Abstract
Little is known about the effects of residue burning or retention on nutrient leaching during the inter-rotation of clonal Eucalyptus grown on the sandy soils of subtropical Zululand, South Africa. A study compared zero-tension nutrient leaching through the top metre of soil at depths of 0.15, 0.5 and 1.0 m in an undisturbed crop with adjacent clearfelled areas subjected to residue burning and residue retention. Leaching at 1.0 m in the undisturbed crop was 80% less than at 0.15 m leaching due to high water use of the mature trees. Loss of nutrients past 1.0 m in the undisturbed crop amounted to 7.0, 13.1, 6.6, 15.1 and 60.7 kg ha−1 of nitrogen (N), potassium (K), calcium (Ca), magnesium (Mg) and sodium (Na) over the period between felling and new crop canopy closure (22 months). Annualised 1.0 m leaching amounted to 4.2, 7.8, 4.0, 9.0 and 36.3 kg ha−1 of N, K, Ca, Mg and Na, respectively. Clearfelling induced an increase in N and cation leaching that was apparent five months after clearfelling and persisted for nine months. Leaching loss declined rapidly in the new crop after planting to levels similar to the undisturbed crop by six months of age. Leaching past 1.0 m soil depth under residue retention amounted to 30.6, 132.0, 82.5, 108.7 and 299.1 kg ha−1 of N, K, Ca, Mg and Na, respectively, between felling and canopy closure. Although some weakly significantly differences were found between residue burning and retention, residue burning did not substantially alter leaching past 1 m soil depth. Burning rather induced a large loss of N (121 kg ha−1) through oxidisation, around half the residue N content. Residue retention or burning followed by rapid re-establishment can therefore be practiced to retain most nutrients on this site. Burning of residues should be practiced conservatively on low N soils or be followed by N fertilisation.
Introduction
The residue and litter layer remaining after clearfelling a plantation forest contains large quantities of nutrients and may need to be retained to ensure present and future nutrient supply (Piatek and Allen Citation1999). Residue and litter stores of carbon and nutrients are more important on sandy soils that are characteristically low in clay, organic matter and fertility (Rosenstrauch Citation1938; Noble et al. Citation2005). The retention, displacement or loss of residues during the inter-rotation phase (clearfelling to tree canopy closure period) can significantly impact the nutrient dynamics of a site (O'Hehir and Nambiar Citation2010). Impacts of such losses can be large on sandy soils where soil nutrient pools are small within the region where fine-root density is greatest (top 0.5 m of the soil [Smethurst and Nambiar Citation1990; Carlyle and Nambiar Citation2001]) and the ability of soil to retain nutrients is low.
The sandy soils of the Zululand coastal ecosystem in South Africa are characterised by low clay and organic matter content, low nutrient and water storage capacity and high drainage rates (Hartemink and Hutting Citation2005). These are characteristics that may ultimately limit nutrient availability and consequently reduce stand productivity. Such soils have limited ability to buffer physical, chemical and biological changes and are at risk of becoming degraded. In addition, the Zululand coast is also characterised by high temperatures and rainfall and low vapour pressure deficit that results in rapid growth of trees, short rotation lengths (6–7 years), a high nutrient demand and rapid litter and residue decomposition (Noble et al. Citation2005; Smith and du Toit Citation2005). The combination of the above factors can lead to accelerated loss of organic matter and nutrients, as nutrients that are not taken up after mobilisation may be transferred through leaching to greater depths in the soil profile or completely lost to deep drainage. Nutrients released from residues, along with increased soil moisture levels during the inter-rotation, can result in accelerated nutrient leaching losses (Fisher and Binkley Citation2000; Gonçalves et al. Citation2007; O'Hehir and Nambiar Citation2010). Nutrient leaching loss can be greater after burning as nutrients in the residues become mobilised, adding to a soil solution already enriched with nutrients from a decomposing root system (Powers et al. Citation2005).
A number of residue management practices are utilised in eucalypt plantations to cater for cost, fire prevention, ergonomics and practicalities around the establishment of a new crop. These practices include residue retention, mulching, displacement (into windrows), removal (for fuel) and onsite residue burning. Residue burning after clearfelling is often practiced as a means of creating a clean site that is easier to plant and manage (Smith and du Toit Citation2005). Residue retention is often avoided as larger fuel-loads increase wildfire risk. However, concerns have been raised within the South African forestry industry around the impacts of residue burning on nutrient loss and tree productivity in current and future rotations. Large losses of organic matter and plant nutrients during residue burning, particularly carbon (C) and nitrogen (N), (and sulphur) can be detrimental to nutrient poor ecosystems (du Toit and Scholes Citation2002).
This study aimed at comparing nutrient leaching (zero tension) within the top metre of the soil between residue retention and burning management practices. The study included plots of undisturbed standing crop of mature clonal Eucalyptus within the same compartment, but adjacent to the clearfelled areas. Nutrient leaching was expected to increase after clearfelling and further increase after residue burning. This was expected to be detrimental to the soil nutrient status of this sandy, low organic matter aeolian soil. The data presented in this study follows from a hydrological description reported in Dovey et al. (Citation2011a).
Materials and methods
Study site
A fast-growth Eucalyptus grandis × E. camaldulensis plantation (mean annual increment 21 m3 ha−1 at seven years) was selected on the nutrient-poor subtopical Zululand coastal plains (northern KwaZulu-Natal, South Africa). The site is located with its centre at 28°17′51″ S and 32°18′55″ E. A more detailed site description is given in Dovey et al. (Citation2011a). Physical and chemical properties of soil samples taken at the start of the study the site confirmed the low organic matter and fertility status, with low cation exchange capacity and high soil acidity ().
Table 1: Basic soil physical and chemical properties of the top 1.0 m (in 0.20 m increments) at the start of the study
Design and layout
The experiment was established at the end of 2007 allowing soil and equipment stabilisation over a year before felling. Felling occurred during late 2008 with timber manually stacked and mechanically extracted. Residues were manually broadcast across the site during felling. A randomised complete block design was utilised with treatments arranged into replicates of four blocks. Replicated treatments were applied as residue retention (broadcast), residue burning, fertilisation (stem wood nutrient loss replacement), residue removal using whole tree harvesting and a standing crop treatment. The crop either side of the felled experimental area was not clearfelled to facilitate replicates of the undisturbed Standing crop treatment (see Dovey et al. Citation2011a for details). Residue burning occurred in March 2009. The site was manually pitted at a 3 m × 2 m espacement and planted to clonal Eucalyptus grandis × E. camaldulensis at the end of July 2009. The inter-rotation period was also prolonged for 10 months to allow for measurable leaching losses to occur.
Due to cost constraints, nutrient leaching under zero tension was recorded on replicates of Standing crop, Burn (residue burning) and No-Burn (residue retention) treatments. This subset of treatments was chosen hypothesised to represent highest and lowest nutrient loss practices, respectively, of the typical residue management operations. The standing crop areas were monitored to compare leaching with and without a harvesting disturbance.
Data collection
Rainfall, air temperature, relative humidity, wind speed and wind direction were monitored using an automatic weather station (Campbell Scientific, Logan, UT, USA). Data were collected at hourly intervals and summarised daily. Atmospheric inputs were monitored as precipitation outside the tree canopy and throughfall plus stemflow under the tree canopy (Dovey et al. Citation2011a, Citation2011b). A soil capacitance profile probe was used to measure volumetric soil water content at depths of 0.1, 0.2, 0.3, 0.4, 0.6, 0.8 and 1.0 m below the soil surface. Soil water was measured each week via five access tubes installed into each plot in five set positions. Probes were positioned at the midpoint of a row, midpoint of an inter-row, midpoint of a row and inter-row, one-third from the stump in a row and one-third from the stump in an inter-row (Dovey et al. Citation2011a). All monitoring was carried out prior to felling, during felling, residue management and re-establishment phases of the plantation cycle until the newly planted trees were 18 months old.
Soil solution collection
Zero-tension plate lysimeters (30 cm × 30 cm) with 3 cm walls on three sides were inserted horizontally at depths of 15, 50 and 100 cm in four replicates of plates at each depth.
Lysimeter plates were used as a cost-effective method to collect samples of drainage water for analysis of the chemical composition of the zero-tension soil solution. The depths were chosen to reflect eucalypt fine-root length and mass density distributions (Knight Citation1999; Laclau et al. Citation2001; Gonçalves and Mello Citation2004; O'Grady et al. Citation2005). Plates were inserted into undisturbed soil in the wall of cresentshaped trenches spanning six tree inter-rows in each plot. Horizontal distances between plates were greater than 60 cm. The method was chosen to ensure a large area of coverage and no soil disturbance above the collection plates to allow comparisons between lysimeter data. Plates were installed at a slight angle to enable drainage to a corner hole. Each plate drained by gravity though a polyurethane tube to a plastic collection bottle. Bottles were stored in a covered pit at 1.3 m belowground for less than a week prior to collection. A more graphically detailed description of this layout and a description of plate insertion are given in Dovey et al. (Citation2011a).
The same solution concentration averaging process was assumed to occur at each plate across the site and represent a flux concentration defined as the mass of solute passing the plate during each drainage event divided by the volume of water that crossed the plate during the same time interval (Laclau et al. Citation2005; Mareschal et al. Citation2013). Plates are reported as having a collection efficiency of about 10% as they generally do not drain as rapidly as the surrounding soil. Low collection efficiency results from plate overflow, water flow around the plate or off the non-walled end, and due to saturation being required at the plate base for drainage to occur (Weihermuller et al. Citation2007). Drainage was therefore interpolated using the one-dimensional Hydrus 1D soil water model (Šimůnek et al. Citation2008) that was parameterised for the study site and matched with in situ daily soil water content at each depth. Zero-tension leaching was determined by multiplying model interpolated drainage volumes with nutrient concentrations in soil solution collected at three depths after Laclau et al. (Citation2005) and Mareschal et al. (Citation2013). Water collected as rainfall (above and below the tree canopy) and predicted soil water drainage are given in Dovey et al. (Citation2011a).
Laboratory analysis
Volume of water draining from each plate was recorded weekly between December 2007 and July 2010, subsampled into 0.25 litre Teflon bottles and placed into a cool box. All subsamples were assessed for pH and electrical conductivity (EC) then refrigerated at 5 °C awaiting bulking and further analysis. Analytical costs necessitated sample bulking. Samples were bulked into four weekly batches prior to analysis by weighting the bulking according to volumes recorded in field.
Total Kjeldahl-N (TKN) (4500-Norg method; Clesceri et al. Citation1998), ammonia N (NH4-N) and nitrate N (NO3-N) (flow injection; Clesceri et al. Citation1998) were determined for each sample. The TKN method included a modification where salicylic acid was added to the catalyst mixture that converts NO3-N to nitric acid, which nitrates the salicylic acid. The nitro-compounds produced were then reduced to ammonia and distilled by adding excess alkali. The cations potassium (K), calcium (Ca), magnesium (Mg) and sodium (Na) were determined using an auto analyser (US EPA Citation1984). Phosphorous (P) and sulphur were initially determined, but later abandoned due to cost and laboratory equipment failure. Organic N was calculated as the difference between TKN and inorganic N (NH4-N + NO3-N).
Tree sampling
Tree biomass and nutrient accretion were determined every six months across the entire site from felling to one year after planting the new crop by destructive harvesting. A dbh (tree diameter at 1.3 m above the ground) size class distribution of all trees on the site was divided into five size classes from which 20 trees were selected. Each harvested tree was separated into foliage, branches, bark and stem wood and wet mass determined. Subsamples were taken of each component and used to determine moisture contents by drying to constant mass at 60 °C. Additional stem discs were dried at 105 °C to account for incomplete drying of larger samples. Wet:dry mass ratios were used to calculate total dry mass of each tree component.
Polynomial and natural log allometric functions were developed to predict tree component masses from individual tree dbh measurements using methods described in Seifert and Seifert (Citation2014).
Litterfall and residue sampling
Standing crop litterfall was collected weekly using five litter traps randomly placed in each treatment plot. Forest litter layer and residue biomass was collected at four weekly intervals using a metal ring (0.34 m diameter) across five random points in each treatment plot. A loss on ignition was used to estimate mass without of soil contamination after oven drying at 60 °C. The same ring method and analyses were used before and after burning to assess biomass and nutrient loss after burning.
Samples from each tree component, litter, residue and ash were individually ground, homogenised and analysed for nutrient concentration (N, P, K, Ca, Mg and Na) at Cedara laboratories, using the methods described in Kalra and Maynard (Citation1991). Nutrient concentrations were multiplied by each tree component to determine nutrient content at the stand level. Stand-level biomass was determined by applying allometric equations developed in this study to stand inventory data.
Soil sampling
Soils were sampled at the start of the study, just before harvesting and at six-monthly intervals thereafter. Soils were collected from five random locations in each treatment plot and bulked according to treatment and sampling depth. Soils were air dried and sieved (2 mm) and analysed for pH, N, P, K, Ca, Mg and Na using the procedures below that are detailed in Donkin et al. (Citation1993). Nitrogen was determined using the Kjeldahl method. Bray-2 extractable P was determined by automatic analysing the filtrate colourimetrically at 880 nm using a segmented flow autoanalyser (SANplus SYSTEM, Skalar Analytical, Breda, The Netherlands) with ascorbic acid colour development. Cations were extracted with ammonium acetate (Ca, Mg, K and Na) extractants diluted with an ionisation suppressant (strontium or caesium) for determination by atomic absorption spectrophotometry (SpectrAA-10, Varian Techtron Pty Ltd, Mulgrave, Victoria, Australia). Soil pH was determined in 1:2.5 soil:1 M potassium chloride solution using a standard glass electrode (Metrohm Hersiau E396B; http://products.metrohm.com).
Statistical analysis
Differences between treatments and sample depths were compared using a general linear model ANOVA for cumulative measures with least significant difference (LSD, 5%) used to determine significance of treatment differences. Standard deviation was calculated on data taken prior to implementation of treatments. Multiple regression was used to develop tree allometric models between individual tree measurements and mass. Statistical probabilities for predictive equations and predictive parameter estimates were all significant at p < 0.001. All models had R2 values greater than 0.96. Error estimates were propagated from the single tree to the stand level using error propagation (Seifert and Seifert Citation2014). These data and models were used to calculated nutrient losses with harvesting and uptake with tree growth, but are not reported here. All statistical analyses were performed using GenStat for Windows, 12th Edition (Payne et al. Citation2011).
Results
Nutrient concentrations in soil solution
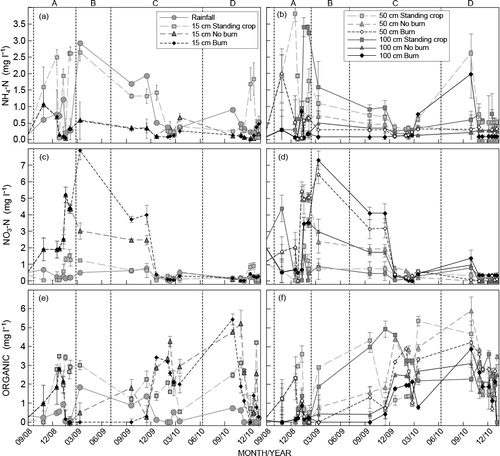
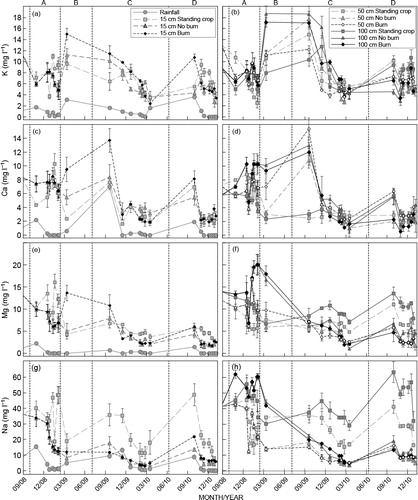
All standing crop treatment concentrations at 0.15 m soil depth followed similar trends to atmospheric input N concentrations (). Base cation concentrations were significantly higher in the soil solution at all depths than in atmospheric inputs (). With few exceptions, cation concentrations were two- to eight-fold higher in the soil solution at all depths than in atmospheric inputs. Soil solution concentrations increased after clearfelling and again after burning for all elements across all clearfelled treatments. This increase was most evident for the cations. Soil solution NH4 +, organic-N and Na concentrations were higher in the standing crop treatment than in the new crop at all depths after felling ( and ). Concentrations of NO3 –, K+, Ca2+ and Mg2+ were lower in the standing crop treatment than in the new crop at all depths after felling ( and ). Soil solution NH4 + and organic-N concentrations were similar between the Burn and No burn treatments at all depths between felling and canopy closure of the new crop. Higher NO3−, K+, Ca2+ and Mg2+ soil solution concentrations occurred in the Burn treatment after residue burning than in the No burn treatment. Concentrations of most elements were similar between treatments around the time of canopy closure of the new crop, except for Na+ and Mg2+ which were higher in the Standing crop than in both new crop treatments. Leachate concentrations were highest for the cations and the largest for Mg followed by K, Ca and N.
Soil solution mass flux of nutrients
Nitrogen
Nitrogen speciation changed from being largely comprised of NH4 ions in the Standing crop treatment to NO3 ions in the new crop between felling and six months after planting (). A significantly larger quantity of NO3-N was leached in the new crop than NH4-N. Speciation in the new crop reverted to similar levels to the Standing crop treatment after the trees reached six months of age. NH4-N leaching was significantly greater in the No burn treatment than the Standing crop treatment between planting and six months after planting. These fluxes decreased after the tree canopy closed, becoming similar across all treatments by 18 months after planting. New crop NO3-N leaching was significantly larger in the new crop treatments than in the Standing crop (). Nitrate leaching was significantly larger in the Burn treatment than in the No burn treatment during the 14-month period after planting. This effect was not significant from six months after planting onward. Organic-N leaching increased significantly in the new crop after clearfelling and again between six months after planting. Significantly less organic-N was leached in the Burn treatment than the No burn treatment from six months after planting. In total, more N was leached beyond the 1.0 m soil depth in the new crop than from the undisturbed Standing crop. While significantly larger in the Burn treatment than in the No burn treatment, the net effect was small, only persisting to six months after planting.
Table 2: Nutrient leaching (kg ha–1) beyond 1.0 m depth for felled and standing crop areas of the study between felling and planting and at six-month intervals after planting. Different superscript letters denote a significant difference between treatments for each period at LSD0.05
Cations
Leaching of cations (K, Ca, Mg and Na) was significantly larger in the new crop than in the standing crop treatment between clearfelling and six months after planting (). A far larger quantity of K leached past the 1.0 m soil depth in the No burn treatment than in the Burn treatment. This effect diminished six months after planting with marginally less K leaching in the No burn treatment than the Burn treatment. A larger loss of K occurred in the No burn treatment than the Burn treatment of the study period, which was significant but relatively small. Calcium leaching was slightly higher in the No burn treatment than the Burn treatment between six and 12 months after planting. Sodium leaching was marginally lower in the No burn treatment than the Burn treatment during the same period. No significant differences were observed in Mg leaching between the No burn and Burn treatments at any age.
Tree growth and nutrient accumulation
Large quantities of nutrients continued to accumulate into the aboveground tree and litter layer biomass in the Standing crop treatment (). Nutrient uptake into the trees of the Standing crop treatment underwent rapid turnover through high rates of litterfall. No litterfall occurred in the new crop over the study period. Residue burning after clearfelling resulted in large losses of N from the residues and relatively smaller losses of the other elements (). Ash was no longer visually present on the soil surface in the Burn treatment by canopy closure. The No burn treatment showed large aboveground N, P, Ca and Mg nutrient pools at the end of the study () compared to the Burn treatment. Limited K retention in the residues resulted in similar aboveground biomass K pools in the Burn and No burn treatments (). Residue treatments in the new crop did not significantly affect tree growth or nutrient uptake, although fertilisation increased growth and uptake by around 15% (data not shown).
Table 3: Aboveground nutrient pools at felling and canopy closure (trees and residues) and nutrient uptake with growth during felling to canopy closure of the new crop. Different superscript letters denote a significant difference (LSD0.05; all P values are <0.001). The propagated SE is shown in parentheses
Soil nutrient pools and fluxes
Soil N pool sizes were significantly smaller in the new crop than in the standing crop at the end of the study period. Exchangeable K soil pools were significantly larger in all treatments of the new crop than in the standing crop, accounted for by large differences in the surface to 0.15 m soil layer (). These K soil pools were significantly larger in the No burn treatment than the Burn treatment. Calcium pool sizes were significantly larger in the Burn treatment than the No burn and Standing crop treatments. This was due to large surface accumulation of Ca after burning. Differences in soil Mg pools were not significant between any of the treatments imposed on the site. The soil Na pool was significantly smaller in the felled treatments at the end of the study than in the Standing crop treatment. The reduced Na pool size in the felled treatments compared with the Standing crop treatment was due to a pronounced decrease in Na concentrations at depth, particularly in the 0.5 to 1.0 m layer. Sodium concentrations in the 0.5 to 1.0 m layer of the No burn and Burn treatments were around 9% and 31% of Standing crop treatment values, respectively, at the end of the study.
Table 4: Soil nutrient pools at incremental depths between 0 and 1.0 m at the end of the study. Significant differences between treatments are shown as different superscripts between total pool sizes for each depth range (LSD0.05)
Discussion
Nutrient leaching in an undisturbed standing crop
Nutrient leaching was small in the undisturbed standing crop and was dependent on rainfall volume and intensity, with drainage constrained by high tree water use (Dovey et al. Citation2011a). A study in Congo on a site with sandy soils similar to our study site showed that rapid nutrient and water uptake by eucalypt tree roots limits drainage volumes under mature eucalypts (Laclau et al. Citation2005). This can result in a relatively small fraction of nutrients leached through the soil.
Mobile anions to facilitate the leaching of base cations would have been present in relative abundance in the form of sulphates and chlorides (not monitored in our study) due to proximity of the study site to the ocean. High levels of litterfall and rapid decomposition at the soil surface added to nutrients in the soil solution, increasing surface soil concentrations. Further additions might have occurred through rapid fine-root turnover, although not recorded in our study. Nitrogen was least as it was immobilised through soil microbial activity under the mature trees (unpublished data). Laclau et al. (Citation2003) also demonstrated that a large proportion of nutrient uptake by clonal eucalypts are derived from litterfall and root turnover.
Nutrient leaching during post felling
The increase in soil nutrient leaching that occurred a few months after clearfelling was due to a lack of water uptake that afforded soil water recharge and more rapid drainage. The long fallow period and low tree water demand during early growth exacerbated nutrient leaching as tree uptake was not sufficient to reduce drainage and utilise nutrients released from residue decomposition or ash. Accelerated soil processes such as N mineralisation and belowground organic matter decomposition also played a role in increasing soil solution concentrations, further increasing leaching loss (Fisher and Binkley Citation2000; Gonçalves et al. Citation2007; O'Hehir and Nambiar Citation2010). It is, however, likely that release of nutrients from the previous crop tree root system was delayed, increasing soil solution concentrations a few months after clearfelling, as observed in and and Powers et al. (Citation2005). A further source of nutrients was from nutrients that had accumulated at depth in the standing crop prior to clearfelling (). This indicates that cations added with atmospheric deposition, canopy exchange and litterfall accumulate in the deeper soil layers though limited leaching loss (Mayer et al. Citation2000; de Vries et al. Citation2003; Dovey et al. Citation2011b). This may explain the high Na concentrations in the soil and soil solution. Lower rainfall (dry periods) have been shown to immobilise soil nutrients and result in an accumulation of nutrients in the soil (Johnson et al. Citation2002).
The short-lived increase in leaching, constrained to the period directly following clearfelling to canopy closure indicates that the inter-rotation period up to canopy closure is most crucial for nutrient loss. This period presents opportunities for nutrient conservation through better management. It is also apparent from the data in this study that clearfelling had a more substantial effect on leaching than residue management. However, the combination of nutrients loss with harvesting and during burning and increased leaching may rapidly reduce soil nutrient levels if such losses are accumulated over successive rotations. This points out the importance of the organic nutrient pools, which are crucial to maintaining nutrient supply to trees on infertile sites (Laclau et al. Citation2003).
Although root growth has been shown to extend beyond 1 m depth after the first year (Bouillet et al. Citation2002), the first metre of soil carries the bulk of nutrient-scavenging fine roots (Laclau et al. Citation2001). Leaching in our study therefore described a transfer of nutrients out of this high-root-density zone. A loss of nutrients from the top 1.0 m of soil can be detrimental to tree growth during the establishment phase of the new crop where soil nutrient levels are low and root uptake from this soil layer is high during early tree growth demand. Nutrient leaching therefore can be limited through adherence to correct management practice during this intensively managed part of the rotation. It may be possible to reduce leaching by minimising the duration of the interrotation period and accelerating early tree growth with faster-growing tree species or fertilisation. This will enable sooner and more rapid water and nutrient uptake, which will conserve site nutrients. Conservation of residue pools will also reduce deep leaching loss beyond 1.0 m and help to conserve nutrients in the long term. This was shown under Pinus radiata grown on sandy soils (Carlyle et al. Citation1998) and eucalypts (Gómez-Rey et al. Citation2008). As the trees grew and matured, deep root exploration extracted nutrients that were lost to deeper regolith layers and redistributed them to shallower soil layers through litterfall and root turnover (McCulley et al. Citation2004; da Silva et al. Citation2011). Clonal eucalypt stands extend coarse roots to regolith depths of around 85% of mean tree height under non-limiting soil conditions (Christina et al. Citation2011).
Conclusion
Leaching loss was accelerated after clearfelling, but was rapidly reduced though new crop growth. These losses were small relative to aboveground nutrient pools and the loss that can occur through increased biomass removal. Large nutrient losses can reduce nutrient levels in the top soil layer, which may negatively impact on early tree growth. Re-establishment of a new crop soon after clearfelling and promoting rapid early growth can be used to facilitate the conservation of soil nutrients. Losses in this study would have been less under standard plantation forest management practices of a 2–3 months interrotation than the prolonged period in this study. Residue burning compared to residue retention did not have a major impact on leaching loss in this study. Burning can pose a direct loss of N through oxidisation and should be practiced conservatively on sites with low soil N reserves. This study demonstrates that a complete synthesis of nutrient inputs and losses and thresholds to soil nutrient depletion under plantation forestry is required on rapidgrowth, low-nutrient sites to enable prediction of nutrientrelated growth decline.
Acknowledgements
The senior author thanks the ICFR member companies who funded the core project (Mondi, Sappi, SiyaQhubeka, NCT and TWK), SiyaQhubeka and staff and contractors for the time and effort they placed in supporting this study, and the Stellenbosch University Forestry Department staff for encouraging me to publish work outside of my field of expertise. My gratitude goes to the Director and staff of the ICFR for their support and encouragement, Jean-Paul Laclau (ESALQ, University of São Paulo, Brazil) for his insight and inspiration, and Colin Smith for his project supervision and support. Special thanks to Denis Oscroft and the Zululand technical team for their excellent management of the field experiment, and to Nkosinathi Kaptein for sample and data management. Finally, I would like to thank the anonymous referees for their generous and encouraging contributions to this manuscript.
References
- Bouillet J-P, Laclau J-P, Arnaud M, M'Bou AT, Saint-André L, Jourdan C. 2002. Changes with age in the spatial distribution of roots of Eucalyptus clone in Congo: Impact on water and nutrient uptake. Forest Ecology and Management 171: 43–57.
- Carlyle JC, Bligh MW, Nambiar EKS. 1998. Woody residue management to reduce nitrogen and phosphorus leaching from sandy soil after clear-felling Pinus radiata plantations. Canadian Journal of Forest Research 28: 1222–1232.
- Carlyle JC, Nambiar EKS. 2001. Relationships between net nitrogen mineralization, properties of the forest floor and mineral soil, and wood production in Pinus radiata plantations. Canadian Journal of Forest Research 31: 889–898.
- Christina M, Laclau J-P, Gonçalves JLM, Jourdan C, Nouvellon Y, Bouillet J-P. 2011. Almost symmetrical vertical growth rates above and below ground in one of the world's most productive forests. Ecosphere 2: art27.
- Clesceri LS, Greenberg AE, Eaton AD. 1998. Standard methods for the examination of water and wastewater (20th edn). New York: American Public Health Association, the American Water Works Association, and the Water Environment Federation.
- da Silva EV, Bouillet J-P, Gonçalves JLM, Abreu CH Jr, Trivelin PCO, Hinsinger P, Jourdan C, Nouvellon Y, Stape JL, Laclau J-P. 2011. Functional specialization of Eucalyptus fine roots: contrasting potential uptake rates for nitrogen, potassium and calcium tracers at varying soil depths, Functional Ecology 25: 996–1006.
- de Vries W, Reinds GJ, Vel E. 2003. Intensive monitoring of forest ecosystems in Europe: 2: Atmospheric deposition and its impacts on soil solution chemistry. Forest Ecology and Management 174: 97–115.
- Donkin MJ, Pearce J, Chetty PM. 1993. Methods for routine soil analysis in the ICFR laboratories. ICFR Bulletin Series 08/1993. Pietermaritzburg: Institute for Commercial Forestry Research.
- Dovey SB, de Clercq W, du Toit B. 2011a. A comparison of soil moisture relations between standing and clearfelled plots with burnt and unburnt harvest residue treatments of a clonal eucalypt plantation on the Zululand Coastal Plain, South Africa. Water SA 37: 483–494.
- Dovey SB, du Toit B, de Clercq W. 2011b. Nutrient fluxes in rainfall, throughfall and stemflow in Eucalyptus stands on the Zululand coastal plain, South Africa. Southern Forests 73: 193–206.
- du Toit B, Scholes MC. 2002. Nutritional sustainability of Eucalyptus plantations: a case study at Karkloof, South Africa. Southern African Forestry Journal 195: 63–72.
- Fisher RF, Binkley D. 2000. Ecology and management of forest soils (3rd edn). New York: John Wiley and Sons.
- Gómez-Rey M, Vasconcelos E, Madeira M. 2008. Effects of eucalypt residue management on nutrient leaching and soil properties. European Journal of Forest Research 127: 379–386.
- Gonçalves JLM, Mello SLM. 2004. The root system of trees. In: Gonçalves JLM, Benedettis V (eds), Forest nutrition and fertilization. Piracicaba: IPEF. pp 223–267.
- Gonçalves JLM, Wichert MCP, Gava JL, Masetto AV, Abreu CH Jr, Serrano MIP, Mello SLM. 2007. Soil fertility and growth of Eucalyptus grandis in Brazil under different residue management practices. Southern Hemisphere Forestry Journal 69: 95–102.
- Hartemink AE, Hutting J. 2005. Sandy soils in southern and eastern Africa: extent, properties and management. In: Proceedings: Management of tropical sandy soils for sustainable agriculture: a holistic approach for sustainable development of problem soils in the tropics, 27 November–2 December 2005, Khon Kaen, Thailand, Bangkok FAO Regional Office for Asia and the Pacific. pp 148–158.
- Johnson DW, Hanson PJ, Todd DE. 2002. The effects of throughfall manipulation on soil leaching in a deciduous forest. Journal of Environmental Quality 31: 204–216.
- Kalra YP, Maynard DG. 1991. Methods manual for forest soil and plant analysis. Information Report NOR-X-319. Edmonton: Forestry Canada, Northwest Region, Northern Forestry Centre.
- Knight JH. 1999. Root distributions and water uptake patterns in eucalypts and other species. In: Landsberg J (ed.), The way trees use water: water and salinity trends. Water and Salinity Issues in Agroforestry no. 5, RIRDC Publication no. 99-37. Kingston, Australia: Rural Industries Research and Development Corporation. pp 66–102.
- Laclau JP, Arnaud M, Bouillet J-P, Ranger J. 2001. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: relationships with the ability of the stand to take up water and nutrients. Tree Physiology 21: 129–136.
- Laclau J-P, Deleporte P, Ranger J, Bouillet J-P, Kazotti G. 2003. Nutrient dynamics throughout the rotation of Eucalyptus clonal stands in Congo. Annals of Botany 91: 879–892.
- Laclau J-P, Ranger J, Deleporte P, Nouvellon Y, Saint-André L, Marlet S, Bouillet J-P. 2005. Nutrient cycling in a clonal stand of Eucalyptus and an adjacent savanna ecosystem in Congo: 3. Input–output budgets and consequences for the sustainability of the plantations. Forest Ecology and Management 210: 375–391.
- Mareschal L, Laclau J-P, Nzila JDD, Versini A, Koutika LS, Mazoumbou JC, Deleporte P, Bouillet J-P, Ranger J. 2013. Nutrient leaching and deep drainage under Eucalyptus plantations managed in short rotations after afforestation of an African savanna: two 7-year time series. Forest Ecology and Management 307: 242–254.
- Mayer R, Liess S, Lopes MIMS, Kreutzer K. 2000. Atmospheric pollution in a tropical rain forest: effects of deposition upon biosphere and hydrosphere II. Fluxes of chemicals and element budgets. Water, Air and Soil Pollution 121: 79–92.
- McCulley R, Jobbágy E, Pockman W, Jackson R. 2004. Nutrient uptake as a contributing explanation for deep rooting in arid and semi-arid ecosystems. Oecologia 141: 620–628.
- Noble AD, Berthelsen S, Mather J. 2005. Changes in soil chemical properties under two contrasting plantation systems on the Zululand coastal plain, South Africa. In: Proceedings: Management of tropical sandy soils for sustainable agriculture: a holistic approach for sustainable development of problem soils in the tropics, 27 November–2 December 2005, Khon Kaen, Thailand, Bangkok. FAO Regional Office for Asia and the Pacific. pp 93–100.
- O'Grady AP, Worledge D, Battaglia M. 2005. Temporal and spatial changes in fine root distributions in a young Eucalyptus globulus stand in southern Tasmania. Forest Ecology and Management 214: 373–383.
- O'Hehir JF, Nambiar EKS. 2010. Productivity of three successive rotations of P. radiata plantations in South Australia over a century. Forest Ecology and Management 259: 1857–1869.
- Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM. 2011. Introduction to GenStat for Windows (14th edition). Hemel Hempstead: VSN International.
- Piatek KB, Allen HL. 1999. Nitrogen mineralization in a pine plantation fifteen years after harvesting and site preparation. Soil Science Society of America Journal 63: 990–998.
- Powers RF, Scott DA, Sanchez Fg, Voldseth RA, Page-Dumroese D, Elioff JD, Stone DM. 2005. The North American long-term soil productivity experiment: findings from the first decade of research. Forest Ecology and Management 220: 31–50.
- Rosenstrauch FJ. 1938. Soil survey: with special reference to the Natal and Zululand Sugar Belt. In: Proceedings of the Annual Congress of the South African Sugar Technologists’ Association, 4–7 April 1938, Durban, South Africa, Durban. South African Sugar Technologists’ Association. pp 175–185.
- Seifert T, Seifert S. 2014. Modelling and simulation of tree biomass. In: Seifert T (ed.), Bioenergy from wood: sustainable production in the tropics. Dordrecht: Springer. pp 43–65.
- Šimůnek J, Šejna M, Saito H, Sakai M, van Genuchten MT. 2008. The Hydrus-1D software package for simulating the movement of water, heat, and multiple solutes in variably saturated media, version 4.0. HYDRUS Software Series 3. Riverside, California: Department of Environmental Sciences, University of California Riverside.
- Smethurst PJ, Nambiar EKS. 1990. Distribution of carbon and nutrients and fluxes of mineral nitrogen after clear felling a Pinus radiata plantation. Canadian Journal of Forest Research 20: 1490–1497.
- Smith CW, du Toit B. 2005. The effect of harvesting operations, slash management and fertilisation on the growth of a Eucalyptus clonal hybrid on a sandy soil in Zululand, South Africa. Southern African Forestry Journal 203: 15–26.
- US EPA 1984. Methods for chemical analysis of water and wastes. EPA-600/4-79-020. Cincinnati: US Environmental Protection Agency.
- Weihermuller L, Siemens J, Deurer M, Knoblauch S, Rupp H, Gottlein A, Putz T. 2007. In situ soil water extraction: a review. Journal of Environmental Quality 36: 1735–1748.
