?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The size-dependent chemical maturity of sediment cycling in a high-altitude glacial/periglacial environment was studied by examining the sediments of the Chorabari Glacier, Mandakini Valley, central Himalaya, India. Sediments evacuated from glacial debris and a periglacial area were studied via particle-size analysis, X-ray fluorescence, and X-ray diffraction of different grain-size fractions. The results suggest a clear control of grain-size fraction over chemical weathering and release of its attendant nutrients from the glacial substrate. Elemental indices suggest the release of nutrients is principally controlled through comminution and sub-glacial chemical weathering of leachable minerals such as feldspar, mica, and apatite. Owing to biogeochemical cycles, the release of nutrients like phosphorous and calcium is predominantly controlled through mineral dissolution process of aluminosilicate rocks containing apatite, carbonate minerals and organic complexes. Overall, the size-dependent chemical maturity of glacial sediments suggests a much more chemically active system than previously thought. This study is of particular importance for downstream weathering tracer studies of detrital sediments.
Editor S. Archfield Associate editor S. Pande
1 Introduction
In high-altitude glacierized basins, grain size is known to be the most effective tool to accurately access mechanisms of sediment production, alteration, and transportation (Owen et al. Citation2003, Haritashya et al. Citation2010). Sediment-discharge studies from the Himalayan glacial environments suggest a significant control of debris over sediment production and downstream supply from most of the glacial systems (Hasnain and Thayyen Citation1999, Singh and Mishra Citation2001, Benn and Owen Citation2002, Haritashya et al. Citation2010, Singh Citation2014a). Sediment is deposited over the glacial surface as debris through fluvial, glacial, aeolian, and mass movement processes within the valley system and enters the subglacial substrate before its final evacuation from the glacial termini (Hammer and Smith Citation1983, Woodward et al. Citation2002, Swift et al. Citation2005). Here, a specific process, i.e. comminution of glacial sediments, applies along the subglacial transport path under conditions of compressive flow regimes and gives rise to different grain-size fractions with distinct chemical signatures. However, surface processes, i.e. flash floods and higher precipitation, could also affect the sediment comminution process through the modification of landforms and sediment distribution, as outlined in previous studies from Gangotri and Kedarnath areas (Singh and Mishra Citation2001, Singh Citation2014a). The production of coarse sediment fractions (>0.5 mm) is likely due to glacial crushing mechanisms, whereas fine sediment fractions (<0.5 mm) are the product of glacial abrasion processes (Boulton Citation1978). The physical disintegration mechanism of the englacial zone could typically be assessed by studying grain size, whereas subglacial sediment modification could only be quantified by the chemical characterization of the sediments. In previous studies, the physical erosion processes of the Himalaya have been given significant consideration (e.g. Thayyen et al. Citation1999, Bhutiyani Citation2000, Singh and Mishra Citation2001, Pandey et al. Citation2002, Swift et al. Citation2005, Haritashya et al. Citation2006, Citation2010, Singh Citation2014a, Kumar et al. Citation2016). However, little attention has been paid to the chemical characterization of sediments, as chemical processes are thought to be negligible in glacial sedimentary environments. Several studies of chemical weathering have evaluated the bulk glacial sediment chemistries but do not permit us to properly evaluate the effects of grain size on source-rock control over sediment composition.
Chemical weathering in general appears to be well understood for solute fluxes (Meybeck Citation1987, Gaillardet et al. Citation1999, Singh and Hasnain Citation2002, West et al. Citation2005, Singh et al. Citation2014b, Citation2015b, Singh and Ramanathan Citation2015a), yet studies of their solid counterparts from glacierized basins are limited (France-Lanord and Derry Citation1997, Gaillardet et al. Citation1999). Appreciable efforts have been made to understand the chemical weathering within the Himalayan system – the largest active orogeny and highest mountain range on Earth (Sarin et al. Citation1992, Derry and France-Lanord Citation1996, Galy and France-Lanord Citation1999, English et al. Citation2000, Karim and Veizer Citation2000, Bickle et al. Citation2001, Citation2005, Dalai et al. Citation2002, Jacobson et al. Citation2002, Oliver et al. Citation2003, Singh et al. Citation2005, Tripathy et al. Citation2010, Lupker et al. Citation2012, Shukla et al. Citation2018b, Citation2020b, Sundriyal et al. Citation2018). Nevertheless, knowledge of the weathering processes within the Himalayan glacial system is scarce, and more data are needed from different catchments to better understand the cumulative effects of physical and chemical processes on sediment production and release. In particular, understanding the effects of physical processes on the major and trace element geochemistry of sediments and their attendant nutrient release is most urgently required.
Therefore, this study evaluates: (a) the effect of comminution on sediment fractions (grain size) and their chemical composition in glacial environments, (b) the limitations of source-rock control across grain-size grades and bulk geochemistry, and (c) the origin and release of micronutrients (i.e. P, Ca, and K) from glacial environments. Our study differs from previous studies by quantifying the chemical maturity of sediment eroded via the glacial/subglacial mechanisms of a single system (i.e. subglacial erosion). We present here a novel approach by assessing the nature of sediment production and post-glacial alteration processes from the glacierized basin of the Himalaya.
2 Study area
The present investigation were conducted over Chorabari Glacier (30°46′20.58″N, 79°2′59.381″E) and Chorabari Lake (30°44′51.26″N, 79°03′38.79″E), situated in the Mandakini Valley, central Himalaya, India ()). The Chorabari Glacier (length ~7.5 km; area ~6.6 km2) is a compound valley-type glacier system that originates at 6420 m a.s.l. and terminates at 3800 m a.s.l. (Kumar et al. Citation2016). The total catchment area of the glacier up to the gauging site is ~15.4 km2 (Kumar et al. Citation2016). The proglacial stream emerging from the Chorabari Glacier is a source of suspended sediments for the downstream.
Figure 1. Location of the study area, representing the catchment area of Chorabari glacier (solid line), Chorabari Lake (dotted lines), and the physiographical setup of the area
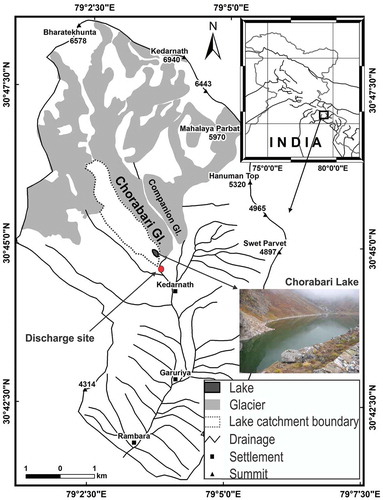
Chorabari Lake is located on the right flank of Chorabari Glacier and is dammed by a glacial moraine (Shukla et al. Citation2020a). The lake inflow is controlled by a stream originating from meltwater of glacier ice and snow, with subordinate contributions from watershed precipitation. The catchment rocks do not support groundwater aquifers, so the effect of groundwater mixing is negligible. Here we studied the sediment deposition patterns of two sets of environments: first, the glacial suspended sediments (GSS) evacuating from the snout of Chorabari Glacier; and, second, the sediments deposited in Chorabari Lake.
2.1 Geological and regional climatic setting
Geologically, the area falls within the complexes of metamorphic and granitic rocks comprising a major thrust system bounded by litho-tectonic units (Valdiya Citation1999b, Yin Citation2006). The rocks are bounded by the Main Central Thrust in the south and the Trans Himadri Fault in the north (Heim and Gansser Citation1939, Valdiya Citation1998). The regional geology comprises the Pindari Formation, which is highly metamorphosed and predominantly made up of calc-silicate gneisses and calc-schists, interbedded with subordinate veins of biotite-psammitic gneisses and granite-pegmatite-apatite (Heim and Gansser Citation1939, Valdiya et al. Citation1999a).
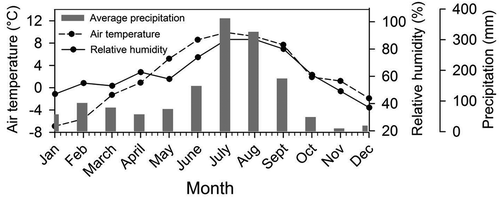
The study area is fed by two contrasting weather systems, namely the Indian Summer Monsoon (ISM) and Western Disturbances (WDs), during the summer and winter months, respectively (Benn and Owen Citation1998). The glacial catchment receives the maximum precipitation during the summer months through ISM (Kumar et al. Citation2016). The climate is characterized by dry–cold winters and humid–temperate summers (Benn and Owen Citation1998). Field-based meteorological data (2011–2013) suggest temperatures remain high between May and October, and remain low from November to April (). The daily mean air temperature fluctuates between +12 and – 1°C (June–October), with the maximum during June and the minimum during January. The relative humidity tends to fluctuate between 87% and 37% during summer and winter months, respectively ().
3 Methodology
3.1 Sampling methods
We sampled an exposed section of Chorabari Lake approximately 12 m thick. A total of 101 sediment samples of different size fractions were collected, and sealed at the site to avoid external contamination (Shukla et al. Citation2020a). The bulk sediment samples varied from clay to coarse particle size throughout the stratigraphy. Additionally, GSS samples were collected at a gauging site about 200 m downstream, near the snout of the glacier, from the proglacial melt water stream (GSS sampling methods detailed in Kumar et al. Citation2016). We also reassessed the GSS and lake sediment samples from the studies of Kumar et al. (Citation2016) and Shukla et al. (Citation2020a), respectively. In addition, eight freshly exposed rock samples from supraglacial debris were collected from Chorabari Glacier catchment during the field survey of 2016. This represents the major lithology of the underlying bedrock. In light of previous studies (Owen and Sharma Citation1998, Singh and Mishra Citation2001) highlighting the inherent complexities in sampling from glaciated terrain (i.e. sediment redistribution due to Glacial Lake Outburst Flood (GLOF), great care was taken to ensure the representativeness of each sample. Singh et al. (Citation2017) explained the differential pattern of glacial retreat at Gangotri Glacier, which is likely due to the collection of secondary samples by different workers. We ensured that the collected samples were not contaminated by any flash flood events.
3.2 X-ray fluorescence analysis
Analysis by X-ray fluorescence (XRF) was performed on the bulk suspended sediment samples, lake sediments, and representative bedrock composition. About 5 g of sample powder was mixed with polyvinyl alcohol solution as binding agent and kept under hydrologic pressure of 2000 kg cm−2 to obtain pellets of ~35 mm diameter. After removal of excess moisture, pellets were placed in a Wavelength Dispersive X-Ray Fluorescence (WD-XRF) Bruker S8 Tiger X-ray spectrometer with an end window 4 kW Rh anode tube (60 kV, 170 mA) to determine the weight of major oxides (%) and concentration of trace elements (ppm). The analytical precision and accuracy were checked with reference standards for soil (SO-1, GSS-1, GSS-4, GXR-2, GXR-6) and sediments (GSD-9, GSD-10). The measurement accuracy was <5% for major elements and <10% for trace elements. The analytical precision and reproducibility of sediments and standards were <2%. The GSS, rock samples, and lake sediment samples were statistically analysed to determine the minimum, maximum, mean, standard deviation (SD), and coefficient of variation (CV, %) for the major and trace elements. All analyses were carried out at the Wadia Institute of Himalayan Geology, Dehradun, India.
3.3 Grain-size analysis
Grain-size fractions of sediments <1 mm diameter were analysed using a Mastersizer 2000MU laser particle size analyser (particle size: 0.02–1000 µm), along with a Hydro-2000 wet dispersion unit. Accuracy of measurement was better than 1% (polydisperse standard), and reproducibility was <1% variation (polydisperse standard). The instrument calculates the volume percentage of the sample and divides it into different class intervals according to the distribution of different size fractions of the grains. However, the samples of larger size (>1 mm in diameter) were analysed using wet sieving and pipette analysis following standard procedures (Carver Citation1971). Based on particle size analysis, we categorized the samples into three major size fractions, viz. clay, sand, and coarser sand fractions. We classified them as follows: if the particle diameter was <0.004 mm, it was categorized as clay; grains between 0.004 and 0.5 mm in size were categorized as (silt+) sand; and the remaining (>0.5 mm) size fraction was the coarse sand fraction. The classification was based on the Wentworth grain-size chart from United States Geological Survey Open-File Report 2006–1195 (Wentworth Citation1922).
3.4 Enrichment factor
Sedimentary data evaluation in the closed reference frame was used to calculate the enrichment factor, by assuming that the sediments were evacuated through the lithology of the same catchment. Non-mobilization and low solubility of Ti were used for elemental evaluation (EquationEquation 1(1)
(1) ). The change in relative percentage of elements is calculated with respect to Ti following the methods of Nesbitt (Citation1979) and Middleburg et al. (Citation1988).
where X denotes the elements in question.
Further, we used the chemical index of alteration (CIA) weathering indices to understand the changes in the relative contributions of chemical and physical weathering in the production of sedimentary detritus. Following Nesbitt and Young (Citation1982) the CIA values from molar proportions of elements can be derived from EquationEquation (2)(2)
(2) :
where CaO* represents the CaO derived from silicate rocks only (Nesbitt and Young Citation1982, Citation1984, Fedo et al. Citation1995). CaO values have been corrected to carbonate, apatite, and Na2O according to McLennan et al. (Citation1993), using EquationEquation (3)(3)
(3) :
We followed the assumption that if molar (CaO) < molar (Na2O), then required CaO* = molar (CaO); whereas if molar (CaO) > molar (Na2O), then required CaO* = molar (Na2O).
Previous studies suggest that grain-size has a significant influence on CIA values even in glacial environments (e.g. Young and Wayne Nesbitt Citation1999; Young et al. Citation2004). The chemical weathering process typically preferentially removes the unstable alkali and alkaline earth elements (i.e. calcium, sodium, and potassium) from feldspar, whereas aluminium remains immobile during alteration (Nesbitt and Young Citation1982). We expect secondary products of feldspar alteration, i.e. aluminous clay minerals (illite and kaolinite), in glacially derived clay-sized fractions.
3.5 X-ray diffraction
For X-ray diffraction (XRD) analysis, clay fractions were separated using the decantation–centrifugation method of Jha et al. (Citation2012). Further, a set of chemical treatments were applied to remove the organic matter, carbonates, and allophanes. The clay fraction was then analysed using a PANalytical EMPYREAN X-ray diffractometer with Cu-Kɑ radiation functioning at an instrument setting of 45 kV and 40 mA to identify the mineral fractions. Samples were prepared through oriented mounts on the glass slides and scanned at a 2θ range of 3 to 35° with a step size of 0.02 and a count time of 1 second per step. We used a semi-quantitative method to identify each mineral peak by its position and relative intensity (Biscaye Citation1965). The identified minerals were confirmed by their diffraction patterns from the powder diffraction database of the International Centre for Diffraction Data (ICDD) (Carrols Citation1970).
3.6 Rock petrography
The petrographic investigation of the representative rock samples was carried out at the polarized light microscope facility of the Wadia Institute of Himalayan Geology Dehradun, India. The minerals were identified based on their optical properties.
4 Analysis and results
4.1 Bedrock composition
One of the main difficulties in weathering-related studies is the selection of a suitable reference frame so that the approach can meet stringent criteria. To overcome this deficit, we used the elemental composition of the underlying bedrock (). This approach calculates the rock-type abundance by assuming that the immobile elements remain conserved within rock composition. The limitation of this approach includes leaching of certain elements by chemical leaching and physical disintegration during transportation that could hamper the representativeness of the rock composition. The statistical parameters are presented in , showing the average composition and degree of variability in major and trace element composition of the parent rocks and sediments of different grain-size fractions. The lithological samples collected from Chorabari Glacier catchment are within the range of the elemental compositions reported from the Higher Himalayan Crystalline (HHC) by Galy and France-Lanord (Citation2001). Most rock types are of silicic composition, except lime-silicate marble and lime-amphibolite, which indicate the dominance of carbonates. The occurrence of calcite in the form of carbonates has also been previously reported in the HHC belt, but not quantified from a compositional viewpoint (Jacobson and Blum Citation2000). Therefore, we attempted to examine the composition in detail along a long stretch of the HHC, representing the origin and presence of carbonate-bearing rocks for quantification of the geochemical processes in this particular lithological setting.
Table 1. Statistical composition parameters of the parent rock used in the present study
Table 2. Statistical parameters for sediments of different grain size, representing trace element concentrations
Table 3. Statistical parameters for sediments of different grain size, representing trace element concentrations
4.1.1 Petrography of the underlying bedrock
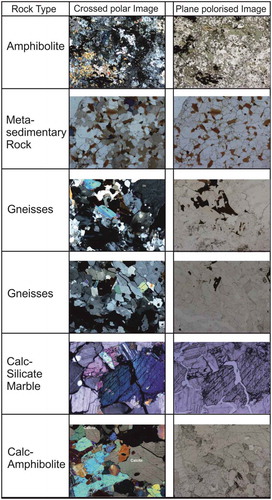
The major mineral assemblage of the major lithology of the Chorabari glacial catchment is predominantly of gneissic composition, consisting of quartz, plagioclase feldspar, biotite, and muscovite (see Supplementary material, Table S1). Of the eight representative rock samples, two showed a gneissic composition, each representative of amphibolites, quartzite, metasedimentary rock, tourmaline-bearing pegmatite, and quartz veins, with two other calcite-bearing rocks, associated with lime amphibolites and lime-silicate marble. Metasedimentary rocks show quartz as the dominant mineral in fine- to coarse-grained fractions with subhedral to anhedral grain morphology. Quartz veins consisted of anhedral to euhedral grains containing a few inclusions of tiny muscovite flakes.
Based on the petrographic properties of minerals and their elemental compositions, quartz, K-feldspar, plagioclase, amphiboles (hornblende), biotite, muscovite, and calcite were identified as the major mineral assemblage at the Chorabari glacial catchment ().
4.2 Concentration and quantitative normalization of major oxides and trace elements
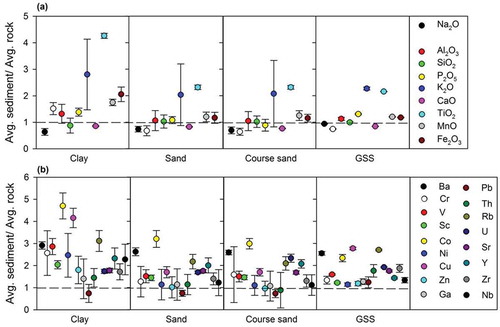
The major oxides and trace elemental composition of each grain-size fraction are presented in . To examine the relative enrichment of the elemental composition compared to the bedrock samples, we plotted the average normalized concentration of oxides and trace elements for the different grain-size fractions against the average underlying bedrock (). The results suggest the variation in elemental composition is likely caused by sample-to-sample noise within different classes or by post-deposition sediment modification. For example, Na and Ca show consistent depletion in all environments; however, K, Fe, Al, P, and Ti are enriched for all grain-size fractions. Conversely, trace elements show relative enrichment in all grain-size fractions except for Sr, likely due to its higher presence in the parent rock type or its rapid dissolution in solute phases (Anderson et al. Citation1997). Similarly, the depleted Mg and Ca concentrations in the coarse size fraction suggest their probable release in the aqueous phase (Shukla et al. Citation2018b). Further, the presence of Mg and Fe in finer fractions (mostly clay) is likely due to the abrasion of mafic minerals.
Figure 4. Distribution of (a) major oxides (wt %) and (b) trace elements (ppm) in sediments of different grain-size classes, normalized to their distribution in the underlying bedrock. Error bars are 2σ standard deviations, reflecting both uncertainty in the bedrock composition and variability in the sampled sediment
4.3 Correlation statistics for major and trace elements
Pearson’s correlation is widely used for correlation statistics; however, it has certain limitations. For example, it assumes a linear relationship among variables with a bivariate normal distribution, and failure of this assumption requires the use of a non-parametric correlation coefficient (Snedecor and Cochran Citation1967). The bedrock samples collected from Chorabari glacial catchment are within the range of the elemental compositions reported from HHC by Galy and France-Lanord (Citation2001). The assumption that Pearson’s correlation is the most appropriate method available to represent the samples seems to be a valid approach for the present study. We estimated the correlation statistics for major and trace elements in all studied grain-size fractions (clay, sand, coarse sand, and GSS).
4.3.1 Major elements
The concentrations of major elements for different grain-size fractions and GSS are presented in Table S2 A–D. We observed some obvious correlations in elemental composition; for example, Mg, K, Ti, and Fe show a positive correlation with Al for sediments of all grain-size fractions, but the sand and GSS also show a positive correlation of Al with P (see Supplementary material, Table S2). Aluminium has low mobility under most environmental conditions, although below pH 5.5 its solubility increases during its release from silicate rocks (Shiller and Frilot Citation1996). Therefore, the role of aluminosilicate minerals controlling the elemental concentration in sediments could be suggested. Further, Ca, Mn, and Fe show a similar mechanism for elemental evacuation, likely explained through weathering of feldspars and mica. Mg is dominant in biotite, and subjected to rapid alteration and incorporation into clay minerals, e.g. vermiculite or illite (Stephens et al. Citation1979), whereas the alteration of K from feldspar and its ability to be absorbed into clay minerals explains the higher concentration of K in the sediments.
Al and Fe remain immobile during weathering, but the redox conditions can affect the mobility of Fe (Rice Citation1973). Na shows a negative relationship with Al for all environments because of its tendency to remain in the solute phase (see Supplementary material, Table S2). Si is also negatively correlated with Na, probably due to its core presence in the detrital-form quartz and feldspars. The chemistry of Mn is similar to that of Fe, as are their contributions in redox reactions. This can be further confirmed by the positive correlation of Fe and Mn in the GSS (see Supplementary material, Table S2).
4.3.2 Trace elements and redox transformation
Among the trace elements, U and Th demonstrate a positive correlation, suggesting a similar origin mechanism (see Supplementary material, Table S2). A positive correlation of Th with P, Y, and Zr indicates the presence of monazite and zircon, and a strong relationship between Zr and Y suggests the presence of zircon (Singh Citation2010). Further, a positive correlation of the heavy metals (Cu, Cr, Ni, and Zn) with each other suggests a common mechanism for their origin, i.e. the oxic-acid phase derivation. A high correlation (R2 = 0.7) of Cu and Zn for all studied grain-size fractions further signifies the co-precipitation of Cu and Zn in an acidic environment, in the presence of Fe hydrous oxides (Lottermoser et al. Citation1999).
Redox transformations applied to the trace elements indicate the solubility/insolubility of elements in oxidizing/reducing conditions to set the state of oxidation in weathering reactions. Mn and Cr show positive correlations for the GSS (0.98), sand (0.34), clay (0.77) and coarse sand (0.60) fractions, as the removal of Mn (II) would be oxidized and precipitated as Mn (IV)-oxyhydroxides and Cr effectively scavenged as Cr (III) or co-precipitated with Fe and Al (Middelburg et al. Citation1988). However, the extreme proximity to the source limits our interpretation and source rock discrimination across grain-size grades for trace elements even in a glacial environment (Eynatten et al. Citation2012).
4.4 Enrichment factor and chemical index of alteration (CIA)
The CIA values for different grain-size fractions range between 50 and 65, typically showing low to moderately chemically weathered detritus. These are typical ranges for unweathered or slightly weathered detritus of glacial weathering regimes (Young et al. Citation2004, Bahlburg and Dobrzinski Citation2011). The CIA values are highest for the clay component of the lake sediments (range 55–65), similar to sand (50–65), while the coarser sand fraction presents lower values (51–61), indicating less alteration of rocks during deposition. Meanwhile, the suspended sediments evacuating through sub-glacial pathways suggest the least alteration (54–55). These values agree with CIA values reported for suspended sediments for Pindari Glacier basin (56.5, Pandey et al. Citation2002) and other parts of the world, i.e. Antarctica, Southwest Islands, the Alps, and other areas (see Shukla et al. Citation2020b). The CIA values and their respective percentage losses compared to the bedrock composition were tested and are presented for all grain-size fractions (). Our detailed study of the percentage loss for the mineral phase suggests that all grain-size fractions had 20–90% element loss in low to medium weathering conditions.
Figure 5. Percentage change for individual elements vs. chemical index of alteration for major elements of clay, sand, coarse sand and glacier suspended sediments (GSS). The percentage change is on the ordinate and the chemical index of alteration is on the abscissa
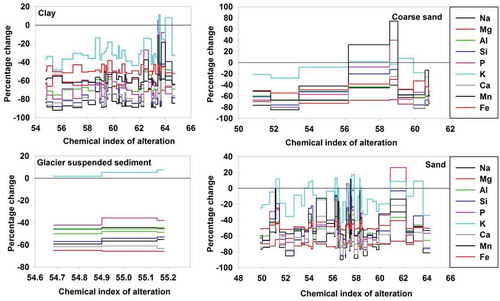
The clay fraction of the lake sediments shows low- to medium-grade alteration (55–65) and higher compositional enrichment compared to the underlying bedrock. We observed an increase in percentage loss with increasing alteration in all major grain-size fractions. As the weathering trends shift from low to medium conditions, K and P start to vary inexplicably (). The intensively altered conditions (CIA = 65) indicate no change in K and P values. However, Na, K, P, and Ca show an increasing trend with progressive alteration. This is likely due to the presence of Na, Ca, and K in feldspar, but the higher K values are indicative of the more rapid dissolution of Ca and Na than of K. However, this hypothesis is subject to additional proxy-based investigations.
The coarse and sand-sized fractions show a similar weathering mechanism, yet the progressive enrichment of a few elements (i.e. Na, K, and P) is observed. P, K, and Na show a lesser degree of immobility and could be correlated with a decreasing enrichment pattern. It is possible that during low-flow conditions (cold and dry), sediments may have begun to alter but a sudden rainfall event in the catchment led to rapid deposition. This hypothesis could be strengthened by the observations of rapid sediment deposition for Chorabari Lake sediments explained in Shukla et al. (Citation2020a).
Conversely, the GSS show lower CIA values (54–55) than all other grain-size fractions. This suggests the least alteration compared to the underlying bedrock (up to 60%). All major elements show a change in composition, except K, which indicates differential weathering for all modes of deposition of the different grain-size fractions. Here the more rapid alteration of plagioclase feldspar compared to K-feldspar is likely and the presence of K in biotite could also enrich the elemental composition. In addition, the possibility of the surface area/volume ratio affecting the elemental composition cannot be neglected here.
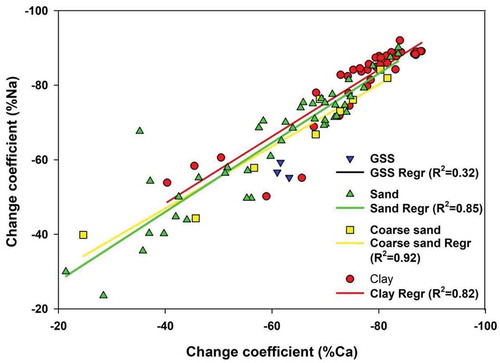
4.5 Geochemical budget during arenization
The feldspar alteration process has been studied through the percentage change in the Na and Ca molar fractions, as they are considered to be the most leached from the parent rock composition. The results suggest Na and Ca losses range from 20 to 90%, where lower values indicate the least alteration, and higher values suggest the complete alteration of the plagioclase feldspars ().
4.6 XRD mineral analysis
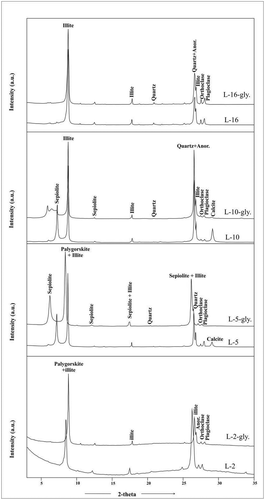
The mineralogical composition of the clay fraction separated out from the bulk representative samples showed the presence of the Mg-rich mineral palygorskite (110) in association with illite (001) at a d-spacing value of 10.4 Å, and sepiolite (110) at 12.2 Å (McLean et al. Citation1972) along with calcite (3.06 Å) which is normally found in the lacustrine environment. Minor amounts of quartz and feldspars are signalled by small peaks at d-spacing of 3.34 and 3.24 Å, and of 3.21 Å, respectively (). Semi-quantification of these diffractograms revealed the dominance of sepiolite and palygorskite over illite and other non-clay minerals. The presence of Mg-rich clay minerals point towards their formation by weathering of Mg-rich but low Fe-bearing minerals.
Figure 7. X-ray diffractograms of the separated clay sized fraction showing palygoskite with associated illite in the clayey sample (L2), whereas in sandy samples sepiolite and illite, along with non-clay minerals (with traces of calcite) and diffraction patterns collected after glycolation, show a peak shift due to -OH adsorption. Anor, anorthite; gly., glycolated
5 Discussion
5.1 Origin and release of glacial/periglacial nutrients
5.1.1 Origin and release of P
We studied the internal P cycle bounded in multiple compound-specific sedimentary phases and interpreted its solubility and reactivity with various chemical solutions (Guidry and Mackenzie Citation2000, Föllmi et al. Citation2009). Acids formed in a sub-glacial environment contain anions which can exchange the phosphate (PO4-P) from its absorption site and could result in total P from superficial sediments. Also, the association of P in mineral lattices is primarily limited to apatite minerals (Ca5 (PO4,CO3)3(F,Cl,OH)) as detrital carbonate-fluorapatite and hydroxyapatite, associated with calcium carbonate (Jensen and Andersen Citation1992, Baldwin Citation1996). P with mineral oxides is found on particle surfaces (on both Fe and Al), and its presence in organic matter is the most abundant form (Jensen and Andersen Citation1992, Baldwin Citation1996).
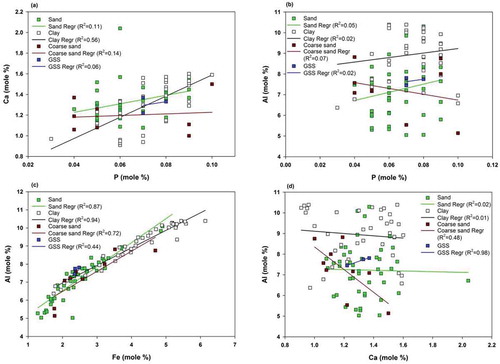
We assessed the relative correlations of P in the assemblage through multiple bivariate plots for different grain-size fractions. P shows weak correlations with various elements, i.e. calcium shows weak correlations for all grain-size fractions (sand, 0.11; coarse sand 0.14, GSS, 0.06) except for clay (R2 = 0.56) ()), whereas Al does not show any correlation ()). Conversely, the correlations of Fe with Al are very high for all grain-size fractions ()). Therefore, the association of P with Fe and Al phases is not possible. However, the plot of P with Ca is indicative of their co-presence in the clay size fractions. A possible explanation is its release from apatite mineral grains during organic matter production. Also, the possibility of orthophosphate anions bonded with the mineral lattice by a specific ligand exchange reaction (anion penetration) is a feasible explanation, but the present data do not allow us to investigate this hypothesis further. However, similar observations have been reported in aquatic environments (White Citation1980, Lukkari et al. Citation2007). Since the similar correlations do not hold for all sediment size fractions (sand, coarse sand, and GSS), and lead towards the co-precipitation of CaCO3 and P with finer particles, thus, we suggest that the control of sediment size on the P dynamics in glacial environments is true only for finer fractions.
Figure 8. Bivariate plot of major elements to determine the extent of correlation for various immobile and mobile elements for all possible weathering cases. (a), P plotted with Ca to determine the association of P with calcsilicate rocks; (b), P plotted with Al to determine the association of P with aluminosilicate rocks; (c), Fe plotted with with Al to understand the association of Fe with aluminosilicate rocks; (d), Ca plotted with Al to determine the association of calcium- and magnesium-phase dissolution with calcsilicate rocks
5.1.2 Origin and release of carbonates
The apparent presence of Ca and Mg in the HHC rocks is considered minor (Anderson et al. Citation1997). However, we observed an abundant Ca concentration in the underlying bedrock, through petrography and elemental composition analysis. The silicate origin of Ca is also identified in the studied parent rock sections (). To support this fact, the bivariate plots for Ca and Al show weak or no correlations for clay (R2 = 0.01) or sand (R2 = 0.02) fractions, whereas they show a good correlation with coarse sand (R2 = 0.48) and GSS (R2 = 0.98) fractions. This suggests that the Ca of detrital origin was transported through glacial melt containing aluminosilicate rocks ()). Similarly, as with phosphorus, the Ca/Mg ratio has been affected via the grain size, therefore we can conclude that these elements are susceptible to show differential mobility with changing climatic settings in HHC rocks.
5.1.3 Origin and release of elements from micaceous minerals
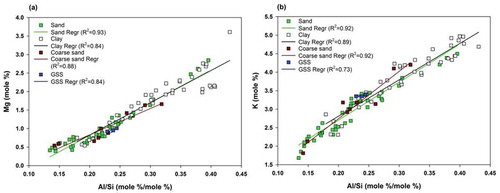
The elemental release from micaceous minerals (i.e. muscovite and biotite) is quantified by assuming that the silica fluxes are principally controlled through silicate weathering. The release of elements like K, Mg, Fe, Ti, Si, and Al from mica minerals is computationally difficult to distinguish, as these elements are released from the underlying bedrock via a similar set of minerals. However, weathering of mica is evident from the higher positive correlation of Al with K and Mg, which is also confirmed by the bivariate plots of molar ratios of Al/Si with K and Mg for all grain-size fractions (). These relations also exist for other glaciers of the Himalaya, such as Pindari Glacier, Kumaon Himalaya, India (Pandey et al. Citation2002). Therefore, a clear control of the grain-size parameter over nutrient release from glacierized basins of the Himalaya is also visible.
Figure 9. Bivariate plots for molar proportions of major elements to study the release of elements from micaceous minerals. (a) Correlation of Mg with Al/Si to determine the association of Mg with minerals containing mica; (b) correlation of K with Al/Si to determine the association of Mg with minerals containing mica
5.2 Chemical maturity of the sediments
A simpler and more robust way to evaluate the trend of mineral weathering is the A-CN-K plot, where A = Al2O3, CN = CaO* + Na2O, K = K2O in molar proportions, and CaO* represents the CaO associated with silicate minerals only (Nesbitt and Young Citation1984, Nesbitt Citation2003). Here, the plagioclase and feldspars are plotted at the 50% line (feldspar line). At an initial stage of weathering, the plot tends to be parallel to the A-CN line, as the Na2O and CaO may have started to leach out first from the dissolution of plagioclase feldspars (). Afew minerals, i.e. biotite, K-feldspar, augite, and amphibole, were plotted near the CN apex, and the clay minerals such as illite and smectite were plotted at the Al apex with 70–85% Al2O3. At a progressive stage of weathering, plagioclase feldspar alteration tends to release CaO and Na2O from the mineral lattice by shifting the overall weathering trends towards the A-K boundary. The loss of Ca and Na from feldspars of up to 90% supports this suggestion (). The removal of K from K-feldspar, along with Al, redirects the weathering trend towards the Al2O3 apex. This weathering reaction indicates K-metasomatism modification of sediments (Fedo et al. Citation1995), either formed due to the conversion of aluminous clay minerals (kaolinite) to illite or added due to the conversion of plagioclase to K-feldspars. The variability for K was also observed at other glacierized basins of the Himalaya and in upper Ganga catchment sediments, which could also be linked with this process (Pandey et al. Citation2002, Singh Citation2010). Deviation of compositional trend and low CIA values clearly support the fact of supplementary K2O during metasomatism of the samples (). Taken together, all grain-size fractions suggest low average CIA values (60.7, 56.8, 57.4, 55 for clay, sand, coarse sand, and GSS, respectively) for the present observations, which are likely to be higher (67, 64, 62, and 60 for clay, sand, coarse sand, and GSS, respectively) if we include the pre-metasomatic K inclusion process. Such variability in the K values reflects the amount of secondary kaolinite formation from plagioclase.
Figure 10. Al2O3-(CaO+Na2O)-K2O diagram of glacial sediments suggesting the weathering history of the catchment. Chl, chlorite; Ka, kaolinite; Gi, gibbsite. The dotted line represents the overall trend of weathering, and the dashed line represents the metasomatic trend and the corresponding pre-metasomatized chemical index of alteration (CIA) values for premetasomatized samples
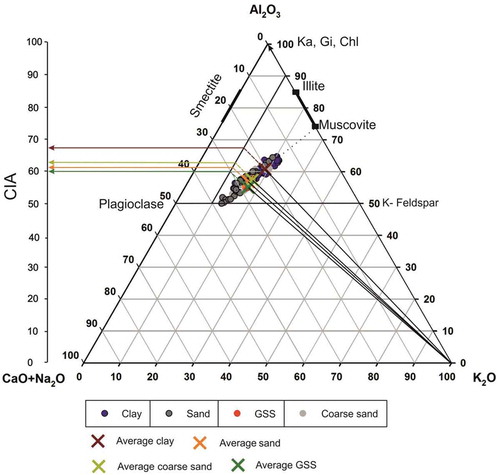
A quantitative comparison of elemental loss suggests that K is the most consistent element in all studied environmental settings except for the coarser sand fraction. We observed the possibility of K adsorption in illite and K-metasomatism using the A-CN-K diagram (). A slight tilt from the A-CN line is also observed in the upper Ganga catchment sediments; this is consistent with our results, as the minor loss of K from mica minerals was also observed previously (Singh Citation2010). Since the acid-derived oxidative weathering reactions in glacial environments have already been reported (Torres et al. Citation2017, Shukla et al. Citation2018b), the major and trace element release derived through acidic weathering could be expected for feldspar and mica minerals. These weathering reactions preferentially leached the elements from solid to liquid phase. For example, despite the dominant presence of calcite in the underlying bedrock samples, the concentration of CaO in particulate form is very low. This could be correlated with higher mobility of Ca in the solute fluxes (Anderson et al. Citation2000). The crux of our discussion lies in the fact that the sediments have been much sorted through physical weathering, and exposure of the fresh surface to chemical weathering increases the maturity and secondary mineralogical processes.
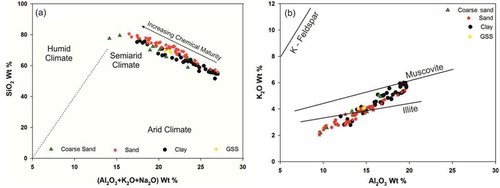
Further, we applied a binary SiO2 wt. % vs. (Al2O3+K2O+Na2O) wt. % model to infer the relation between changing climatic conditions and the resulting sedimentation (Suttner and Dutta Citation1986). We applied a similar approach to observe the depositional environment of different grain-size fractions and their chemical maturity under changing climatic settings ()). The Al2O3 and K2O bivariate plot shows that the major clay minerals in the sediments are close to the illite line, suggesting decomposition of K-feldspars and muscovite during progressive weathering ()). K has an aqueous mobility, but tends to be conserved in clay minerals due to its stable mineral phase during illite formation. The presence of illite in glacial sediments is also confirmed by the XRD data (). Previous results from Chorabari basin (Mandakini Valley) suggested the dominance of medium silt- (70–80%) to fine sand-sized (8–10%) fractions in glacial suspended sediments with (<1%) clay fractions (Kumar et al. Citation2016). The modern erosion rates of 0.6–2.3 mm y−1 also coincide with the peak suspended sediments evacuation during extreme rainfall events. Therefore, the geochemical characteristics of sand-sized grain fractions studied here replicate the geochemical behaviour and transport mechanism of first-order glacial basins in downstream sediment supply.
Figure 11. (a). Chemical maturity of sediments in all sets of weathering environments, suggesting a link between changing climatic conditions and the resulting sedimentation in the catchment. (b) Bivariate plots of Al2O3 and K2O depicting the decomposition of K-feldspars and muscovite during progressive weathering, and the possible origin of sediments close to the illite line (Cox and Lowe Citation1995)
5.3 Downstream implications of glacial/periglacial sediment release
It is argued that the large Himalayan rivers carry a substantial amount of suspended and dissolved load to the oceans, that regulates the weathering process associated with nutrient cycling. Nonetheless, it has been noted that, even for the most mobile elements, the dominant form of nutrient transport is the solid counterpart (Gaillardet et al. Citation2003). This implies that sediments are not only a sink (Walling et al. Citation2003) but also a secondary source for pollutants, because sediments can release pollutants back to the ecosystem. For example, changes in pH and redox potential may increase the potential for pollutants to be desorbed from particles into the dissolved phase (Förstner and Wittmann Citation1981, Hessler and Lowe Citation2006). The current knowledge of sediment-discharge relationships in the Himalayan system suggests that the export of nutrients such as P, Ca, Na, and K during rain events (as represented by the Chorabari glacial system) could significantly increase the downstream nutrient concentrations in particulate form. As a consequence, sediment pollution can lead to undesirable ecosystem conditions and water quality deterioration. Combining the previous observations from Chorabari Glacier catchment, the increased sediment supply during June and July of peak discharge could significantly decrease the reservoir storage capacity and energy production (Kumar et al. Citation2016). We presented the sediment geochemical potential from the Chorabari Glacier basin controlled through different grain-size fractions, which have a significant influence over downstream nutrient release and detrital phase studies in river systems.
5.4 Regional correlation
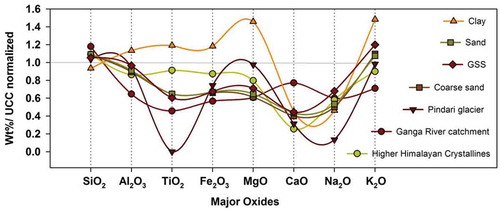
We compared our geochemical data with other basins of the Himalaya to strengthen our observations. We used UCC normalized ratios for the Pindari Glacier (Pandey et al. Citation2002), Ganga River catchment (Singh Citation2010), and HHC rock composition (Galy and France-Lanord Citation2001) for comparison (). The clay-sized fractions of the present study suggest the highest variability in proglacial settings of the Chorabari Glacier. The GSS composition is similar to the Ganga River catchment sediments, suggesting sediment sorting through a common mechanism of physical/chemical processes (sediment sorting and chemical reactions) exporting out from the first-order catchments. However, it should be noted that the sediment production and geochemical sorting processes are operating at a steady state relative to modern sediment fluxes. The lower CIA values (45–54) obtained by source rock studies of Ganga River sediments accord well with the present study and signify the role of the glacial catchments in controlling physical weathering/erosion mechanisms (Singh Citation2010). The differentiation of clay, sand, and coarse sand fractions quantifies the mean composition of sediments releasing from the glacierized catchment. The high elemental concentrations of Na, K, and Ca found in the present study are in agreement with the sediment composition of the upper Ganga catchment (Singh Citation2010) and the suspended sediments of Pindari Glacier (Pandey et al. Citation2002). Depletion in the oxides of Al, Ti, Ca, Na, and K compared to UCC was also observed in the upper Ganga catchment due to the dilution effect of silica (Singh Citation2010). However, the enrichment of U, Th, and Zr in the present study may be related to the occurrence of minerals such as monazite, apatite, and zircon. The correlation of trace elements, e.g. Ba, Sr, Th, and Zr, was similarly observed in upper Ganga catchment sediments (Singh Citation2010). This is possible in glacial environments as the chemical denudation rates are 0.1–0.01 times lower than the physical denudation rates (Jacobson et al. Citation2002, West et al. Citation2005). Therefore, the sediments in mountain river systems are likely to be similar in geochemical composition to the bedrock (Hilton et al. Citation2010).
Figure 12. UCC normalized ratio of major oxide composition by weight %. Ganga River catchment suspended sediment data from Singh (Citation2010); Higher Himalayan Crystalline data from Galy and France-Lanord (Citation2001); Pindari Glacier suspended sediment data from Pandey et al. (Citation2002); clay, sand, coarse sand, and glacial suspended sediments (GSS) data from the present study
The sediments are produced through physical weathering (comminution), transported through the meltwater, and deposited in lakes and/or ultimately in the oceans. Under non-glacierized and pro/periglacial conditions, the compositions were modified after the sediments were deposited into the lake basin. However, under the present glacierized conditions, these sediments traversed through crevasses, supraglacial channels, moulins, and subglacial transport, hence providing a suitable environment for chemical weathering.
6 Conclusions
We present a novel approach to determine variations in the geochemical and mineralogical patterns of sediment evacuated from glacial/subglacial environments. The present study bridges the scale of theory, natural observations, and statistical relationships among elements originating through comminution in a glacial catchment. The study presents the following insights:
The quantitative assessment of elemental mobility in different grain-size fractions points to inconsistency in sediment sorting under glacial environments. Nevertheless, the chemical reactions indicate contrasting weathering patterns at all scales and a clear control of grain size over nutrient release from the Himalayan glacierized basin.
K is identified as the most consistent element among the studied grain-size fractions, possibly adsorbed in illite and other clay minerals. We observed the possibility of K-metasomatism and chemical modification at Himalayan glacial sedimentary environments.
The correlations of major and trace elemental loss are ascribed to the mineral sorting and sub-glacial acidic reactions, leading to secondary mineral deposition. In contrast, the elemental loss in the watershed is mainly derived through leachable minerals such as feldspars, micas, and apatite.
Owing to the biogeochemical reactions of micronutrient cycling, P is associated mainly with Ca and organic material. These elements (Ca and Mg) do not show differential mobility for changing climatic settings in the HHC rocks. However, Ca is mainly derived from aluminosilicate rocks through the process of carbonate dissolution.
The mechanism of sediment origin and changes in composition through comminution presented here are useful for downstream weathering tracer studies. We provided a better implementation of sediment control strategies in glacierized basins of the Himalaya. However, further information on the factors controlling the temporal and spatial variation of nutrient release through sediment comminution is needed to accomplish future legislation requirements of water quality and treatment studies.
Supplemental Material
Download MS Excel (32.8 KB)Supplemental Material
Download PDF (345.8 KB)Acknowledgements
The authors are thankful to the Director, Wadia Institute of Himalayan Geology, Dehra Dun, India, for providing the necessary facilities for this research. We are also thankful to the Department of Science and Technology and Ministry of Earth Sciences, Government of India, for financial support to carry out this work. We convey our sincere thanks to the scientific editor, Dr Saket Pande, and three anonymous reviewers for their detailed reviews and constructive comments, which greatly improved the manuscript. Meenakshi thanks IUAC, India, for the use of the XRD facility, procured under the Geochronology Project [MoES/P.O.(Seismic)8(09)-Geochron/2012].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Anderson, S.P., et al., 2000. Chemical weathering in the foreland of a retreating glacier. Geochimica et Cosmochimica Acta, 64 (7), 1173–1189. doi:10.1016/S0016-7037(99)00358-0.
- Anderson, S.P., Drever, J.I., and Humphrey, N.F., 1997. Chemical weathering in glacial environments. Geology, 25 (5), 399–402. doi:10.1130/0091-7613(1997)025<0399:CWIGE>2.3.CO;2.
- Bahlburg, H. and Dobrzinski, N., 2011. Chapter 6 A review of the Chemical Index of Alteration (CIA) and its application to the study of Neoproterozoic glacial deposits and climate transitions. Geological Society, London, Memoirs, 36 (1), 81–92. doi:10.1144/M36.6.
- Baldwin, D.S., 1996. The phosphorus composition of a diverse series of Australian sediments. Hydrobiologia, 335 (1), 63–73. doi:10.1007/BF00013684.
- Benn, D.I. and Owen, L.A., 1998. The role of the Indian summer monsoon and the mid-latitude westerlies in Himalayan glaciation: review and speculative discussion. Journal of the Geological Society, 155 (2), 353–363. doi:10.1144/gsjgs.155.2.0353.
- Benn, D.I. and Owen, L.A., 2002. Himalayan glacial sedimentary environments: a framework for reconstructing and dating the former extent of glaciers in high mountains. Quaternary International, 97-98, 3–25. doi:10.1016/S1040-6182(02)00048-4
- Bhutiyani, M.R., 2000. Sediment load characteristics of a proglacial stream of Siachen Glacier and the erosion rate in Nubra valley in the Karakoram Himalayas, India. Journal of Hydrology, 227 (1–4), 84–92. doi:10.1016/S0022-1694(99)00174-2.
- Bickle, M.J., et al., 2001. Controls on the 87Sr/86Sr ratio of carbonates in the Garhwal Himalaya, headwaters of the Ganges. The Journal of Geology, 109 (6), 737–753. doi:10.1086/323192.
- Bickle, M.J., et al., 2005. Relative contributions of silicate and carbonate rocks to riverine Sr fluxes in the headwaters of the Ganges. Geochimica et Cosmochimica Acta, 69 (9), 2221–2240. doi:10.1016/j.gca.2004.11.019.
- Biscaye, P.E., 1965. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geological Society of America Bulletin, 76 (7), 803–832. doi:10.1130/0016-7606(1965)76[803:MASORD]2.0.CO;2.
- Boulton, G.S., 1978. Boulder shapes and grain-size distributions of debris as indicators of transport paths through a glacier and till genesis. Sedimentology, 25 (6), 773–799. doi:10.1111/j.1365-3091.1978.tb00329.x.
- Carrols, D., 1970. Clay minerals: a guide to their x-ray identification. Bulletin of the Geological Society of America, 126, 48–65.
- Carver, R.E., 1971. Procedures in sedimentary petrology. New York: John Wiley & Sons Incorporated.
- Cox, R. and Lowe, D.R., 1995. A conceptual review of regional-scale controls on the composition of clastic sediment and the co-evolution of continental blocks and their sedimentary cover. Journal of Sedimentary Research, 65 (1), 1–12.
- Dalai, T.K., Krishnaswami, S., and Sarin, M.M., 2002. Major ion chemistry in the headwaters of the Yamuna river system: chemical weathering, its temperature dependence and CO2 consumption in the Himalaya. Geochimica et Cosmochimica Acta, 66 (19), 3397–3416. doi:10.1016/S0016-7037(02)00937-7.
- Derry, L.A. and France-Lanord, C., 1996. Neogene Himalayan weathering history and river87Sr86Sr: impact on the marine Sr record. Earth and Planetary Science Letters, 142 (1–2), 59–74. doi:10.1016/0012-821X(96)00091-X.
- English, N.B., et al., 2000. Geologic control of Sr and major element chemistry in Himalayan Rivers, Nepal. Geochimica et Cosmochimica Acta, 64 (15), 2549–2566. doi:10.1016/S0016-7037(00)00379-3.
- Eynatten, H.V., Tolosana-Delgado, R., and Karius, V., 2012. Sediment generation in modern glacial settings: grain-size and source-rock control on sediment composition. Sedimentary Geology, 280, 80–92. doi:10.1016/j.sedgeo.2012.03.008
- Fedo, C.M., Wayne Nesbitt, H., and Young, G.M., 1995. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology, 23 (10), 921–924. doi:10.1130/0091-7613(1995)023<0921:UTEOPM>2.3.CO;2.
- Föllmi, K.B., et al., 2009. Weathering and the mobility of phosphorus in the catchments and forefields of the Rhône and Oberaar glaciers, central Switzerland: implications for the global phosphorus cycle on glacial–interglacial timescales. Geochimica et Cosmochimica Acta, 73 (8), 2252–2282. doi:10.1016/j.gca.2009.01.017.
- Förstner, U. and Wittmann, G.T.W., 1981. Heavy metals in the aquatic environment. Berlin: Springer Verlag.
- France-Lanord, C. and Derry, L.A., 1997. Organic carbon burial forcing of the carbon cycle from Himalayan erosion. Nature, 390 (6655), 65–67. doi:10.1038/36324.
- Gaillardet, J., et al., 2003. Geochemistry of the suspended sediments of circum-himalayan rivers and weathering budgets over the last 50 Myrs. In EGS-AGU-EUG Joint Assembly, Nice France, 6–11 April 2003, Abs. ID. 13617.
- Gaillardet, J., Dupré, B., and Allègre, C.J., 1999. Geochemistry of large river suspended sediments: silicate weathering or recycling tracer? Geochimica et Cosmochimica Acta, 63 (23–24), 4037–4051. doi:10.1016/S0016-7037(99)00307-5.
- Galy, A. and France-Lanord, C., 1999. Weathering processes in the Ganges–Brahmaputra basin and the riverine alkalinity budget. Chemical Geology, 159 (1–4), 31–60. doi:10.1016/S0009-2541(99)00033-9.
- Galy, A. and France-Lanord, C., 2001. Higher erosion rates in the Himalaya: geochemical constraints on riverine fluxes. Geology, 29 (1), 23–26. doi:10.1130/0091-7613(2001)029<0023:HERITH>2.0.CO;2.
- Guidry, M.W. and Mackenzie, F.T., 2000. Apatite weathering and the Phanerozoic phosphorus cycle. Geology, 28 (7), 631–634. doi:10.1130/0091-7613(2000)28<631:AWATPP>2.0.CO;2.
- Hammer, K.M. and Smith, N.D., 1983. Sediment production and transport in a proglacial stream: Hilda Glacier, Alberta, Canada. Boreas, 12 (2), 91–106. doi:10.1111/j.1502-3885.1983.tb00441.x.
- Haritashya, U.K., et al., 2006. Suspended sediment from the Gangotri Glacier: quantification, variability and associations with discharge and air temperature. Journal of Hydrology, 321 (1–4), 116–130. doi:10.1016/j.jhydrol.2005.07.037.
- Haritashya, U.K., Kumar, A., and Singh, P., 2010. Particle size characteristics of suspended sediment transported in meltwater from the Gangotri Glacier, central Himalaya — an indicator of subglacial sediment evacuation. Geomorphology, 122 (1–2), 140–152. doi:10.1016/j.geomorph.2010.06.006.
- Hasnain, S.I. and Thayyen, R.J., 1999. Discharge and suspended-sediment concentration of meltwaters, draining from the Dokriani glacier, Garhwal Himalaya, India. Journal of Hydrology, 218 (3–4), 191–198. doi:10.1016/S0022-1694(99)00033-5.
- Heim, A. and Gansser, A., 1939. Central Himalaya. Delhi: Hindustan Publishing.
- Hessler, A.M. and Lowe, D.R., 2006. Weathering and sediment generation in the Archean: an integrated study of the evolution of siliciclastic sedimentary rocks of the 3.2Ga Moodies Group, Barberton Greenstone Belt, South Africa. Precambrian Research, 151 (3–4), 185–210. doi:10.1016/j.precamres.2006.08.008.
- Hilton, R.G., et al., 2010. The isotopic composition of particulate organic carbon in mountain rivers of Taiwan. Geochimica et Cosmochimica Acta, 74 (11), 3164–3181. doi:10.1016/j.gca.2010.03.004.
- Jacobson, A.D. and Blum, J.D., 2000. Ca/Sr and 87Sr/86Sr geochemistry of disseminated calcite in Himalayan silicate rocks from Nanga Parbat: influence on river-water chemistry. Geology, 28 (5), 463–466. doi:10.1130/0091-7613(2000)28<463:SASGOD>2.0.CO;2.
- Jacobson, A.D., Blum, J.D., and Walter, L.M., 2002. Reconciling the elemental and Sr isotope composition of Himalayan weathering fluxes: insights from the carbonate geochemistry of stream waters. Geochimica et Cosmochimica Acta, 66 (19), 3417–3429. doi:10.1016/S0016-7037(02)00951-1.
- Jensen, H.S. and Andersen, F.O., 1992. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of four shallow, eutrophic lakes. Limnology and Oceanography, 37 (3), 577–589. doi:10.4319/lo.1992.37.3.0577.
- Jha, S.K., Shrivastava, J.P., and Bhairam, C.L., 2012. Clay mineralogical studies on Bijawars of the Sonrai Basin: palaeoenvironmental implications and inferences on the uranium mineralization. Journal of the Geological Society of India, 79 (2), 117–134. doi:10.1007/s12594-012-0028-9.
- Karim, A. and Veizer, J., 2000. Weathering processes in the Indus River Basin: implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chemical Geology, 170 (1–4), 153–177. doi:10.1016/S0009-2541(99)00246-6.
- Kumar, A., et al., 2016. Hydroclimatic influence on particle size distribution of suspended sediments evacuated from debris-covered Chorabari Glacier, upper Mandakini catchment, central Himalaya. Geomorphology, 265, 45–67. doi:10.1016/j.geomorph.2016.04.019
- Lottermoser, B.G., Ashley, P.M., and Lawie, D.C., 1999. Environmental geochemistry of the Gulf Creek copper mine area, north-eastern New South Wales, Australia. Environmental Geology, 39 (1), 61–74. doi:10.1007/s002540050437.
- Lukkari, K., et al., 2007. Fractionation of sediment phosphorus revisited. I: fractionation steps and their biogeochemical basis. Limnology and Oceanography: Methods, 5 (12), 433–444.
- Lupker, M., et al., 2012. Predominant floodplain over mountain weathering of Himalayan sediments (Ganga basin). Geochimica et Cosmochimica Acta, 84, 410–432. doi:10.1016/j.gca.2012.02.001.
- McLean, S.A., et al., 1972. The occurrence of sepiolite and attapulgite on the southern High Plains. Clays and Clay Minerals, 20 (3), 143–149. doi:10.1346/CCMN.1972.0200305.
- McLennan, S.M., et al., 1993. Geochemical approaches to sedimentation, provenance, and tectonics. Geological Society of America, Special Papers, 284, 21–40.
- Meybeck, M., 1987. Global chemical weathering of surficial rocks estimated from river dissolved loads. American Journal of Science, 287 (5), 401–428. doi:10.2475/ajs.287.5.401.
- Middelburg, J.J., van der Weijden, C.H., and Woittiez, J.R.W., 1988. Chemical processes affecting the mobility of major, minor and trace elements during weathering of granitic rocks. Chemical Geology, 68 (3–4), 253–273. doi:10.1016/0009-2541(88)90025-3.
- Nesbitt, H. and Young, G.M., 1982. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature, 299 (5885), 715–717. doi:10.1038/299715a0.
- Nesbitt, H.W., 1979. Mobility and fractionation of rare earth elements during weathering of a granodiorite. Nature, 279 (5710), 206–210. doi:10.1038/279206a0.
- Nesbitt, H.W., 2003. Petrogenesis of siliciclastic sediments and sedimentary rocks. Geochemistry of Sediments and Sedimentary Rocks, 4, 39–51.
- Nesbitt, H.W. and Young, G.M., 1984. Prediction of some weathering trends of plutonic and volcanic rocks based on thermodynamic and kinetic considerations. Geochimica et Cosmochimica Acta, 48 (7), 1523–1534. doi:10.1016/0016-7037(84)90408-3.
- Oliver, L., et al., 2003. Silicate weathering rates decoupled from the 87Sr/86Sr ratio of the dissolved load during Himalayan erosion. Chemical Geology, 201 (1–2), 119–139. doi:10.1016/S0009-2541(03)00236-5.
- Owen, L.A., Derbyshire, E., and Scott, C.H., 2003. Contemporary sediment production and transfer in high-altitude glaciers. Sedimentary Geology, 155 (1–2), 13–36. doi:10.1016/S0037-0738(02)00156-2.
- Owen, L.A. and Sharma, M.C., 1998. Rates and magnitudes of paraglacial fan formation in the Garhwal Himalaya: implications for landscape evolution. Geomorphology, 26 (1–3), 171–184. doi:10.1016/S0169-555X(98)00057-9.
- Pandey, S.K., SINGH, A.K., and HASNAIN, S.I., 2002. Grain-size distribution, morphoscopy and elemental chemistry of suspended sediments of Pindari Glacier, Kumaon Himalaya, India. Hydrological Sciences Journal, 47 (2), 213–226. doi:10.1080/02626660209492925.
- Rice, C.M., 1973. Chemical weathering on the Carnmenellis granite. Mineralogical Magazine, 39 (304), 429–447. doi:10.1180/minmag.1973.039.304.06.
- Sarin, M.M., et al., 1992. Major ion chemistry of the Ganga source waters: weathering in the high altitude Himalaya. Proceedings of the Indian Academy of Sciences-Earth and Planetary Sciences, 101 (1), 89–98.
- Shiller, A.M. and Frilot, D.M., 1996. The geochemistry of gallium relative to aluminum in Californian streams. Geochimica et Cosmochimica Acta, 60 (8), 1323–1328. doi:10.1016/0016-7037(96)00002-6.
- Shukla, T., et al., 2018b. Carbonate and silicate weathering in glacial environments and its relation to atmospheric CO 2 cycling in the Himalaya. Annals of Glaciology, 59 (77), 159–170. doi:10.1017/aog.2019.5.
- Shukla, T., et al., 2020a. Late-Holocene climate response and glacial fluctuations revealed by the sediment record of the monsoon-dominated Chorabari Lake, Central Himalaya. The Holocene, 30 (7), 953–965. doi:10.1177/0959683620908654.
- Shukla, T., et al., 2020b. Misinterpreting proxy data for paleoclimate signals: a reply to Srivastava and Jovane, 2020. The Holocene, 30 (12), 1874–1883. doi:10.1177/0959683620950481
- Singh, A.K. and Hasnain, S.I., 2002. Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrological Processes, 16 (4), 835–849. doi:10.1002/hyp.367.
- Singh, D.S., 2014a. Surface processes during flash floods in the glaciated terrain of Kedarnath, Garhwal Himalaya and their role in the modification of landforms. Current Science, 106 (4), 594–597.
- Singh, D.S., et al., 2017. Pattern of retreat and related morphological zones of Gangotri Glacier, Garhwal Himalaya, India. Quaternary International, 444, 172–181. doi:10.1016/j.quaint.2016.07.025.
- Singh, D.S. and Mishra, A., 2001. Gangotri glacier characteristics, retreat and processes of sedimentation in the Bhagirathi valley. Geological Survey of India Special Publication, 65, 17–20.
- Singh, P., 2010. Geochemistry and provenance of stream sediments of the Ganga River and its major tributaries in the Himalayan region, India. Chemical Geology, 269 (3–4), 220–236. doi:10.1016/j.chemgeo.2009.09.020.
- Singh, S.K., Sarin, M.M., and France-Lanord, C., 2005. Chemical erosion in the eastern Himalaya: major ion composition of the Brahmaputra and δ13C of dissolved inorganic carbon. Geochimica et Cosmochimica Acta, 69 (14), 3573–3588. doi:10.1016/j.gca.2005.02.033.
- Singh, V.B., et al., 2014b. Seasonal variation of the solute and suspended sediment load in Gangotri glacier meltwater, central Himalaya, India. Journal of Asian Earth Sciences, 79, 224–234. doi:10.1016/j.jseaes.2013.09.010.
- Singh, V.B. and Ramanathan, A.L., 2015a. Assessment of solute and suspended sediments acquisition processes in the Bara Shigri glacier meltwater (Western Himalaya, India). Environmental Earth Sciences, 74 (3), 2009–2018. doi:10.1007/s12665-015-4584-3.
- Singh, V.B., Ramanathan, A.L., and Sharma, P., 2015b. Major ion chemistry and assessment of weathering processes of the Patsio glacier meltwater, Western Himalaya, India. Environmental Earth Sciences, 73 (1), 387–397. doi:10.1007/s12665-014-3432-1.
- Snedecor, G.W. and Cochran, W.G., 1967. Statistical methods. 6th ed. Ames, IA: Iowa State University, 135–317.
- Stephens, et al., 1979. Structural and chemical aspects of metamorphic layering development in metasediments from Clunes, Australia. American Journal of Science, 279 (2), 129–160. doi:10.2475/ajs.279.2.129.
- Sundriyal, S., et al., 2018. Deposition of atmospheric pollutant and their chemical characterization in snow pit profile at Dokriani Glacier, Central Himalaya. Journal of Mountain Science, 15 (10), 2236–2246. doi:10.1007/s11629-017-4817-x.
- Suttner, L.J. and Dutta, P.K., 1986. Alluvial sandstone composition and paleoclimate, I. Framework mineralogy. Journal of Sedimentary Research, 56 (3), 329–345.
- Swift, D.A., Nienow, P.W., and Hoey, T.B., 2005. Basal sediment evacuation by subglacial meltwater: suspended sediment transport from Haut Glacier d’Arolla, Switzerland. Earth Surface Processes and Landforms, 30 (7), 867–883. doi:10.1002/esp.1197.
- Thayyen, R.J., GERGAN, J.T., and DOBHAL, D.P., 1999. Particle size characteristics of suspended sediments and subglacial hydrology of Dokriani Glacier, Garhwal Himalaya, India. Hydrological Sciences Journal, 44 (1), 47–61. doi:10.1080/02626669909492202.
- Torres, M.A., et al., 2017. Glacial weathering, sulfide oxidation, and global carbon cycle feedbacks. Proceedings of the National Academy of Sciences, 114 (33), 8716–8721. doi:10.1073/pnas.1702953114.
- Tripathy, G.R., et al., 2010. Temporal variations in Sr and 87Sr/86Sr of the Ganga headwaters: estimates of dissolved Sr flux to the mainstream. Hydrological Processes: An International Journal, 24 (9), 1159–1171. doi:10.1002/hyp.7572.
- Valdiya, K.S., 1998. Dynamic himalaya. Hyderabad, India: Universities Press, 178, ISBN 81-7371-094- 5.
- Valdiya, K.S., et al., 1999a. Tectonic and lithological characterization of Himadri (Great Himalaya) between Kali and Yamuna rivers, central Himalaya. Himalayan Geology, 20 (2), 1–17.
- Valdiya, K.S., 1999b. Rising Himalaya: advent and intensification of monsoon. Current Science, 76, 514–524.
- Walling, D.E., et al., 2003. Storage of sediment-associated nutrients and contaminants in river channel and floodplain systems. Applied Geochemistry, 18 (2), 195–220. doi:10.1016/S0883-2927(02)00121-X.
- Wentworth, C.K., 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology, 30 (5), 377–392. doi:10.1086/622910.
- West, A.J., GALY, A., and BICKLE, M., 2005. Tectonic and climatic controls on silicate weathering. Earth and Planetary Science Letters, 235 (1–2), 211–228. doi:10.1016/j.epsl.2005.03.020.
- White, R.E., 1980. Retention and release of phosphate by soil and soil constituents. In: P.B. Tinker, ed. Soils and agriculture. Critical Reports on Applied Chemistry Volume 2. Society of Chemical Industry. Oxford: Blackwell Scientific Publications, 71–114.
- Woodward, J.C., et al., 2002. Composite suspended sediment particles and flocculation in glacial meltwaters: preliminary evidence from Alpine and Himalayan basins. Hydrological Processes, 16 (9), 1735–1744. doi:10.1002/hyp.361.
- Yin, A., 2006. Cenozoic tectonic evolution of the Himalayan orogen as constrained by along-strike variation of structural geometry, exhumation history, and foreland sedimentation. Earth-Science Reviews, 76 (1–2), 1–131. doi:10.1016/j.earscirev.2005.05.004.
- Young, G.M., MINTER, W., and THERON, J., 2004. Geochemistry and palaeogeography of upper Ordovician glaciogenic sedimentary rocks in the table Mountain Group, South Africa. Palaeogeography, Palaeoclimatology, Palaeoecology, 214 (4), 323–345. doi:10.1016/S0031-0182(04)00399-2.
- Young, G.M. and Wayne Nesbitt, H., 1999. Paleoclimatology and provenance of the glaciogenic Gowganda Formation (Paleoproterozoic), Ontario, Canada: a chemostratigraphic approach. Geological Society of America Bulletin, 111 (2), 264–274. doi:10.1130/0016-7606(1999)111<0264:PAPOTG>2.3.CO;2.