ABSTRACT
Background: It is widely known that for many students it is very difficult to correctly predict how thermal expansion affects the appearance of a metal plate with a circular hole. Interviews with school teachers show that the source of this difficulty could stem from the fact that students’ internal visualizations of an arbitrary object’s thermal expansion often boil down to visualizing changes along one dimension only.
Purpose: In this study, we investigated how students’ mental models about one-dimensional expansion can be extended for purposes of running mental simulations about expansion along two dimensions.
Sample: To that end a pretest-posttest quasi-experiment has been conducted, with 100 students in the control group and 95 students in the experimental group.
Design and methods: Whereas control group students received traditional instruction with a focus on formal representations, in the experimental group the students were led to draw an analogy between heating of a straight rod and a circular rod of same length, whereby the internal structure of the rods was represented by springs.
Results: Eventually, it has been found that students from the experimental group were significantly more successful at predicting the effects of thermal expansion, especially within contexts of objects with holes.
Conclusion: Analogies and extreme case reasoning can be effectively used for helping the students to correctly transfer their mental models about one-dimensional expansion to situations that require reasoning about expansion along two dimensions.
1. Introduction
Phenomena related to temperature changes are an inevitable part of everyday experience because temperature differences between day and night can reach 15℃ or more, while temperature variations over the course of a year are even more pronounced (Bueno et al. Citation2012). One important result of temperature change is the expansion or contraction of objects. Knowledge about thermal expansion is applied in numerous branches of engineering and science – it is equally important for building bridges as it is for choosing adequate dental fillings, and designing thermometers and thermostats (Tipler and Mosca Citation2008; Giancoli Citation2005; Halliday, Resnick, and Walker Citation2011). Consequently, we could conclude that understanding of thermal expansion represents an important aspect of scientific literacy. Therefore, it is not surprising that the topic of thermal expansion has found its place in science curricula at all educational levels. Learning about thermal expansion does not only potentially result with a better understanding of a broad spectrum of its applications in engineering and medicine, but it is also very important for learning of other physics topics, such as heat transfer by convection, anomaly of water, thermoelectric effect, Nernst effect and measurement of temperature. Most of the studies about students’ misconceptions in thermodynamics have been focused on students’ conceptual understanding of heat, temperature and internal energy (Liew and Treagust Citation1995; Yeo and Zadnik Citation2001; Lewis and Linn Citation1994; Sözbilir Citation2003; Reiner et al. Citation2000). Probably one of the most important findings from these studies is that students often fail to differentiate between heat, temperature and internal energy. At the same time, it should be noted that there were not many studies on students’ understanding of thermal expansion. One widely known students’ misconception is that thermal expansion results from an increase in atoms’ volumes which indicates that many students tend to transfer their macro-world observations to the micro-world level (Yeo and Zadnik Citation2001; Duit Citation2015). It has been also found that students often think that temperature does not affect the height of liquid in a container which seems to indicate that many students think that expansion of the container exactly balances the expansion of the liquid (Kartal, Öztürk, and Yalvaç Citation2011). In other words, it seems that many students fail to take into account the difference between coefficients of thermal expansion when reasoning about heating of a system consisting of objects made of different materials. Furthermore, a widely known misconception about thermal expansion is that in objects with holes the diameter of the holes decrease as a result of heating the objects (McHugh and McCauley Citation2016; Watkins and Mazur Citation2013). McHugh and McCauley (Citation2016) suggested that in thermodynamics instruction it is very useful to explicitly discuss this misconception because it triggers student attention and interest and can be used to promote conceptual understanding based upon thermodynamics. However, they did not offer explanations about possible causes of the given misconception nor strategies how to prevent/overcome it.
In order to identify possible causes of that misconception, we have to consider the characteristics of the students’ mental models of thermal expansion. Here we define a mental model as ‘a structural analog of a real-world or imaginary situation, event or process that a person constructs to reason with in the mind’ (Nersessian Citation1995). If the mental model includes iconic representations of the physical situation, it can be used as a basis for running mental simulations, i.e. vivid thought experiments (Nersessian Citation2008; Craik Citation1943). To construct and/or manipulate the physical model through mental simulation, it is necessary for the individual to have implicit and explicit domain knowledge of the entity, behavior and processes, and especially causal knowledge (Nersessian Citation2008). Thereby, an effective way for developing causal knowledge is to enrich the mental model with micro–macro relationships (Eylon and Ganiel Citation1990). Nonformal interviews that had been conducted with physics teachers from Bosnia and Herzegovina within the context of a professional development seminar showed that even some teachers have inadequate mental models of thermal expansion. For example, while discussing the plate with a hole, one of the teachers drew (on blackboard) the plate as consisting of numerous straight strips and noted that these strips’ right and left ends expand in different directions when heated resulting in a decrease of the diameter of the hole. These nonformal interviews led us to conclude that some teachers (and probably also many students) reason about thermal expansion of a plate with a hole by mentally simulating this situation based on a model of one-dimensional expansion of multiple mutually independent straight strips. In other words, they use the thermal expansion of a straight strip as a cognitive anchor but they obviously misapply it by assuming that the strips can be regarded as mutually independent for an object such as a plate. Consequently, they exclusively reason about expansion along the x-axis disregarding completely the expansion along the y-axis which directly results in the observed misconception that the diameter of the hole decreases when the plate is heated. From the cognitive load perspective, it could be noted that for students it is relatively easy to reason about the effects of heating a straight thin strip/rod and they tend to transfer this kind of reasoning to different contexts. Simultaneous reasoning about expansion along both dimensions of a plate induces a higher intrinsic load which could explain students’ tendency to reason about expansion along one dimension only (Sweller Citation1994; Sorden Citation2005).
We can conclude that students have a natural tendency to think about expansion problems by using the one-dimensional case as an analogical anchor and by perceiving arbitrary plates as extreme cases consisting of numerous mutually independent, straight strips. In other words students’ natural approach to thinking about thermal expansion is characterized by combinations of analogies and extreme case reasoning. Here analogy is defined as a ‘transfer of information or meaning from a particular source domain to a target domain’ and we say that extreme case reasoning is at work when, ‘in order to facilitate reasoning about a situation A (the target), a situation E (extreme case) is suggested, in which some aspect of situation A has been maximized or minimized’ (Podolefsky and Finkelstein Citation2006; Stephens and Clement Citation2009). In our opinion, the students’ natural approach to reasoning about thermal expansion of a plate with a hole needs only slight modifications in order to become scientifically acceptable. Consequently, a cognitive bridging strategy could be an effective choice for organizing conceptualization of this situation because bridging strongly builds on already existing intuitive knowledge of the students (Redish Citation2003). As a matter of fact, the main issue with students’ intuitive approach is related to the fact that it completely fails to take into account simultaneous reasoning about expansion along both dimensions of the plate. This issue could be potentially resolved by encouraging the students to compare the thermal expansion of a thin, straight rod (analogical anchor) to the thermal expansion of a thin circular rod (extreme case obtained from bending the given straight rod) of same length. Drawing conclusions about the effects of heating a circular rod facilitates visualizing the effects of thermal expansion along both dimensions of the plate. Generally, students can be led to see that it is more appropriate to divide a plate into thin two-dimensional geometric figures that resemble the appearance of the plate (or its hole) than to divide it into one-dimensional shapes, that is, straight strips. This should help the students to realize that, generally, in thermal expansion the interparticle distances do not increase only along one dimension. When thinking of a plate as consisting of numerous two-dimensional geometric shapes (of continually increasing perimeters), we necessarily reason about thermal expansion along both, the x- and y-axis.
Taking into account the usefulness of including visualizations of microscopic mechanisms into reasoning about macroscopic phenomena (Greca and Moreira Citation2000), we can conclude that a mental model about thermal expansion should also include inter-particle interactions which are traditionally represented by periodically arranged springs. Consequently, it can be noted that the experimental approach presented in this paper uses a combination of analogies and extreme case reasoning, enriched by external visualizations that are meant to reduce cognitive load as well as to represent the microscopic mechanisms of thermal expansion (Mayer and Moreno Citation2003). The effectiveness of combining visualizations, analogies and extreme case reasoning has been proven many times throughout history of science. For example, it helped Maxwell in his endeavor to describe electromagnetic phenomena and Galileo in his formulation of the inertia law (Nersessian Citation1999; Einstein and Infeld Citation1938). In educational studies, it has been found that combining analogies with extreme case reasoning helps the students to develop mental models that foster deeper conceptual understanding of the physics phenomena (Stephens and Clement Citation2009, Citation2010; Clement Citation1988, Citation1993; Zietsman and Clement Citation1997).
2. Purpose
In this study we performed a pre-post quasi-experiment with the aim to investigate whether teaching by combining analogies and extreme cases with corresponding visualizations of microscopic mechanisms of thermal expansion can help the students to be more successful in predicting the effects of thermal expansion. In our opinion the significance of this study is related to the fact that we are presenting a powerful example of how analogies and extreme cases can be effectively used for establishing cognitive bridging from students’ intuitive models to scientifically acceptable models. It should be noted that the presented teaching approach emphasizes the usefulness of nurturing nonformal reasoning in physics (Clement Citation1993; Voss, Perkins, and Segal Citation1991). Furthermore in this study, it is also shown how we could prevent the occurrence of some widely known misconceptions about thermal expansion, such as the misconception that in plates with holes the holes shrink when the plates are heated. In this paper we also describe some misconceptions that have not been reported in earlier research.
3. Design and methods
3.1. Participants and curriculum
The sample for our study consisted of all 195 students (mostly 19 year-olds) who were enrolled in the first year introductory physics course at the Faculty of Chemical Engineering and Technology at the University of Zagreb. We conducted the study within the context of the regular first year curriculum. Thereby, the total sample has been divided into six subgroups, whereby three control subgroups were supposed to receive the traditional treatment (nc = 100) while the remaining three subgroups were assigned to the experimental treatment (ne = 95). In none of the six subgroups the number of assigned students was above 35. In addition, it should be noted that the gender distribution was similar across all subgroups – in all subgroups there was a larger share of females (approximately: 70%: 30%).
Before their university education, students from our sample finished an eight-year primary school and four-year high school, were they also had the opportunity to learn about thermal expansion. In their first year at the university, the students are enrolled in an introductory physics course where they receive two hours of lectures and two hours of recitations per week, in both the first and second semester. Generally, the teaching approach within the course could be characterized as traditional – in lectures the focus is on facts and procedures, whereas in recitation sessions the focus is on solving numerical problems. Thereby, in traditional recitation sessions most of the time the teaching assistant is modeling the solution of physics problems to a relatively small class of students (Redish Citation2003). In Croatia, traditional recitation sessions typically include one teaching assistant and 30–40 students.
After finishing the five-year study program at Faculty of Chemical Engineering and Technology (3 + 2) the students have access to jobs in industry or they can continue their education at the PhD level.
3.2. Treatment
Our study was situated within the context of the regular curriculum. Students from all subgroups received the same traditional lectures about heat phenomena, thermal expansion included. However, in the three experimental subgroups the traditional format of the recitation sessions has been enriched to include analogies, extreme cases and visual representations of microscopic mechanisms that are at heart of thermal expansion. The three control subgroups received the usual traditional treatment characterized by solving and discussing numerical problems. In recitation sessions for both, the control and experimental group, the same concepts were covered, that is, concepts related to heat including thermal expansion. All subgroups were taught by the same teaching assistant who is also the first author of this article. It should be also noted that he was their regular teaching assistant and that at the time of the experiment he had five years of teaching experience.
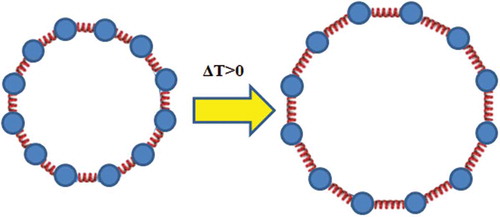
For all subgroups the teaching treatment lasted 90 min, and besides thermal expansion phenomena it also included some tasks related to ideal gas laws and calorimetry. In control subgroups the teaching assistant solved numerical problems on the blackboard and led the corresponding classroom discussions. Specifically, in the control subgroups’ recitations the teaching assistant discussed with his students about the problem solving process and final results for problems from . In the experimental subgroups the same concepts have been covered as in the control subgroups but in the experimental subgroups the treatment was also enriched by analogies, extreme cases and a visualization of microscopic mechanisms that are at heart of thermal expansion. Because significant time had to be devoted to these cognitive bridging activities, in the experimental subgroups less numerical problems were solved than in the control subgroups, that is, Problem 4 and Problem 5 from were only solved in the control subgroups. As a matter of fact, prior to solving numerical problems in the experimental subgroup, the teaching assistant showed external visualizations of various objects’ internal structures by metaphorically representing their constituent particles by little spheres and interparticle bonds by springs. In the next step, the interparticle bonds were additionally discussed on a more abstract level within the context of a diagram that showed how potential energy changes with interparticle distance. Relationship between the concrete external visualization (i.e. spheres and springs) with the diagram of potential energy has been firstly discussed for a straight metal rod (see ). Thereby, it has been emphasized that an increase of temperature implies a higher average velocity of the particles (i.e. little spheres) which in combination with the fact that the potential well is asymmetric leads to larger equilibrium interparticle separations (i.e. spring lengths). Then the students were guided to realize that the total length increase equaled to the sum of the length increments of individual springs (i.e. interparticle separations). At the same time the students could see that an increase of temperature, that is increase of average velocity of the spheres (i.e. particles) does not affect their size. Taking into account that this one-dimensional expansion of a straight rod is for most students easily comprehensible, it was used as a cognitive anchor for developing understanding about more complex situations. Thus, after having discussed about thermal expansion of a straight rod we moved on to discussing a rectangular frame, that is, a frame that consists of four thin, straight rods. The students could easily predict that the perimeter of the rectangular frame will increase as a result of heating, whereby the length of the longer sides will increase by a larger amount. Thereafter we led the students to realize that in the extreme case a rectangular plate can be obtained by ‘melting together’ a large number of rectangular frames, whereby perimeters of each two subsequent rectangular frames differ by an infinitesimal amount. This helped us to develop in our students an understanding about the increase of surface area as a result of heating – the surface area increases, because perimeters of all the rectangular frames increase. Finally, we showed the students how the introduced reasoning strategy can be used to predict the effects of thermal expansion on thin circular rods (i.e. rings). Again the thin straight rod has been used as a cognitive anchor. If we bend the straight rod of length l, in the extreme case we get a ring of circumference l. Then we led the students to conclude that in the same way as springs of the straight rod elongate due to heating, there must be also an increase of the spring lengths in the circular rod – the mere process of bending the rod cannot change ‘how the springs react to a change of temperature’ (see ).
Table 1. Short description of problems that were solved in the recitation sessions. An asterisk denotes problems that were solved only in control subgroups.
Figure 1. Lengths of individual springs increase when ΔT > 0 and the total length increase of the rod equals the sum of length increases of individual springs.
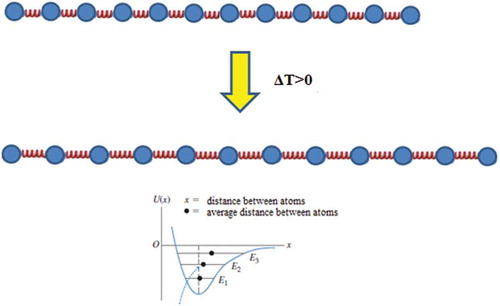
Figure 2. Lengths of springs increase when ΔT > 0 and this results in an increment of ring’s diameter.
After the students were exposed to analogies, extreme cases and visualizations of microscopic mechanisms of thermal expansion, the problem solving session was opened. During the course of the problem solving process, the teaching assistant encouraged the students from the experimental group to apply analogies, extreme cases and visualizations within the corresponding classroom discussions.
3.3. Instruments
In order to lower the pretest-treatment threat to internal validity (see Ary et al. Citation2010), we decided to use different instruments for pretest and posttest. However, for both instruments we can say that they measure conceptual understanding of thermal expansion. Most test items in both, the pretest and posttest, require the test taker to use knowledge of thermal expansion in order to provide explanations, predictions or solutions to problems. According to Michael and Modell (Citation2003), making acceptable predictions about scientific phenomena and processes is a very good indicator of understanding the corresponding scientific topic. Consequently, within the posttest special attention has been devoted to assessing whether the students are able to predict how thermal expansion will change the appearance of objects. In our opinion these items are typically solved by means of mental simulations (Nersessian Citation2008). How successful the mental simulations are depends on the characteristics of students’ mental models, especially on the internal visual representations which are part of these models. Therefore, in our opinion, the differences in posttest scores can inform us at least partly about corresponding differences in characteristics of students’ mental models about thermal expansion. In addition, the posttest items were chosen/designed in a way that provides us with the opportunity to assess whether the experimental treatment is more successful than the control treatment in preventing the occurrence of some widely known misconceptions.
The pretest and posttest versions of the Basic Understanding of Thermal Expansion Survey (BUTES) consisted of 5 and 10 conceptual questions, respectively. Both versions are provided in the Supplemental Online Material. Most pretest questions were adapted from existing literature. Questions 1 and 2 were adopted from Physics: Principles with applications by Giancoli (Citation2005) and Question 4 was adopted from Fundamentals of Physics by Halliday, Resnick, and Walker (Citation2011). On the other hand, a significant part of the posttest questions is original. Question 2 has been adopted from Physics by Walker (Citation2017), question 4 is from Fundamentals of Physics by Halliday, Resnick, and Walker (Citation2011), question 7 is from Physics: Principles with applications by Giancoli (Citation2005) and question 9 has been adopted from TIMSS 2007 released items by Foy and Olson (Citation2009).
On each test item, both in pretest and posttest, a correct answer was rewarded with one point. The small number of test items at pretest and posttest can be justified by the fact that the measured latent trait is relatively narrow and for narrow constructs validity of the instrument can be already established by using a relatively small number of test items (Abell, Springer, and Kamata Citation2009). Short descriptions of pretest and posttest items are provided in and .
Table 2. Short description of pretest items.
Table 3. Short description of posttest items.
The Cronbach’s alpha coefficient for the posttest amounted to 0.65, which can be considered as acceptable (Bowling and Ebrahim Citation2005; McKagan, Perkins, and Wieman Citation2010). The average item difficulty index for posttest was 0.45 which is close to the optimal value (see Cohen and Swerdlik Citation2009). However, it should be also noted that Item 2 and 4a on the posttest proved to be very difficult with difficulty indices of 0.03 and 0.13, respectively. When it comes to item discrimination, on the posttest only for Item 2 the corrected item-total correlation was below 0.2 which is considered to be the lower boundary of acceptable values (Jarrett, Ferry, and Takacs Citation2012; Ebel Citation1979). When it comes to the pretest version of BUTES, it should be noted that the test items proved to be very difficult for our students – the average item difficulty index amounted to 0.34. Particularly difficult were the Item 3 and Item 4 with item difficulty indices of 0.1 and 0.04, respectively. Consequently, the average item-total correlation and Cronbach’s alpha for pretest were relatively low with values of 0.12 and 0.23, respectively. The aim of administering the pretest was to get information about possible pretreatment between-group differences related to the dependent variable. From the fact that the pretest was too difficult for all subgroups, we can conclude that all subgroups entered our teaching treatments with a relatively low (on average) level of understanding of thermal expansion.
3.4. Research design
For the purpose of investigating the effectiveness of our experimental teaching treatment a pretest-posttest quasi-experiment has been implemented. The total student sample has been divided into two groups (control and experimental) and these two groups were further subdivided into six subgroups. The three control subgroups received traditional recitation sessions, whereas in the experimental subgroups the traditional recitation sessions were enriched by analogies, extreme cases and visual representations of microscopic mechanisms that are at heart of thermal expansion. The students received the teaching treatments in their natural learning environment by their regular teaching assistant. All subgroups wrote the pretest one week before the start of the experiment and they wrote the posttest immediately after the treatment. For the pretest and posttest, we allocated 15 and 20 min, respectively.
4. Results
In this section, we first are going to describe the pretest and posttest results across the control and experimental subgroups. Then, the subgroups data will be collapsed for purposes of conducting further analyses of differences between the control and experimental group. Concretely, an analysis of covariance (ANCOVA) will be conducted. Finally, we are going to provide an item-level analysis with the aim of identifying the easiest and most difficult items, as well as the most often chosen distractors in the pretest and posttest contexts.
4.1. Pretest and posttest scores across subgroups
From it can be seen that the experimental subgroups performed similarly to the control subgroups on the pretest. At the pretest, EG2 had the highest and EG3 had the lowest average score. On the other hand, on the posttest, even the lowest scoring experimental subgroup EG3 outperformed the highest scoring control subgroup CG1 (t(64) = 2.34, p = 0.02).
Table 4. Average pretest and posttest percent-correct scores for experimental subgroups (EG) and control subgroups (CG) are provided. Standard deviations are given in parentheses.
Taking into account that at the posttest experimental subgroups consistently outperformed the control subgroups, we decided to collapse the data for individual subgroups to keep the analyses concise and plain as possible. Consequently, the analyses below are provided for data that was collapsed across individual experimental and control subgroups.
4.2. Between-group differences in score distributions in the pretest and posttest contexts
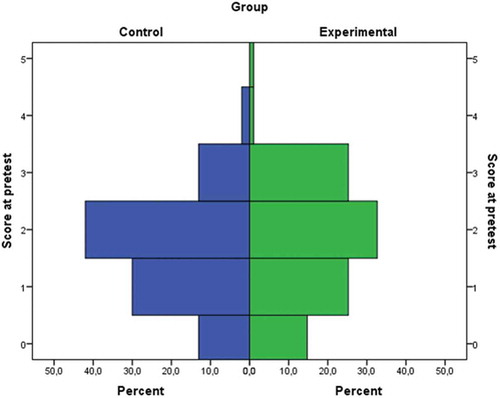
shows the distribution of pretest scores in the control and experimental group. In both groups the most prevalent score at the pretest was two out of five points. Although for the two groups the shapes of the distributions of scores were relatively similar to each other, it could be noticed that in the experimental group there was a higher incidence of extreme scores (zero and five points). However, the percentage of students who scored 2 points or lower on the pretest was 83% in the control group and 72.6% in the experimental group.
Figure 3. Distribution of pretest scores in experimental and control group. Theoretically, the pretest scale ranges from 0 to 5.
shows the distribution of posttest scores for the experimental and control group. It is easy to notice that the between-group differences at posttest are much more pronounced than that was the case at pretest.
Figure 4. Distribution of posttest scores in experimental and control group. Theoretically, the posttest scale ranges from 0 to 10.
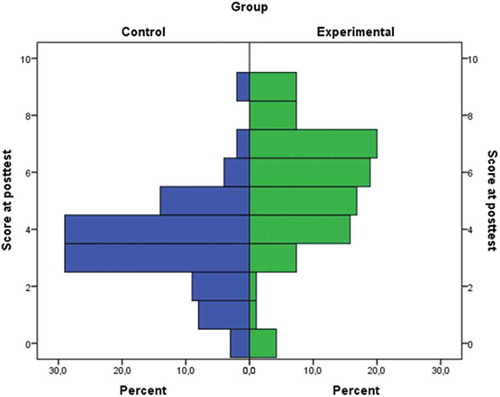
Concretely, in the distribution of posttest scores of the experimental group there is much higher incidence of higher scores than in the control group. As a matter of fact, in the experimental group the most frequent student scores were 6 and 7 out of 10, whereas in the control group the most frequent scores were 3 and 4 out of 10. It is interesting to notice that in the experimental group we had again a higher incidence of extreme scores (zero or nine points). However, generally the percentage of students who scored 2 points or lower at the posttest was equal to 20% in the control group and 6.4% in the experimental group.
provides a summarized overview of students’ achievement on pretest and posttest.
Table 5. A summarized overview of average pretest and posttest percent-correct scores for experimental and control group is provided. Standard deviations are given in parentheses.
4.3. Investigating the significance of the observed between-group differences
For purposes of investigating the statistical significance of the observed between-group differences on posttest, we implemented an analysis of covariance (ANCOVA) which allowed us to control for between-group differences on pretest (Field Citation2009). Before running ANCOVA we could show that the assumption of independence of the covariate (pretest score) and treatment variable (‘group’ as teaching treatment variable) was met (t(193) = 1.02, p = 0.31), that is, the between-group differences on pretest scores were not significant. In addition, the interaction between the covariate and treatment variable (F(1, 191) = 0.015, p = 0.9) proved to be highly non-significant. The normality assumption has been checked by a visual inspection of Q-Q plots for the control and experimental group (David Citation2013), whereby it has been found that this assumption has been approximately met. Unfortunately, the Leven’s statistic (F(1, 193) = 6.22, p = 0.013) proved to be significant which indicates a violation of the homogeneity of variances assumption. However, taking into account the fact that the sample size was large, as well as the facts that the observed between-group differences on posttest were large (approximately 20%) and that the ANCOVA is very robust to violations of the normality and homogeneity of variances assumptions (Glass, Peckham, and Sanders Citation1972; Bradley Citation1978), eventually we decided to go on with using ANCOVA for investigating the significance of between-group differences.
ANCOVA analysis showed that there was a significant effect of teaching treatments on students’ posttest scores after controlling for pretest scores, F(1, 192) = 52.33, p < 0.001, partial η2 = 0.21. Furthermore, a planned contrast revealed that students from the experimental group significantly outperformed their colleagues from the control group, t(193) = 7.34, p < 0.001, r = 0.46.
4.4. Item-level analyses
provides a summarized overview of between-group differences on individual posttest items.
Table 6. Percentages of correct answers on individual posttest items are provided.
From we can conclude that at the posttest experimental group students outperformed their peers from the control group on all test items. Most prominent differences in favor of the experimental group were found on Item 5 (how a circular plate with a hole changes due to heating), Item 9 (how heating influences size and spatial arrangement of particles inside a metal), Item 6 (how heating influences the appearance of two concentric circular plates made of same material) and Item 1 (how length increment depends on initial length). Both groups showed very low achievement on Item 2 (how level of the liquid depends on the difference in coefficients of expansion of the liquid and the container) and Item 4 (how vertical heights and surface areas of different plates change due to heating).
The most frequently made errors at pretest and posttest are presented in and , respectively. Obviously, similar posttreatment misconceptions were detected in both groups.
Table 7. Most frequent errors at the pretest.
Table 8. Most frequent errors at the posttest.
5. Discussion
First of all, it should be noted that the pretest scores were very low across all subgroups. Taking into account the fact that the pretest questions were not outside the scope of primary and secondary school curricula on thermal expansion, we could also conclude that the quality of achieved curricula in Croatia is not at a satisfactory level, at least when it comes to developing conceptual understanding about thermal expansion. This result indicates that in Croatia the physics instruction is mostly traditional, at all educational levels, which is in line with earlier findings from Croatia (Planinic, Ivanjek, and Susac Citation2010; Marušić and Sliško Citation2012; Hake Citation1998). Consequently, when students entered our teaching treatments their level of conceptual understanding was very low which means that the posttreatment level of understanding can be mostly associated with the mere teaching treatment that took place in recitation sessions. Thereby, it should be noted that the performance of control subgroups stayed low even after the treatment (approximately 35%), whereas the experimental subgroups showed at posttest an average performance that is close to the 60% boundary which is in some contexts considered to be the entry threshold for basic understanding (Hestenes and Halloun Citation1995). From a statistical perspective, it is useful to point out that the extremely low performance on Item 2 lowered the average score for both groups.
It is important to once more emphasize that at the posttest even the lowest achieving experimental subgroup significantly outperformed the highest achieving control subgroup. This is a strong statistical evidence on the superiority of the experimental teaching treatment over the control teaching treatment, when it comes to developing conceptual understanding about thermal expansion. Our finding was further supported by results of ANCOVA where we detected a significant, medium to large effect in favor of the experimental group (Field Citation2009). The main difference between the two teaching treatments was related to the fact that in addition to discussions about numerical problems the experimental treatment also included use of analogies, extreme cases and visualizations of microscopic mechanisms that underlie the process of thermal expansion. On the other hand, within the control treatment the students had the opportunity to discuss about two more problems compared to their peers from the experimental group. The very low result of the control group at the posttest could be explained by the fact that students entered the discussion about numerical problems without having some intuitively and physically acceptable, as well as visually enriched mental models about thermal expansion. This made their discussions mostly dominated by the use of formal representations and language of mathematics. Earlier research shows that solving thousands of numerical problems is sometimes not sufficient to develop deep conceptual understanding (Kim and Pak Citation2002). On the other hand, students from the experimental groups entered the classroom discussions about numerical problems only after they had developed some basic intuitive understanding and internal visualizations of the given phenomena. According to Greca and Moreira (Citation1997) for developing deep conceptual understanding about physical phenomena and processes, it is very important to develop the corresponding internal visualizations. After they were introduced to vivid analogies, extreme cases and visualizations of microscopic mechanisms of thermal expansion, the students from the experimental subgroups were able to discuss the given problems by using a language that goes beyond the language of mathematics. This sequence of developing and consistent using of conceptual knowledge for purposes of discussing numerical problems probably led to superior performance of experimental subgroups at the posttest. Our findings are in line with the idea that using analogies and extreme cases helps students to create imageable and intuitively grounded mental models of physical phenomena (Stephens and Clement Citation2009, Citation2010; Clement Citation1988, Citation1993; Zietsman and Clement Citation1997). By enriching the presentation of analogies and extreme cases with corresponding sequences of logically linked static images, we allowed the students to reason about the presented topics step by step. This helped us to prevent cognitive overload that otherwise could have occurred as a result of relatively small capacity and short duration of working memory (Mayer and Moreno Citation2003; Luck and Vogel Citation1997; Redish Citation2003; Sweller Citation1994).
Next, we are going to discuss between-group differences on individual posttest items. From we can see that students from the experimental group outperformed their peers from the control group on all items. As we have already noticed earlier, very large differences in favor of experimental group have been detected for Item 5, Item 9, Item 6 and Item 1. As a matter of fact, on these items the performance differences ranged from 29% (Item 1) to 45% (Item 5).
In Item 5 students were shown a circular plate with a circular hole and they were asked to predict the appearance of the plate after heating. Within the experimental treatment, students were trained to imagine plates of certain shapes as consisting of numerous frames with increasing perimeters, whereby all frames resemble the shape of the mere plate. They also learned to draw an analogy between one-dimensional expansion of a thin, straight rod of length l and a circular rod of same length that is obtained by bending the straight rod. In other words, experimental group students were trained to correctly bridge from thinking about one-dimensional expansion to expansion along two dimensions. On the other hand, by comparing the student answers in Item 3 (pure one-dimensional expansion) and Item 5 (circular plate with a circular hole), we could come to the conclusion that students from the control group attempted to perform bridging between the one-dimensional situation (a straight rod expands to the left and to the right) and two-dimensional situation, too. However, similarly to the teachers from our nonformal interviews they failed to realize that the straight rods that constitute the plate, are not independent from each other – there is also expansion along the y-axis, not only along the x-axis. The very high percentage of correct answers on Item 3 (pure one-dimensional expansion; 92% vs. 88%) once more confirms that students are able to correctly mentally simulate how a thin, straight rod expands due to heating which justifies taking this phenomenon to be our cognitive anchor.
In Item 9, students were expected to show understanding that increase of object’s volume in thermal expansion is not a result of an increase in volume of the atoms but rather it is a result of an increase of average interparticle distances. In our opinion, the external visualizations that represented how thermal expansion results in longer springs (with spheres retaining their size) helped to prevent the development of the misconception of enlarged atoms in experimental group students. In Item 6, students were shown two concentric hollow discs. The discs were made of the same material and between them there was air. Students were expected to predict the appearance of the discs after their temperature increased by the same amount. Taking into account that rings of larger circumference should expand by a larger amount, their radii will also expand by a larger amount than radii of rings of smaller initial circumference. If we imagine the discs as consisting of thin rings, then we easily come to the conclusion that the air-filled space increases as a result of heating the discs to the same higher temperature. It is interesting to note that in Item 8 the between-group differences were relatively small, although it differed from Item 6 only to the point that the outer disc was made from a material with larger coefficient of expansion. In this situation, the air-filled space between the discs experiences an even larger increase due to heating than in Item 6. From comparison of student answers on Item 6 and Item 8 it follows that some students believe that the amount of thermal expansion only depends on the coefficient of expansion, that is, some students fail to take into account the initial length/area/volume of the object. Consequently, an increase/decrease of the air-filled space between concentric discs is for them more intuitively acceptable if the coefficients of expansion of the discs differ. Difficulties related to failing to take into account the ‘third variable’ are well known in physics education research (Leinonen, Asikainen, and Hirvonen Citation2013; Matijašević, Korolija, and Mandić Citation2016) and our finding is in line with results of that research. Issues with taking into account the ‘third variable’ (i.e. initial length) can be clearly identified from students’ answers on Item 1. As a matter of fact, even 51% of students from control group believed that two rods made of same material will experience the same amount of expansion due to same increase of temperature, although their initial lengths differ. When thinking about the amount of expansion in Item 1, the students from experimental group probably visualized the total expansion as a result of the sum of length increments of individual springs. Since objects that are initially longer have more springs, they experience greater expansion for the same temperature increase. In our opinion, such a reasoning line could explain the superiority of experimental group students on Item 1. At first glance, the low achievement of control group students on Item 1 seems surprising because Item 1 could have been easily solved by mere application of the corresponding physical formula. Consequently, we could also say that our finding for Item 1 is in line with earlier evidence on the inertness of mathematical representations – visually enriched mental models seem to be activated in a broader range of contexts than mental models that are solely based on decontextualized propositional representations (Bransford et al. Citation1989; Gallagher Citation2000; Vermunt and Verloop Citation1999).
Finally, it should be also noted that students from both groups scored extremely low on Item 2. In Item 2, students were required to explain why the alcohol level in an alcohol-in-glass-thermometer first drops and then rises when the thermometer is put into a hot bath. In our opinion, the extremely low result on this item could be explained by the fact that this item required the students not only to mentally simulate the effect of different coefficients of expansion of alcohol and glass on the level of alcohol in the glass tube, but also to think about thermal conductivity and rate of heat transfer. Our finding is in line with earlier research in which it has been shown that students exhibit significant difficulties when required to predict the level of liquid in a heated container (Kartal, Öztürk, and Yalvaç Citation2011).
Next, we will discuss the identified misconceptions at pretest. The pretest data were collapsed because all subgroups were exposed to exactly the same lectures before the teaching treatment and consequently the student answers across subgroups were similar at the pretest. In what follows we will speak of a misconception in such cases in which a distracter has been chosen much more frequently compared to other distracters. We already have such a situation in Item 2 of the pretest where the wrong answer B was most common (57%). In that test item, students were asked how temperature influences the accuracy of a steel tape measure and it seems that the majority of students believe that a steel tape measure will provide accurate measurements at any temperature.
When it comes to Item 3 of pretest, the most common wrong answer was C (85%). In other words, a large majority of students believed that in a steel bottle filled to the brim with water the water level will decrease as a result of exposing the bottle to sunlight. The students’ poor performance on Item 3 is particularly surprising in the light of the fact that already in secondary school the students from our sample were taught that liquids generally expand faster than solids. However, it seems that this knowledge often fails to be activated in everyday contexts. Past studies showed that students think that metals attract the heat better than other matter objects (Erickson Citation1979; Yeo and Zadnik Citation2001; Lewis and Linn Citation1994). Probably students from our sample also believed that the steel bottle received more heat and expanded more than water leading to a decrease of the water level. An alternative explanation would be that many students thought that evaporation of water ‘prevails’ over the effect of thermal expansion of water which eventually results in decrease of the water level. A similar misconception has been already identified by Reiner et al. (Citation2000) and it probably stems from overgeneralization of everyday experiences (McCloskey Citation1983). It should be noted that decrease of water level due to evaporation is highly unlikely, given the relatively small rate of heat transferred to the water and relatively small free surface of the water.
On 4th item, the students were given information on initial length, temperature increase and length increase of four rods and they were asked to rank the coefficients of linear expansion of these rods. Only 5% of students provided a correct answer. Many students believed that it is sufficient to think only about two variables (temperature increase and length increase), disregarding the importance of the third variable (initial length). The students’ tendency to disregard the importance of the ‘third variable’ has been already identified in earlier research on other physics topics (Leinonen, Asikainen, and Hirvonen Citation2013; Matijašević, Korolija, and Mandić Citation2016).
Finally, we are going to discuss the misconceptions exhibited by the students at the posttest (see ). Here the student answers will be discussed separately for control and experimental group in order to allow for discussion of treatment effects. Generally, it should be noted that on most items the most frequently chosen distracter was the same for both groups.
Student answers on Item 1 indicate that even after the treatment the students from the control group did not take into account the initial length when reasoning about length increase, whereas most students from the experimental group seem to have overcome the ‘third variable’ issue. As already mentioned earlier, in our opinion the superiority of the experimental group could be explained by the fact that in the experimental treatment the total expansion has been visually associated with the sum of length increments of individual springs. For objects with larger initial length, there are ‘more springs’ and consequently their length increases by a larger amount. The results for Item 1 once more show that decontextualized mathematical representations are more inert than visual representations (Bransford et al. Citation1989; Gallagher Citation2000; Vermunt and Verloop Citation1999). In Item 2 students were required to explain why the alcohol level in the alcohol-in-glass thermometer immersed in hot liquid firstly drops and then rises. Even 99% of students from control group and 96% of students from experimental group provided a wrong answer to this item. Students could not explain at all why there was an initial drop of the alcohol level in the alcohol-in-glass thermometer. This result is in line with the research by Liew and Treagust (Citation1995) who used a similar task and found that none of his 18 high-performing students predicted an initial drop of the level of the liquid. In that study students provided some explanations for water level rise (liquid expands when heated, molecules move quicker, molecules move faster and take up more space etc.) but they did not provide fully explanation for initial drop of liquid.
In items 4a and 4b students were showed four different rectangular plates that experienced the same temperature increase and students were explicitly required to rank the amount of increase of vertical height (Item 4a) and surface area (Item 4b) for these plates. In other words, the stems of these items now explicitly required the students to think also about the initial dimension(s) of the plates and not only about the temperature increase variable. However, it seems that in such a context students tend not only to reason about the variable of interest (vertical height in Item 4a and size of surface area in Item 4b) but they also include into their reasoning other variables. For example, many believed that increase in vertical height does not only depend on temperature increase and initial vertical height, but also on the size of initial surface area and/or narrowness of the object. Similarly, many believed that for objects with mutually equal initial surface areas, the amount of increase in surface area is larger for objects with larger initial height although the initial height was already incorporated within the initial surface area variable. Such a reasoning is similar to the misconception that both the resultant force and its components act on an object independently of each other – force components are taken into account twice (Aviani, Erceg, and Mešić Citation2015). The high degree of difficulty of items 4a and 4b could be also accounted for by the fact that in both items there were even four objects that had to be compared which combined with significant amount of irrelevant load (e.g. horizontal lengths of the plates and different surface areas in 4a) resulted often with cognitive overload. These findings are in line with earlier research (Sorden Citation2005). Finally, it should be noted that, although on items 4a and 4b students from both groups scored relatively low, on both items experimental group students outperformed their control group peers by 13%.
In Item 5, students were required to answer how a circular plate with a hole changes as a result of heating. Obviously, the misconception that heating results with shrinking of the hole was much more pronounced in the control group (53%) than in the experimental group (25%). The existence of this misconception is well known to teachers and researchers (McHugh and McCauley Citation2016; Watkins and Mazur Citation2013).
In items 6 and 8, the students were required to think about the thermal expansion of two concentric discs made of the same/different material, respectively. For both items, the most common answer was D which corresponded to the same misconception (i.e. hole that shrinks due to heating) as already discussed for Item 5.
In Item 9, students were required to reason about the effect of heating on the particles in a metal. The most frequently chosen wrong answer in the control group reflects the erroneous belief that thermal expansion is not only the result of an increase in interparticle distances but is also related to the ‘enlargement of the atoms’. The identification of the described misconception supports the findings from earlier research (Yeo and Zadnik Citation2001). Here it could be interesting to note that in earlier studies it has been also shown that students think that gas particles increase in size with the change of physical state from solid to gas (Yalcınkay and Boz Citation2015).
Finally, it should be noted that findings from our study are delimited to contexts similar to the one described in our study, that is, to introductory physics courses for scientists and engineers at the university level. When it comes to psychometric features of the posttest, we could say that they are mostly acceptable. However, for Item 2, which proved to be extremely difficult for both groups, it should be noted that it measures not only understanding of thermal expansion but also understanding of thermal conduction. That item should be modified in a future version of the BUTES. From a statistical perspective there is also a limitation related to the violation of the homogeneity of variances assumption. However, taking into account the fact that the sample size and between-group differences on posttest were very large, as well as the fact that the lowest scoring experimental subgroup significantly outperformed the highest scoring control subgroup, we can conclude that the probability of making a Type I error was extremely unlikely in our study.
6. Summary and conclusion
In this study, we aimed to investigate whether enriching recitation sessions with analogies, extreme cases and visualizations of microscopic mechanisms that underlie thermal expansion can facilitate development of understanding about thermal expansion and secure prevention of some widely known misconceptions. Therefore we decided to conduct a pretest-posttest quasi-experiment with three control and three experimental subgroups. In recitation, sessions for the control group the dominant activities were problem solving and discussions about the problem solving process, whereas in the experimental group the students were presented with analogies, extreme cases and external visualizations before proceeding with discussing and solving physics problems.
Based on the results of this study we came to the following conclusions:
Analogies, extreme cases and external visualizations of microscopic mechanisms can be effectively combined to facilitate development of imageable and intuitively grounded mental models about thermal expansion. Mental models with such characteristics allow for running mental simulations about thermal expansion phenomena. These conclusions are in line with findings by Nersessian (Citation2008) and Clement (Citation1993).
Analogies and extreme cases can promote cognitive bridging from reasoning about the well-understood one-dimensional expansion to the less understood two-dimensional expansion. By relating thin, straight rods to thin rings of same length, and by representing the circular plate as consisting of numerous rings of increasing circumferences, we can effectively prevent the occurrence of the shrinking-hole misconception. This supports the idea of cognitive bridging as an effective technique of conceptualization (Clement Citation1993; Redish Citation2003; Zietsman and Clement Citation1997).
By presenting the internal structure of objects with springs and by relating the total linear expansion of objects to the sum of length increments of individual springs, we can often prevent the issue of not taking into account the initial length of an object when reasoning about the relationship between temperature change and total length increment.
Even university students only rarely activate mathematical representations when faced with a conceptual problem or everyday situation (see Item 1 at posttest). On the other hand, it seems that visual representations are much more often activated. This additionally supports the importance of cognitive bridging and nurturing of nonformal reasoning in physics instruction (Clement Citation1993; Redish Citation2003; Voss, Perkins, and Segal Citation1991).
In future studies we are going to use qualitative methods in order to study some of the identified misconceptions in more detail.
Supplemental Material
Download MS Word (41.4 KB)Supplemental Material
Download MS Word (52.7 KB)Acknowledgments
The authors would like to thank Dr. sc. Iva Movre Šapić from Faculty of Chemical engineering and Technology, Zagreb for her contribution in data analysis
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data can be accessed here.
References
- Abell, N., D. W. Springer, and A. Kamata. 2009. Developing and Validating Rapid Assessment Instruments. Oxford: Oxford Press.
- Ary, D., L. C. Jacobs, C. Sorensen, and A. Razavieh. 2010. Introduction to Research in Education. Belmont: Wadsworth Cengage.
- Aviani, I., N. Erceg, and V. Mešić. 2015. “Drawing and Using Free Body Diagrams: Why It May Be Better Not to Decompose Forces.” Physical Review Special Topics-Physics Education Research 11: 20137. doi:10.1103/PhysRevSTPER.11.020137.
- Bowling, A., and S. Ebrahim. 2005. Handbook of Health Research Methods: Investigation, Measurement and Analysis. Maidenhead: Open University Press.
- Bradley, J. V. 1978. “Robustness?” British Journal of Mathematical and Statistical Psychology 31: 144–152. doi:10.12691/ajams-1-5-4.
- Bransford, J. D., J. J. Franks, N. J. Vye, and R. D. Sherwood. 1989. “New Approaches to Instruction: Because Wisdom Can’t Be Told.” Similarity and Analogical Reasoning 470: 497.
- Bueno, A. C. R., D. A. Prudente, E. C. Machado, and R. V. Ribeiro. 2012. “Temperature Amplitude Affects the Vegetative Growth and Carbon Metabolism of Orange Trees in a Rootstock-Dependent Manner.” Journal of Plant Growth Regulation 31: 309–319. doi:10.1007/s00468-017-1553-3.
- Clement, J. 1988. “Observed Methods for Generating Analogies in Scientific Problem Solving.” Cognitive Science 12: 563–586. doi:10.1207/s15516709cog1204_3.
- Clement, J. 1993. “Using Bridging Analogies and Anchoring Intuitions to Deal with Students Preconceptions in Physics.” Journal of Research in Science Teaching 30: 1241–1257. doi:10.1002/tea.3660301007.
- Cohen, R. J., and M. Swerdlik. 2009. Psychological Assessment: An Introduction to Tests and Measurements. Boston: McGraw-Hill Higher Education.
- Craik, K. 1943. The Nature of Explanation. Cambridge: Cambridge University Press.
- David, H. 2013. Statistical Methods for Psychology. Belmont: Cengage Learning.
- Duit, R. 2015. “Alltagsvorstellungen und Physik lernen.” In Physikdidaktik: Theorie und Praxis, edited by E. Kircher, R. Girwidz, and P. Häußler, 657–680. Heidelberg: Springer Berlin.
- Ebel, R. 1979. Essentials of Educational Measurement. 3rd ed. Englewood Cliffs: Prentice-Hall.
- Einstein, A., and L. Infeld. 1938. The Evolution of Physics. London: Scientific Book Club.
- Erickson, G. L. 1979. “Children’s Conceptions of Heat and Temperature.” Science Education 63 (2): 221–230. doi:10.1088/0031-9120/20/4/309.
- Eylon, B.-S., and U. Ganiel. 1990. “Macro-Micro Relationships: The Missing Link between Electrostatics and Electrodynamics in Students’ Reasoning.” International Journal of Science Education 12 (1): 79–94. doi:10.1080/0950069900120107.
- Field, A. 2009. Discovering Statistics Using SPSS. London: SAGE.
- Foy, P., and J. F. Olson. 2009. TIMSS 2007 International Database and User Guide. Released Items. Mathematics–Fourth Grade. Chestnut Hill, MA: TIMSS & PIRLS International Study Center, Boston College.
- Gallagher, J. J. 2000. “Teaching for Understanding and Application of Science Knowledge.” School Science & Mathematics 100 (6): 310–318. doi:10.1111/j.1949-8594.2000.tb17325.x.
- Giancoli, D. 2005. Physics Principles with Applications. 6th ed. UK: Prentice-Hall International.
- Glass, G. V., P. D. Peckham, and J. R. Sanders. 1972. “Consequences of Failure to Meet Assumptions Underlying the Fixed Effects Analyses of Variance and Covariance.” Review Of Educational Research 42 (3): 237–288. doi:10.3102/00346543042003237.
- Greca, I. M., and M. A. Moreira. 1997. “The kinds of mental representations–models, propositions and images–used by college physics students regarding the concept of field.” International Journal of Science Education 19: 711–724. doi: 10.1080/0950069970190607.
- Greca, I. M., and M. A. Moreira. 2000. “Mental Models, Conceptual Models, and Modelling.” International Journal of Science Education 22: 1–11. doi:10.1080/095006900289976.
- Hake, R. R. 1998. “Interactive Engagement versus Traditional Methods: A Six-Thousand-Student Survey of Mechanics Test Data for Introductory Physics Courses.” American Journal of Physics 66: 64–74. doi:10.1119/1.18809.
- Halliday, D., R. Resnick, and J. Walker. 2011. Fundamentals of Physics. 9th ed. NJ: Wiley.
- Hestenes, D., and I. Halloun. 1995. “Interpreting the Force Concept Inventory: A Response to March 1995. Critique by Huffman and Heller.” The Physics Teacher 33: 502. doi:10.1119/1.2344278.
- Jarrett, L., B. Ferry, and G. Takacs. 2012. “Development and Validation of a Concept Inventory for Introductory-Level Climate Change Science.” International Journal of Innovation in Science and Mathematics Education 20 (2): 25–41.
- Kartal, T., N. Öztürk, and H. G. Yalvaç. 2011. “Misconceptions of Science Teacher Candidates about Heat and Temperature.” Procedia Social and Behavioral Sciences 15: 2758–2763. doi:10.1016/j.sbspro.2011.04.184.
- Kim, E., and S. Pak. 2002. “Students Do Not Overcome Conceptual Difficulties after Solving 1000 Traditional Problems.” American Journal of Physics 70: 759–765. doi:10.1119/1.1484151.
- Leinonen, R., M. A. Asikainen, and P. E. Hirvonen. 2013. “Overcoming Students’ Misconceptions Concerning Thermal Physics with the Aid of Hints and Peer Interaction during a Lecture Course.” Physical Review Special Topics-Physics Education Research 9: 20112. doi:10.1103/PhysRevSTPER.9.020112.
- Lewis, E. L., and M. C. Linn. 1994. “Heat Energy and Temperature Concepts of Adolescents, Adults, and Experts: Implicationsfor Curricular Improvements.” Journal Of Research In Science Teaching 31 (6): 657–677. doi:10.1002/tea.3660310607.
- Liew, C. W., and D. Treagust. 1995. “A Predict-Observe-Explain Teaching Sequence for Learning about Students’ Understanding of Heat and Expansion Liquids.” Australian Science Teachers Journal 41 (1): 68–71.
- Luck, S. J., and E. K. Vogel. 1997. “The Capacity of Visual Working Memory for Features and Conjunctions.” Nature 390: 279–281. doi:10.1038/36846.
- Marušić, M., and J. Sliško. 2012. “Influence of Three Different Methods of Teaching Physics on the Gain in Students’ Development of Reasoning.” International Journal of Science Education 34 (2): 301–326. doi:10.1080/09500693.2011.582522.
- Matijašević, I., J. N. Korolija, and L. M. Mandić. 2016. “Translation of P = kT into a Pictorial External Representation by High School Seniors.” Chemistry Education Research and Practice 17 (4): 656–674. doi:10.1039/C6RP00030D.
- Mayer, R. E., and R. Moreno. 2003. “Nine Ways to Reduce Cognitive Load in Multimedia Learning.” Educational Psychologist 38: 43–52. doi:10.1207/S15326985EP3801_6.
- McCloskey, M. 1983. “Intuitive Physics.” Scientific American 248: 122–130. doi:10.1038/scientificamerican0483-122.
- McHugh, M., and V. McCauley. 2016. “Getting Hooked on Physics!” The Physics Teacher 54: 548–550. doi:10.1119/1.4967896.
- McKagan, S. B., K. K. Perkins, and C. E. Wieman. 2010. “Design and Validation of the Quantum Mechanics Conceptual Survey.” Physical Review Special Topics-Physics Education Research 6: 20121. doi:10.1103/PhysRevSTPER.6.020121.
- Michael, J., and H. I. Modell. 2003. Active Learning in Secondary and College Science Classrooms: A Working Model for Helping the Learner to Learn. London: Routledge.
- Nersessian, N. 1995. “Should Physicists Preach What They Practice? Constructive Modeling in Doing and Learning Physics.” Science & Education 4: 203–226. doi:10.1007/978-1-4615-1921-8_6.
- Nersessian, N. 1999. Model-Based Reasoning in Scientific Discovery. New York: Springer.
- Nersessian, N. 2008. Creating Scientific Concepts. Cambridge, MA, and London: MIT Press.
- Planinic, M., L. Ivanjek, and A. Susac. 2010. “Rasch Model Based Analysis of the Force Concept Inventory.” Physical Review Special Topics-Physics Education Research 6 (1): 10103. doi:10.1103/PhysRevSTPER.6.010103.
- Podolefsky, N. S., and N. D. Finkelstein. 2006. “Use of Analogy in Learning Physics: The Role of Representations.” Physical Review Special Topics-Physics Education Research 2: 20101. doi:10.1103/PhysRevSTPER.2.020101.
- Redish, E. 2003. Teaching Physics with the Physics Suite. Hoboken: Wiley.
- Reiner, M., J. D. Slotta, M. T. H. Chi, and L. B. Resnick. 2000. “Naive Physics Reasoning: A Commitment to Substance Based Conceptions.” Cognition and Instruction 18: 1–34. doi:10.1207/S1532690XCI1801_01.
- Sorden, S. D. 2005. “A Cognitive Approach to Instructional Design for Multimedia Learning.” Informing Science Journal 8: 264–279.
- Sözbilir, M. 2003. “A Review of Selected Literature on Students’ Misconceptions of Heat and Temperature.” Boğaziçi University Journal of Education 20 (1). doi:10.1016/j.sbspro.2011.02.074.
- Stephens, A. L., and J. J. Clement. 2009. “Extreme Case Reasoning and Model Based Learning in Experts and Students.” In Proceedings of the NARST 2009 Annual Meeting.
- Stephens, A. L., and J. J. Clement. 2010. “Documenting the Use of Expert Scientific Reasoning Processes by High School Physics Students.” Physical Review Special Topics-Physics Education Research 6: 20122. doi:10.1103/PhysRevSTPER.6.020122.
- Sweller, J. 1994. “Cognitive Load Theory, Learning Difficulty, and Instructional Design.” Learning and Instruction 4: 295–312. doi:10.1016/0959-4752(94)90003-5.
- Tipler, P., and G. Mosca. 2008. Physics for Scientists and Engineers. 1 vol. 6th ed. New York: Worth Publishers.
- Vermunt, J., and N. Verloop. 1999. “Congruence and Friction between Learning and Teaching.” Learning and Instruction 9: 257–280. doi:10.1016/S0959-4752(98)00028-0.
- Voss, J., D. Perkins, and J. Segal. 1991. Informal Reasoning and Education. NJ: Lawrence Erlbaum Associates, Inc., Publishers.
- Walker, J. 2017. Physics. 5th ed. USA: Pearson.
- Watkins, J., and E. Mazur. 2013. “Retaining Students in Science, Technology, Engineering, and Mathematics (STEM) Majors.” Journal of College Science Teaching 42 (5): 36–41.
- Yalcınkay, E., and Y. Boz. 2015. “The Effect of Case-Based Instruction on 10th Grade Students’ Understanding of Gas Concepts.” Chemistry Education Research and Practice 16: 104–120. doi:10.1039/C4RP00156G.
- Yeo, S., and M. Zadnik. 2001. “Introductory Thermal Concept Evaluation: Assessing Students Understanding.” The Physics Teacher 39: 496–504. doi:10.1119/1.1424603.
- Zietsman, A., and J. Clement. 1997. “The Role of Extreme Case Reasoning in Instruction for Conceptual Change.” The Journal of the Learning Sciences 6: 61–89. doi:10.1207/s15327809jls0601_4.
