?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The physico-chemical and hydrochemical characteristics of run-off of the Neglinka River Basin (northwest Russia) monitored for a year are different for the upstream and downstream sections. The river known hydrologically as the Neglinka, consists hydrochemically of two different streams: one represented by the upstream part of the basin, and the other one by the downstream. The upstream water is characterized by low mineralization (water hardness 0.08–0.43 mmol L−1) and low δ53Cr values (+0.30 to +0.42‰), whereas the lower part is characterized by high mineralization (water hardness 0.37–3.46 mmol L−1) and high δ53Cr values (+0.92 to +1.73‰). The difference in chemical composition of the upstream and downstream waters could be due to the underground discharge input. Aqueous chromium (Cr) mobilized from weathering profiles may have been reduced from soluble Cr(VI) to insoluble Cr(III) during the riverine transportation. Partial removal of Cr from the water balance resulted in a decrease in Cr concentration and an increase in δ53Cr values.
Editor R. WoodsAssociate editor G. Chiogna
1 Study area
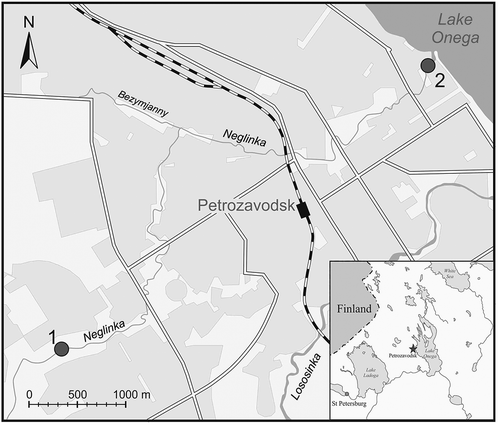
The short (14-km-long) Neglinka River originates in a small forest lake and in its lower part runs through the city of Petrozavodsk (). The average annual water discharge in the estuary of the river is 0.47–0.51 m3 s−1 (Ieshina et al. Citation1987, Kalinkina et al. Citation2012, Borodulina Citation2013). Most of the Neglinka basin (46.1 km2) is located in forested lowland, but the downstream reaches of the Neglinka River run through the hilly environment of the Lake Onega shore. The riverbed stretches along the Petrozavodsk depression in a crystalline basement. The depression is filled with a sedimentary complex of late Proterozoic quartzite-sandstone, and is covered with a thick layer (up to 100 m) of Quaternary glacial sediments (Borodulina Citation2006, Karpechko Citation2013). The bedrock and glacial sediments are covered in places with a thin (<1 m) soil layer.
Figure 1. Schematic location map of the sampling sites: 1 is the upstream (university) site, and 2 is the downstream (city) site. Inset: Location of the sampling area (star) in the border area between Russia and Finland.
Geochemical studies conducted so far have shown that the run-off water in the downstream section of the Neglinka River Basin was characterized by concentrations of basic cations (Ca2+, Mg2+, Na+ and K+) in the range of 6–70 mg L−1, with a total mineralization level varying from 35 to 400 mg L−1 throughout the year (Borodulina Citation2013). Biogenic elements and compounds such as Pmin, Ptot, and N-NH4+ displayed changes over the long-term observations. Whereas average yearly concentrations of N-NH4+ decreased from 0.18 mg L−1 in 1976 to 0.07 mg L−1 in 2016, concentrations of Pmin and Ptot increased for the same period from 59 to 148 μg L−1, and from 76 to 235 μg L−1, respectively (Pirozhkova Citation1981, Dzjubuk and Kljukina Citation2015, Sabylina and Efremova Citation2017). Annual observations showed that electrical conductivity (EC; a proxy to water mineralization) at the river mouth varied from ~200 to ~400 μS cm−1 during most of the year depending on the amount of atmospheric precipitation, but dropped sharply to 35 μS cm−1 with the beginning of the spring snowmelt (Borodulina Citation2013). The acid-alkaline features of water in the river mouth changed similarly: relatively steady pH values (7.5–7.8) were typical for winter, then a sharp decrease in pH (down to 5.8) was observed in April–May, while significant variations in pH were observed during summer and autumn (6.6–8.2; Borodulina Citation2013).
Studies conducted on the Neglinka River Basin run-off to date have mostly dealt with changes in concentrations of basic cations and biogenic elements, and with physico-chemical properties of water (e.g. Pirozhkova Citation1981, Ieshina et al. Citation1987, Borodulina Citation2006, Citation2013). Dzjubuk and Kljukina (Citation2015) reported some limited information on the Neglinka River water composition and physico-chemical properties from three sampling sites (upstream, midstream and downstream) for the period 2013–2014, but water samples were collected three times per year only. Data on concentrations of Zn, Cu and Pb in upstream and downstream Neglinka River Basin run-off samples collected in August 1999 are given in Komulaynen and Morozov (Citation2007). Two most comprehensive works concerning the issues raised in this study are those by Litvinenko and Regerand (Citation2013) and Slukovskii (Citation2014). To the best of our knowledge, no detailed monthly two-site observations of the Neglinka River Basin run-off over an extended period of time were ever conducted.
The information published to date on water parameters in the Neglinka River has allowed us to hypothesize that involvement of underground discharge might have influenced the chemical and physical properties of run-off water significantly (cf. Borodulina Citation2013, Borodulina et al. Citation2015). Since mineralization of groundwater in the region is usually several times higher than that of the surface water (Ieshina et al. Citation1987, Borodulina Citation2006), a significant increase in water mineralization for the Neglinka River downstream section may point to groundwater input. To test this hypothesis, we studied the chemical composition of run-off water samples added by a limited number of Cr isotope data by monitoring the water properties for a period of almost a year.
2 Sampling and analyses
Run-off samples were collected at two sites on the Neglinka River stream, separated by some 7 km. The first site is located in the upstream section outside the city limits, and the second site is located approx. 50 m upstream of the river mouth within the limits of the city of Petrozavodsk (). These two sampling sites were chosen because a comparison of the observations would allow us to make suggestions about the contribution of different solutes to the water balance along the river. One additional sample was collected from the Bezymjanny tributary, which enters the Neglinka River in the middle of the city ().
2.1 Non-isotopic analyses
Chemical analyses of run-off water were performed in the chemical laboratory of the Northern Water Problems Institute (Petrozavodsk, Russia). Samples prepared for analysis of dissolved metals were filtered through a 0.45-μm membrane filter and then acidified with ultrapure concentrated HNO3 to pH <2. Total iron (Fetot) was analysed from unfiltered samples. Before the analysis, the well-mixed sample was digested in the microwave for 30 min in the presence of concentrated HNO3 and H2O2 to obtain a totally transparent solution. Concentrations of Cr, Cu, Fe and Mn were measured with the use of an AA-6800 Shimadzu atomic adsorption spectrophotometer (https://www.shimadzu.com/). While concentrations of Fe and Mn were measured with the use of the atomic absorption method (flame atomization), concentrations of Cu and Cr were measured using a deuterium-corrected electro-thermal atomization method (e.g. Price Citation1979, Lozovik and Efremenko Citation2017). Concentrations of suspended iron were calculated by subtraction of dissolved iron (Fedis) from concentrations of Fetot.
Concentrations of major ions (Na+, K+, Ca2+, Mg2+, SO42- and Cl−) were measured in unacidified subsamples filtered through the 0.45-μm membrane filter. Sodium, K, Ca and Mg concentrations were measured on an atomic-adsorption spectrophotometer (AA 6200 Shimadzu). While Na and K were measured in the atomic emission mode, Ca and Mg were measured in the atomic adsorption mode (e.g. Price Citation1979, Lozovik and Efremenko Citation2017). Photometric methods with application of Sulfonazo III Na salt, and Cl− with Hg thiocyanate and Fe(III) nitrate were used to determine SO42- (Lozovik and Efremenko Citation2017).
Unfiltered and unacidified samples were used to determine EC and pH of water along with concentrations of biogenic elements and compounds (Pmin, Ptot, N-NH4+). Electrical conductivity was measured using an Agilent 3200C conductometer (https://www.agilent.com). A pH analyser (PHM 410) was used to determine pH by potentiometric method. Biogenic components were identified by photometric methods (Murphy and Riley Citation1962, Boeva Citation2009, Lozovik and Efremenko Citation2017). All photometric analyses were conducted on a Portlab 501 spectrophotometer. Dissolved organic carbon (DOC) concentrations were determined using an experimental set-up of a standard UV/peroxidosulphate method (Zobkov and Zobkova Citation2015).
2.2 Isotopic analyses
Isotope measurements were performed in laboratories of the Czech Geological Survey (Prague, the Czech Republic). Immediately after sampling, water samples were adjusted to pH ≥ 8 by ultrapure NH4OH in order to maintain Cr(VI) stability (cf. Cadkova and Chrastny Citation2015), and then filtered through the 0.45-μm membrane filters. Elution chromatography, modified from Chrastny et al. (Citation2011) and Farkas et al. (Citation2013), was applied for Cr(VI) purification. Volumes of sample containing ~1 μg Cr were loaded on columns filled with 2 mL of the anion exchange resin AG1-X8 (100–200 mesh, Bio-Rad, USA). Cations were eluted by washing the resin with 0.1M HCl. Chromium was eluted from the resin by washing with 6M HCl. Collected Cr samples were evaporated and re-diluted in 2% HNO3 to be analysed. The NIST® (National Institute of Standards and Technology) SRM (standard reference material) 979 chromium isotopic standard was processed through the columns along with samples to ensure lack of fractionation during the elution procedure. The procedural blank was negligible (15–20 ng Cr) as compared to the amount of Cr in samples.
The Cr isotope measurements were performed on a multi-collector inductively coupled plasma-mass specrometer (MC-ICP-MS) Neptune (Thermo) operating in a high-resolution mode (M/∆M(95%) = 10 000). The applied instrumental set-up is described in Farkas et al. (Citation2013). Because Cr has four stable isotopes, it was possible to utilize a 50Cr-54Cr double spike for mass bias and isobaric interference corrections. The analytical solution was introduced into the plasma using an ARIDUS II (Cetac, USA) desolvating nebulizer system and an ASX-112 FR (Cetac, USA) autosampler. All Cr isotopes and elements with isobaric overlaps were measured simultaneously. Polyatomic interferences were avoided by making measurements on the Cr peak shoulders. A NIST® SRM 979 standard doped with a double spike was analysed after every four samples. A blank measurement was made between each sample and/or NIST® SRM 979 standard.
Chromium isotope compositions are expressed in the per mil (‰) notation relative to the Cr standard, as follows:
The δ53Cr value of the NIST 979 doped with the double spike was monitored over the course of the measurements, and the average value obtained was 0.02 ± 0.01‰ (2σ) (n = 10). The isotope composition of all samples was corrected to this value. The average δ53Cr value for the NIST 979 standard processed through the chromatographic columns was 0.03 ± 0.01‰ (2σ) (n = 8). The average reproducibility of δ53Cr measurements for samples was ±0.05‰ (2σ) (n = 12).
3 Results
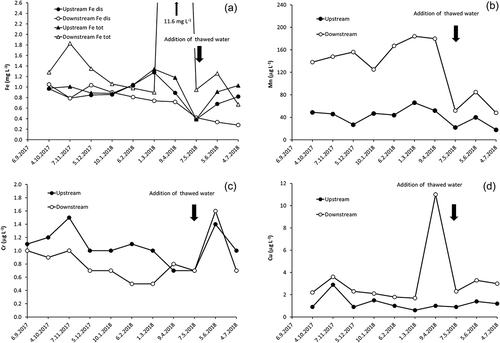
The physico-chemical, chemical and isotopic characteristics of water from the two studied sampling sites were found to be significantly different (cf. Dzjubuk and Kljukina Citation2015). Concentrations of major ions were about an order of magnitude higher in samples from the downstream section of the Neglinka River than from its upstream section (). Concentrations of most measured metals were also higher in downstream samples (; ). While, in upstream samples, the behaviour of dissolved iron (Fedis) was somewhat erratic, with concentrations varying from 390 to 1280 μg L−1, downstream samples displayed a steady decrease in Fedis concentrations (except for November 2017) from 1050 to 280 μg L−1 over the course of observation. In all cases, total iron (Fetot) concentrations remained mostly higher in downstream samples, with a large peak (>11 mg L−1) in April 2018. For the upstream site, most Fe is represented by its dissolved variety. Such a difference in Fe behaviour was due to high amounts of suspended iron (Fesus) as compared to the downstream samples. Manganese concentrations varied from 18 to 66 μg L−1 for upstream samples and from 48 to 184 μg L−1 for downstream samples, depending on the season (a drop in concentrations was noted for downstream samples in April 2018). Concentrations of Cu varied from 0.6 μg L−1 for the upper part of the river to 10 μg L−1 for the lower part. Chromium concentrations display strong seasonal changes for both upstream and downstream samples ()). For most of the year, concentrations of Cr (unlike other metals) are higher in the upper part of the stream (1.0–1.5 μg L−1) than those measured in the lower part of the stream (0.5–1.0 μg L−1). However, starting from April 2018, the picture changed and concentrations became nearly the same for water from the both parts of the river: 0.7–1.4 μg L−1 of Cr in the upstream section and 0.7–1.6 μg L−1 in the downstream.
Table 1. Characteristics of run-off water samples from the Neglinka catchment. Sample locations, US: upstream; DS: downstream. B: Bezymjanny. EC: electrical conductivity; Hard.: hardness; Turb.: turbidity; SD: standard deviation.
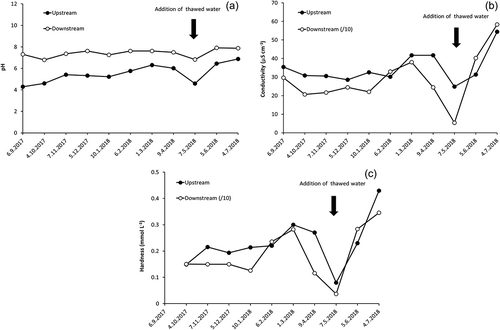
This study confirms observations made by Borodulina (Citation2013) about changes in water hardness along the stream: the water hardness of upstream samples varied from 0.08 to 0.43 mmol L−1, whereas that of downstream samples varied from 0.37 to 3.46 mmol L−1, depending on the season (; )). Biogenic elements and compounds (Pmin, Ptot, N-NH4+) were also much more abundant in downstream samples, with concentrations peaking in April 2018 (). Physico-chemical water parameters were also different for samples collected from the two sites (; ). Electrical conductivity of upstream samples varied from 24.8 to 54.4 μS cm−1, whereas it was almost an order of magnitude higher in downstream water (206–583 μS cm−1 over the year dropping to 53.4 μS cm−1 for samples collected in early May 2018; cf. Borodulina Citation2013). Upstream water displayed a wide range of pH values, varying over the period of observation from acidic to neutral (pH = 4.3–6.9), whereas the acidity was roughly neutral (pH = 6.8–7.9) in downstream samples.
Figure 3. Variation in (a) pH, (b) electrical conductivity, and (c) water hardness values over the period of observation. For the conductivity and water hardness, values for the city samples are divided into 10 for the sake of better presentation.
Pilot analyses of Cr isotopic systematics for the Neglinka River showed that δ53Cr values are different for the two studied sites (up to ~1.3‰). Downstream water samples displayed much higher δ53Cr values (from +0.92 to +1.73‰) than upstream water samples (from +0.30 to +0.42‰), i.e. the downstream water was much richer in the heavier isotope 53Cr (). The range of upstream δ53Cr values is comparable to that reported by Qin and Wang (Citation2017) for values from elsewhere (mostly around +0.3 ‰), whereas downstream water displayed very high δ53Cr values (cf. Farkas et al. Citation2013).
4 Discussion
One of the observed features of the Neglinka water was a sharp increase in concentration of biogenic elements/compounds, Cu and Fe for downstream samples collected in early April 2018 (). During that time, drainage of organic, dust and technogenic pollutants from the city snow cover and soil occurred (it is known that snow cover in the city of Petrozavodsk has elevated concentrations of Cu, Mn, Pb, Zn and other trace metals) (Krutskikh and Kritchevtsova Citation2011). This resulted, first, in a severe increase of particulate load in the river water within the city limits. While, over most of the year, the water turbidity had varied between 0.6 and 1.0 mg L−1, it increased sharply in early April 2018 to 74 mg L−1, and then slowly decreased in May and June 2018 to 10–11 mg L−1. Second, a strong increase in concentrations of biogenic elements was observed during the same period for downstream water samples (Pmin: 316 μg L−1, Ptot: 544 μg L−1 and N-NH4+: 0.86 mg L−1). That clearly suggests strong pollution of the Neglinka River with biogenic solutes. Third, typical for the Neglinka upstream samples, low pH values and a low amount of suspended particles, accompanied by high concentrations of organic compounds, are overall favourable for the existence of iron in dissolved form (Linnik and Nabivanets Citation1986).
For the downstream water samples, however, the city water drainage into the Neglinka River changes the physico-chemical properties as well as the amount of suspended particles. As a result, redistribution of metallic forms occurred, and the proportion of suspended Fe significantly increased. Since the snow cover of Petrozavodsk city has high concentrations of various metals (Krutskikh and Kritchevtsova Citation2011), a very sharp increase in concentrations of Cu and Fetot noted in April 2018 in the downstream Neglinka River (10 μg L−1 of Cu and >11 mg L−1 of Fetot) could have resulted from the city drainage system being overwhelmed by polluted snowmelt products. That is consistent with the observations of Slukovskii (Citation2014, Citation2015) and Slukovskii and Svetov (Citation2016), who conducted a study of the Neglinka River sediments, and concluded that the sediments from the urban part of the river are moderately to heavily polluted with metals.
Run-off from the upstream section of the Neglinka River (the rural part), on the other hand, did not display an increase in turbidity in April 2018 (0.6–2.9 mg L−1 over the course of observations). Since no urban pollution occurred here, no sharp changes in concentrations of organic and particulate load took place in April 2018. In early May 2018, sharp drops in both concentrations of all measured ions and values of physico-chemical parameters were observed for both upstream and downstream water samples (; and ). The reason for this is quite obvious: extensive snowmelt occurred in the region; therefore, all river water was strongly diluted with the products of the snowmelt (cf. Borodulina Citation2013).
4.1 Non-isotopic study
From the analytical results obtained, it appears that two different water streams are present within the single river system studied. It is interesting that in July 2011, the hardness of the water (another proxy to its mineralization) was measured along the Neglinka and Lososinka rivers (two major streams running through Petrozavodsk; ) (Borodulina Citation2013). While the water hardness in the Lososinka River remained quite steady over the whole length of the stream (0.82–0.95 mmol L−1), that in the Neglinka River increased by an order of magnitude in the downstream direction (from 0.28 to 3.76 mmol L−1). If the sources of mineralization were atmospheric precipitation and/or industrial pollution, then the change in mineralization level would be similar for the two closely located streams. However, a significant increase in water hardness for the Neglinka River vs steady hardness for the Lososinka River suggests an influence of underground discharge for the Neglinka River. The presence of underground springs along the downstream reaches of the Neglinka River and along the left-side tributary, the Bezymjanny (Borodulina Citation2013), and the fact that mineralization of groundwater is usually significantly higher than mineralization of the surface water (Ieshina et al. Citation1987, Borodulina Citation2006) are consistent with such a suggestion.
In addition to monitoring the composition of the Neglinka River water, we conducted several analyses of chemical and physico-chemical properties of water from the Bezymjanny tributary (). The tributary (~750 m long) originates in a small spring characterized by EC of 220–255 μS cm−1 and pH of 6.9–7.3, i.e. values similar to those measured for the Neglinka downstream section. The average annual concentration of Cr in the spring water was 0.5 μg L−1, and that of Cu was 0.8 μg L−1 (Borodulina Citation2006). Concentrations of Cr and Cu in the Bezymjanny water samples remained about twice lower than in the Neglinka samples. In early June 2018, Cr was 0.9 μg L−1 for the Bezymjanny tributary and 1.6 μg L−1 for the downstream Neglinka River, while Cu was 1.4 and 3.3 μg L−1, respectively. On the other hand, concentrations of the other elements and compounds in the Bezymjanny tributary were very close to those measured in the downstream Neglinkla samples (). The physico-chemical properties of water from the Bezymjanny tributary were also very close to those in the Neglinka downstream section: pH values for the Bezymjanny tributary varied from 6.9 to 7.5, and EC varied from 199 to 405 μS cm−1 (Borodulina Citation2013, Dzjubuk and Kljukina Citation2015; this study; cf. for the Neglinkla River). These values are very close to those in the Bezymjanny spring source. Such a distribution of the parameters for different parts of the Neglinka River is consistent with the suggestion that, although the Neglinka River is a single system in hydrological terms, hydrochemically it is represented by a combination of two independent systems: (i) its lower part (along with the Bezymjanny tributary), and (ii) its upstream part (from the source to approximately the place where the Bezymjanny tributary enters).
We noted that, for most of observation period, concentrations of Cr were approx. 10–30% higher in water collected from the upstream section than in that collected from the downstream section, including the Bezymjanny tributary. This suggests that the addition of Cr to the downstream water was insignificant (even from the underground discharge), i.e. the main source of Cr was located in the upper part of the river. In particular, a very low Cr concentration in the spring source of the Bezymjanny tributary is consistent with this suggestion. Deviations from the main-observed Cr concentration pattern occurred in April and May 2018, when Cr concentrations decreased in upstream run-off samples and increased in downstream samples ()). In June 2018, both the Neglinka sampling sites displayed a sharp increase in concentrations of Cr (1.4–1.6 μg L−1). Such an increase for the downstream section might have been due to limited incorporation of industrial Cr-bearing pollutants (cf. Komulaynen and Morozov Citation2007), whereas the Cr concentration in the upstream section likely just came back to the natural seasonal level. Although overall Cu concentrations are higher in the urban part of the river than in the rural part, the consistent behaviour of Cu concentrations in both parts ()) suggests that no significant industrial Cu input occurred. The only obvious urban Cu input was noted in April 2018, when its concentration in the downstream water reached 10 μg L−1 (cf. Komulaynen and Morozov Citation2007).
4.2 Isotopic study
In order to better understand the behaviour and origin of Cr in the Neglinka River, we measured Cr isotopic composition in six samples from both the upstream and downstream sections of the river. Overall, Cr exists in water-insoluble [Cr(III)] and water-soluble [Cr(VI)] oxidation states (e.g. Qin and Wang Citation2017). Reactions of Cr(III) oxidation to Cr(VI) and/or Cr(VI) back-reduction to Cr(III) result in Cr fractionation (D´Arcy et al. Citation2016 and references therein). During oxidation of soil/bedrock Cr(III), the heavier 53Cr isotope would be released preferentially into a soluble Cr(VI) form. This Cr(VI), enriched in the heavier 53Cr isotope, can be mobilized to water and become a subject of preferential reduction of the lighter 52Cr isotope. Therefore, further enrichment of the remaining aqueous Cr(VI) in the heavier isotope (increase of a δ53Cr value) would occur as a result of back-reduction. Although δ53Cr values for river run-off from elsewhere average mostly around +0.3‰ (Qin and Wang Citation2017), a number of recent studies have reported δ53Cr values of streams and rivers ranging between – 0.17 and +1.68‰. The highest values could result from fractionation due to Cr oxidation followed by back-reduction (D´Arcy et al. Citation2016 and references therein).
A significant difference in δ53Cr values between run-off samples from the upstream and downstream sections of the Neglinka River (+0.30 to +0.42‰ and +0.92 to +1.73‰, respectively) implies at least two major scenarios of aqueous Cr generation and evolution: (i) two independent sources with different Cr isotopic characteristics supplied Cr for the two different parts of the river; and (ii) a sole source supplied Cr, but aqueous Cr(VI) reduction occurred during the riverine transport. The first scenario would easily explain the difference in δ53Cr values for the two parts of the river by incorporation of materials with different Cr isotopic characteristics. Industrially polluted groundwaters might have been the one of the Cr sources for the first scenario. Izbicky et al. (Citation2012) and Novak et al. (Citation2014, Citation2017a) showed that polluted groundwater in industrial areas might have produced δ53Cr values of dissolved Cr(VI) varying from +1.6 to +3.9‰, which are significantly higher than technological solutions themselves. Unlike in the native waters, the positive δ53Cr shift in industrially contaminated water is believed to be solely due to Cr(VI) reduction (Novak et al. Citation2017b). Waters related to bodies of ultramafic rocks could be the second possible Cr source in the first scenario. Farkas et al. (Citation2013) demonstrated that serpentinite catchment streams may display δ53Cr values of up to +3.9‰. Therefore, addition of serpentinite-related groundwater would increase δ53Cr values of the river water. However, no serpentinite bodies exist, either in the vicinity of the city of Petrozavodsk or in the Neglinka catchment. Therefore, incorporation of Cr from serpentinite-related groundwater seems to be unlikely in the case considered. An additional objection to the first scenario is that both industrially polluted groundwater and groundwater from serpentinite catchments have much higher concentrations of Cr than the concentrations measured for any water sample from the Neglinka River (Novak et al. Citation2014, Citation2017a, Citation2017b). Therefore, incorporation of either industrially polluted groundwater or serpentinite-related groundwater would increase the concentration of Cr, which is not the case for the Neglinka downstream water.
The second scenario suggests that the overall decrease in Cr concentrations could be due to partial spontaneous reduction of aqeous Cr(VI) to Cr(III) during riverine transport in the Neglinka River. Insoluble Cr(III) precipitated from water would decrease Cr concentration in the run-off and increase its δ53Cr value. Data for the Neglinka River waters are more consistent with this second scenario. A wide range of δ53Cr values (from +0.30‰ to +1.73‰) appears to be due to variable redox conditions existing in the catchment. Some previous works have shown that, while, during the oxidation, δ53Cr values shift to higher numbers by just a few tenths of the per mil value, the reduction shifts the δ53Cr of the residual reactant to higher values, by 2.2–5.4‰ (Ellis et al. Citation2002, Zink et al. Citation2010, Kitchen et al. Citation2012, Basu et al. Citation2014). Although there are no data on the rate of such reduction in the environment yet, a work of Cadkova and Chrastny (Citation2015) showed that aqueous Cr(VI) is unstable over even short time periods. In experimental solutions containing various reducing agents, δ53Cr values increased by 1.0–1.8‰ over a period of <72 h. Such change in δ53Cr values was accompanied by a reduction of 27–38% of Cr(VI).
In the case considered, Cr is assumed to be leached from local weathering profiles and delivered to the source forest lake. Although we do not have exact information on Cr isotopic composition of the Neglinka catchment bedrock as yet, bedrock is usually slightly depleted in 53Cr, displaying δ53Cr values of around 0 to – 0.1‰ (cf. Zhu et al. Citation2018). Since, during oxidation of Cr(III) to water-soluble Cr(VI) δ53Cr, values of the resulting reagent [Cr(VI)] shift to higher values only slightly, some reduction of Cr(VI) mobilized to the Neglinka waters might have occurred during the riverine transport from the Cr source to the upstream sampling site (δ53Cr values of upstream samples are +0.30 to +0.42‰). The accumulation of decomposed vegetation in the forested part of the Neglinka catchment resulted in an increase in DOC concentrations in local run-off. Run-off from the forested part of the catchment has about twice the DOC concentrations (29–31 mg L−1) of the downstream section of the river (5–20 mg L−1). Overall, samples with the lowest δ53Cr values (upstream) have higher Cr and DOC concentrations, and lower pH values as compared to the downstream water (cf. D’Arcy et al. Citation2016). The observed change in δ53Cr values from on average +0.4‰ for the upstream to on average +1.5‰ for the downstream seems to be consistent with the reduction of Cr(VI) to Cr(III) in the aqueous environment. Therefore, the second scenario explaining the origin and evolution of aqueous Cr in the Neglinka River seems to be more realistic.
In spite of the obvious breakthrough in data collection for the Neglinka River waters, the newly obtained data are still limited for comprehensively answering the question about the influence of underground discharge and urban pollution on the chemical balance of the Neglinka River waters. In order to fulfil this gap in our knowledge, it would be necessary to extend the existing dataset with new observations, including trace metals, and traditional and selected non-traditional stable isotopes. Nevertheless, we were able to make some conclusions on the basis of the collected non-isotopic and isotopic data.
5 Conclusions
We suggest that the river hydrologically known as Neglinka, consists hydrochemically of two different streams: one represented by the upper section of the river, and the other by the lower section. The upstream section was characterized by overall low water mineralization and low values of physico-chemical parameters (pH, electrical conductivity), whereas the downstream section displayed higher water mineralization and higher physico-chemical parameters. Second, the overall strong increase in water mineralization in the downstream section of the river could be mostly due to the input of underground discharge (mineralization of groundwater in the region is usually several times higher than that of the surface water). And third, we can make a preliminary suggestion that Cr(VI) leached from a source in the uppermost part of the Neglinka River Basin may have been partly reduced to insoluble Cr(III) form, starting after mobilization to water and then further during the riverine transport. Precipitation of Cr(III) from water during riverine transport resulted in a decrease in Cr concentration and an increase of δ53Cr values of run-off. Overall low concentrations of Cr in the water samples studied were likely due to low concentrations of Cr in the catchment bedrock. The Neglinka River waters contained Cr(VI) as the main-dissolved Cr species, with an isotopic composition enriched in the heavier 53Cr isotope. Reduction during riverine transport resulted in the observed difference in δ53Cr values between upstream and downstream samples (+0.30 to +0.42‰ for the upstream vs +0.92 to +1.73‰ for the downstream). Detailed study of groundwater released to the surface in springs along the Neglinka stream may help to estimate more precisely the influence of underground discharge on the elemental and isotopic balance of the Neglinka River.
Acknowledgments
Two anonymous reviewers are thanked for critical reading the earlier version of the manuscript, and for their invaluable comments and advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Basu, A., Johnson, T.M., and Sanford, R.A., 2014. Cr isotope fractionation factors for Cr(VI) reduction by a metabolically diverse group of bacteria. Geochimica et Cosmochimica Acta, 142, 349–361. doi:10.1016/j.gca.2014.07.024
- Boeva, L.V., 2009. Manual to chemical analysis of surface waters from the landmasses. Part 1. Rostov on Don, Russian: NOK Publishing House, 1044 p.
- Borodulina, G., Tokarev, I., and Avramenko, I., 2015. Investigation of small river watershed hydrology in Karelia (north-west Russia) by high-resolution record of δ2H and δ18O in precipitation and river discharge, including experimental estimates of evaporation. In: International Symposium on Isotope Hydrology: Revisiting Foundations and Exploring Frontiers. 11–15 May 2015 Vienna, 174–176.
- Borodulina, G.S., 2006. Groundwater quality. In: N.N. Filatov and T.I. Regerand, eds. Water resources of Republic of Karelia and their use for drinking water supply. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 127–144. (in Russian).
- Borodulina, G.S., 2013. Groundwaters. In: A.V. Litvinenko and T.I. Regerand, eds. Water objects of the city of Petrozavodsk. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 31–42. (in Russian).
- Cadkova, E. and Chrastny, V., 2015. Isotope evidence of hexavalent chromium stability in ground water samples. Chemosphere, 138, 74–80. doi:10.1016/j.chemosphere.2015.05.057
- Chrastny, V., et al., 2011. Method of chromium (Cr) separation and establishing of measurement of its isotopic composition with the multicollector mass-spectrometer MC ICP MS. CGS Open File Report #TACR 010210055, 19 p. (in Czech).
- D´Arcy, J., et al., 2016. Processes controlling the chromium isotopic composition of river water: constrains from basaltic river catchments. Geochimica et Cosmochimica Acta, 186, 296–315. doi:10.1016/j.gca.2016.04.027
- Dzjubuk, I.M. and Kljukina, E.A., 2015. Dynamic of the Neglinka River water quality during its transition through the city of Petrozavodsk. Modern Problems of Science and Education, 5, on-line journal (in Russian).
- Ellis, A.S., Johnson, T.M., and Bullen, T.D., 2002. Chromium isotopes and the fate of hexavalent chromium in the environment. Science, 295, 2060–2062. doi:10.1126/science.1068368
- Farkas, J., et al., 2013. Chromium isotope variations (δ53Cr) in mantle-derived sources and their weathering products: implications for environmental studies and the evolution of δ53Cr in the Earth´s mantle over geologic time. Geochimica et Cosmochimica Acta, 123, 74–92. doi:10.1016/j.gca.2013.08.016
- Ieshina, A.V., et al., 1987. Resources and geochemistry of groundwater of Karelia. Petrozavodsk: Karelian branch of the USSR Academy of Sciences, 151 p. (in Russian).
- Izbicki, J.A., et al., 2012. δ53Cr isotopic composition of native and contaminated groundwater, Mojave Desert, USA. Applied Geochemisry, 27, 541–853.
- Kalinkina, N.M., et al., 2012. Complex assessment of urban rivers influence on the Petrozavodskaya Bay of Onego Lake. In: Ext Abst. 1st I.S. Rivers International Conference, C2 – Rivers as a pollution vector. Lyon, France, 3 p.
- Karpechko, V.A., 2013. Hydrographic and hydrologic characteristics of the streams. In: A.V. Litvinenko and T.I. Regerand, eds. Water objects of the city of Petrozavodsk. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 25–26. (in Russian).
- Kitchen, J.W., et al., 2012. Chromium isotope fractionation factors for reduction of Cr(VI) by aqueous Fe(II) and organic molecules. Geochimica et Cosmochimica Acta, 89, 190–201. doi:10.1016/j.gca.2012.04.049
- Komulaynen, S.F. and Morozov, A.K., 2007. Variations in phytoperiphyton structure in small rivers flowing over urbanized areas. Water Resources, 34, 332–339. doi:10.1134/S0097807807030116
- Krutskikh, N.V. and Kritchevtsova, M.V., 2011. Analysis of technogenic pollution of snowcover of Petrozavodsk. In: Ecologic geology: theory, practice and regional problems. Materials of the second international scientific-applied conference. Voronezh, 32–34.
- Linnik, I.A. and Nabivanets, B.I., 1986. Forms of metals migration in fresh surface waters. Leningrad: Hydrometeoizdat, 272 p. (in Russian).
- Litvinenko, A.V. and Regerand, T.I., 2013. Water objects of the city of Petrozavodsk. Petrozavodsk: Karelian Science Center, Russian Academy of Science, 109 p. (in Russian).
- Lozovik, P.A. and Efremenko, N.A., 2017. Analytical, kinetic and calculative methods in hydrochemical practice. St.-Petersburg: Nestor-History Publishing, 270 pp. (in Russian).
- Murphy, J. and Riley, J.P., 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36. doi:10.1016/S0003-2670(00)88444-5
- Novak, M., et al., 2014. Common occurrence of a positive δ53Cr shift in Central European waters contaminated by geogenic/industrial chromium relative to source values. Environmental Science & Technology, 48, 6089–6096. doi:10.1021/es405615h
- Novak, M., et al., 2017a. Chromium isotope fractionations resulting from electroplating, chromating and anodizing: implication for groundwater pollution studies. Applied Geochemistry, 80, 134–142. doi:10.1016/j.apgeochem.2017.03.009
- Novak, M., et al., 2017b. Temporal changes in Cr fluxes and δ53Cr values in runoff from a small serpentinite catchment (Slavkov Forest, Czech Republic). Chemical Geology, 472, 22–30. doi:10.1016/j.chemgeo.2017.09.023
- Pirozhkova, G.N., 1981. Hydrochemical regime of Petrozavodsk Bay. Petrozavodsk Bay of the Onezhskoye Lake. Petrozavodsk: Karelian Branch of the Academy of Science of the USSR, 192–223.
- Price, W.J., 1979. Spectrochemical analysis by atomic absorption. London, Philadelphia: Heyden, 392 p.
- Qin, L. and Wang, X., 2017. Chromium isotope geochemistry. Reviews in Mineralogy and Geochemistry, 82, 379–414. doi:10.2138/rmg.2017.82.10
- Sabylina, A.V. and Efremova, T.A., 2017. Tendencies in changes of input of chemical compounds with waters of small rivers along the South-Western shore of the Onezhskoye Lake over the last 50 years. Ecologic Chemistry, 26, 333–339. (in Russian).
- Slukovskii, Z.I., 2014. Ecologic-geochemical analysis of bottom sediment conditions in small rivers of urbanized territories (on example of the city of Petrozavodsk). Thesis (PhD). Petrozavodsk state University, 142 p. (in Russian).
- Slukovskii, Z.I., 2015. Geoecological assessment of small rivers in the big industrial city based on the data on heavy metal content in bottom sediments. Russian Meteorology and Hydrology, 40, 420–426. doi:10.3103/S1068373915060084
- Slukovskii, Z.I. and Svetov, S.A., 2016. Geochemical indicators of technogenic pollution of bottom sediments of small rivers in an urbanized environment. Geography and Natural Resources, 37, 32–38. doi:10.1134/S1875372816010054
- Zhu, J.-M., et al., 2018. An improved method of Cr purification for high precision measurement of Cr isotopes by double spike MC-ICP-MS. Journal of Analytical Atomic Spectrometry, 33, 809–821. doi:10.1039/C8JA00033F
- Zink, S., Schoenberg, R., and Staubwasser, M., 2010. Isotopic fractionation and reaction kinetics between Cr(III) and Cr(VI) in aqueous media. Geochimica et Cosmochimica Acta, 74, 5729–5745. doi:10.1016/j.gca.2010.07.015
- Zobkov, M.B. and Zobkova, M.V., 2015. A device for determination of organic carbon in water with photochemical persulfate oxidation in the system of continuous gas flow and FTIR spectrometric detection. Factory laboratory. Diagnostics of Materials, 81, 10–15. (in Russian).