Abstract
A total of 40 water samples were collected from five sites in the Danjiangkou Reservoir, an important drinking water source in China. Over the period November 2004–June 2006, eight field surveys were conducted and water temperature, pH, EC, major ions (Na+, K+, Ca2+, Mg2+, Cl-, and
) and Si were monitored to determine their temporal and spatial patterns across the reservoir. The controlling factors were analysed using the stoichiometry of the water chemicals and multivariate statistics including a correlation matrix and analysis of variance. The results revealed that waters in the reservoir are sub-alkaline and have a low solute load, and most water variables exhibit notable spatial and temporal variations. The major ion chemistry of the reservoir was controlled by carbonate weathering (of limestone) with the dominant ions Ca2+ and
contributing 63–81% and 73–81% to the major cation and anion budgets, respectively. Carbonate dissolution was produced by both sulfuric and carbonic acids; in particular carbonic acid and silicate contributed little to the reservoir waters, while
was important and originated primarily from anthropogenic inputs. This research will help water quality conservation in the Danjiankou Reservoir, China.
Citation Li, S. & Zhang, Q. (2010) Major ion chemistry and weathering processes of the Danjiangkou Reservoir, China. Hydrol. Sci. J. 55(8), 1385–1395.
Résumé
Quarante échantillons d'eau ont été prélevés en cinq sites dans le Réservoir Danjiangkou, une source importante d'eau potable en Chine. Au cours de la période November 2004-Juin 2006, huit campagnes de terrain ont été conduites et la température, le pH, la CE, les ions majeurs (Na+, K+, Ca2+, Mg2+, Cl-, et
) et Si de l'eau ont été suivis afin de déterminer les patrons temporels et spatiaux au sein du réservoir. Les facteurs de contrôle ont été analysés á l'aide de la stoechiométrie deséléments chimiques de l'eau et de statistiques multivariées, dont une matrice de corrélation et une analyse de variance. Les résultats montrent que les eaux dans le réservoir sont sub-alcalines et ont une faible charge en solutés, et que la plupart des variables hydrologiques présente des variations spatiales et temporelles notables. La chimie des ions majeurs du réservoir est contrôlée par l'altération des carbonates (du calcaire) avec une dominante des ions Ca2+ et
qui contribuent á hauteur de 63-81% et de 73-81% aux bilans des cations et des anions majeurs, respectivement. La dissolution des carbonates est produite par les acides sulfurique et carbonique; l'acide carbonique et les silicates contribuant peu á l'eau duréservoir, alors que
est important et d'origine anthropique. Cette recherche va contribuer á la conservation de la qualit é de l'eau dans le Réservoir Danjiankou, en Chine.
INTRODUCTION
The geochemical study of reservoir water reveals the character of weathering and other natural (i.e. evaporation and precipitation) and anthropogenic processes in the vicinity (Gibbs, Citation1970; Chen et al., Citation2002). Water chemistry provides a means of probing into the biogeochemical cycles of major ions at the regional, national and global levels, and characterizes the behaviour of the major ions and stream properties. Thus, the key impact of hydrochemical composition on chemical weathering processes in the drainage basin has been widely accepted (Hu et al., Citation1982; Stallard & Edmond, Citation1983, Citation1987, Citation1987; Meybeck et al., Citation1987; Sarin et al., Citation1989; Chen et al., Citation2002; Anshumali & Ramanathan, Citation2007; Li & Zhang, Citation2008, 2009; Li et al., Citation2009d).
In China, studies on major ion chemistry have mainly focused on the Changjiang and Yellow rivers (Hu et al., Citation1982; Zhang et al., Citation1990, Citation1995; Chen et al., Citation2002; Li & Zhang, Citation2005; Li & Zhang, Citation2008, 2009; Li et al., Citation2009d), and a few lake catchments such as Lake Qinghai (Xu et al., Citation2010). Danjiangkou Reservoir on the Han River, a tributary of the Changjiang River, is in the water source area of the Middle Route of China's South-to-North Water Transfer Project (SNWTP), which supplies water to northern China, including the municipalities of Beijing and Tianjin, for domestic, industrial and irrigation usages (Li et al., Citation2008a, 2008b, Citation2009a). Thus, its water chemistry has been of increasing concern. Past studies have focused on its water quality and trace metals (Li et al., Citation2008b, Citation2009a), water quality (Li et al., Citation2009c; Li & Zhang, Citation2010a,Citationb), hydro-geochemistry (Li & Zhang, Citation2008, 2009; Li et al., Citation2009d), the interaction between land use and water quality (Li et al., Citation2008a, Citation2009b) in its drainage basin, and reporting of the main contaminants of chemical oxygen demand, nitrogen and several heavy metals such as As, Pb, Sb and Se. Examining the processes of mechanisms controlling the waters in the Reservoir will represent a significant advance in our understanding of hydrochemistry, physical and chemical weathering, and ionic cycles at local and larger scales.
In this study, a comprehensive and systematic study of the Danjiangkou Reservoir was completed to determine the temporal and spatial variations of the major ions and chemical composition as a sequel to the previous studies dealing with the physico-chemistry and trace elements (Li et al., Citation2008b, Citation2009a), as well as to examine the mechanisms controlling its major ion chemistry.
STUDY AREA
The Danjiangkou Reservoir (32°36′–33°48′N, 110°59′–111°49′E) is situated at the junction of Hubei and Henan provinces and has a water surface area of 745 km2 (Li et al., Citation2008b, Citation2009a; ). It has a drainage area of approx. 95 200 km2 which includes the upper Han River and Dan River basins. Geologically, strata of Cretaceous, Tertiary and Quaternary age occur in the catchment (Zhang et al., Citation1996). The basement rocks of the Reservoir are overlain by sandstones, shales, schists and limestones (Zhang et al., Citation1996), and are mainly composed of carbonates with coal-bearing strata (Chen et al., Citation2002; Li & Zhang, Citation2008).
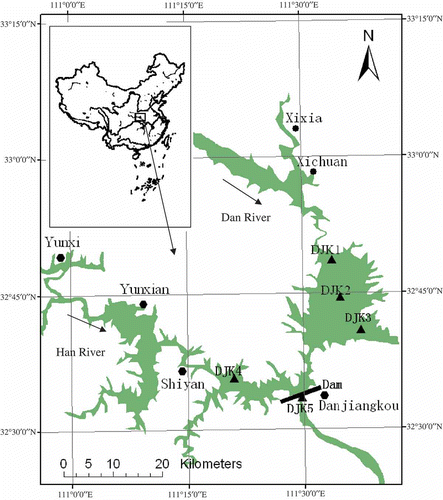
Fig. 1 Locations of five sampling sites in the Danjiangkou Reservoir, China (DJK1, DJK2 and DJK3 are located in the Dan River zone, DJK4 in the Han River zone, and DJK5 in the downstream of the dam).
The Reservoir drains a region of northern sub-tropical monsoon climate with distinct transitional climatic characteristics (Li et al., Citation2009a). The annual mean temperature is 15–16°C. Average annual precipitation ranges between 800 and 1000 mm with large interannual variability; about 80% of precipitation falls during May to October (Li et al., Citation2009a). The monthly maximum precipitation is about 193.7 mm and the minimum is 31.0 mm (Liu et al., Citation2001). In the period from 1930 to 2002, the flood peak was 34 300 m3/s (Liu et al., 2001), the mean maximum monthly flow reached 7500 m3/s in the wet season, and the minimum flow was 64 m3/s in the dry season (Jin & Zhao, Citation2004). During the present study (November 2004–June 2006), the high flow period was August to November 2005, with a peak flow of 14 000 m3/s, and the remainder of the study period was dry season. Thus, the interacting influences of hydrology and seasonal anthropogenic input variations (spring, summer, autumn and winter) on the survey were considered.
MATERIALS AND METHODS
Sampling and sample preparation
Eight surveys were conducted from 2004 to 2006 (November 2004, January, April, June, August and November 2005, April and June 2006) at five sampling sites, geo-located using a portable Global Position System (Model eTrex Summit, USA) in the Reservoir (). Three sites (DJK1, DJK2 and DJK3) were located in the Dan River zone of the reservoir (water surface area 343.4 km2) and DJK3 was at the dike for the SNWTP's Middle Route. One site (DJK4) was located in the Han River zone (water surface area 401.6 km2) for comparison of the water geochemistry between the two rivers. DJK5 was located downstream of the Danjiangkou Dam to assess the influence of the dam on aquatic geochemistry.
A total of 120 grab water samples (3 litres), consisting of three replicates within a 200-m diameter circle, were collected from 50 to 100 cm depth using previously acid-washed high-density polyethylene (HDPE) bottles with Teflon-lined caps. Of these, 40 samples (10 samples at high flows) were pre-treated for laboratory analysis after mixing with replicates. A separate 500 ml sample was immediately filtered in situ, using a portable manual vacuum pump, through a previously acid-washed 0.45 μm pore millipore nitrocellulose membrane filter. The initial portion of the filtrate was discarded to clean the membrane. A small portion of the filtrate was used for measuring Cl- and , while another portion was acidified with ultra-pure concentrated nitric acid, to pH < 2, for measuring Na+, K+, Ca2+, Mg2+ and Si. The samples were stored at 4°C in HDPE bottles until analysis. Cleaning of plastic bottles and plastic bags was carried out by soaking in 15% (v/v) HNO3 for 24 h and then double rinsing with milli-Q water.
Analytical methods
The pH and EC were determined in situ using YSI 6920 (YSI Inc., Yellow Springs, Ohio, USA) equipment. The pH sonde was calibrated at pH 7 and 10 before field sampling. The concentration of was measured on site by acid titration using hydrochloric acid. Major cations (Na+, K+, Ca2+ and Mg2+) and Si were determined using an Inductively Coupled Plasma Atomic Emission Spectrometer (ICP-AES; IRIS Intrepid II XSP DUO, USA). Anions (Cl- and
) were measured using a Dionex Ion Chromatograph (IC) (Dionex Corp., Sunnyvale, California, USA). High purity reagents and milli-Q water were used for all analyses. Reagent and procedural blanks were determined in parallel to the sample treatments using identical procedures. Each calibration curve was evaluated by analyses of the quality control standards before, during and after the analyses of a set of samples. The analytical precision was within ±5% except for Na; for which it was equal to 5.7% (Li & Zhang, Citation2008). Major ions were determined for all the samples, while the concentrations of Si were only measured in August and November 2005, and April and July 2006.
Statistical analyses
Relationships among major ions were tested using Pearson's correlation with statistical significance set at p < 0.05. Analysis of variance (ANOVA) was used to compare spatial and temporal differences in major ion concentrations with the significance level set at p < 0.05 (least-significant difference, LSD). All the processes were performed using SPSS 15.0 for Windows.
RESULTS
The chemical compositions of the samples are presented in . The waters were all mildly alkaline with pH values ranging from 7.64 to 8.85, reflecting the importance of the dissolution of limestone and dolomite in the drainage basin. The EC values varied from 230.7 to 448.0 μS/cm with an average of 292.3 μS/cm. The total cationic charge (TZ
+ = K++Na++2Ca2++2Mg2+ in μeq/L) varied between 2102.6 and 4223.3 μeq/L with an average of 2950.8 μeq/L, twice as high as the average of world rivers (TZ
+ = 1250 μeq/L; Meybeck, Citation1981) but comparable to the average values of the Changjiang (2800 μeq/L; Han & Liu, 2004) and Han rivers (2674 μeq/L; Li et al., Citation2009d). The total anionic charge ( in μeq/L) ranged from 2144.9 to 4392.9 μS/cm with an average of 3202 μS/cm. The extent of Tz
+--Tz
- charge imbalance was characterized by the normalized inorganic charge balance (NICB = (Tz
+ – Tz
-)/Tz
+), and related to the contribution of other cations, and indicated the small contribution of organic ligands to the charge balance.
Table 1 Chemical composition of the Danjiangkou Reservoir, China (unit in mg/L except T in °C, EC in μS/cm, pH, Tz+ and Tz- in in μeq/L)
All the major ions displayed notable temporal and spatial differences, except for and Si among surveys, and K+ and Ca2+ among sites (). Generally, most of the water variables tended to exhibit maximum concentrations in April, such as EC (330.2 ± 67.3 μS/cm), K+ (2.15 ± 0.37 mg/L) and Si (2.85 ± 0.53 mg/L) in April 2006, and Cl- (7.25 ± 1.12 mg/L),
(40.18 ± 5.16 mg/L),
(158.60 ± 31.99 mg/L), Ca2+ (49.92 ± 2.47 mg/L) and Mg2+ (11.16 ± 2.41 mg/L) in April 2005. Only the minimum concentrations of EC (268.5 ± 19.3 μS/cm),
(132.98 ± 11.73 mg/L) and Ca2+ (32.96 ± 2.07 mg/L) were observed during high flows (August 2005, November 2005) (). also shows that all the highest physicochemical parameter values occurred in the Dan River, with concentrations decreasing downstream from DJK1 to DJK3; the lowest values were monitored in the Han River at DJK4.
Table 2 Temporal and spatial variations of water chemical data statistics using analysis of variance: main variables (T, pH, EC), Si and major ion compositions in the Danjiangkou Reservoir, China. (units in mg/L except T in °C, EC in μS/cm and pH). The different letters indicate statistical difference among sampling month at p < 0.05; LSD test)
Calcium was the dominant cation, contributing 63–81% to the major cation budget, while Mg contributed 12–25%, and Na+ + K+, 3–12% (). Anion chemistry was dominated by constituting 73–81% of the total anions, and 15–25% by
. Thus, water composition was dominated by
and Ca2+ (). Generally, the major ion concentrations were in the following order:
> Ca2+ >
> Mg2+ > Cl- > Na+ > Si > K+ (). Overall, waters in the Reservoir were of a Ca2+ and
type, and had mild alkalinity with an average pH of 8.2 and low dissolved salt concentrations, as indicated by the EC measurements ().
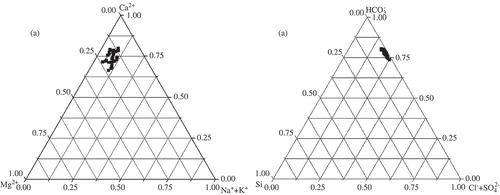
Fig. 2 Ternary diagrams showing: (a) cation, and (b) anion compositions of water in the Danjiangkou Reservoir, China.
The results of correlation analysis among the variables are shown in : ,
, Ca2+ and Mg2+ have positive correlations with each other, and their significant contributions to the hydrochemistry are shown by their correlations with EC. Also, EC shows strong correlations with Cl- and K+ (p < 0.01, r > 0.64). Cl- was positively correlated to
, K+ and the sum of Na+ plus K+, while Si was only significantly correlated to Ca2+.
DISCUSSION
The Danjiangkou Reservoir collects runoff from the Dan and Han rivers, i.e. a vast area (95 200 km2) with much variability in the landscape and geological setting. Previous studies have reported that the Reservoir's hydrochemistry is controlled by chemical weathering (Li & Zhang, Citation2008; Li et al., Citation2009d). Thus, we ascribed the significant variability in major ion compositions to the different lithology of the two river basins (). In the Dan River zone, our results indicated a decrease in major ion concentrations along the water flow, which was largely due to dilution in the reservoir. Compared to the Dan River (i.e. DJK1-3) in the reservoir, concentrations of major ions below the dam (i.e. at DJK5) were relatively lower, although higher than those in the Han River (DJK4). This might be attributable to a combination of ion exchange, biological uptake and other complex biogeochemical process (Olias et al., Citation2006; Li et al., Citation2009a).
The maximum (329.6 mg/L) and minimum (187.9 mg/L) solute loads were found in April and November 2005, respectively (), and the seasonal values of major ions showed maxima in April and minima in August 2005 (). These variations might result from seasonal chemical weathering rates and anthropogenic activities, and specifically photosynthetic and respiratory processes in spring and summer (growing season) (Olias et al., Citation2006; Li et al., Citation2009a). Comparing major ions in June and August of 2005, indicates that the large precipitation input and consequent dilution was responsible for the lower solute load in August 2005 (Anshumali & Ramanathan, Citation2007; Li & Zhang, Citation2009). There were also large annual variations in major ions and their averages tended to decrease, cf. November 2004 and 2005, April 2005 and 2006, and June 2005 and 2006 (). In particular there was obvious variability in precipitation in November. In the other seasons differences were probably associated with local anthropogenic activities and recent reforestation up-stream. Further investigation is needed of the long-term evolution of the hydro-geochemistry of the reservoir.
Since the precipitation is concentrated in the wet season (August–November 2005), we have hypothesized that major ion constituents have significant temporal variability with lower concentrations in high flows. Yet, the results indicated that only EC and Ca2+ showed the lowest concentrations in the rainy season, but their variation factors (the ratio of maximum to minimum) were smaller than 2 (). We concluded that these were attributable to the enhanced dissolution of carbonates and detrital calcite by intense soil erosion, particularly during the wet season, which balanced the dilution effect (Chen et al., Citation2002), and was opposite to the important influence of rain runoff on water quality parameters such as nitrogen, chemical oxygen demand, suspended particulate matter and dissolved oxygen in the reservoir (Li et al., Citation2009a) and its upper streams (Li et al., Citation2008a, 2009c).
Table 3 Pearson correlation coefficients among the water physicochemistry values of the Danjiangkou Reservoir, China
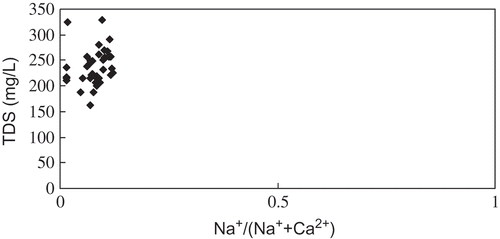
The plotting of total dissolve solid (TDS) concentrations versus the weighted ratio of Na+/(Na+ + Ca2+) revealed the typical rock weathering-dominated character of waters in the reservoir by the very low ratio of Na+/(Na+ + Ca2+) and a moderate concentration of TDS (; Gibbs, Citation1970). Since different parent rock (i.e. carbonates, silicates and evaporites) contribute to a variety of dissolved constituents (Stallard & Edmond, Citation1983; Chen et al., Citation2002), ternary plots of anions, Si and cations () were prepared to study the relative importance of different weathering types. On the cation plot ((a)), samples fell near the Ca2+ axis and towards the Ca2+ apex. On the anion and silica plot, samples fell in a cluster near the apex. Thus, the major ion chemistry of the Reservoir is likely to be dominated by the chemical weathering of carbonates, which is consistent with the prevalence of carbonate rocks in its drainage basin (Chen et al., Citation2002; Li & Zhang, Citation2008). Also, strong geochemical relationships between EC,
, Ca2+ and Mg2+ (r > 0.71, p < 0.01; ) point to the dominance of carbonate weathering (Gibbs, Citation1972; Li & Zhang, Citation2008).
Fig. 3 The Gibbs graph of major ion compositions of water (TDS is the sum of major ions) in the Danjiangkou Reservoir, China.
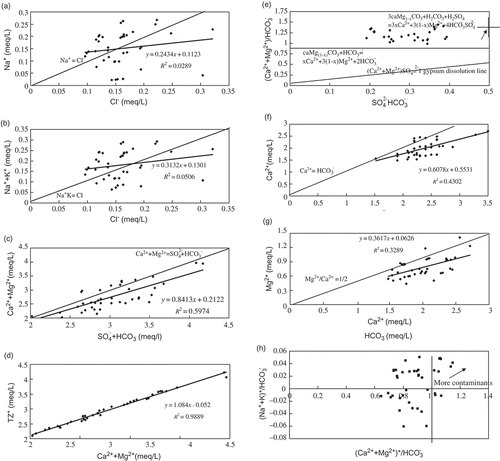
Approximately half of the water samples (20) exhibited equivalent mole ratios of Na+/Cl and (Na+ + K+)/Cl- larger than unity, showing excess of Na+ and (Na+ + K+) over Cl- (.(a) and (b)), and indicating other sources, e.g. anthropogenic and silicate weathering. Most of the water samples had equivalent ratios showing excess () relative to (Ca2+ + Mg2+) (.(c)), which indicated that dissolution of Na+ and K+ silicate minerals was needed to account for this anion excess. However, the ratio of (Ca2+ + Mg2+)/Tz+ of around 1:1 (.(d)) and the low equivalent ratio of
(<0.12) () imply a minimal contribution of silicates to the water chemistry.
Fig. 4 Scatter plots of: (a) Na versus Cl, (b) Na+K versus Cl, (c) Ca + Mg versus SO4 + HCO3, (d) Tz+ versus Ca + Mg, (e) (Ca+Mg)/HCO3 versus SO4/HCO3, (f) Ca versus HCO3, (g) Mg versus Ca, and (h) (Na+K)*/HCO3 versus (Ca+Mg)*/HCO3 in the Danjiangkou Reservoir, China.
In the Danjiangkou Reservoir's drainage basin there is no geological evidence of the presence of evaporite strata but wide coal-containing strata exposure. The co-variation of the equivalent ratios of
versus
((e)) demonstrate that the water samples locate notably above the gypsum dissolution line, indicating the small contribution of gypsum to sulfate. A previous study reported the very small contribution (1%) of precipitation to solutes in the basin (Li & Zhang, Citation2009). The
concentration, showing large seasonality, was much higher than the global average (Meybeck & Helmer, Citation1989) and higher than the average in the Changjiang River (Chen et al., Citation2002). Thus,
in the reservoir waters is interpreted as mainly due to anthropogenic emissions of
by the extensive and intensive usage of sulfur-enriched coal and power production via coal combustion in the drainage basin. Another possible source of the
is the oxidation of sulfide minerals in the catchment, given that the catchment is rich in coal-formations and the coal is rich in sulfides.
According to the stoichiometric relations of these chemical reactions, more than 60% of was balanced by Ca2+ ((f)) while the (Ca2+ + Mg2+)/HCO3 equivalent ratio fell in the range 1–1.5 ((e)), which, with the low equivalent ratio of Mg2+/Ca2+ (<0.5) ((g)), indicates that the water geochemistry was controlled by carbonate dissolution such as dissolution of dolomite and in particular limestone.
The Ca2+ and Mg2+ were greatly in excess with respect to , and sources additional to carbonate weathering are needed to account for the excess. Dissolution of carbonate minerals could take place due to both attack by H2CO3 derived from dissolution CO2 in water, and by sulfuric acid originating from anthropogenic inputs or the oxidation of sulfide minerals in the coal-containing strata upstream of the Danjiangkou Reservoir. The chemical reactions that were likely responsible for the chemical compositions of the reservoir waters are illustrated in the (e). The following reactions describe the dissolution of carbonates by
, and by both H2CO3 and H2SO4.
From the stoichiometry of the water chemicals, waters with equivalent ratios of of around one and the low
ratios indicate that the carbonate mineral dissolution by carbonic acid controlled the chemical weathering. If carbonate mineral dissolution by both carbonic and sulfuric acids was occurring and reached equilibrium, the
and
equivalent ratios in the waters should be 0.5 and 1.5, respectively, as shown in Equationequations (1)
(1) and Equation(2)
(2) and (e). If gypsum dissolution occurred, the waters should have a higher
equivalent ratio and a
equivalent ratio of one, and the data points would be distributed close to the gypsum dissolution line. Our results demonstrate that the water samples are located between what is expected for carbonate weathering by carbonic acid and that by both sulfuric and carbonic acids, indicating a combined chemical weathering of carbonates. However, there were no positive associations between
and
, which might result from the dominance of carbonate dissolution by carbonic acid, confirmed by the stronger relations between Ca2+ and Mg2+ and
().
We assumed that was only balanced by Ca2+ and Mg2+, thus (Ca2+ + Mg2+)*, which was obtained by subtracting
from the total (Ca2+ + Mg2+) equivalences, should originate from weathering of carbonate and/or silicate minerals by carbonate acid. Consequently, the (Ca2+ + Mg2+)*/HCO3 equivalent ratio should be smaller than one. Accordingly, assuming that Cl- was only balanced by K+ and Na+, (Na+ + K+)*, given by subtracting Cl− from the total (Na+ + K+) equivalences, should account for carbonate and/or silicate weathering. Co-variation of
versus
in these waters showed the relative importance of contributions by carbonate and silicate weathering to the water chemical compositions ((h)).
Compared to rivers draining karst-dominated terrain controlled by carbonate dissolution in the Changjiang River (Han & Liu, 2004), most of the samples in our study fall close to the two lines of and
, suggesting the dominance of carbonate dissolution and little contribution from silicate weathering to water chemistry. However, some samples fell in the first quadrant ((h)) showing the excess of (Ca2+ + Mg2+)* over
; this may be associated with anthropogenic inputs, such as phosphate and nitrate, causing an excess of cations in the waters rather than mineral weathering.
CONCLUSIONS
Waters in the Danjiangkou Reservoir are mildly alkaline and have low dissolved loads which are dominated by the weathering of carbonates. In general, the major ion constituents were of the following order: > Ca2+ >
> Mg2+ > Cl- > Na+ > Si > K+. The dominant ions, Ca2+ and
contributed 63–81% and 73–81% to the total cations and total anions, respectively. Most of the major ions exhibited remarkable temporal and spatial variations. Water variables including EC,
and Ca2+ had the lowest values/concentrations in high flows, while the highest values for EC, Cl-,
,
, K+, Ca2+, Mg2+ and Si occurred in April. All physico-chemical parameters showed maximum concentrations in the Dan River zone with a decrease downstream, while the minimum concentrations were found in the Han River. Further studies should focus on understanding of the persistent trend of the major ions.
The stoichiometry indicated that the water geochemistry was dominated by carbonate dissolution of dolomite and in particular limestone. Further, there was a mixture of chemical weathering of carbonates by both sulfuric and carbonic acids, and especially by carbonic acid, but only a small contribution of silicate weathering to the reservoir water chemistry. The in the reservoir waters was primarily attributable to anthropogenic emissions and the oxidation of sulfide minerals in the drainage basin as there is no geological evidence of the presence of evaporite strata, but coal-containing strata are wide spread.
Acknowledgements The research is funded by the National Key Sciences Research Program of China (2008CB418000), and the “Hundred-talent Project” of the Chinese Academy of Sciences (O629221C01). We would like to thank Sheng Gu, Jia Li, Lianfa Li, Sha Mu and Yiping Wang for their assistance with field sampling, and Hongyin Han of the Chinese University of Geosciences for the assistance on the major ion analysis. Appreciation is also due to the editor and two anonymous reviewers for their constructive comments and suggestions.
REFERENCES
- Anshumali and Ramanathan , A. L. 2007 . Seasonal variation in the major ion chemistry of Pandoh Lake, Mandi District, Himachal Pradesh, India . Appl. Geochem. , 22 : 1736 – 1747 .
- Chen , J. , Wang , F. , Xia , X. and Zhang , L. 2002 . Major element chemistry of the Changjiang (Yangtze River) . Chem. Geol. , 187 : 231 – 255 .
- Gibbs , R. J. 1970 . Mechanisms controlling world water chemistry . Science , 170 : 1088 – 1090 .
- Gibbs , R. J. 1972 . Water chemistry of the Amazon River . Geochim. Cosmochim. Acta , 36 : 1061 – 1066 .
- Han , G. and Liu , C. 2004 . Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China . Chem. Geol. , 204 : 1 – 21 .
- Hu , M. , Stallard , R. F. and Edmond , J. M. 1982 . Major ion chemistry of some large Chinese rivers . Nature , 298 : 550 – 553 .
- Jin , Y. & Zhao , W. ( 2004 ) Preliminary analysis on regulation of hydrology and meteorology of Autumn flood season in the Danjiangkou Reservoir . Express Water Resour. Hydropower Inf. 22 – 24 .
- Li , J. and Zhang , J. 2005 . Chemical weathering processes and atmospheric CO2 consumption of Huanghe River and Changjiang River basins . Chin. Geogr. Sci. , 15 : 16 – 21 .
- Li , S. , Cheng , X. , Xu , Z. , Han , H. and Zhang , Q. 2009a . Spatial and temporal patterns of the water quality in the Danjiangkou Reservoir, China . Hydrol. Sci. J. , 54 : 124 – 134 .
- Li , S. , Gu , S. , Liu , W. , Han , H. and Zhang , Q. 2008a . Water quality in relation to the land use and land cover in the Upper Han River basin, China . Catena , 75 : 216 – 222 .
- Li , S. , Gu , S. , Tan , X. and Zhang , Q. 2009b . Water quality in the upper Han River basin, China: The impacts of land use/land cover in riparian buffer zone . J. Hazard. Mater. , 165 : 317 – 324 .
- Li , S. , Liu , W. , Gu , S. , Cheng , X. , Xu , Z. and Zhang , Q. 2009c . Spatio-temporal dynamics of nutrients in the upper Han River basin, China . J. Hazard. Mater. , 162 : 1340 – 1346 .
- Li , S. , Xu , Z. , Cheng , X. and Zhang , Q. 2008b . Dissolved trace elements and heavy metals in the Danjiangkou Reservoir, China . Environ. Geol. , 55 : 977 – 983 .
- Li , S. , Xu , Z. , Wang , H. , Wang , J. and Zhang , Q. 2009d . Geochemistry of the upper Han River basin, China. 3: anthropogenic inputs and chemical weathering to the dissolved load . Chem. Geol. , 264 : 89 – 95 .
- Li , S. and Zhang , Q. 2008 . Geochemistry of the upper Han River basin, China, 1: spatial distribution of major ion compositions and their controlling factors . Appl. Geochem. , 23 : 3535 – 3544 .
- Li , S. and Zhang , Q. 2009 . Geochemistry of the upper Han River basin, China. 2: seasonal variations in major ion compositions and contribution of precipitation chemistry to the dissolved load . J. Hazard. Mater. , 170 : 605 – 611 .
- Li , S. and Zhang , Q. 2010a . Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques . J. Hazard. Mater. , 176 : 579 – 588 .
- Li , S. and Zhang , Q. 2010b . Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China . J. Hazard. Mater. , 181 : 1051 – 1058 .
- Liu , M. , Xu , Y. , Zhu , L. , Xu , S. & Xu , S. ( 2001 ) Study on hydrology and climate in the flood causing of Danjiangkou Reservoir stream . Hubei Meteorol. 15 – 16 .
- Meybeck , M. 1981 . “ Pathways of major elements from land to ocean through rivers ” . In River Inputs to Ocean Systems , Edited by: Martin , J. M. , Burton , J. D. and Eisma , D. 18 – 30 . New York : United Nations Press .
- Meybeck , M. 1987 . Global chemical weathering of surficial rocks estimated from river dissolved loads . Am. J. Sci. , 287 : 401 – 428 .
- Meybeck , M. and Helmer , R. 1989 . The quality of rivers: from pristine stage to global pollution . Palaeogeogr. Palaeoclimatol. Palaeoecol. , 75 : 283 – 309 .
- Olias , M. , Ceron , J. C. , Moral , F. and Ruiz , F. 2006 . Water quality of the Guadiamar River after the Aznalcollar spill (SW Spain) . Chemosphere , 62 : 213 – 225 .
- Sarin , M. M. , Krishnaswamy , S. , Dilli , K. , Somayajulu , B. L. K. and Moore , W. S. 1989 . Major ion chemistry of Ganga-Brahmaputra river system: weathering processes and fluxes of the Bay of Bengal . Geochim. Cosmochim. Acta , 53 : 997 – 1009 .
- Stallard , R. F. and Edmond , J. M. 1983 . Geochemistry of the Amazon. 2. The influence of geology and weathering environment on the dissolved load . J. Geophys. Res. , 88 ( C14 ) : 9671 – 9688 .
- Stallard , R. F. and Edmond , J. M. 1987 . Geochemistry of the Amazon. 3. Weathering chemistry and limits to dissolved inputs . J. Geophys. Res. , 92 ( C8 ) : 8293 – 8302 .
- Xu , H. , Hou , Z. , An , Z. , Liu , X. and Dong , J. 2010 . Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet plateau, China . Quat. Int. , 212 : 35 – 43 .
- Zhang , J. , Huang , W. and Letolle , R. 1995 . Major element chemistry of the Huanghe, China: Weathering processes and chemical fluxes . J. Hydrol. , 168 : 173 – 203 .
- Zhang , J. , Huang , W. and Liu , M. 1990 . Drainage basin weathering and major element transport of the Chinese rivers (Huanghe and Changiiang) . J. Geophys. Res. , 95 : 13277 – 13288 .
- Zhang , L. , She , Z. and Zhang , S. 1996 . Study of Chemical Elements in Water Environment , Beijing : Chinese Environment Science Press .