Abstract
The southern part of Mozambique is vulnerable to drought, affecting the country's food production, and thus requires more drought-tolerant crops. Four local cowpea landraces, Massava nhassenje, Timbawene moteado, Namarua and Tete-2, which are currently widely used by local Mozambican farmers, were evaluated for their drought tolerance with the aim to identify the most drought-tolerant landrace and also a phenotypic marker easily applicable for drought-tolerance selection under local Mozambican conditions. Above- and below-ground plant characteristics, including biomass, protein content, proteolytic activity, symbiotic nitrogen fixation and nodule number, were measured in a greenhouse under well-watered and drought conditions using vermiculite as the plant growth medium. The key finding was that variability exists among the landraces for growth under drought with Timbawene moteado displaying significantly higher leaf dry biomass, leaf and nodule protein content, and symbiotic nitrogen fixation and the lowest increase in proteolytic activity compared to all other landraces. Timbawene moteado might be suitable for inclusion into a future cowpea breeding program in Mozambique and might also be tested in other areas in Mozambique experiencing drought stress. Furthermore, leaf dry biomass might be selected as a simple and informative marker for future screening of the Mozambican cowpea germplasm for drought tolerance.
Introduction
Cowpea (Vigna unguiculata (L.) Walp.) is an important protein and mineral source in Mozambique (Aykroyd and Doughty Citation1982; Doto et al. Citation1993; Badiane et al. Citation2004). It also fixes atmospheric nitrogen by root nodule bacteria. The latter characteristic avoids the widespread use of chemicals, saves existing soil nitrogen supplies from depletion and improves the organic content of soil by acting as a green soil fertiliser, thus contributing greatly to sustainable agriculture.
In Mozambique, drought considerably reduces cowpea productivity, especially in areas where the agricultural system is dependent on rainfall. Drought is among the most difficult challenges faced by resource-poor farmers (Serraj et al. Citation1999). Plants have numerous morphological, physiological, metabolic and molecular responses to water stress (Chaves et al. Citation2003). However, significant differences exist among cowpea genotypes in their response to drought (Watanabe et al. Citation1997; Mai-Kodomi et al. Citation1999). Stomatal closure has been identified as a common response to drought in cowpea (Ogbannaya et al. Citation2003; Anya and Herzog Citation2004; Souza et al. Citation2004; Hamidou et al. Citation2007).
The Mozambican National Gene Bank has recently started collecting the country's national heritage of plant genetic resources. A considerable number of cowpea accessions (144) have so far been collected in 31 districts across Mozambique. Landraces are generally defined as a population of cultivated plants with a historical background and identity but without any breeding improvement (Camacho Villa et al. Citation2005). Resource-poor farmers grow these unimproved landraces despite the availability of improved cultivars that often perform worse under certain environmental conditions, involve high seed cost as well as require substantial fertiliser input (Almekinders et al. Citation1994; Keleman et al. Citation2009; Bellon and Hellin Citation2011). The aim of this study was to compare landrace performance under drought and to possibly identify a morphological, physiological or biochemical marker easily applicable under local Mozambican conditions using locally available skills and equipment. Four cowpea landraces obtained from the gene bank were tested in a greenhouse in South Africa at the Forestry and Agricultural Biotechnology Institute, University of Pretoria, using vermiculite as planting medium. Characteristics for landrace evaluation were primarily selected on the basis that they can also be easily measured under local conditions in Mozambique. The four evaluated cowpea landraces are the most commonly grown landraces in different parts of Mozambique characterised by low and unpredictable precipitation and low soil fertility. Landraces used in our study were Namarua (grown in the Zambézia, Nampula and Cabo Delgado provinces in the centre and north of Mozambique), Massava nhassenje (grown in the Inhambane province in the south of Mozambique), Timbawene moteado (grown in the Maputo and Gaza province in the south of Mozambique) and Tete-2 (grown in the central semi-arid very warm areas prone to drought). Timbawene moteado is also known to have limited seed shattering, a common cowpea problem, and Tete-2 has been previously classified in a field study as high yielding and drought tolerant (Chiulale Citation2010).
Materials and methods
Cowpea seeds of landraces (Massava nhassenje, Timbawene moteado, Namarua and Tete-2) were obtained from the Mozambican Germplasm Collection Bank. Temperature-controlled greenhouse experiments were carried out at the Forestry and Agricultural Biotechnology Institute, University of Pretoria, South Africa, at a light intensity of 600 mmol m-2 s-1 with a 13 h photoperiod. Additional artificial light for 3 h in the evening was provided by metal-halide lamps for elongation of day length to 13 h. A day/night temperature of 27 °C/17 °C and 60% of relative humidity were used. The environmental conditions in the growth room were monitored regularly to ensure that adequate growth conditions were maintained.
Seeds were placed into a small hole containing 0.5 g Bradyrhizobium powder (Stimuplant CC, South Africa) in 17.5 cm × 20 cm diameter pots (one seed per pot) containing fine grade vermiculite (Mandoval PC, South Africa) after being presoaked with deionised water. Plants were grown in vermiculite to allow easier analysis of root nodules. Plants grown in vermiculite were watered twice daily (morning and evening) with normal tap water to maintain a field capacity of 100%, which was determined by a gravimetric method. Since vermiculite does not contribute to fertility, plants were fertilised twice per week with a nitrogen-free Hoagland nutrient solution (Hoagland and Arnon Citation1950), instead of tap water, to allow nodule formation.
The experimental design was a complete randomised block with four landraces (four plants per landrace for each individual treatment) and two watering regimes (well-watered and drought treated). During the experimental period pots were rearranged periodically. Drought stress was introduced when plants had grown to the third foliar stage. In the experiment, half of experimental plants at the third foliar stage were maintained for two weeks under well-watered conditions (100% field capacity) whereas half of the experimental plants were subjected to drought stress by completely withholding the supply of water and nutrient solution for two weeks. After two weeks drought exposure, the experiment was terminated and all plants (well-watered and drought treated) were harvested to carry out the analysis. The complete experiment with all landraces was repeated once resulting in eight different treatment combinations per experiment.
Cowpea crown nodules were harvested from well-watered and drought-treated plants and crown nodule number counted and biomass (fresh weight) was determined by weighing. The dry weight of leaves and roots was determined after drying the plant material at 80 °C for 48 h. For determination of symbiotic nitrogen fixation (SNF), all formed nodules (crown and lateral) were harvested and the acetylene reduction assay according to Turner and Gibson (Citation1980) using a gas chromatograph (Varian, Walnut Creek, CA, USA) was applied.
Total protein was determined from leaves and crown nodules with a Bradford protein determination assay kit (Bio-Rad, Watford, UK) with bovine serum album as a standard (Bradford Citation1976). Cysteine protease activity was also measured from leaves and crown nodules as an additional biochemical marker with a fluorimeter (Fluostar Galaxy, BMG, Offenburg, Germany) with an excitation and emission wavelength of 355 and 460 nm, respectively. Substrate-specific protease activity was monitored with the synthetic fluorogenic MCA substrate Z-Phe-Arg-MCA (cathepsin L-like substrate; Sigma-Aldrich) by monitoring the substrate hydrolysis progress curve in 100 mM citrate phosphate buffer, pH 6.0 (Salvesen and Nagase Citation1989).
Data were analysed with the JMP 5.0 statistical package (SAS Institute, Inc., Cary, NC, USA, 2011). The significance level was set at 5%.
Results
When plants were grown in a temperature-controlled greenhouse in vermiculite under either well-watered or drought conditions (), leaf biomass decreased significantly (p ≤ 0.05) in all landraces after exposure to drought (). In comparison, root biomass significantly (P ≤ 0.05) increased, except for Tete-2, in all landraces as a response to drought treatment. When nodule performance was measured, nodule biomass, nodule number and SNF were significantly (p ≤ 0.05) lower in drought-treated than in well-watered landraces (). When comparing individual landraces, drought-treated Timbawene moteado had higher leaf and root dry biomass as well as higher leaf and nodule protein content ( and ). At the same time this landrace had also the highest nodule biomass and nodule number and significantly (P < 0.05) higher SNF under drought (). In contrast, Massava nhassenje had the lowest biomass (leaf and root) and also protein content (leaf and nodule) ( and ) as well as the lowest nodule biomass and SNF, together with Namarua, and a significantly (p ≤ 0.05) lower nodule number when compared to all other landraces ().

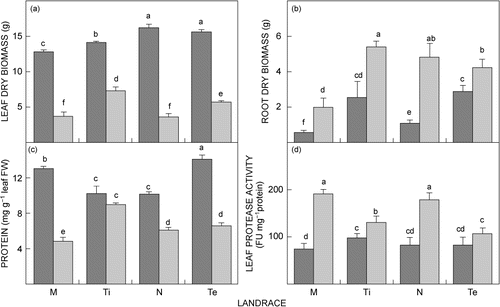
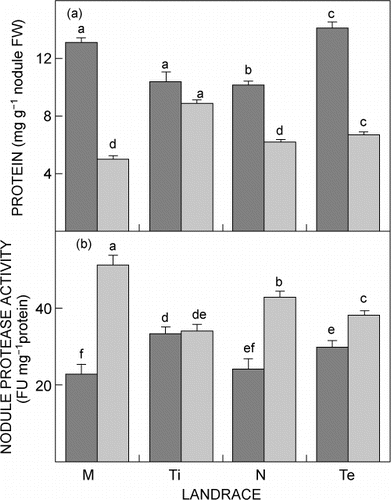
Table 1: Nodule biomass (fresh weight), nodule number and symbiotic nitrogen fixation (SNF) in four cowpea landraces grown for two weeks under well-watered (WW) and drought (D) conditions in a temperature-controlled greenhouse. Means followed by the same superscript letter within a column are not significantly different as determined by a Tuckey HSD test (P ≤ 0.05). Data are the means of four plants ± SE for each landrace
Given that cowpea is an important protein source, with young leaves a dietary staple food where access to animal protein is limited, the effect of drought stress in the protein content of leaves of landraces was determined. Drought stress significantly (p ≤ 0.05) decreased the leaf protein content in all landraces except in Timbawene moteado which showed almost no decrease. The greatest decrease was found in landrace Massava nhassenje (). Protein content, as a result of the proteolysis, was also investigated. Leaves of all landraces, except for Timbawene moteado and Tete-2, showed significant (P ≤ 0.05) increase in cysteine protease activity after exposure to drought, with the lowest increase in Timbawene moteado and the highest in Massava nhassenje (). Similarly, nodules showed, after exposure to drought stress, lowest protein decrease in Timbawene moteado and Tete-2 and the highest decrease in Massava nhassenje (). Under drought conditions, Timbawene moteado had the highest total nodule protein content (P ≤ 0.05) of all landraces ( and ).
Discussion
Screening and selection of different cowpea landraces for drought tolerance using phenotypical and physiological traits are important for the development of new drought-tolerant cowpea cultivar(s) in Mozambique. Our study has greatly contributed to selection of potential landraces for any future breeding program by evaluating several phenotypic and physiological traits in well-used landraces currently grown in Mozambique. Except for Tete-2, which was recently evaluated for drought tolerance in a field study (Chiulele Citation2010), none of the landraces investigated in our study have, to our knowledge, been investigated in more detail for their drought tolerance. Data for cowpea landraces obtained in our study for biomass production, SNF, protein content and proteolytic activity in above- and below-ground plant parts therefore extend currently available landrace data, consisting predominantly on seed characteristics, in the Mozambican national gene bank.
Although drought affected the performance of all landraces, variability was found among the investigated landraces. Massava nhassenje was the most drought-sensitive landrace with lowest biomass accumulation, lowest protein content and highest increase in proteolysis under drought conditions. In contrast, landrace Timbawene moteado performed best of all landraces tested under drought. This landrace had higher leaf biomass and lowest increase in proteolytic activity, which was directly related to higher leaf protein content. Characteristics correlated with higher nodule number and biomass as well as SNF. It can therefore be postulated that stable nodule performance in Timbawene moteado, even under drought conditions, enables this landrace to fix more nitrogen, supply more nitrogen compounds via xylem to the shoot, which finally allows better growth. Significant relationships exist between SNF and leaf parameters, such as photosynthetic CO2 assimilation rates, stomatal conductance values and intracellular CO2 concentration, as recently found for soybean (Fenta et al. Citation2012). Symbiotic nitrogen fixation activity is, however, rapidly inhibited in dry soil, affecting the life-span of nodules and causing premature nodule senescence, limiting the nitrogen supply for plants (Fenta et al. Citation2011, Citation2012). A relationship between drought tolerance and the presence of larger nodules has also been previously reported by King and Purcell (Citation2001). Overall, our study has confirmed the existence of a close relationship between the plants capacity for nitrogen acquisition in nodules and the above-ground performance under different environmental conditions.
In a recent field assessment of drought affecting yield of Mozambican cowpea landraces, Tete-2 was identified as a relatively high-yielding landrace under drought conditions (Chiulele Citation2010). However, Timbawene moteado, tested in our study, was not included in this field evaluation. In our greenhouse study, Tete-2 was surprisingly rather moderately drought tolerant when compared to Timbawene moteado. In our opinion, Timbawene moteado, originating from an area characterised by low and unpredictable precipitation, therefore would be well-suited for growth under drought conditions. Our results also indicate the importance of characterising landraces in Mozambique to prevent loss of favoured traits. Timbawene moteado, despite that the darker seed colour of the landrace is less preferred by Mozambican farmers, has, in our opinion, potential as a source for breeding programs and should also be tested in the future in the field particularly in the central, semi-arid very warm areas of Mozambique prone to drought where Tete-2 is performing well. In comparison, Massava nhassenje is poor-yielding in the southern part of Mozambique where periods of drought are experienced. Our study confirmed such poor performance under drought. In our opinion, this landrace might therefore be better suited for areas under irrigation and not for rain-fed areas.
Overall, characterisation of landraces is crucial for effective conservation and exploitation of genetic resources in any crop improvement program. To our knowledge, this study was the first more detailed investigation on the phenotypical and physiological characterisation of Mozambican cowpea landraces for drought tolerance under greenhouse conditions. Comparing the performance parameters measured in our study, particularly above-ground biomass (leaf dry biomass) determination, was a simple method for cowpea germplasm screening. Shenkut and Brick (Citation2003) already demonstrated that shoot biomass accumulation highly correlates with seed yield. Measuring only shoot biomass in cowpea might, therefore, provide direct first information on the expected seed yield. Future research with more landraces has, however, to demonstrate if simple measurement of leaf dry biomass indeed can be applied as a useful marker for drought tolerance. In this regard, we also recently carried out a tunnelhouse experiment in Mozambique where these landraces were grown in soil in an open-sided tunnel-house and exposed for four weeks to drought conditions (Martins unpublished data). In this tunnelhouse study, we could confirm that Massava nhassenje is the most drought-sensitive and Timbawene moteado again the most drought-tolerant landrace. Therefore, rapid and inexpensive screening of the gene bank's existing cowpea germplasm for drought tolerance only in a tunnelhouse, and applying methods used in our study, might ultimately be sufficient and not require more sophisticated equipment and growth facilities.
Acknowledgements
This work was funded by Cooperação UEM-Sida/SAREC and also partially by the British Council via a Development Partnerships in Higher Education grant to the University of Eduardo Modlane in Mozambique (DelPHE 18). CMM thanks Sida/SAREC for providing a PhD fellowship. The authors would also like to thank Dr Urte Schlüter at the University of Düsseldorf, Germany, for helping with the design of the experiments, for fruitful research discussions, critically reading the manuscript and training provided to CMM. We also thank Dr Berhanu Fenta for technical assistance and the Mozambican gene bank for providing cowpea seeds.
References
- Almekinders CJM, Louwaars NP, Bruijn GH. 1994. Local seed systems and their importance for an improved seed supply in developing countries. Euphytica 78: 207–216.
- Anya AO, Herzog H. 2004. Genotypic variability in drought performance and recovery in cowpea under controlled environment. Journal of Agronomy Crop Science 190: 151–159.
- Aykroyd WR, Doughty J. 1982. Legumes in human nutrition. FAO Nutritional Studies no. 20. Rome: Food and Agriculture Organization of the United Nations.
- Badiane FA, Diouf D, Sane D, Diouf O, Goudiaby V, Diallo N. 2004. Screening cowpea (Vigna unguiculata (L.) Walp.) varieties by inducing water deficit and RAPD analyses. African Journal of Biotechnology 3: 174–178.
- Bellon MR, Hellin J. 2011. Plant hybrids, keeping landraces: agricultural modernization and tradition among small-scale farmers in Chapas, México. World Development 39: 1434–1443.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye-binding. Analytical Biochemistry 72: 248–254.
- Camacho Villa TC, Maxtel N, Scholten M, Ford-Lloyd B. 2005. Defining and identifying crop landraces. Plant Genetic Resources 3: 373–384.
- Chaves M, Maroco MJP, Pereira JS. 2003. Understanding plant responses to drought - from genes to the whole plant. Functional Plant Biology 30: 239–264.
- Chiulele RM. 2010. Breeding cowpea (Vigna unguiculata (L.) Walp.) for improved drought tolerance in Mozambique. PhD thesis, University of KwaZulu-Natal, South Africa.
- Doto AL, Honwana C, Santos LA. 1993. Report of cowpea research. Maputo: Eduardo Mondlane University.
- Fenta B, Schlűter U, Garcia BM, du Plessis M, Foyer CH, Kunert KJ. 2011. Identification and application of phenotypic and molecular markers for abiotic stress tolerance in soybean. In: Krezhova D (ed.), Soybean: genetics and novel techniques for yield enhancement. Croatia: InTech. pp 181–200.
- Fenta BA, Driscoll SP, Kunert KJ, Foyer CH. 2012. Characterization of drought tolerance traits in nodulated soybeans. Journal Agriculture and Crop Science 198: 92–103.
- Hamidou F, Zombre G, Braconnier S. 2007. Physiological and biochemical responses of cowpea genotypes to water stress under glasshouse and field conditions. Journal of Agronomy and Crop Science 193: 229–237.
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. California Agricultural Experimental Station Circular no. 347 (revised edn). Berkeley: University of California, College of Agriculture.
- Keleman A, Hellin J, Bellon MR. 2009. Maize diversity, agricultural policy, and farmers’ practices: Lessons from Chiapas, Mexico. Geographical Journal 175: 52–70.
- King CA, Purcell LC. 2001. Soybean nodule size and relationship to nitrogen fixation response to water deficit. Crop Science 41: 1099–1107.
- Mai-Kodomi Y, Singh BB, Myers O Jr, Yopp JH, Gibson PJ, Terao T. 1999. Two mechanisms of drought tolerance. Indian Journal of Genetics 59: 309–316.
- Ogbonnaya CI, Sarr B, Brou C, Diouf O, Diop NN, Roy-Macauley H. 2003. Selection of cowpea genotypes in hydroponics, pots and field for drought tolerance. Crop Science 43: 1114–1120.
- Salvesen G, Nagase H. 1989. Inhibition of proteolytic enzymes. In: Beynon RJ, Bond JS (eds), Proteolytic enzymes: a practical approach. New York: IRL Press. pp 83–104.
- Serraj R, Sinclair T, Purcell L 1999. Symbiotic N2 fixation response to drought. Journal of Experimental Botany 50: 143–155.
- Shenkut AA, Brick MA. 2003. Traits associated with dry edible bean (Phaseolus vulgaris L.) productivity under diverse soil moisture environments. Euphytica 133: 339–347.
- Souza RP, Machad EC, Silva JAB, Lagon AMMA, Silveira JAG. 2004. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environmental and Experimental Botany 51: 45–56.
- Turner GL, Gibson AH. 1980. Measurement of nitrogen fixation by indirect means. In: Bergersen FJ (ed.), Methods for evaluating biological nitrogen fixation. New York: John Wiley and Sons. pp 111–138.
- Watanabe IS, Hakoyama S, Terao T, Singh BB. 1997. Evaluation methods for drought tolerance of cowpea. In: Singh BB, Mohan Raj DR, Dashiell K, Jackai LEN (eds), Advances in cowpea research. Ibadan: International Institute of Tropical Agriculture/ Japan Research Center International Agriculture. pp 87–98.
