Abstract
This study was carried out from 2003 to 2007 to understand the hydrogeochemical processes and the solute sources of the meltwaters of the Chhota Shigri Glacier, Himalaya. The meltwater is almost neutral to slightly alkaline in nature: bicarbonate and sulphate are the dominant anions, while calcium and magnesium are the dominant cations. Bicarbonate is found to be derived from carbonate weathering and partly from silicate weathering. Rock weathering followed by precipitation are the main controlling factors that influence the meltwater chemistry of this region. The relatively high values of pCO2 reflect a higher rate of solubility in comparison to release of excess CO2 gas to the atmosphere. The presence of active hydrogeochemical processes and sediment–water interaction results in excess solute transport through the meltwater to the Chandra River that feeds the Chenab, one of the great Himalayan river systems, and ultimately flows into the ocean. This study is the first of its kind to understand in detail the hydrogeochemical process and resultant solute load transport in this Himalayan glacier.
Citation Sharma, P., Ramanathan, A.L., and Pottakkal, J., 2013. Study of solute sources and evolution of hydrogeochemical processes of the Chhota Shigri Glacier meltwaters, Himachal Himalaya, India. Hydrological Sciences Journal, 58 (5), 1128–1143.
Editor Z.W. Kundzewicz
Résumé
Cette étude a été réalisée de 2003 à 2007 pour comprendre les processus hydrogéochimiques et les sources de solutés des eaux de fonte du glacier Chhota Shigri, Himalaya. L'eau de fonte est presque neutre à légèrement alcaline dans la nature. Les anions dominants sont les bicarbonates et les sulfates, et le calcium et le magnésium sont les cations dominants. Le bicarbonate est dérivé de l'altération des carbonates et en partie de celle des silicates. L'altération des roches suivie de précipitation sont les principaux facteurs qui influencent la chimie des eaux de fonte de cette région. Les valeurs les plus élevées de pCO2 reflètent un taux plus élevé de solubilité par rapport au dégagement de CO2 en excès dans l'atmosphère. La présence de processus hydrogéochimiques actifs et d'interaction eau-sédiments a pour résultat un excès de transport de soluté par l'eau de fonte de la rivière Chandra qui alimente le Chenab, l'un des grands systèmes fluviaux himalayens, et, finalement, se jette dans l'océan. Cette étude est l'une des premières du genre pour comprendre en détail les processus hydrogéochimiques et le transport de charge résultante en soluté dans ce glacier himalayen.
Citation Sharma, P., Ramanathan, A.L., and Pottakkal, J., 2013. Study of solute sources and evolution of hydrogeochemical processes of the Chhota Shigri Glacier meltwaters, Himachal Himalaya, India. Hydrological Sciences Journal, 58 (5), 1128–1143.
Editor Z.W. Kundzewicz
INTRODUCTION
Meltwater hydrochemistry and geochemistry of glaciers are significant for the water resource management of Himalayan rivers, and also help us to understand the complex weathering dynamics operating in the glacier system. The chemical characteristics of meltwater discharge from glaciers can be differentiated from other aqueous environments by their chemical activity. Geochemical analysis of meltwater near the snout generally reveals enrichment in concentration of chemical species. The amount of dissolved ion concentration present shows the intensity of effective hydrochemical reaction within the glacier system and particularly at the interface with the glacier bedrock. Silicate minerals at near-zero and sub-zero temperatures react with the water very rapidly; high dissolution of atmospheric CO2 in this cold water supplies H+ ions needed for acid hydrolysis of the minerals, during which bicarbonate, cations and dissolved silica will be released to the water (Raiswell Citation1984). The snow-fed streams of the Himalayas are the major source of water in most of the major river systems in the Indian subcontinent; hence, it becomes imperative to understand the hydrogeochemical processes occurring here, along with their solute transport capabilities, which in turn are likely to alter the solute loads of global ocean waters. Further, the changing snowfall pattern, lack of intense monsoonal rain and global climate change will also alter the quantity and quality of the Himalayan river systems.
Few studies have been undertaken on the hydrochemistry of glacier meltwater in the Indian Himalayas (Hasnain et al. Citation1989, Citation2001, Hasnain and Chauhan Citation1993, Nijampurkar et al. Citation1993, Bahadur Citation1996, Hasnain and Thayyen Citation1996, Citation1999, Singh and Hasnain Citation1998, Pandey Citation1999, Ahmad and Hasnain Citation2000, 2001), especially with regard to understanding the hydrochemical evolution of meltwaters. For Chhota Shigri Glacier, there is very limited documented work available on the hydrogeochemistry, and that is based on only a few days’ data of a single year. The present study attempts to understand the hydrogeochemical evolution and solute acquisition process over space and time using five years’ data. This is probably the first attempt to understand the hydrogeochemical processes occurring in this high-altitude Himalayan glacier over a five-year period, and in a climatic zone less influenced by monsoon (monsoon–arid transition zone).
The glaciers in the Central and Eastern Himalaya are mostly sustained by the Indian Summer Monsoon system which is rooted in the larger atmospheric phenomenon, the Inter-tropical Convergence Zone (ITCZ) that arises because of the seasonal temperature and pressure differences in the northern and southern hemispheres, while the Western and Trans-Himalayan regions receive precipitation mostly during the winter months (December–March) due to Westerly Disturbances. These varying weather systems give rise to precipitation gradients along and across the Himalaya due to mechanical and thermal forcing. Due to their relative importance in bringing moisture to the high peaks of the Himalaya and the Trans-Himalaya, they have played an important role in defining the spatial and temporal distribution of glaciers in the past and the present.
A classification by Vohra (Citation1996), based on the precipitation pattern along the Himalayan arc, describes three classes:
| a. | dominant monsoon precipitation areas of the Eastern Himalaya; | ||||
| b. | equal to sub-equal monsoon and winter precipitation areas, including the Ganga basin and parts of Himachal Pradesh (Chhota Shigri Glacier falls in this precipitation regime); and | ||||
| c. | dominant winter precipitation, including areas of Ladakh, eastern Spiti and Tibet. | ||||
Although, most of the international literature reveals that the major component of accumulation in the glaciers of the Indian Himalaya takes place in summer (summer accumulation type), coincident with the ablation season (Fujita and Ageta Citation2000), for the glaciers in the Central, Western and Northwest Himalaya, the accumulation patterns are more varied and complex, and there are very few data on temperature or accumulation parameters at glacier elevations.
During the monsoon, two general rainfall gradients are observed in the Himalaya:
| a. | a decreasing east–west gradient with higher rainfall occurring near the moisture source, the Bay of Bengal, and | ||||
| b. | a strong decreasing south–north gradient across the range from its rain-drenched southern flank to the semi-arid Tibetan Plateau. | ||||
The south–north gradient is a consequence of orographic rainfall, whereby rising topography in the face of prevailing winds causes mechanical lifting of the humid air, cooling of the air column, condensation and precipitation. Heavier precipitation is thus induced on the windward sides of the mountain ranges as compared to the leeward side. Chhota Shigri Glacier is on the leeward side and receives less monsoon precipitation.
In the winter months (December–March), the Western Himalaya receive precipitation due to the weather systems known as Westerly Disturbances that originate over the Mediterranean Sea/Black Sea/Caspian Sea as extra-tropical frontal systems (Singh and Hatwar Citation2005). In these months, these mid-latitude disturbances move to their lowest latitudes and follow a pathway across the northern and central parts of India, travelling in a phased manner from west to east, disturbing the normal features of the circulation pattern (Yadav et al. Citation2009); they account for snow at the higher elevations of northwest India and winter rainfall in the plains of northern and central India. Most precipitation during October–March at Chhota Shigri Glacier is due to Westerly Disturbances.
The complex precipitation patterns and their interaction with the local geological, topographic and biological systems have produced varied snow climatic zones in the Himalaya (Sharma and Ganju Citation1999) and have a profound impact on meltwater chemistry and discharge. Annual winter snowfall varies from 100 to >1600 cm, the highest snowfall occurring in the Pir Panjal range with higher ranges receiving progressively less snowfall (Bhutiyani et al. Citation2007).
STUDY AREA
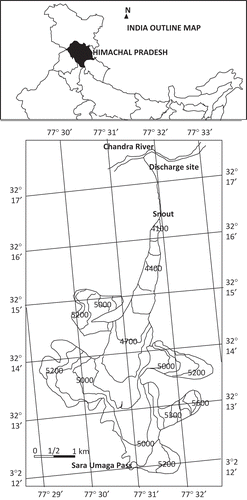
Chhota Shigri Glacier, located between 32°11′–32° 17′ N and 77°30′–77°32′ E, with altitude varying from 4100 to 6000 m a.m.s.l., is a valley-type glacier, debris-covered in the lower ablation zone that lies in the Chandra-Bhaga River basin on the northern ridge of the Pir Panjal range in the Lahaul-Spiti valley of Himachal Pradesh (). The glacier extends 9 km from the snout (at around 4000 m a.m.s.l.) to the accumulation zone near the Sara Umga Pass (4900 m), and its width varies from 0.5–1.5 km in the ablation zone to about 4.5 km above the equilibrium line (Kumar et al. Citation1987, Dobhal et al. Citation1995). The area of Chhota Shigri Glacier, including all tributaries, is 15.7 km2, with a total glacerized basin area of 16.4 km2 (Wagnon et al. Citation2007), and the equilibrium line ranges between 4800 and 5100 m a.m.s.l. (Sharma Citation2008). The Chhota Shigri Glacier drains into the River Chandra. The total drainage area of the glacier basin is about 36 km2 and the glacier occupies about 47% of the drainage area. Several supraglacial water streams are formed in the ablation zone, most of which terminate in moulins or crevasses.
Fig. 1 Location of Chhota Shigri Glacier in the Himalayan arc and map of the glacier showing the discharge site (sampling site), Lahul-Spiti Valley, Himachal Pradesh, India.
Climate
There is great variability in precipitation across the Himalayas and even different ranges in the Northwestern Himalaya receive different snowfall amounts, ranging from about 100 to >1600 cm (Bhutiyani et al. Citation2010). Further, the Western Himalaya is characterized by land surface/topographic heterogeneity and, during winter, eastward-moving low-pressure synoptic weather systems, the Western Disturbances. The glacier is in the monsoon–arid transition zone and considered to be a potential indicator of the northern limits of the intensity of the monsoon (Dobhal et al. Citation1995); thus it is influenced by both the Asian monsoon in summer and westerlies in winter. Most of the precipitation falls in summer (July–September) due to the Indian southwest monsoon, but there is also a significant amount of precipitation in winter (November–February/March) due to the mid-latitude westerlies. The Chandra River valley, where the glacier is situated, is drier than the southern slopes of the Pir Panjal range due to the leeward effect of the west–east oriented main ridge that prevents part of the monsoon flux from reaching the valley (Bookhagen and Burbank Citation2006).
During the study period, it was observed that, in the morning, the sky was generally clear, while a strong surface wind began to blow in the afternoon. Cumulus clouds formed during afternoons and were replaced by thick stratus clouds drifting through the Sara Umga Pass from the south that, by evening, had covered the glacier completely, reducing visibility. Winds were generally light and south to southwesterly in the morning, and gained momentum in the afternoon. Rainfall was generally little in quantity but high in frequency.
Geology
Chhota Shigri Glacier lies within the central crystalline axis of the Pir Panjal range of the Himachal Himalaya. This crystalline axis is composed mostly of meso- to ketazonal metamorphites, migmatites and gneisses (Kumar et al. Citation1987). In a few places, granitic rocks of different composition and younger age indicate rejuvenation, but 3 km upstream of Chhota Dara, in the upper Chandra valley, older Palaeozoic granitic rocks are exposed (Kumar et al. Citation1987, Rawat and Purohit Citation1988). The Haimanta Formation overlies these with a tectonic break where black slates, phyllites and fine-grained biotite-schists are exposed. The slates and phyllites show a well-developed thrust tectonic contact, which forms the crest of the northern ridge. Box-type folds with décollement are quite prominent in the Haimanta Formation. The Haimantas, which rest directly on basement rocks, are highly metamorphosed metasediments and show intense folding and shearing. The brown biotite, with a fine-grained texture, shows intense heating effects, which indicate periodic re-heating of the granite rocks below (Rawat and Purohit Citation1988). The various types of granite and gneiss rocks present in the basement also indicate this. Schistose gneiss and augen gneiss have developed in the granite without any distinct margins. In Chhota Shigri, Rohtang gneiss is dominant throughout the glacier bed, while some chalcopyrite was found in the lateral moraines up to an altitude of 4700 m, and veins of stibnite were found in the granitic rocks along the right lateral moraine (Katoch Citation1989).
MATERIAL AND METHODS
Meltwater samples were collected from the discharge sites (3800 m a.m.s.l.), i.e. 2 km from the snout (terminal of glacier, approx. 4100 m a.m.s.l.) each year from 2003 to 2007. Due to lack of funds, only one expedition was made in September–October of every year to obtain annual mass balance data. This was the main reason for selecting this period for sampling. In addition, September–October marks the end of monsoon and beginning of westerlies precipitation, i.e. the glacier experiences monsoon precipitation as well as that from Westerly Disturbances, so this was a chance to get the signature of both. In 2005, there was heavy snowfall prior to our field expedition and this is reflected in the water chemistry of the meltwater.
Meltwater samples were collected in 250-ml polyethylene bottles which were prewashed in distilled water and also washed with meltwater. Each year about 40 samples were collected during 10–15 days (last week of September–first week of October) and brought to the laboratory for further analysis. In the field, pH, EC and bicarbonate were measured immediately after collecting the samples, but were also verified in the laboratory. The pH of all samples was measured by a Consort microcomputer (P-307) ion meter and electrical conductivity (EC) was measured using a Pentex EC meter. Bicarbonate was measured by the potentiometer titration method. Suspended sediments were separated from the meltwater samples in the field by using 0.45-μm Millipore cellulose membrane filters of 47 mm diameter. Chloride ion concentration was measured by the mercury (II) thiocynate method (Florence and Farrar Citation1971). This involves the reaction of chloride with mercury (II) thiocynate to form chloromercurate (II) complex ion with the liberation of thiocynate ions, which then react with iron (III) to give a light red colour. Sulphate concentration was measured by the turbidimetric method (APHA 1985), dissolved silica by the molybdosilicate method (APHA 1985), phosphate by the ascorbic acid method (APHA 1985) and nitrate by the brucine-sulphanilic acid method (APHA 1985). The content of major cations, calcium, magnesium, sodium and potassium, was determined on a GBC 906 atomic spectrophotometer (AAS). Calcium and magnesium concentrations were determined in absorbance mode and sodium and potassium in emission mode. Three replicates were run for all samples and instruments were recalibrated after every 15 samples. Reference solutions were run periodically during all analyses. The analytical precision for cations was 5% and for anions 8–10%. The cationic and anionic charge balance (10%) is added proof of the precision of the data, although in some samples the charge balance is greater than 10%, it is still less than 15%.
RESULTS AND DISCUSSION
The weathering of the rock-forming minerals, dry and wet deposition (snow, rainfall and dust) and limited anthropogenic sources are considered to be the major sources of ions in the meltwater of glaciers (Collins Citation1978, Trudgill Citation1986). In general, the hydrochemistry of meltwaters helps in understanding of the overall natural solute acquisition processes.
Yearly variation
The charge balance between cations and anions of Chhota Shigri Glacier meltwater was calculated using the formula:
The charge balance of most of the samples was between 1% and 10%, and a very few samples were in the range 10–15% ( and Appendix Table A1).
Table 1 Major ion composition (in meq/L) of Chhota Shigri Glacier meltwater during September–October, 2003–2007
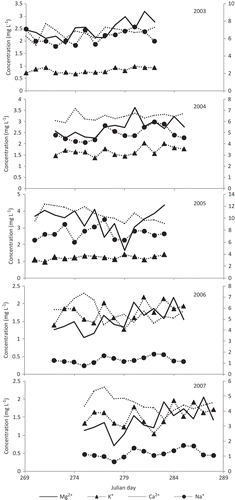
The meltwaters are slightly alkaline, with average pH values of 7.24, 7.4, 7.4, 7.2 and 7.1 in 2003–2007, respectively (). During acid hydrolysis meltwater becomes alkaline through its interaction with the basement rocks, where the H+ ion is consumed due to higher dissolution rates in the sedimentary environment. Meybeck (Citation1980) suggested that dissolved load is greater in sedimentary environments than in igneous and metamorphic rock environments. In 2005, snowfall was very high, which might have resulted in the high pH variability that year, since snowmelt initially reduces the pH value of meltwater (Ahmad and Hasnain Citation2001). If the snow pack stays on the ground surface for a long time during winter, temperature change causes preferential leaching of ions into the meltwater.
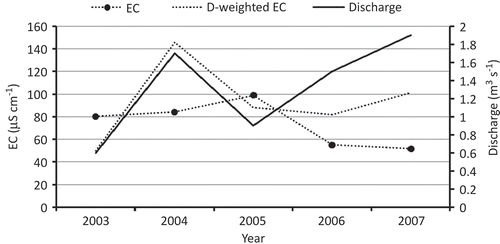
Average electrical conductivity (EC) values for 2003–2007 were 81, 84, 109, 54 and 51 μS/cm, respectively (). In 2005, EC was high due to high snowfall that increased the albedo of the glacier surface, reducing the ablation rate and resulting in low discharge, which, in turn, affects dilution of the meltwater. Thus the variation in EC is related to discharge variation due to snow and ice melting processes. High temperature leads to more snow- and icemelt, resulting in dilution of the solutes in the streamwater, while low temperature leads to less snow- and icemelt, resulting in higher solute loads in the meltwater stream (Chauhan and Hasnain Citation1993, Singh and Hasnain Citation1998).
Electrical conductivity is a measure of total dissolved solids (TDS) and depends on the ionic strength of the solution. The TDS varied from 51 to 71 mg/L with an average of 58 mg/L in 2003; 56–71 with average of 63 mg/L in 2004, 70–87 with average of 79 mg/L in 2005, 33–49 with average 39 mg/L in 2006 and 28–43 with average 35 mg/L in 2007. The ratio of TDS values in ppm to EC is generally accepted as 0.7 (Meybeck Citation1984) for freshwater. In this study, the average TDS/EC ratio was 0.723 in 2003, 0.743 in 2004, 0.735 in 2005, 0.735 in 2006 and 0.681 in 2007, indicating good water quality. The average TDS/EC ratio was 0.72 ± 0.21, confirming the reliability and quality of the analytical results. This slight difference may be either due to non-determination of some dissolved ions and/or due to the role of discharge variations in controlling cations rather than anions, as well as the diversity of ionic sources in the bulk meltwaters, including snow/icemelt and rock weathering.
The maximum TDS of meltwater was found in 2005 to be due to lower discharge; however, fluctuations in TDS were large in 2004 probably due to diurnal variation. More dilution of meltwater occurs in high-discharge compared to lower-discharge periods, and dilution has reduced ionic concentration of meltwater; thus discharge has an inverse relationship to electrical conductivity and in turn to TDS.
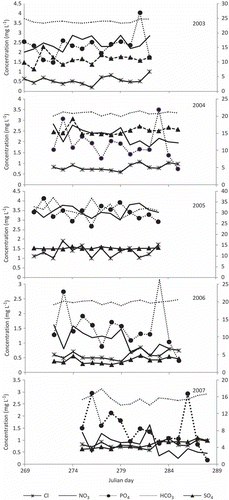
Temporal variations of anions and cations are shown in and , respectively. In 2003, bicarbonate (52.3%) and sulphate (30.5%), followed by phosphate (9.7%), nitrate (5.2%) and chloride (2.05%), contributed to anions, and calcium (48.3%), followed by magnesium, sodium and potassium (29.2, 13.5 and 8.6%, respectively) to cations. In 2004, bicarbonate (43.2%) and sulphate (41.5%), followed by phosphate, nitrate and chloride (7.6, 4.6, 2.9%), contributed to anions, and calcium (43.9%), followed by magnesium, sodium and potassium (30.2, 14.2, 11.5%), to cations. In 2005, the trend did not change much: bicarbonate (52.6%) and sulphate (30.3%), followed by phosphate, nitrate and chloride (9.7, 5.2, 2.1%), for anions, and calcium (48.5%), followed by magnesium, sodium and potassium (29.1, 13.6, 8.7%), for cations. In 2006, bicarbonate (68.4%), followed by sulphate, phosphate, nitrate and chloride (14.4, 9.9, 3.7 and 3.4%, respectively), contributed to anions, and calcium (55.5%), followed by magnesium, sodium and potassium (24.3, 11.2, 8.7%), to cations, whereas in 2007, bicarbonate (58.7%) and sulphate (22.5%), followed by phosphate, chloride and nitrate (10.7, 5.2, 3.1%), contributed to anions, and calcium (53.0%), followed by magnesium, sodium and potassium (25.9, 12.0, 9.0%), to cations.
The dissolved silica concentration varied as follows: 3.2–8.1 mg/L in 2003, 4.2–8.3 mg/L in 2004, 4.3–7.2 mg/L in 2005, 1.4 –5.5 mg/L in 2006, and 1.1–5.1 mg/L in 2007. The concentrations of dissolved silica are much higher than those of PO4 2−, NO3 − and Cl−, which probably indicates the negligible contribution from anthropogenic sources and the dominance of silicate weathering induced by the alkaline pH. The slow silicate dissolution as meltwater traverses through supraglacial, englacial and subglacial pathways, having variable residence times and mixing ratios in space and time, is the cause of the observed variability in dissolved silica concentration.
Trilinear classification
A trilinear diagram (), also frequently referred to as a Piper diagram (Piper Citation1944), provides a convenient method to classify and compare water types based on the ionic composition of different water samples (Hem Citation1985) using Aqua Chem 5.1 software. Cation and anion concentrations for each meltwater sample are plotted as percentages of their respective totals in two triangles and then projected into a quadrilateral polygon that describes the water type or hydrochemical facies.
Fig. 4 Trilinear diagram showing water types for average of meltwater samples collected from discharge site of Chhota Shigri Glacier for the years 2003–2007.
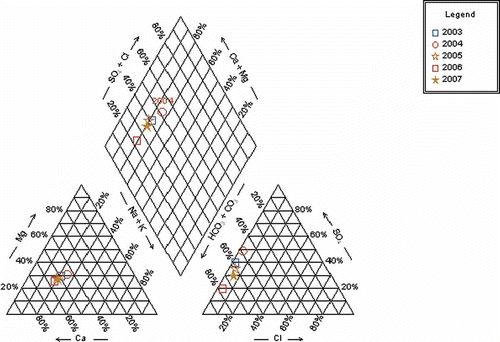
In samples collected from Chhota Shigri meltwater from 2003 to 2007, the dominant cation is calcium, and the dominant anion bicarbonate, i.e. they would be classified as calcium-bicarbonate type waters. It is clear from the graph that most of the samples fall into the normal earth alkaline water group with prevailing bicarbonate and sulphate. This type of water originates through natural processes by the dissolution of carbon dioxide (CO2) from the atmosphere and from the soil horizon, which causes the dissolution of the carbonate minerals, calcite CaCO3 and dolomite (CaMg)(CO3)2 of the aquifer (Suk and Lee Citation1999). The sample from 2004 shows it is earth alkaline water with increased portions of alkalis (prevailing bicarbonate and sulphate in equal amounts); this water type is characterized by its high SO4 2−, which might be an indication of pyrite or chalcopyrite dissolution along with carbonate dissolution.
Overall, the water chemistry shows that bicarbonate and sulphate are the dominant anions and calcium and magnesium the dominant cations in the meltwater of Chhota Shigri Glacier. This is similar to available world data on meltwater chemistry compiled by Raiswell (Citation1984), which shows that half of the world glacier meltwaters are dominated by bicarbonate followed by sulphate. The range of percentage of cations tabulated by Collins (Citation1979) from different drainage sources suggests that Ca2+ is the dominant cation in approximately 88% of the Earth's meltwaters and comprises more than 70% of the total equivalents (Raiswell Citation1984).
In the years 2003–2007, bicarbonate and sulphate contributed more than 75% of the total anions in Chhota Shigri Glacier meltwater, whereas (Ca2+ + Mg2+) contributed more than 75% of total cations. The Ca2+ is released to the meltwater more readily and was greater in absolute quantities than other ions derived from partial dissolution of the suspended load. The Ca2+ and Mg2+ may be derived from carbonates and silicates such as pyroxene, carbonate and dolomite. Additionally, a possible source of Ca2+ is granitic rock through weathering of biotite and feldspar (Kumar Citation1989). Bicarbonate is generally the dominant ion followed by sulphate, indicating the nearly equal dominance of weathering, atmospheric precipitation and melting on the hydrochemcial evolution of the meltwater. The relative proportions of the various ions in solution depend on their relative abundance in the bedrock and their solubility.
The chemical composition of meltwater of other Indian Himalayan glaciers () reveals that bicarbonate is the dominant anion and calcium the dominant cation, except for Kafni Glacier where sulphate dominated over bicarbonate and sodium, and potassium dominated over calcium.
Table 2 Average chemical composition of meltwater of some Himalayan glaciers in India (EC in μS/cm, cation and anions in μeq/L)
Table 4 Averages of different ion ratios for Chhota Shigri Glacier meltwater, 2003–2007
Solute sources
Solute is defined as all ions, including base cations (e.g. Ca2+, Mg2+, K+ and Na+), aqueous protons (H+(aq)) and anions (HCO3 −, SO4 2−, Cl−, PO4 −, NO3 −) and neutral species (e.g. O2, CO2, H4SiO4) dissolved in water (Stumm and Morgan Citation1981). In Chhota Shigri meltwater, the ratio of calcium, magnesium and bicarbonate is almost 1:0.6:1.3, which is close to the dolomite reaction. Pyrites were present in small veins of antimony traversing the granite bedrock, while chalcopyrite was found in the lateral moraine of Chhota Shigri Glacier (Kumar et al. Citation1987, Katoch Citation1989). Despite pyrite being a minor component of the bedrock, its dissociation kinetics is of several orders of magnitude more rapid than those of most other rock forming minerals (Tranter Citation1982). Among the anions, bicarbonate is derived from the dissociation of atmospheric CO2 and the dissolution of carbonates—dolomite and calcite—as well as weathering of primary and secondary minerals. The dissolved CO2 produced bicarbonate as follows:
The carbonic acid reacts with carbonate and silicate to produce bicarbonate:
The HCO3 − in the natural waters results from dissolution of carbonate and silicate rocks. The stochiometry of carbonate weathering reactions in the meltwater of Chhota Shigri indicates that carbonate derived from (HCO3)-C is moderately in excess of (Ca2+ + Mg2+). In (Ca2+ + Mg2+) vs HCO3 − for 2003, 2005, 2006 and 2007, the excess of bicarbonate over (Ca2+ + Mg2+) requires that part of the alkalinity should be balanced by alkali metals (Na+ + K+). This HCO3 − might have derived from silicate weathering in addition to carbonate weathering which is compensated by (Na+ + K+). The high concentration of bicarbonate and its positive correlation with calcium and magnesium indicate carbonate dissolution as a possible source of bicarbonate, calcium and magnesium. The (Ca2+ + Mg2+)/TZ+ ratio is >0.7 in all years: 2003 (0.776), 2004 (0.74), 2005 (0.796), 2006 (0.79) and 2007 (0.78); thus it can be used as an index of significant contribution by silicate weathering (Sarin et al. Citation1989). The molar abundance ratio in the silicate of the upper crust is generally 1.0 (Taylor and McLennan Citation1985, Singh and Bengtsson Citation2005) and the observed (Ca2+ + Mg2+)/(Na+ + K+) ratio ranges from 2 to 5, which indicates that the source of these ions in the meltwater might be due to the combined influence of carbonate and silicate weathering. The estimated bicarbonate contribution from carbonate and silicate weathering using the method suggested by Raymahasay (Citation1986) indicates that most HCO3 − (79–95%) comes from carbonate weathering (). The different ion ratios shown in also confirm the dominance of carbonate weathering, followed by silicate weathering, in these meltwater samples.
Table 3 Average carbonate and silicate weathering (in %) for 2003–2007
Sulphate is the second dominant anion. The presence of pyrite and chalcopyrite in the bedrock (Kumar et al. Citation1987, Katoch Citation1989) indicates that oxidation of sulphides is a major source of sulphate in the meltwater of Chhota Shigri Glacier. In 2004, as in all years studied, the sulphate contribution (41.5%) almost equals that of bicarbonate (43.3%) due to prominent weathering of pyrite (Garrels and Mackenzie Citation1971), and additional contributions from dissolution of atmospheric aerosols (Wake et al. Citation1992). The high concentration of sulphate ions also suggests the oxidation of sulphide minerals which provide H+ ions to the system for the chemical weathering (Garrels and Mackenzie Citation1971). Hence the supply of the CO2 and O2 in the streamwater is assumed to be a fundamental control on the rate and the extent of weathering processes in the high-altitude basin.
The relative dominance of the HCO3 − and SO4 2− in the meltwater reflects the relative contribution of the major source of H+ deriving reaction (Brown et al. Citation1996). The ratio of HCO3 − to (HCO3 − + SO4 2−) (c-ratio, ) is also used to characterize the relative importance of two major proton producing reactions, carbonization and oxidation of sulphides. The value of the ratio of HCO3 − to (HCO3 − + SO4 2−) varies from 0.5 to 0.6 in 2003–2005, suggesting the combined input of carbonate rock weathering and oxidation of sulphide, and from 0.7 to 0.8 in 2006 and 2007, suggesting the dominant input to be from carbonate weathering with less activity of sulphide oxidation (Singh and Hasnain Citation1998, Ahmad and Hasnain Citation2001) in these years.
Table 5 Average c-ratio (HCO3 − vs (HCO3 − + SO4 2−)) for Chhota Shigri Glacier meltwater, 2003–2007
Other anions, such as Cl− and NO3 −, have predominantly atmospheric sources (Tranter et al. Citation1993) in almost every studied year. The Cl− comes originally from sea spray then through atmospheric deposition, whereas NO3 − comes from anthropogenic sources then wet atmospheric deposition (Wake et al. Citation1992). Although the contribution of phosphate anions is high, the source is not confirmed. A possible source might be phosphatic nodules, but this has not been reported so far in the available literature on Chhota Shigri Glacier region.
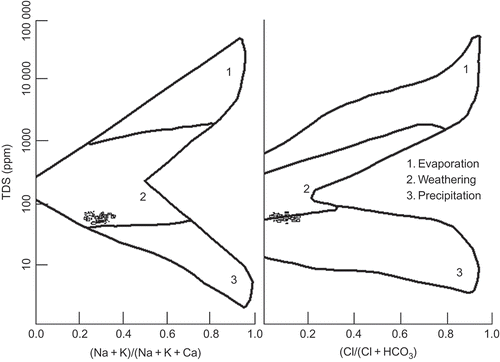
The (Na+ + K+) vs Cl− ratio in Chhota Shigri meltwater shows that the values are moderately high (), indicating a lower input from sea spray and evaporate deposits. The meltwater shows a higher Na+/Cl− ratio: from an average of >6 in 2003 to 2.5 in 2007 (), suggesting that the sodium in meltwater resulted from silicate weathering. The Na+/Cl− ratio is >3, because there is little sodium in the precipitation in this region due to the distance from the sea. Sarin et al. (Citation1992) suggested that the atmospheric contribution of Na and K in the Himalayan region are almost 20%, whereas Ca and Mg are limited to 5%. Data for atmospheric inputs show Na+ and K+ ratios with Cl− are 1.0 and 0.2, respectively (Pandey Citation1999), which are very low relative to 2–6 in Na+/Cl− and 2–3 in K+/Cl− (not shown) from our results. The Na+/Cl− ratio is >1 in the majority (80%) of samples where the sodium input of meltwater results from silicate weathering, but a few samples (20%) have a ratio <1 showing the contribution from aerosols, which could be of marine origin or from anthropogenic sources and dry deposition processes. In the meltwater of Chhota Shigri Glacier, potassium is least abundant among cations in all sampling years (2003–2007). According to Meybeck (Citation1984), in silicate weathering a quarter of the potassium comes from igneous and metamorphic silicates, so the source of potassium should be weathering of silicate minerals and clay.
The (Na+ + K+) / (Na+ + K+ + Ca2+) vs TDS plot (Gibbs Citation1970, ) shows that precipitation, followed by rock weathering, control the solute concentration of this meltwater. The role of chemical weathering in controlling meltwater chemistry may be attributed to high meltwater flushing rates, an abundance of finely-ground, geochemically-reactive suspended sediment resulting from physical abrasion and crushing, turbulent meltwaters capable of mobilising these sediments and providing a rough water surface for air–meltwater gas exchange, and the generally low buffering capacity of meltwaters.
Diurnal variation
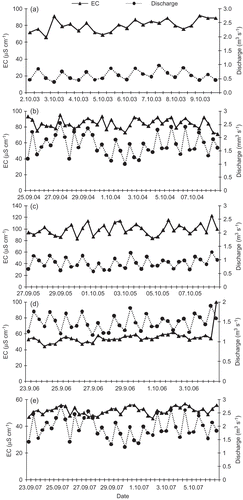
Analyses of instantaneous observations of electrical conductivity and discharge at the discharge site indicated an approximately inverse relationship between electrical conductivity and discharge, but detailed analysis showed that the relationship was not perfectly inverse (Appendix, Table A1). The diurnal cycles followed the same pattern in every year of data collection, with low discharge in the morning and the highest in the afternoon; sometimes evening discharge rates were similar to afternoon discharge. The EC was found to be highest in the morning, followed by a gradual decrease due to change in temperature and resulting melting. These inverse relationships between EC and discharge are shown in . This might be due to dilution; in high discharge periods meltwater is more diluted than in lower discharge periods. The variation in EC is related to snow and ice melting processes: high temperature leads to a higher degree of snow and ice melting and results in dilution of the solute in the streamwater, and vice versa (Chauhan and Hasnain Citation1993, Singh and Hasnain Citation1998). The intra-annual relationship between EC and discharge follows the same trend, but when compared with the inter-annual relationship, it is more complex and difficult to find any existing trend.
Variation between years
There is less variation in EC even though discharge variation is very high (). In 2003, 2004 and 2005, EC varied very little, while discharge varied almost three-fold (). This variable discharge is probably controlled by melting rate, as well as englacial reservoirs that flooded and contributed to discharge. For these years, there is no simple relationship between EC and discharge. These shifts were usually gradual, but, because of the absence of long periods of continuous data, are difficult to explain. Generally, EC represents the mixing of englacial and subglacial flow components of roughly constant conductivity. Thus these changes can be interpreted in terms of the gradual re-routing of one or both of these components before they reach the discharge site of the glacier. To fully understand re-routing of flow, we would need continuous data sets for englacial and subglacial hydrology. The changes in EC during the study period 2003–2007, may be explained by re-routing of the englacial flow component. This hypothesis is reinforced by the fact that, during this period, a clear increase in discharge was observed at the discharge site. Moreover, the abrupt complementary changes in conductivity and the observed sudden increase in discharge could be explained by a rapid form of the same mechanism; i.e. a sudden re-routing of englacial water including temporary storage of some of the water during this re-routing.
Effective CO2 pressure
The “partial effective CO2 pressure” or “internal CO2 pressure” (logpCO2) in Chhota Shigri Glacier over five years was computed from the pH values and HCO3 concentration (). The average pCO2 value for meltwater of Chhota Shigri Glacier was slightly higher (10−2.7–10−3.0) than the atmospheric value (10−3.5), indicating open system weathering. It is universally observed that streamwaters show disequilibrium with the atmosphere (Garrels and Mackenzie Citation1971, Raymahasay Citation1986). The slightly higher values of pCO2 could be explained by the relatively higher rate of solubility in comparison to release of excess of CO2 gas in a turbulent and low-temperature environment (Stumm and Morgan Citation1970). High pCO2 values are also likely to arise from the coupling of sulphide oxidation and carbonate dissolution. Further, in glacial environments (extreme cold), CO2 has a relatively higher rate of solubility in comparison to release of excess of CO2 gas to the atmosphere (Stumm and Morgan Citation1981). Holland (Citation1978) also observed that the release of excess CO2 gas to the atmosphere in turbulent water is very low in glacial environments.
Table 6 Average of logmHCO3 and logpCO2 for Chhota Shigri Glacier meltwater, 2003–2007
CONCLUSIONS
The data presented herein relate to observations recorded during the months of September and October over five years. Diurnal observations show that discharge is lower in the morning and increases in the afternoon due to the change in temperature, which has a great influence on snow- and icemelt at the glacier surface. During low discharge, EC is high, but it is less during high-discharge periods due to dilution. In 2005, high snowfall (positive mass balance) induced low discharge and the average EC was high. During high snowfall, the albedo of glacier surfaces increases, resulting in less melting. Calcium is the dominant cation, as in other Indian glaciers. Sodium is dominant over potassium, due to its high mobility and the weathering of silicate minerals of the igneous and metamorphic rocks. Sodium and chloride ratios are high, indicating low contributions from atmospheric input (especially for Na). Most of the sodium comes from weathering of sodic plagioclase, but the chloride source is atmospheric. Bicarbonate is the dominant anion, followed by sulphate. In three years (2003, 2005 and 2007), the ratio Ca–Mg–HCO3 was 1:0.7:1.3, indicating their similar sources, i.e. weathering of primary and secondary minerals. Most of the SO4 2− in all years is derived from the oxidation of sulphides such as pyrite and chalcopyrite. The SO4 2− is approximately in equal proportion to bicarbonate suggesting that some sulphate might have been derived from dissolution of atmospheric sources, i.e. due to release of protons. The meltwater is almost neutral to slightly alkaline in nature, induced by bedrock weathering. Most of the carbonate is derived from carbonate weathering, followed by silicate weathering. The high ratios of (Ca2+ + Mg2+) to (Na+ + K+), and (HCO3)-C to (HCO3)-Si, indicate that the meltwater chemistry is controlled by complex weathering processes. In general, chemical weathering seems to dominate and control the meltwater chemistry. Also, all cation and anion concentrations were at their maxima in the morning and at their minima in the afternoon during the study period, indicating the role of meltwater flushing rates through the glacier and variable mixing of different flow components.
Acknowledgements
The authors are grateful to SES, Jawaharlal Nehru University, New Delhi, for providing the facilities to analyse the samples. The authors also wish to thank Mr Adhikari and porters for their support in Chhota Shigri Glacier field work. Thanks are also due to the DST, Government of India and SAC (ISRO) for the partial financial support for this study.
REFERENCES
- Ahmad , S. and Hasnain , S.I. 2000 . Hydrochemical interaction of an alpine stream with sub alpine environment in Ganga headwater region, Garhwal Himalaya, India . Geological Society of India , 56 : 431 – 439 .
- Ahmad , S. and Hasnain , S.I. 2001 . Snow and stream water chemistry of Ganga headwater basin, Garhwal Himalaya, India . Hydrological Sciences Journal , 46 : 103 – 111 .
- APHA-AWWA-WPCF . 1985 . Standard methods for the examination of water and wastewater , 16th , 1268 Washington , DC : APHA . ed
- Bahadur , J. 1996 . “ On ecohydrological investigation over the Himalayas. In ” . In Ecohydrology of high mountain areas (international conference) Edited by: Chalise , N.R. Khanal and S.R. 392 Kathmandu: ICIMOD
- Bhutiyani , M.R. , Kale , V.S. and Pawar , N.J. 2007 . Long-term trends in maximum, minimum and mean annual air temperature across the Northwestern Himalaya during twentieth century . Climate Change , 85 (1–2) : 159 – 177 .
- Bhutiyani , M.R. , Kale , V.S. and Pawar , N.J. 2010 . Climate change and the precipitation variations in the northwestern Himalaya: 1866–2006 . International Journal of Climatology , 30, 535–548
- Bookhagen , B. and Burbank , D.W. 2006 . Topography, relief, and TRMM-derived rainfall variations along the Himalaya . Geophysical Research Letters , 33 ( 8 ) : L08405 10.1029/2006GL026037
- Brown , G.H. , Sharp , M. and Tranter , M. 1996 . Subglacial chemical erosion—seasonal variations in solute provenance, Haut glacier d'Arolla, Valais, Switzerland . Annals of Glaciology , 22 : 25 – 31 .
- Chauhan , D.S. and Hasnain , S.I. 1993 . “ Chemical characteristics, solute and suspended sediments load in meltwater draining Satopanth and Bhagirathi Kharak glaciers, Ganga basin, India. In ” . In Snow and glacier hydrology , Edited by: Young , G.J. Vol. 218 , 2 – 10 . Wallingford : IAHS Press, IAHS Publ .
- Collins , D.N. 1978 . Hydrology of an alpine glacier as indicated by the chemical composition of meltwater . Zeitschrift für Gletscherkunde und Glaziageologie , 13 ( 1/2 ) : 219 – 238 .
- Collins , D.N. 1979 . Hydrochemistry of meltwaters draining from an alpine glacier . Arctic Alpine Research , 11 : 307 – 324 .
- Dobhal , D.B. , Kumar , S. and Mundepi , A.K. 1995 . Morphology and glacier dynamics studies in monsoon–arid transition zone: an example from Chhota Shigri Glacier, Himachal-Himalaya, India . Current Science , 68 ( 9 ) : 936 – 944 .
- Florence , T. and Farrar , Y.J. 1971 . Spectrophotometric determination of chloride at the parts-per-billion level by the mercury(II) thiocynate method . Analytica Chimica Acta , 54 : 373 – 377 .
- Fujita , K. and Ageta , Y. 2000 . Effect of summer accumulation on glacier mass balance on the Tibetan Plateau revealed by mass-balance model . Journal of Glaciology , 46 : 244 – 252 .
- Garrels , R.M. and Mackenzie , F.T. 1971 . Evolution of sedimentary rocks , New York : Norton .
- Gibbs , R.J. 1970 . Mechanism controlling world water chemistry . Science , 170 : 1088 – 1090 .
- Hasnain , S.I. and Chauhan , D.S. 1993 . Sediment transfer in glacio-fluvial environment—a Himalayan perspective . Environmental Geology , 22 : 205 – 211 .
- Hasnain , S.I. , Subramanian , V. and Dhanpal , K. 1989 . Chemical characteristics and suspended sediment load of meltwaters from a Himalayan glacier in India . Journal of Hydrology , 106 : 99 – 108 .
- Hasnain , S.I. and Thayyen , R.J. 1996 . Discharge and suspended sediment concentration of meltwaters draining from Dokriani glacier, Garhwal Himalaya, India . Journal of Hydrology , 218 : 191 – 198 .
- Hasnain , S.I. and Thayyen , R.J. 1999 . Factors controlling suspended sediments transport in Himalayan Glacier meltwaters . Journal of Hydrology , 181 : 49 – 62 .
- Hasnain , S.I. 2001 . Character of subglacial drainage system in ablation area of Dokriani Glacier, India: as revealed by dye tracer studies . Journal of Hydrology , 248 : 216 – 223 .
- Hem , J.D. 1985 . Study and interpretation of the chemical characteristics of natural water , 3rd ed Reston, VA: USGS, Water-Supply Paper 2254
- Holland , H.D. 1978 . The chemistry of atmosphere and ocean , New York : John Wiley .
- Katoch , K.C. 1989 . Study of moraines with special reference to metallic minerals in Chhota Shigri glacier in Lahaul and Spiti District, Himachal . New Delhi: Department of Science and Technology, Technical Report , 3 : 299 – 301 .
- Kumar , S. 1989 . “ Structural analysis of the central crystalline between Rohtang-Manali area, H.P., India ” . In Recent researches in geology , Vol. 7 , 90 – 117 . New Delhi : Hindustan Publishing Corporation . In:
- Kumar , S. , Rai , H. , Purohit , K.K. , Rawat , B.R.S. and Mundepi , A.K. 1987 . Chhota Shigri glacier . New Delhi: Department of Science and Technology, Technical Report , 1 : 1 – 29 .
- Meybeck , M. 1980 . “ Pathways of major elements from land to ocean through river ” . In D. Burton, D. Eisma, and J.M. Martin, eds. IOC – Unesco Workshop on River inputs to ocean systems (RIOS), Rome, March 1979 , 18 – 30 . UNEP/UNESCO Report . In:Paris: IOC Unesco.
- Meybeck , M. 1984 . “ Atmospheric inputs and river transport of dissolve substances; dissolved loads of rivers and surface water quantity/quality relationships. In ” . In Dissolved loads of rivers and surface water quantity/quality relationships , Edited by: Webb , B. Vol. 141 , 173 – 192 . Wallingford : IAHS Press, IAHS Publ .
- Nijampurkar , V.N. , Sarin , M.M. and Rao , D.K. 1993 . Chemical composition of snow and ice from Chhota Shigri glacier, Central Himalaya . Journal of Hydrology , 151 : 19 – 34 .
- Pandey , S.K. 1999 . Aspect of weathering processes and chemical characteristics of Pindari glacier meltwater, Kamaon Himalaya, India , Thesis (PhD), Jawaharlal Nehru University .
- Piper , A.M. 1944 . A graphic procedure in the geochemical interpretation of water analysis . Transactions of the American Geographical Union , 25 : 914 – 923 .
- Raiswell , R. 1984 . Chemical models of solute acquisition in glacier meltwaters . Journal of Glaciology , 30 : 35 – 43 .
- Rawat , B.S. and Purohit , K.K. 1988 . Geology of the area around Chhota Shigri glacier, Lahul and spiti district (H.P.) . New Delhi: Department of Science and Technology, Technical Report , 2 : 152 – 157 .
- Raymahasay , B.C. 1986 . Geochemistry of bicarbonate in river water . Journal of Geological Society of India , 27 : 114 – 118 .
- Sarin , M.M. 1989 . Major ion chemistry of Ganga-Brahmputra river system; weathering processes and fluxes to the bay of Bengal . Geochimica et Cosmochimica Acta , 53 : 997 – 1009 .
- Sarin , M.M. 1992 . Major ion chemistry of Ganga source water: weathering in high altitude Himalaya . Proceedings of the Indian Academy of Science (Earth and Planetary Science) , 101 : 89 – 98 .
- Sharma , P. 2008 . Mass balance and chemical characteristics of Chhota Shigri Glacier-B, Lahaul-Spiti Valley, Himachal Pradesh , Thesis (PhD), Jawaharlal Nehru University .
- Sharma , S.S. and Ganju , A. 1999 . Complexities of avalanche forecasting in Western Himalaya—an overview . Cold Regions Science and Technology , : 95 – 102 .
- Singh , A.K. and Hasnain , S.I. 1998 . Major ion chemistry and weathering control in a high altitugde basin—Alaknanda River, Garhwal Himalaya, India . Hydrological Sciences Journal , 43 : 825 – 844 .
- Singh , O.P. and Hatwar , H.R. 2005 . Response of sea state to the monsoon onset . Mausam , 56 : 59 – 64 .
- Singh , P. and Bengtsson , L. 2005 . Impact of warmer climate on melt and evaporation for the rainfed, snowfed and glacierfed basins in the Himalayan region . Journal of Hydrology , 300 : 140 – 154 .
- Stumm , W. and Morgan , J.J. 1970 . Aquatic chemistry , New York : John Wiley-Interscience .
- Stumm , W. and Morgan , J.J. 1981 . “ An introduction emphasizing chemical equilibria in natural waters. In ” . In Aquatic chemistry , 2nd , Edited by: Stumm , W. and Morgan , J.J. New York : Wiley-Interscience . ed
- Suk , H. and Lee , K. 1999 . Characterization of a ground water hydrochemical system through multivariate analysis: clustering into groundwater zones . Ground Water , 37 : 358 – 366 .
- Taylor , S.R. and McLennan , S.M. 1985 . The continental crust: its composition and evolution , Oxford: Blackwell Scientific Publications, Geosciences texts .
- Tranter , M. 1982 . Controls on the chemical composition of Alpine glacial meltwaters , Thesis (PhD), University of East Anglia .
- Tranter , M. 1993 . A conceptual model of solute acquisition by alpine glacial meltwaters . Journal of Glaciology , 39 ( 133 ) : 573 – 581 .
- Trudgill , S.T. 1986 . Solute processes , New York : Wiley .
- Vohra , C.P. 1996 . “ Himalayan glaciers. In ” . In Harnessing the Eastern Himalayan rivers , Edited by: Iyer , R. 120 – 142 . New Delhi : Konark Publishers Pvt. Ltd .
- Wagnon , P. 2007 . Four years of mass balance on Chhota Shigri Glacier, Himachal Pradesh, India, a new benchmark glacier in the Western Himalaya . Journal of Glaciology , 53 ( 183 ) : 603 – 611 .
- Wake , C.P. , Mayawaski , P.A. and Wang , P. 1992 . Anthropogenic sulphate and Asian dust signal in snow from the Tien Shan, Northwest China . Annals of Glaciology , 16 : 45 – 52 .
- Yadav , R.K. , Kumar , R.K. and Rajeevan , M. 2009 . Out-of-phase relationships between convection over northwest India and warm pool region during winter season . International Journal of Climatology , : 1330 – 1338 . 29 (9)
APPENDIX