers .
- Imamura JS , Higaki T , Kunisaki J. 1986 . Interactions of culture, medium and fertilizer on Dendrobium Jacquelyn Thomas . HITAHR Research Series 050 . Manao : College of Tropical Agriculture and Human Resources, University of Hawaii .
- Jackson , ML. 1973 . Soil chemical analysis , New Delhi : Prentice Hall of India .
- Lee N , Lin MG. 1987 . Controlling the flowering of Phalaenopsis . In: Chang LR (ed.), Proceedings of the symposium on forcing culture of horticulture crops . Special Publication no. 10 . Taichung , , Taiwan : Taichung District Agricultural Improvement Station . pp 27 – 43 .
- Lunt , OR and Kofranek , AM. 1961 . Exploratory nutritional studies on cymbidiums using two textures of fir bark . American Orchid Society Bulletin , 30 : 297 – 302 .
- Miles , K. 1982 . Growing equitant oncidiums . American Orchid Society Bulletin , 51 : 155 – 160 .
- Moss , RC. 1955 . Effect of soil mixtures on root growth of Cymbidium orchids . American Orchid Society Bulletin , 24 : 313 – 314 .
- Naik , SK , Barman , D and Medhi , RP. 2010 . Response of Cymbidium Pine Clash Moon Venus to major nutrient at vegetative growth stage . Journal of Ornamental Horticulture , 13 : 182 – 188 .
- Poole , HA and Seeley , JG. 1978 . Nitrogen, potassium and magnesium nutrition of three orchid genera . American Orchid Society Bulletin , 104 : 485 – 488 .
- Poole , HA and Sheehan , TJ. 1973 . Chemical composition of plant parts of Cattleya orchids . American Orchid Society Bulletin , 42 : 889 – 895 .
- Powell , CLL , Caldwell , KI , Littler , RA and Warrington , I. 1988 . Effect of temperature regime and nitrogen fertilizer level on vegetative and reproductive bud development in Cymbidium orchids . Journal of the American Society of Horticultural Science , 133 : 552 – 556 .
- Sheehan TJ. 1958 . Orchid potting media . In: Dillon GW (ed.), Proceedings of the Second World Orchid Conference , 19–23 September 1957 , Honolulu , Hawaii . Cambridge , Massachussetts : Harvard University Printing Office . pp 226 – 230 .
- Sheehan , TJ. 1961 . Effect of nutrition and potting media on growth and flowering of certain epiphytic orchids . American Orchid Society Bulletin , 30 : 289 – 292 .
- Subbiah , BV and Asija , GL. 1956 . A rapid procedure for the determination of available nitrogen in soils . Current Science , 25 : 259 – 260 .
- Tang , S and Qi , Z. 1999 . Study on the nutrition properties of Dendrobium and the fertilization technique . Acta Horticulturae Sinica , 26 : 184 – 187 .
- Wang , YT and Gregg , LL. 1994 . Medium and fertilizer affect the performance of Phalaenopsis orchids during two flowering cycles . HortScience , 29 : 269 – 271 .
- Wang , YT. 1996 . Effect of six fertilizers on vegetative growth and flowering of phalaenopsis orchids . Scientia Horticulturae , 65 : 191 – 197 .
- Wang , YT. 1998 . Impact of salinity and media on growth and flowering of a hybrid Phalaenopsis orchid . HortScience , 33 : 247 – 250 .
- Watanabe , FS and Olsen , SR. 1965 . Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil . Soil Science Society of America Journal , 29 : 677 – 678 .
- Wright , RD. 1986 . The pour-through nutrient extraction procedure . HortScience , 21 : 227 – 229 .
- Yoneda , K , Usui , M and Kubota , S. 1997 . Effect of nutrient deficiency on growth and flowering of Phalaenopsis . Journal of the Japanese Society for Horticultural Science , 66 : 141 – 147 .