Abstract
A model for systematic changes in patterns of inter-individual variation in affective responses to physical activity of varying intensities is presented, as a conceptual alternative to the search for a global dose – response curve. It is theorized that trends towards universality will emerge in response to activities that are either generally adaptive, such as moderate walking, or generally maladaptive, such as strenuous running that requires anaerobic metabolism and precludes the maintenance of a physiological steady state. At the former intensity the dominant response will be pleasure, whereas at the latter intensity the dominant response will be displeasure. In contrast, affective responses will be highly variable, involving pleasure or displeasure, when the intensity of physical activity approximates the transition from aerobic to anaerobic metabolism, since activity performed at this intensity entails a trade-off between benefits and risks. Preliminary evidence in support of this model is presented, based on a reanalysis of data from a series of studies.
Introduction
The dose – response relationship between the intensity of physical activity and the accompanying affective responses has become one of the prominent research foci within exercise psychology in recent years. In their review of this literature, Ekkekakis and Petruzzello (Citation1999) identified 31 studies that compared the effects of multiple levels of intensity, conducted over a period of 27 years, from 1971 to 1998. Since then, the production of new information on this topic has accelerated dramatically, resulting in at least 17 additional studies in less than 5 years (citations available upon request). The primary reason for this growing interest is the expectation that this research will elucidate the mechanisms underlying the negative relationship between intensity and adherence (e.g. Cox, Burke, Gorely, Beilin, & Puddey, Citation2003; Lee et al., Citation1996; Perri et al., Citation2002). People generally tend to do what makes them feel good and avoid what makes them feel bad, so it is critical to understand how the intensity of physical activity influences affective responses, as these responses might reduce the intrinsic motivation for physical activity, and thus lead to decreased adherence and increased likelihood of dropout. According to the text of the Healthy People 2010 programme in the USA, “each person should recognize that starting out slowly with an activity that is enjoyable … is central to the adoption and maintenance of physical activity behaviour” (US Department of Health and Human Services, Citation2000, pp. 22 – 24). Yet, in actuality, the hypothesized causal chain linking “starting out slowly”, enjoyment and the “adoption and maintenance of physical activity behaviour” remains poorly understood.
One of the most prominent obstacles in the continued effort to increase our understanding of this causal chain is the absence of an adequate conceptual framework for the critical first link, the relationship between intensity and affect. It appears that the goal of most published dose – response studies has been to identify a global dose – response curve similar to the one that has been proposed for the biological outcomes of physical activity (Haskell, Citation1994). However, some reviewers have expressed the concern that the relationship between the intensity of physical activity and affective responses might not be amenable to conventional or traditional (i.e. biomedical) dose – response models, whereby the relationship can be represented by a single curve with global or nomothetic applicability (Biddle & Mutrie, Citation2001, p. 153; Ekkekakis & Petruzzello, Citation1999; Rejeski, Citation1994). The reason is that affective responses to physical activity are subject to a multitude of interacting personal, social, physical and physiological factors, to which individuals tend to respond “as ‘active’ rather than ‘passive’ agents” (Rejeski, Citation1994, p. 1040). Although there is consensus that individuals differ in how they respond to varying intensities of physical activity, deciphering the implications of this observation for theory and practice poses a challenge and opinions begin to diverge. For example, one viewpoint is that, if we accept individual differences and the “inevitable existence of complex biopsychosocial interactions”, then “it follows that no single dose of activity will ever have universal appeal” (Rejeski, Citation1994, p. 1053). Another viewpoint is that, although “future research should take both the individual's psychological and physiological profiles into account” and it would not make sense to prescribe physical activity that is aversive to a certain individual, it may still be “possible to defend a single exercise prescription for all individuals (e.g., 70% of [Vdot]O2max)” (Morgan, Citation1997, p. 11). Here, we (a) present an alternative conceptual model of the relationship between the intensity of physical activity and affective responses and (b) review data that support this formulation.
A brief definition of affect
Affect is defined here as the most basic or elementary characteristic component of all valenced (positive or negative, pleasant or unpleasant) responses, including, but not limited to, emotions and moods (for a more detailed discussion, see Ekkekakis & Petruzzello, Citation2000). In that sense, affect is a broader concept than emotion. The chief difference is that an emotion (e.g. pride or shame) requires a cognitive appraisal of a stimulus as having positive or negative implications for one's goals or well-being. On the other hand, affect (e.g. pleasure or displeasure) may occur as one of the components of an emotion (e.g. shame is unpleasant) or independently, even in the absence of a cognitive appraisal (e.g. the immediate, “automatic” or cognitively unmediated displeasure associated with pain). Although affect is commonly defined in terms of both valence (pleasure – displeasure) and activation, emphasis here is placed on valence as the dimension that is generally considered of greater value for survival and adaptation (Cabanac, Citation1971, Citation1979; Panksepp, Citation1998a,Citationb). Thus, an “affective response” is defined here as a change in self-reported pleasure – displeasure.
Physical activity, affective responses and the unshakable legacy of the inverted-U
As demonstrated in hundreds of studies to date, bouts of physical activity can induce significant affective responses (for reviews, see Tuson & Sinyor, Citation1993; Yeung, Citation1996). The most oft-cited assumption in this literature regarding the shape of the dose – response relationship between the intensity of physical activity and affect is that it approximates an inverted-U or inverted-J (). Specifically, it has been assumed that moderately vigorous intensity (not “too low”, not “too high”) optimizes the conditions for positive affective changes, whereas low intensity is insufficient to produce significant changes in affect and high intensity is either ineffective or experienced as aversive (Berger & Motl, Citation2000; Kirkcaldy & Shephard, Citation1990; Ojanen, Citation1994).
Figure 1. The inverted-J (solid part of the line) and inverted-U hypotheses of the relationship between the intensity of physical activity and affective benefits. An intensity that is “too low” is believed to be ineffective in producing significant affective benefits, whereas an intensity that is “too high” may be ineffective or aversive. An intensity approximating 60 – 70% of maximal aerobic capacity (i.e. not “too low” and not “too high”) is believed to provide the optimal stimulus for positive affective changes.
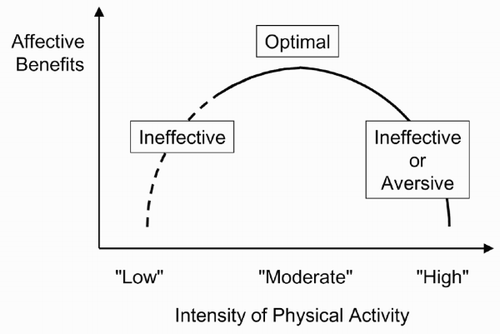
There are two main problems with the inverted-U or inverted-J. First, despite the popularity of these models and associated assumptions, they are not consistent with the extant evidence (Ekkekakis & Petruzzello, Citation1999). For example, several studies have shown that low-intensity activity, such as walking at a self-chosen pace (less than 25% of heart rate reserve), results in increased self-rated pleasure among young participants (Ekkekakis, Hall, Van Landuyt, & Petruzzello, Citation2000; Saklofske, Blomme, Kelly, Citation1992; Thayer, Citation1987a,Citationb; Thayer, Peters, Takahashi, & Birkhead-Flight, Citation1993). Moreover, during a 30-min bout of cycle ergometry at 60% of estimated maximal aerobic capacity ([Vdot]O2max), a mid-range intensity (not “too low”, not “too high”), some participants reported a gradual improvement but others reported a gradual decline (Van Landuyt, Ekkekakis, Hall, & Petruzzello, Citation2000).
A second problem with global dose – response models in general, and the inverted-U or inverted-J in particular, is that they “espouse a stimulus – response paradigm” (Rejeski, Citation1994, p. 1040) and, as such, fail to account for patterns of inter-individual variation. Yet, these patterns of inter-individual differences appear to be systematic and might be of considerable psychological significance. Despite sporadic calls for increased attention to inter-individual differences from within exercise psychology (Ekkekakis & Petruzzello, Citation1999; Gauvin & Brawley, Citation1993; Rejeski, Citation1994; Van Landuyt et al., Citation2000) and similar calls in other areas, including exercise science (Bouffard, Citation1993), general psychology (Thorngate, Citation1986) and behavioural ecology (Bennett, Citation1987), neither concepts nor methods in the study of the exercise – affect relationship have changed in any substantive way in the last 35 years. A possible reason for this apparent unwillingness to treat inter-individual differences as a phenomenon worthy of systematic psychological study is the absence of a conceptual framework for approaching and interpreting this variability.
Two methodological pitfalls
Two crucial methodological problems pertaining to dose – response investigations became apparent in Ekkekakis and Petruzzello's (Citation1999) review of this literature. Both can critically distort the outcomes of dose – response studies and, therefore, warrant scrutiny.
The first problem, which can be dubbed the “now you see it, now you don't” illusion, relates to the timing of assessments of affective responses. In most published studies, affective changes were assessed from before to various time points after the activity, based on the apparently false assumption that any change in the interim would be linear. However, assessments of affective responses during the activity bout have shown that, as intensity increases, there are consistent decreases in self-rated pleasure and, eventually, increases in displeasure during the activity (Acevedo, Kraemer, Haltom, & Tryniecki, Citation2003; Acevedo, Rinehardt, & Kraemer, Citation1994; Bixby, Spalding, & Hatfield, Citation2001; Ekkekakis, Hall, & Petruzzello, Citation2004; Hall, Ekkekakis, & Petruzzello, Citation2002; Hardy & Rejeski, Citation1989; Parfitt & Eston, Citation1995; Parfitt, Eston, & Connolly, Citation1996; Parfitt, Markland, & Holmes, Citation1994). Upon cessation of the intense activity, the typically negative changes are followed by rapid positive changes, leading to a post-activity state that is more pleasant than the pre-activity one (Bixby et al., Citation2001; Hall et al., Citation2002). Thus, the consequence of this problem is that a dose – response pattern that is apparent during the activity may quickly dissipate as soon as the activity is terminated.
The second problem, which can be dubbed the “what's in a name?” imbroglio, relates to the nomenclature used to describe different levels of physical activity intensity. It is probably fair to say that, on this topic, chaos and confusion reign supreme, as there is no consistency in the methods used to determine intensity levels or in the terms used to describe them. One encounters methods as diverse as a fixed number of watts, a fixed heart rate, a fixed rating of perceived exertion, or various, arbitrarily selected percentages of measured or estimated maximal heart rate, heart rate reserve, or maximal oxygen uptake. Or one may come across terms as unspecific and enigmatic as low, mild, high, vigorous, strenuous, and perhaps the most polysemous of them all, moderate. It should be apparent that such inconsistency renders the integration of findings an essentially impossible task.
One possible solution would be to follow the conventional classification scheme proposed by the American College of Sports Medicine (ACSM, Citation2000, p. 150). In this scheme, for example, “moderate” intensity is conventionally defined as between 40% and 59% of heart rate reserve, between 55% and 69% of maximal heart rate, or a rating of perceived exertion of 12 or 13. However, new problems soon become apparent. First, this solution is based on convention, not physiology, and conventions often change to reflect the spirit of the times. For example, just 5 years earlier, the term “moderate” was used by ACSM (Citation1995, p. 162) to describe a higher range of intensities, namely between 60% and 79% of maximal heart rate. Second, this solution does not take into account the balance between the underlying metabolic processes (aerobic and anaerobic), which may differ between two individuals exercising at the same percentage of their maximal capacity, even if these individuals are of the same sex and of similar age, health and activity status (e.g. Dwyer & Bybee, Citation1983; Katch, Weltman, Sady, & Freedson, Citation1978). Consequently, it does not provide an adequate standardization of the exercise stimulus across individuals, particularly if one considers the multitude of cardiovascular, biochemical, neuroendocrine and, as we will show, affective changes that take place as the organism transitions between the aerobic and anaerobic modes of operation. Therefore, in this paper, as will be explained later, we follow a three-domain typology of intensity, the basis of which was proposed as early as the 1950s (Wells, Balke, & Van Fassen, Citation1957) and which has since been endorsed by experts in exercise physiology (Gaesser & Poole, Citation1996; Wasserman, Hansen, Sue, Casaburi, & Whipp, Citation1999).
Physical activity from an evolutionary and adaptational perspective
A central thesis underpinning the proposed model is that physical activity must be considered from an evolutionary and adaptational perspective, a potentially insightful conceptual vantage point that has been all but ignored in exercise psychology. Physical activity has been an integral part of life for the human species throughout its evolutionary history (Åstrand, Citation1986, Citation1992a,Citationb, Citation1994; Bortz, Citation1985; Cordain, Gotshall, & Eaton, Citation1997, Citation1998; Eaton, Shostak, & Konner, Citation1988; Malina, Citation1991, Citation1998). According to one assessment, “exercise is arguably the most important factor in driving evolution” (Woakes, Citation1991, p. ii). Consequently, locomotion and physical performance have been focal areas of research in evolutionary biology, physiology and anthropology for many years. The knowledge base that has been developed as a result of these efforts contains information of potentially tremendous value for improving the current understanding of the dynamics of inter-individual variation in affective responses to physical activity. Even though direct empirical evidence is difficult to obtain, physical performance capacity is believed to be closely related to adaptation and Darwinian fitness, the relative reproductive success of a genotype (Arnold, Citation1983; Bennett, Citation1991; Bennett & Huey, Citation1990; Garland & Lossos, Citation1994; Irschick, Citation2002; Irschick & Garland, Citation2001; Leonard & Ulijaszek, Citation2002; Plaut, Citation2001; Pough, Citation1989). According to Bennett and Ruben (Citation1979, p. 206):
The selective advantages of increased activity capacity are not subtle but rather are central to survival and reproduction. An animal with greater stamina has an advantage that is readily comprehensible in selective terms. It can sustain greater levels of pursuit or flight in gathering food or avoiding becoming food. It will be superior in territorial defense or invasion. It will be more successful in courtship and mating.
It is clear that certain aspects of the adaptational significance of physical activity behaviour for humans have changed over evolutionary time (e.g. in most cases, it is no longer necessary to exert oneself to obtain food or to fight off predators). It is also clear that there is inherent risk in drawing analogies between humans during the Pleistocene (i.e. the period that shaped the evolution of Homo sapiens, lasting approximately from 1.8 million to 10,000 years ago) and modern humans, who live much longer and generally succumb to different causes of death. Nevertheless, many of the adaptational implications of physical activity appear to have remained invariant. Importantly, human anatomy and physiology have remained essentially unchanged since the later palaeolithic era (which ended approximately 15,000 years ago).
Darwinian fitness consists of two major components, namely survival and reproductive success, and physical activity is relevant to both. First, physical activity is relevant to survival, since activity can both promote it and endanger it. In other words, as hunters and gatherers (i.e. for approximately 99% of their natural history), humans had to be active to stay alive and stay alive while being active. On the one hand, as numerous modern epidemiological studies have demonstrated, regular moderate physical activity can protect against a wide range of chronic diseases, significantly decreasing mortality rates, whereas inactivity appears to constitute a harmful disuse of the body (Balady, Citation2002; Booth, Chakravarthy, Gordon, & Spangenburg, Citation2002; Myers et al., Citation2002). This evidence is consistent with findings from other species (Holloszy, Citation1993; Holloszy & Smith, Citation1987). On the other hand, physical activity of excessive intensity entails risk, leading to an increased incidence of sudden cardiac death (e.g. Albert et al., Citation2000; Cadroy et al., Citation2002), musculoskeletal injuries (e.g. Hootman et al., Citation2001) and suppression of immune function (e.g. Nieman, Citation1997). Second, with respect to reproductive success, among contemporary men, physical activity has been linked to enhanced sexual behaviour, such as frequency of encounters and reliability of adequate functioning (White, Case, NcWhirter, & Mattison, Citation1990), as well as maintenance of reproductive health (Bacon et al., Citation2003; Derby et al., Citation2000). Similar findings have come from the animal literature, showing that chronic wheel running results in enhanced copulatory behaviour among male rats (Chambliss, Van Hoomissen, Holmes, Bunnell, & Dishman, Citation2004; Yoo, Tackett, Crabbe, Bunnell, & Dishman, Citation2000). Conversely, excessive physical activity has been linked to reproductive problems in both men (DeSouza, Arce, Pescatello, Scherzer, & Luciano, Citation1994) and women (Jasienska, Citation2003). In the following sections, we make links between the adaptational significance of different levels of physical activity intensity and the patterns of affective responses that accompany the activity.
Fundamental assumptions
Our conceptual formulation is based on a series of core assumptions, drawing mainly from principles of evolutionary theory and prominent views in evolutionary and affective psychology. First, we consider physical activity to constitute an essential component of the human Environment of Evolutionary Adaptedness (EEA; Tooby & Cosmides, Citation1990a), the environment that shaped human evolution. Unlike previous views of what the EEA encompasses, here we place emphasis on the internal environment of the body. Human physical performance, being a trade-off between speed and endurance, is subject to certain constraints, including morphological and metabolic design features that have remained invariant during human evolution (Conley, Kemper, & Crowther, Citation2001).
Second, we consider affective responses as manifestations of evolved psychological mechanisms, selected for their ability to promote health and well-being or to solve recurrent adaptational problems (Nesse, Citation1990) within the particular EEA of physical activity. Thus, we view affective responses to physical activity as analogous to affective responses that accompany other activities of vital adaptational importance (e.g. tasting foods, smelling odours, engaging in sexual intercourse, etc.). Furthermore, we accept that physical activity is unique among survival-critical activities, as it can induce bidirectional changes in affect (i.e. pleasure or displeasure). We consider pleasure to signify utility and displeasure to signify danger (Cabanac, Citation1971, Citation1979, Citation1995; Panksepp, Citation1998a,Citationb). As summarized earlier, different levels of physical activity intensity may entail either utility or danger. Following Bartley (Citation1970), Cabanac (Citation1971, Citation1995), Damasio (Citation1995, Citation1999, Citation2000), Schulze (Citation1995) and others, we believe that pleasure and displeasure are tied to the maintenance of homeostasis. We follow the rationale expressed in Schneirla's (Citation1959, Citation1965) seminal theory of approach-withdrawal motivation, adopting the belief that both the absence of stimulation and the presence of intense stimulation are stressful. Based on this, Schneirla proposed that, at least for organisms at an early ontogenetic stage, low intensities of species-specific, survival-important stimulation tend to evoke approach reactions, whereas high intensities tend to evoke withdrawal reactions with reference to the source of the stimulation. In his words, “the high road of evolution has been littered with the remains of species that diverged too far from these rules” (Schneirla, Citation1959, p. 3). Presumably, approach responses are accompanied by pleasure and withdrawal responses by displeasure (Tobach, Citation1970).
Third, consistent with emerging evidence from affective psychology and neuroscience, we assume that affective responses, including those that originate in the body (Craig, Citation1996, Citation2002, Citation2003; Damasio, Citation1995, Citation1999), depend on a hierarchically organized system involving multiple layers of control. This system ranges from oligosynaptic, subcortical and evolutionarily primitive pathways that underlie survival-critical, automatic or obligatory responses at the bottom and polysynaptic, evolutionarily recent, cortical pathways producing complex, flexible and highly individualized responses at the top (Berntson, Boysen, & Cacioppo, Citation1993; Berntson & Cacioppo, Citation2000; Damasio, Citation1995; LeDoux, Citation1986; Toates, Citation2002). Based on this, we accept that some types of affective responses can be induced directly by somatic afferent cues and some require cognitive elaboration (Cacioppo, Berntson, & Klein, Citation1992). This view is also consistent with Schneirla's (Citation1959, Citation1965) position that, in adult organisms, approach towards or withdrawal from stimuli (and, presumably, pleasure or displeasure, respectively) are driven by two interacting forces: (a) the fundamental tendency to approach weak and avoid intense stimuli, and (b) an increasingly influential superimposed component that reflects the learning and experience accumulated over the course of one's life. Within this hierarchical system, control can shift from more complex to simpler mechanisms and vice versa, depending on which can provide the appropriate response to a given situation (Berntson & Cacioppo, Citation2000; den Dulk, Heerebout, & Phaf, Citation2003; Toates, Citation1998, Citation2002). The occasional relegation of control to the less flexible mechanisms at the bottom of the hierarchy can confer an adaptive advantage because the multivariate inferential (i.e. cognitive) processes of the higher levels are slower and, by intervening between direct perception and response, may introduce options that are maladaptive. According to Griffiths (Citation1990), “it is vital for an organism to be able to accept data which contradict even its most firmly held beliefs” (p. 186). This is because “a condition for the reliability of perception … is that it generally sees what's there, not what it wants or expects to be there. Organisms that don't do so become deceased” (Fodor, Citation1983, p. 68). Although reliance on low-level pathways may lead to some false positives, “false positive responses … probably have more survival value than false negative responses” (LeDoux, Citation1986, p. 241). According to Cabanac (Citation1995), “one great advantage of this mechanism is that it does not take rationality or a high level of cognition to produce a behaviour adapted to biological goals” (p. 413).
In the particular case of the affective responses to physical activity, the shifts between different levels of control appear to be systematic and dependent largely upon the intensity of the activity (Ekkekakis, Citation2003). Specifically, affective responses to high-intensity activity have been shown to correlate closely with indices of physiological strain, indicating a direct link between somatic afferents and the affective centres of the brain. On the other hand, affective responses to physical activity performed at an intensity that may be challenging but does not pose an imminent threat correlate primarily with cognitive variables (such as physical self-efficacy), suggesting cortical involvement.
Fourth, we assume that “more primitive phylogenetic structures and functions, being the successful outcome of eons of adaptation, display less variation from individual to individual” (Reber, Citation1993, p. 7), whereas structures and functions that are evolutionarily recent show higher plasticity and are mostly shaped by individual developmental histories (Geary & Huffman, Citation2002). In general, within a particular ecological niche, “natural selection is a process that eliminates variation” (Tooby & Cosmides, Citation1990b, p. 37), whereas the presence of variation “generally signals a lack of adaptive significance” (Tooby & Cosmides, Citation1990b, p. 38). If a trait conferred a consistent adaptive advantage, it would have spread through the population, and thus it would exhibit limited heritable variation. Conversely, if it proved consistently maladaptive, it would have been eliminated, and thus it would also show limited heritable variation. According to Tooby and Cosmides (Citation1990b), “people display more diversity in their preferences for hat color or in their beliefs about gods and spirits than in their desire to continue breathing, their attraction to sex, or their desire to avoid pain” (p. 58). Nevertheless, although there is a general “desire to avoid pain”, there is also considerable heritable variation in pain sensitivity and tolerance (Mogil, Citation1999), as well as in the preferred level of sensory stimulation (Eysenck, Citation1983; Fulker, Eysenck, & Zuckerman, Citation1980). Presumably, this variability exists because these traits are, ultimately, adaptively neutral, involving a trade-off between benefits and risks. Consider Zuckerman's (Citation1990, p. 314) comments on the adaptational implications of individual differences in sensation seeking:
Sensation seeking and sensation avoidance, as extremes of a continuous behavioural trait dimension, may represent two different strategies for adaptation to a dangerous environment in which novel stimuli can be either sources of biological reward or a threat to survival. The sensation seeker among our hominid ancestors was probably more exploratory and more adventurous than the sensation avoider. This trait pattern would provide the advantage of increased access to new potential food sources and mates, but a disadvantage in terms of the risks entailed in such activities. The sensation avoider would tend to avoid the risks at the expense of the loss of foraging and reproductive advantage.
Returning to the idea of pleasure as indicative of utility and displeasure as indicative of danger, the presence of variability in affective responses (i.e. pleasure responses in some individuals, displeasure in others) could be interpreted as an indication that the situation entails neither definite benefit nor definite danger (but perhaps a trade-off between benefit and risk). On the other hand, whenever all or most individuals respond in the same manner, either with pleasure or with displeasure (within reasonable quantitative variation), we assume that the situation is likely one that has significant, either positive or negative, implications for adaptation.
Physical activity intensity domains and their adaptational significance
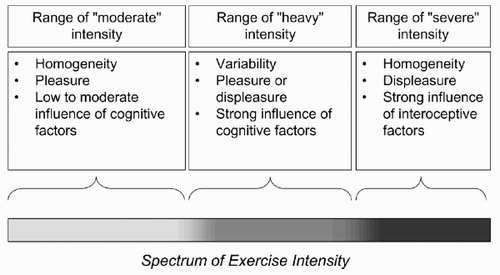
As noted earlier, in this paper, we follow a classification of physical activity intensity based on three domains with distinct metabolic requirements (Gaesser & Poole, Citation1996). We presume that distinct metabolic requirements also entail differences in adaptational significance. In the following sub-sections, we consider each domain in turn, followed by a consideration of the adaptational significance of the immediate post-activity phase.
Domain of “moderate” intensity
First, there is a broad range of intensity, characterized as moderate, which encompasses the intensities below the lactate threshold (i.e. this threshold being the lowest workload at which the rate of lactate appearance in the blood starts to exceed the rate of removal). In this domain, aerobic metabolism is the predominant source of energy. This is enormously important from an adaptational standpoint because the abundant energy stores available for aerobic metabolism in the human body (muscle and liver glycogen, fats, proteins) entail that activities in the moderate domain can be maintained for a long period of time while permitting the maintenance of a physiological steady state. Activities that typically fall in this range include walking, gardening and moderate swimming. Most of the hunting, scavenging and gathering activities preponderant during human evolution (Cordain et al., Citation1998) also fall within this range of intensity. An excerpt from Åstrand (Citation1994, p. 102), on his observations from a contemporary group of hunters and gatherers, illustrates this point:
Some years ago, I visited the bushmen in the Kalahari desert, probably the last remaining Stone-Age people. They followed the lifestyle of the true hunters and food gatherers. Gathering sufficient food meant trudging long distances for the men in their hunting efforts and for the women and children in their collection of berries, melons, roots, and various plants. This walking, stopping, and squatting to dig, and walking again, is physically demanding. When the women gather enough and return home, they still have to collect and carry firewood for the cooking and the night fire … To get enough to eat, the bushmen have to exercise for hours almost every day … I never saw an adult bushman out jogging, but the walking was fast!
These anecdotal observations are supported by several anthropological studies of hunter-gatherer societies, including the Kung of the Kalahari Desert, the Ache of Paraguay and others (Cordain et al., Citation1998; Hill, Kaplan, Hawkes, & Hurtado, Citation1985; Hurtado, Hawkes, Hill, & Kaplan, Citation1985; Leonard & Robertson, Citation1997; Norgan, Ferro-Luzzi, & Durnin, Citation1974; Sorensen & Leonard, Citation2001). For example, Leonard and Robertson (Citation1997) reported that walking and light work take up 6.8 and 5.5 h daily for Ache men and women, and 5.0 and 5.5 h daily for Kung men and women, respectively. The respective ranges covered are 14.9, 9.1, 19.2 and 9.2 km. Even in modern societies, activities in the moderate domain of intensity, predominantly walking, are the strongest predictors of total daily energy expenditure (Westerterp, Citation2001).
The amount of time and proportion of energy resources invested in physical activity of moderate intensity in primitive human societies are an indirect indication of the importance of such activities for promoting Darwinian fitness. Direct evidence linking the ability for physical activity within the moderate or aerobic range to survival and reproduction in human populations is scarce and mostly anecdotal (Carrier, Citation1984; Nabokov, Citation1981). In a preliminary observational study of a population of contemporary hunter-gatherers (the Ache of eastern Paraguay), Kaplan and Hill (Citation1985) found that, among males, hunting ability (presumed to be a composite of skill and the ability to pursue prey) was associated with increased reproductive success, defined as survivorship of offspring. Additional support comes from animal research (Hayes & O'Connor, Citation1999; Jayne & Bennett, Citation1990). Studies show that the amount of time a constant speed can be sustained (i.e. presumably within the moderate domain of intensity) is predictive of (a) the amount of time an animal spends moving in open-field conditions and the distance covered (Garland, Citation1999), and (b) social dominance (Robson & Miles, Citation2000; Sinervo, Miles, Frankino, Klukowski, & De Nardo, Citation2000). Access to large territories in conjunction with a dominant social status eventually can lead to increased encounters with potential mates and greater reproductive success.
Although activity in animal and primitive human societies, unlike modern societies, was motivated mainly by the need to access food and mates, considering the consequences of the (mostly theoretical then, all too real now) possibility of persistent physical inactivity is still suggestive of the necessity of activity for sustaining life. The modern sedentary lifestyle essentially constitutes a vast natural experiment for this. Regular participation in moderate physical activity has consistently been shown to be associated with reduced all-cause mortality, even after controlling for other risk factors directly or indirectly associated with the modern lifestyle, such as smoking, obesity, adult-onset diabetes and hypertension. In a study of retired men followed for 12 years, the all-cause mortality rate for those who walked less than 1 mile per day was nearly twice that of those who walked 2 or more miles per day (Hakim et al., Citation1998). Data from human (Myers et al., Citation2002) and animal (Jayne & Bennett, Citation1990) studies alike also demonstrate that endurance capacity is a significant predictor of mortality. Meyers et al. (2002) found that the risk of death from any cause for participants whose exercise capacity was less than 5 MET was roughly double that of participants whose exercise capacity was more than 8 MET. Moreover, every 1 MET increase in exercise performance was associated with a 12% improvement in survival.
Be it due to starvation or disuse and decay, it is clear that inactivity increases the risk of premature death and decreases Darwinian fitness. This invites an intriguing possibility: Could there be an evolved mechanism that promotes regular, moderate-intensity physical activity? Essentially, this proposition is an extension of the widely accepted and uncontroversial notion that the pleasure associated with eating tasty food, smelling inviting odours or engaging in sexual intercourse is a manifestation of precisely such mechanisms. According to Panksepp (Citation1998a), “pleasure is nature's way of telling the brain that it is experiencing stimuli that are useful – events that support the organism's survival by helping to rectify biological imbalances” (p. 182). What we are suggesting here is that the pleasure associated with moderate-intensity physical activity is one such example.
Along similar lines, other authors have suggested that humans and other animals may have an innate propensity for moderate physical activity, evolved to maintain a healthy balance between energy intake and expenditure and to sustain overall health, strength and mobility (Rowland, Citation1998; Thorburn & Proietto, Citation2000). When we started developing the idea for this article, we could only locate two opinion pieces, in which the authors had taken the extra step, highlighting the pleasure responses associated with moderate-intensity physical activity as the psychological mechanism that evolved to reward – and thus promote – this behaviour (Eikelboom, Citation1999; Sher, Citation1998). This situation has been changed by the publication of two recent studies that were based on the same rationale. Based on a long-term study examining the adaptations associated with the artificial genetic selection for high wheel-running behaviour in mice, Rhodes, Garland and Gammie (Citation2003) set out to identify brain regions associated with the motivation to run and the regulation of the amount of running performed. Their hypothesis was that, as a result of genetic selection, adaptations must have evolved in the brains of runner-mice, specifically in areas known to be associated with reward. Indeed, the researchers found several such areas, including the nucleus accumbens (a part of the dopamine reward system). In a study of two large human cohorts, Simonen et al. (Citation2003) investigated the relationship between physical activity and a polymorphism in a gene that encodes for the D2 type of dopamine receptor (DRD2). The rationale for this study was again based on the fundamental assumption that the amount of physical activity that humans perform is associated with the pleasure derived from the activity. Indeed, they found a link (particularly consistent among White women) between the DRD2 polymorphism and physical activity participation over the previous year. These studies definitely help to legitimize what might have initially appeared like an untenable or counterintuitive idea, namely that there is a genetically determined pleasure-based mechanism that has evolved to reward and promote physical activity.
What makes the idea seem untenable or counterintuitive to some is one indisputable fact, namely that an alarmingly high percentage of modern humans, living in industrialized countries, are physically inactive. Admittedly, this counter-argument seems compelling at first. However, we submit that it might be somewhat misleading. What we are proposing is only that pleasure responses to moderate physical activity represent a psychological adaptation that evolved to reward and promote such activity. It is not logical to argue that the veracity of this proposition hinges upon whether modern humans are physically active. Making such an extrapolation neglects the fact that both modern humans themselves (i.e. their unprecedentedly high levels of obesity and low levels of weight-adjusted physical fitness) and their living conditions have changed dramatically over the last century and, therefore, what they now do (which may be maladaptive in an evolutionary sense) is not a reliable indication of their genetic heritage. One example, offered by Irons (Citation1998), is the widespread use of vasectomies and contraception in an effort to lower lifetime fertility, certainly a strange behaviour from the standpoint of Darwinian fitness! However, this does not diminish our conviction that the tendency to procreate is “in our genes” and that the pleasure of intercourse has evolved to promote this behaviour. We just accept the fact that there are novel constraints (e.g. related to career or financial planning) that alter behaviour.
As Irons (Citation1998) notes, “the use of addictive drugs, modern eating habits, and the sedentary nature of urban life are other candidates for nonadaptive behaviours” (p. 203). Exercise psychology research has discovered several novel barriers that preclude physical activity participation (e.g. perceived lack of time, a low sense of self-efficacy and high social-physique anxiety, all shaped by modern cultural standards). Here, we add one other possibility. Modern humans may not be willing to participate in physical activity because they themselves have changed in ways that make the activity unpleasant. Unlike their ancestors who, based on skeletal remains, were characterized by robust physical abilities (Cordain et al., Citation1997, Citation1998; Eaton et al., Citation1988), modern humans suffer from the unprecedented combination of low endurance and high body weight. This combination has probably resulted in a moderate domain of physical activity intensity whose range is narrower now than it has ever been. Consequently, maintaining the intensity of the activity within the moderate domain, as this was defined previously, might be difficult or even unattainable for some. As an imaginary but not unrealistic example, a middle-aged individual weighing 120 kg, with a peak oxygen uptake of 25 ml · kg−1 · min−1 and a level of aerobic – anaerobic transition at 50% of peak capacity, will likely exceed the moderate domain of intensity very soon after starting to walk, even at what most people would consider a “slow” pace. Moreover, the affective response of this individual to the activity will be influenced not only by the cardiorespiratory cues but also by muscular and skeletal aches and pains, not to mention social-cognitive factors, such as perceived physical inefficacy and social physique anxiety. Therefore, the proposition that the pleasure response to moderate physical activity represents an evolutionary adaptation should not be judged by whether or not modern humans are physically active, but rather by whether this proposition holds up in contexts that are free of the unprecedented peculiarities of modern humans and their lifestyles.
Here we focus on two pertinent examples: (a) spontaneous activity in a wide range of animals, including wheel running in rodents; and (b) locomotor play (as distinct from other forms of play, such as object play, social play, or rough-and-tumble play, which have additional attributes and, possibly, functions) as a pervasive phenomenon across species, including humans. The fact that both of these phenomena lack an apparent immediate “function” has caused legions of researchers to speculate about their origins and significance (Martin & Caro, Citation1985; Sherwin, Citation1998). However, the debate is over the so-called “distal causation” (i.e. what their functions are over the long haul) and not over “proximal causation”. Proximally, there is consensus that both of these forms of physical activity are driven by the fact that they are apparently pleasurable and thus self-reinforcing.
In the following paragraphs, we discuss diverse evidence from the literatures that deal with voluntary running and play, in an effort to substantiate the claim of an evolutionarily ancient inherent tendency for physical activity in the moderate domain of intensity. For both phenomena, we follow a similar line of argumentation. We wish to show that: (a) both voluntary running and play are ubiquitous behaviours, at least among mammalian species; (b) animals spend considerable proportions of their time and energy budgets on these activities, despite the cost and associated risks; (c) both activities predominantly involve aerobic metabolism; (d) the distal function of both activities appears to be the maintenance of health and the promotion of activity capacity and, therefore, of Darwinian fitness; and (e) both activities appear to be inherently pleasurable, as evidenced by their successful application as rewards in conditioning studies and the fact that they involve brain mechanisms of established relevance to pleasure and reward.
According to a review of the voluntary wheel-running literature by Sherwin (Citation1998), most animal species will use running wheels when these are provided. Rodents, whose spontaneous running behaviour has been studied more often than other species, have been found to run several kilometres per day (Koteja, Swallow, Carter, & Garland, Citation1999b; Mather, Citation1981). The distances per 24-h period that have been reported in the literature range from 3.5 km for weasels to 43 km for rats (Sherwin, Citation1998). This amount of activity entails substantial investments of time and energy budgets. Although the amount of running performed can differ dramatically (in one study, some individuals were found to perform up to 37 times more revolutions per hour than others; Premack & Schaeffer, Citation1963), the tendency to engage in such activity seems remarkably consistent across species and individuals.
Most wheel running “is presumed to be primarily aerobic” (Houle-Leroy, Guderley, Swallow, & Garland, Citation2003, p. R433), based on analyses of the intensity and patterning of running and the type of physiological adaptations that occur as a result (Garland et al., Citation2002; Girard, McAleer, Rhodes, & Garland, Citation2001; Houle-Leroy, Garland, Swallow, & Guderley, Citation2000; Koteja et al., Citation1999b; Swallow, Garland, Carter, Zhan, & Sieck, Citation1998; Zhan et al., Citation1999). It is also of interest that animals that run more as a result of artificial selection for high activity do so by running faster and more intermittently (Girard et al., Citation2001; Koteja et al., Citation1999b). The purpose of pausing is to maintain the intensity near the high end of the aerobic range and prevent fatigue by (a) allowing the resaturation of myoglobin and haemoglobin, (b) allowing the restoration of phosphate stores, (c) limiting the depletion of glycogen stores and (d) preventing the concentrations of lactate, hydrogen ions and inorganic phosphates from rising continuously (Edwards & Gleeson, Citation2001; Girard et al., Citation2001; Kramer & McLaughlin, Citation2001; Weinstein & Full, Citation1999).
The distal function of voluntary wheel running has been debated extensively without a consensus being reached (Mather, Citation1981; Sherwin, Citation1998). According to Sherwin (Citation1998), this activity appears to have a proximal function (i.e. it is pleasurable and thus self-reinforcing) but lacks an adaptive distal function and its frequent excessiveness can only be an artifact of the laboratory environment. However, others disagree, offering explanations that focus on the maintenance of health and greater access to resources. Even Sherwin (Citation1998) acknowledged that running has several health benefits, including the fact that animals appear to adjust their running to balance caloric intake and expenditure. For Eikelboom (Citation1999), the health benefits of wheel running suffice to substantiate the claim that its distal function is, in fact, the promotion and maintenance of health. For Mather (Citation1981), wheel running represents a behavioural analogue to “exploratory migration”, a reflection of the redirected drive to seek resources in the wild. A variation of the same idea is also supported by Koteja, Garland, Sax, Swallow and Carter (Citation1999a), who view running as being representative of a tendency to travel distances and thus encounter more resources. Although Sherwin's (Citation1998) opinion that the aetiology of wheel running is multifaceted is probably correct, the few examples in which running develops into a maladaptive behaviour cannot outweigh the evidence of health benefits or eliminate the possibility of other important gains in Darwinian fitness.
What is undisputed is that wheel running appears to be a highly rewarding and self-reinforcing activity. Wheel running has been used as an effective reinforcer in numerous studies (e.g. Belke, Citation1997; Iversen, Citation1993) and, as reported by Sherwin (Citation1998), the animals will alter other important behaviours, even eating and drinking, in order to maintain access to running. An intriguing new line of research shows that wheel running can gradually replace the self-administration of drugs of addiction, such as cocaine (Cosgrove, Hynter, & Carroll, Citation2002), amphetamine (Kanarek, Marks-Kaufman, D'Anci, & Przypek, Citation1995) and ethanol (McMillan, McClure, & Hardwick, Citation1995). Like other apparently pleasurable and self-reinforcing activities, wheel running has been shown to involve the brain opioids (Boer, Epling, Pierce, & Russell, Citation1990; Hoffmann, Terenius, & Thorén, Citation1990; Lett, Grant, & Koh, Citation2001; Sisti & Lewis, Citation2001) and the mesolimbic dopaminergic network and nucleus accumbens (Sabol, Richards, & Freed, Citation1990; Werme et al., Citation2002; Wilson & Marsden, Citation1995).
Play is ubiquitous in mammals, evident in most birds and, as Burghardt (Citation1998) demonstrated, also apparent in non-avian reptiles (ancestral to both mammals and birds), suggesting a very long phylogenetic history. The physiological intensity of play spans the entire range, including (a) brief (up to 30 s) periods of strenuous activity involving primarily anaerobic metabolism, (b) slightly longer periods of high-intensity activity (1 – 3 min) involving mostly aerobic metabolism, and (c) prolonged periods of activity (several minutes to an hour or more) of submaximal intensity, also involving aerobic metabolism (Fagen, Citation1976, Citation1981). Play of the first type is associated with higher risk and cost than the latter two types (Burghardt, Citation1984). This might explain why play behaviour is not as common in reptiles, which depend heavily on anaerobic metabolism, as it is in mammals, which depend mostly on aerobic metabolism. For example, by week 12, pronghorn fawns, whose play consists primarily of running, average approximately 600 m per bout of running (Miller & Byers, Citation1991).
According to Burghardt (Citation1984), all the postulated functions of play can be classified into three categories: motor training, socialization and enhanced cognitive abilities. However, importantly, of these three, “locomotor play seems to be the most physiologically based, widespread and ontogenetically earliest type of play” (p. 7). Locomotor play involves running, jumping, kicking and so on and, although it may be performed concurrently by multiple animals, it is essentially an individual activity, not directed towards other individuals of the species. When the question about the distal function of play focuses specifically on locomotor play, then most theorists converge on variants of the so-called “motor training” hypothesis, originally proposed by Brownlee (Citation1954). The core of this idea is that play provides a stimulus for physical training, which, in turn, has the deferred benefit of increasing the chances of long-term survival (Bekoff, Citation1988; Byers, Citation1984; Byers & Walker, Citation1995; Fagen, Citation1976, Citation1981; Pellegrini & Smith, Citation1998). Although it is clear that “physical training alone cannot account for all play behaviour” (Fagen, Citation1981, p. 307), “motor training is the most plausible ancestral function of play” (Byers, Citation1984, p. 60). According to Siviy and Atrens (Citation1992), “play may have evolved partly as a means to ensure that the young of a species engage in some form of exercise” (p. 146).
Progress in the search for the neuroanatomical and neurophysiological substrates of play-induced pleasure, which could strengthen the argument for an inherent activity drive, is hindered by the fact that studies have failed to distinguish between the different types of play (locomotor, social, rough-and-tumble, etc.). In fact, at this stage, most of the information refers to social and rough-and-tumble play and not locomotor play. With this caveat in mind, it is still important to note that play (a) appears to be pleasurable and rewarding, and (b) involves the brain structures and neurotransmitter networks known to regulate other apparently pleasurable behaviours, including voluntary wheel running. Evidence that play is pleasurable is provided by studies showing that rats will readily perform other tasks, such as learning to traverse a maze, in order to earn the opportunity to play (Calcagnetti & Schechter, Citation1992; Humphreys & Einon, Citation1981; Normansell & Panksepp, Citation1990). Like other self-rewarding behaviours, play has been shown to involve the brain dopaminergic (Siviy, Citation1998) and opioid systems (Panksepp, Siviy, & Normansell, Citation1984; Siviy, Citation1998; Vanderschuren, Niesink, & Van Ree, Citation1997). Opioid agonists generally increase play behaviour, whereas opioid antagonists decrease it (Beatty & Costello, Citation1982; Normansell & Panksepp, Citation1990). Importantly, it appears that the neocortex is not necessary for the initiation of play behaviour, as neonatal decortication does not affect its appearance or its vigour (Murphy, MacLean, &Hamilton, Citation1981; Panksepp, Normansell, Cox, & Siviy, Citation1994). On the other hand, there is extensive evidence of subcortical involvement, particularly of the amygdala (Meaney, Dodge, & Beatty, Citation1981; Panksepp et al., Citation1984), areas of the thalamus and brainstem (Siviy & Panksepp, Citation1985, Citation1987), as well as the tectum, periaqueductal gray and hypothalamus (Gordon, Kollack-Walker, Akil, & Panksepp, Citation2002).
In summary, there are at least two phenomena, voluntary wheel-running and play, that appear consistent with the idea of an inherent tendency for activity. Both types of activity entail substantial energy costs and, therefore, some risk. Yet a wide range of animals perform them, as they derive the proximal benefit of pleasure and, presumably, the distal benefit of gains in Darwinian fitness. In humans, if one accepts the argument that physical activity performed within the moderate domain of intensity is consistently beneficial, the expectation is that affective responses to such activity will exhibit signs of homogeneity, with most individuals reporting increased pleasure.
Domain of “heavy” intensity
The second range of physical activity intensity, termed the domain of heavy activity, extends from the lactate threshold to the highest work rate at which blood lactate can be stabilized (also referred to as the maximal lactate steady state). In this domain, lactate appearance and removal rates can regain balance over time, but at elevated lactate concentrations. As a result of a continuous upward drift in oxygen uptake that appears in this domain, the oxygen cost per unit of work is increased compared with the moderate domain. The activity cannot be continued indefinitely, but it can be continued for a certain period of time. From an adaptational standpoint, the physiological events in this domain present a challenge, as considerable changes must take place to allow the maintenance of the work rate. Together with the rise in lactic acid concentration, there is the beginning of exponential increases in the frequency and depth of ventilation, catecholamine concentration, rate – pressure product and muscle fibre recruitment. These changes generate a barrage of interoceptive information that enters conscious awareness, alerting to the potentially critical perturbation of homeostasis (Craig, Citation1996, Citation2002, Citation2003).
Specifically, entering the anaerobic range is accompanied by a rapid accumulation of lactate and hydrogen ions dissociated from lactic acid. These, in turn, have been linked to several processes that contribute to fatigue, including the accelerated breakdown of creatine phosphate (McCann, Mollé, & Caton, Citation1995), the inhibition of glycolysis and glycogenolysis (Spriet, Lindinger, McKelvie, Heigenhauser, & Jones, Citation1989), the inhibition of lipolysis (Boyd, Giamber, Mager, & Lebovitz, Citation1974) and the interference with the calcium triggering of muscle contractions (Favero, Zable, Bowman, Thompson, & Abramson, Citation1995). In addition, lactic acidosis stimulates the release of catecholamines (Goldsmith, Iber, McArthur, & Davies, Citation1990), and thus the lactate threshold has been found to occur in close proximity to a catecholamine threshold (Urhausen, Weiler, Coen, & Kindermann, Citation1994; Weltman et al., Citation1994). In turn, catecholamines have widespread effects that further push the organism towards its functional limits, including a breakpoint in the relationship between the rate – pressure product and work rate (Riley et al., Citation1997; Tanaka et al., Citation1997). The intensity that exceeds the lactate threshold has also been shown to be associated with the appearance of abnormalities in left ventricular function in both healthy untrained individuals (Boucher et al., Citation1985; Tanaka et al., Citation1986) and in heart disease patients (Koike, Itoh, Taniguchi, & Hiroe, Citation1989). Moreover, to compensate for metabolic acidosis, above the point of transition to anaerobic metabolism, there is an increase in the frequency and depth of ventilation (Wasserman, Citation1978). Finally, the transition to anaerobic metabolism is accompanied by the recruitment of low-efficiency fast-twitch muscle fibres (Shinohara & Moritani, Citation1992), thus increasing the oxygen cost of work and disrupting coordination.
Presumably, like the ability to tolerate pain, the ability to tolerate these bodily cues, which may depend on individual differences in somatosensory modulation or cognitive factors (e.g. physical self-efficacy), would, on average, be adaptively neutral. On the one hand, this tolerance could aid a hunter or gatherer to perform more physical work or persist under adverse environmental conditions. On the other hand, the higher level of exertion would make one more susceptible to injury or exhaustion. Consistent with the hypothesis that individuals differ in their responses when the situation entails neither a consistent benefit nor a consistent threat, studies with human twins have shown that there is more heritable variation in participation in and preference for physical activities of vigorous rather than mild intensity (Beunen & Thomis, Citation1999; Lauderdale et al., Citation1997; Maia, Thomis, & Beunen, Citation2002).
Data from animal studies appear consistent with the notion of a trade-off between advantages and risks in the case of vigorous activity. In one study, physical endurance to the point of exhaustion, a trait that shows reliable evidence of heritable genetic variation and depends to a considerable extent on an individual's tolerance for fatigue, was found to be associated with higher growth rates and lower parasite loads in lizards, but also with high risk of being injured by predators (Clobert et al., Citation2000). In another study, male lizard morphs characterized by high endurance and high activity were found to defend large territories with many females, but also suffer low survival rates (Sinervo et al., Citation2000) due to the increased demands for energy intake or the increased risk of succumbing to predators.
In humans, the research on individual differences in tolerance for fatigue-related somatic cues has been surprisingly limited. Nevertheless, it is reasonable to assume that a higher level of tolerance for somatic cues would enable one to perform more activity and cover greater distances and, at the same time, expose one to heightened risk not only from predatory attacks, but also from one's own body. As an example, higher self-efficacy is associated with lower ratings of perceived exertion (e.g. Pender, Bar-Or, Wilk, & Mitchell, Citation2002; Rudolph & McAuley, Citation1996). Although this might enable one to perform more challenging activities, it might also lead to levels of intensity that pose a danger for cardiorespiratory problems (Ewart et al., Citation1986). For instance, the cognitive suppression or underestimation of the seriousness of a situation are responsible for delaying the call for medical assistance during cardiac episodes, often resulting in death (Burnett, Blumenthal, Mark, Leimberger, & Califf, Citation1995; Meischke, Ho, Eisenberg, Schaeffer, & Larsen, Citation1995).
In summary, physical activity in the heavy domain, although not uncommon and frequently necessary, is probably neither consistently adaptive nor consistently maladaptive. Exceeding the lactate threshold and entering the range of intensity that requires tapping into the limited resources available for anaerobic metabolism could help one perform more intense physical work but, at the same time, is not without some potential risks. Consequently, given the absence of consistent utility or danger, affective responses within the domain of heavy intensity are expected to vary from individual to individual, with some reporting pleasure and some reporting displeasure.
Domain of “very heavy” or “severe” intensity
The final range of intensity, termed the domain of very heavy or severe physical activity, extends from the maximal lactate steady state to the level of maximal exercise capacity. In this range, oxygen consumption and blood lactate rise continuously until the activity is terminated due to exhaustion. Energy supply relies heavily on the limited resources available to anaerobic metabolism (mainly the phosphagen pool and anaerobic glycolysis). This brings about an accelerated accumulation of lactate, which, in turn, leads to further complications by accelerating the breakdown of creatine phosphate, inhibiting glycolysis and lipolysis and interfering with the calcium triggering of muscle contractions. If the intensity of the activity is not reduced, the available energy stores will be depleted and the muscles will go into rigour. What prevents this from happening is the activation of a protective mechanism that precedes the failure in neuromuscular excitation and manifests itself as powerful perceptions of fatigue and displeasure.
Several authors have noted that the primary means by which critical disruptions in homeostasis enter consciousness is through salient surges of displeasure (Cabanac, Citation1971, Citation1979, Citation1995; Damasio, Citation1995, Citation1999, Citation2000; Panksepp, Citation1998a,Citationb). According to Damasio (Citation1999), compared with our almost unlimited ability to adapt to varied social conditions, our ability to survive changes in physiological parameters is very limited: “The permissible range is indeed so small and the need to respect its limits so absolute for survival that organisms spring forth equipped with an automatic regulation system to ensure that life threatening deviations do not occur or can be rapidly corrected” (p. 141).
Affect is at the core of this life-preserving “automatic regulation system”. Emphasis should be placed on the significance of the term “automatic”. Damasio (Citation1995) makes an important distinction between primary and secondary emotions. Primary emotions are “synonymous to innate, preorganised, or Jamesian emotions … Organisms, including the human organism, no doubt are ready to respond with emotion early in life, in preorganised fashion, when certain features of external or internal stimuli are perceived alone or conjunctively … The evaluative component in primary emotions is rudimentary” (p. 21). According to Damasio, certain configurations of the body's internal state (such as during a heart attack or, presumably, during severe-intensity exercise) are characteristic examples of internal stimuli that may induce primary emotional responses. On the other hand, “the evaluative component is an important aspect of secondary emotions” (p. 21). When this notion is applied to affective responses to physical activity, the “rudimentary evaluative component” of “primary emotions” entails that these responses probably depend on pathways that link somatosensory afferents to the affective centres of the brain (i.e. the amygdala, anterior cingulate and insular cortex) directly, bypassing the cortex and thus allowing little or no influence from top-down cognitive factors (Ekkekakis, Citation2003). What this presumed reliance upon lower-level (i.e. subcortical) networks implies is that there will be limited variability in affective responses, with most individuals experiencing displeasure.
Immediate post-activity phase
The instantaneous reversal from displeasure to pleasure that typically occurs immediately after physical activity in the heavy or severe ranges is also of interest from an adaptational standpoint. This pattern of change (i.e. baseline – unpleasant phase – pleasant phase – baseline) has the characteristics of what Solomon (Citation1980, Citation1991) described as the “affective contrast” phenomenon. According to Solomon, this phenomenon is the manifestation of an innate and automatic affective opponent-process mechanism, which is “brought into play whenever significant departures from affective equilibrium occur” and whose function is to “suppress or reduce all excursions from hedonic neutrality” (Solomon & Corbit, Citation1974, p. 143). Keeping affect “in check” is necessary if the individual is to regain a clear sense of perspective and return to an ordinary prioritization of goals.
What an individual experiences in response to a bout of intense physical activity, according to the opponent-process model, is the result of two processes that carry opposite valence signs, one negative (associated with displeasure, called the primary or a-process) and one positive (associated with pleasure, called opponent or b-process). The a-process “closely tracks the stimulus intensity” (Solomon, Citation1980, p. 710), whereas the b-process “has a long latency, a sluggish course of increase, and a sluggish course of delay” (p. 710) after the a-process is terminated. The nature and intensity of the a- and b-processes are fed into a summing device, which constantly computes | a - b | and determines the quality and intensity of affective experience at any moment. Solomon (Citation1991) postulated that “opponent-processes are not tripped into action until the a-process reaches a critical intensity” (p. 339).
Although Solomon did not discuss the biological underpinnings of his theory in detail, he did note the possibility of the opponent-process being driven by endogenous opioids, primarily beta-endorphin (Solomon, Citation1980, p. 708). This speculation has since been substantiated by research on the phenomenon of stress-induced analgesia, first discovered in animals in the mid-1970s and later confirmed in humans (see Amit & Galina, Citation1986, for a review). It is now known that descending opioidergic neurons, originating mainly in the periaqueductal grey, can exert an inhibitory effect on ascending sensory cues from joints, muscles and viscera in response to stressful or aversive stimuli (Yamada & Nabeshima, Citation1995). Furthermore, via connections with hypothalamic nuclei and the ventrolateral medulla, activation of the periaqueductal grey can also have cardiovascular and respiratory effects that mimic those of the parasympathetic system, slowing heart rate and reducing blood pressure and ventilation (Keay et al., Citation1997; Workman & Lumb, Citation1997). Strenuous physical activity, in the form of forced swimming (e.g. Mogil, Sternberg, Balian, Liebeskind, & Sadowski, Citation1996) and forced walking (e.g. Nakagawasai et al., Citation1999), has been found to induce stress-induced analgesia, and artificial selection experiments have shown that this phenomenon has a genetic basis (Panocka, Marek, & Sadowski, Citation1986).
Interestingly, a hypothesis linking the exercise-induced brain opioid response to the dampening of cardiovascular and respiratory responses, and thus to reduced perceptions of exertion and discomfort, was proposed several years ago by Hatfield and Landers (Citation1987): “It appears that endorphin levels may reduce the sense of physical effort and discomfort associated with [perceived exertion] and thereby promote a psychological effect” (p. 361). After a series of failed attempts to demonstrate that opioids could significantly influence exercise tolerance and perceived exertion, Sgherza et al. (Citation2002) recently showed the administration of the opioid receptor blocker naloxone led to reduced exercise capacity and higher ratings of perceived exertion than placebo. Although post-exercise assessments of affective states were not conducted, this finding is consistent with the presence of an opponent process during exercise, which was attenuated by opioid blockade.
If opioids, in fact, drive the b-process, then what drives the a-process? In the case of exercise, the primary factor underlying the a-process appears to be metabolic acidosis (Taylor et al., Citation1994). Especially important for the perspective of the present conceptual model is the fact that, consistent with Solomon's speculation that a “critical intensity” of the a-process must be reached for a b-process to be elicited, Sgherza et al. (Citation2002) observed that the threshold for the release of endogenous opioids coincided with the lactate and ventilatory thresholds (p. 2025). In other words, it appears that, for an opioid-mediated opponent-process to be elicited, the intensity of physical activity must be within the heavy or severe domains.
The adaptive significance of stress-induced analgesia has been discussed by several authors (Amit & Galina, Citation1986; Gillman & Katzeff, Citation1988; Harris, Citation1996). In line with Solomon's position on the need to suppress departures from the affective equilibrium, Amit and Galina (Citation1986) wrote that “to act appropriately and perhaps survive after exposure to severe stress, the [individual] must focus [his or her] attention on escaping the threat to [his or her] integrity and not on dealing with the pain” (p. 1110). The affective opponent-process in the context of intense physical activity might play a similar role, allowing the rapid return of attention to pressing matters of survival, such as developing an alternative strategy for dealing with a predator or trapping prey, and thus discontinuing the taxing activity. If we accept that the rebound from affective negativity to positivity that has been observed during the minutes following the termination of heavy or severe physical activity is under selection pressure, it follows that it should be consistent across individuals, showing little or no qualitative variation.
An alternative model of dose – response: Basic postulates
Based on the fundamental assumptions and the adaptational significance of the three-domain typology of physical activity intensity described in the previous sub-sections, we now summarize the basic postulates of an alternative model of the dose – response relationship between the intensity of physical activity and affective responses (see ). First, during activity performed within the moderate domain of intensity, affective responses are expected to be primarily positive, with a relatively low inter-individual variability. The reason is that activity within this range is believed to be consistently adaptive, as it poses no substantial threat to homeostasis or the maintenance of a physiological steady state, it draws from a vast reservoir of energy resources and it provides the primary means of promoting Darwinian fitness within the human Environment of Evolutionary Adaptedness.
Figure 2. An alternative dose – response model based on the three-domain typology of physical activity intensity. Within the moderate domain of intensity, there is a trend towards homogeneously positive affective changes. At intensities that approximate the heavy domain, variability emerges, with some individuals reporting changes towards pleasure and some reporting changes towards displeasure. Within the severe domain of intensity, the affective changes tend to be homogeneously negative. See text for definitions of the three intensity domains.
Second, during activity performed within the domain of heavy intensity (i.e. approximating the transition from aerobic to anaerobic metabolism), affective responses are expected to exhibit marked inter-individual variability, such that some individuals may report changes towards pleasure, whereas others may report changes towards displeasure. The reason is that physical activity performed within this range probably entails neither definite benefit nor definite danger but, instead, presents a trade-off between potential benefits and potential risks. Furthermore, at this level of intensity, affective responses are expected to depend heavily on individual-difference factors, such as cognitive parameters (e.g. self-efficacy) and personality dimensions (e.g. somatosensory modulation).
Third, during activity performed within the domain of severe intensity, affective responses are expected to be negative in most individuals. The reason is that physical activity at this level of intensity poses a substantial threat to survival (e.g. cardiovascular complications, risk of injury, suppression of immune function) and can only be continued for a relatively brief period of time, as it relies on a small reservoir of energy resources.
Finally, after heavy or severe activity, the predominant response is expected to be a robust rebound towards pleasure. The reason, according to Solomon (Citation1991), is because of the adaptational benefit associated with the removal or termination of a noxious or aversive stimulus and the return to affective equilibrium.
Supporting evidence: A reanalysis of data
To test the aforementioned postulates, the data from a series of published investigations on the affective changes associated with bouts of physical activity of various levels of intensity were reanalysed. Specifically, two sets of studies were examined: one involving bouts of activity of increasing intensity performed by different groups of individuals (between-participant comparisons) and one involving bouts of activity of increasing intensity performed by the same individuals (within-participant comparisons). The participants in all of these studies were young (average age 20 – 25 years), healthy and mostly physically active volunteers. Except for the first study mentioned below, all testing was done individually in a laboratory. For further methodological details, the readers are referred to the original sources.
All the data reported were collected using the Feeling Scale (Hardy & Rejeski, Citation1989), an 11-point, single-item measure of pleasure – displeasure. The scale ranges from − 5 to + 5. Anchors are provided at 0 (“neutral”) and at all odd integers, ranging from “very good” ( + 5) to “very bad” (−5). The analyses focus on the percentages of participants that reported an improvement, a decline, or no change in affective valence (pleasure – displeasure).
In the between-participant set, changes in valence were examined from baseline to immediately post-exercise. The set included: (a) a 10-min bout of self-paced walking performed outdoors at approximately 15% of heart rate reserve (data from Study I of Ekkekakis et al., Citation2000); (b) a 15-min bout of self-paced walking performed on a treadmill at approximately 22% of heart rate reserve (data from Study III of Ekkekakis et al., Citation2000); (c) a 30-min bout of cycle ergometry at 60% of estimated maximal aerobic capacity (data from Van Landuyt et al., Citation2000); (d) an incremental treadmill test performed until volitional exhaustion (data from Hall et al., Citation2002); and (e) a bout of cycle ergometry performed at 75% [Vdot]O2max until exhaustion under conditions of dehydration in the heat (data from Ekkekakis et al., Citation1997). Although these studies did not include assessments of the lactate or ventilatory thresholds, given the nature of the tests and the recorded intensities, we assumed that sessions (a) and (b) represented physical activity near the low to mid-point of the moderate domain, session (c) represented activity at an intensity approximating the heavy domain, since the lactate and ventilatory thresholds have been found to occur at approximately 60% [Vdot]O2max during cycle ergometry in samples similar to the one used in our study (e.g. Sgherza et al., Citation2002), and sessions (d) and (e) represented activity in the severe domain, as both were terminated due to exhaustion. Based on our model, we expected to find trends towards homogeneously positive changes in sessions (a) and (b), variable changes in session (c) and trends toward homogeneously negative changes in sessions (d) and (e).
The within-participant set included two studies. In the first study, we compared affective changes across the phases of an incremental treadmill test performed until volitional exhaustion (data from Hall et al., Citation2002). Specifically, we examined: (a) changes from baseline to the early part of the test (after a 3-min warm-up walk at 4.8 km · h−1 and 2 min of running at 8 km · h−1); (b) changes from the second minute of running to 1 min after the participants exceeded their ventilatory threshold; (c) changes from 1 min after the ventilatory threshold to the fatigue-induced termination of the test; (d) changes over the first minute of a cool-down walk at 4.8 km · h−1; (e) changes over the second minute of a cool-down walk at the same pace; and (f) changes over a 20-min seated recovery. In this case, we assumed that phase (a) represented activity near the mid to high end of the moderate domain, phase (b) represented activity at an intensity approximating the entry into the heavy domain, phase (c) represented activity in the severe domain, phase (d) represented the immediate post-activity phase, and phases (e) and (f) represented the gradual return to baseline. Based on our model, we expected to find some positive changes but also the first emerging signs of variability in phase (a), variable responses in phase (b), a trend towards homogeneously negative responses in phase (c), an immediate and homogeneous affective rebound from negativity to positivity in phase (d), and the gradual re-emergence of variability in phases (e) and (f).
In the second study, we compared changes during and following 15-min runs at three intensities proximal to the ventilatory threshold: (a) one at 20% [Vdot]O2max below the ventilatory threshold; (b) one at the ventilatory threshold; and (c) one at 10% [Vdot]O2max above the ventilatory threshold (data from Ekkekakis, Hall, & Petruzzello, unpublished). Specifically, we examined affective changes over: (i) 5-min warm-up walks at 4.8 km · h−1, (ii) the first 6 min of the runs, (iii) the last 6 min of the runs, (iv) the entire 15-min duration of the runs, (v) 5-min cool-down walks at 4.8 km · h−1 and (vi) 20-min seated recovery. Given the intensities involved and the fact that there was upward drift in physiological parameters (heart rate and oxygen uptake) during the runs, we assumed that run (a) represented the transition from the high end of the moderate domain to the low end of the heavy domain, run (b) represented the transition from the heavy to the severe domain, and run (c) represented activity in the low to mid point of the severe domain (i.e. without reaching exhaustion, since none of the participants stopped prematurely). Based on our model, we expected to find the development of some variability during run (a), the emergence of trends towards homogeneously negative changes during runs (b) and (c), rebounds from negativity to positivity during the cool-downs following all three runs, but greater rebounds with higher intensities and the re-emergence of some variability during recovery.
The results are generally consistent with the hypotheses. In the between-participant set (see ), the 10-min and 15-min walks, which were presumably in the moderate domain, led to improvements in valence in 77% and 74% of participants, respectively. Importantly, most of the remaining participants reported no change and very few (only two in one study) reported minor declines. On the other hand, the two exhaustive exercise stimuli, which were presumably in the severe domain, led to declines in valence in 90% and 100% of the participants, respectively. The 30-min bout of cycling at 60% [Vdot]O2max, the intensity of which presumably approximated the heavy domain, despite a negligible overall average change (mean ± s = 0.05 ± 2.00), led to the most variable pattern of responses at the individual level, with 48% of the participants reporting improvements and 35% reporting declines.
Table I. Percentages of participants reporting an improvement, no change, or a decline in affective valence as measured by the Feeling Scale in five studies
In the first within-participant study (see ), we examined changes over much shorter periods of time, in response to increases in exercise intensity that occurred every 2 min. In response to the 3-min warm-up walk and the first 2 min of running, 55% of the participants reported improvements in valence and 17% reported declines. Although this was not a homogenous response, like the ones we found in the previous set with longer bouts (10 and 15 min) of just walking, we again found that the majority of participants reported improvements. The fact that fewer participants reported improvements and more reported declines compared with the bouts of walking may be attributed to both the shorter duration and the higher intensity of this study. In other words, it is possible that (a) a minimum period of time of activity within the moderate domain is necessary to induce a significant positive change and (b) running (in this case, at 8 km · h−1), although still within the moderate domain of intensity, may initiate a transition or approach to the heavy domain, and thus elicit some variability. During the period of time from the second minute of running to 1 min after the ventilatory threshold, presumably corresponding to the heavy domain of intensity, we found variability (21% reporting improvements, 41% reporting no change and 38% reporting declines), but also a growing trend towards displeasure. This trend was transformed into a homogeneous (90%) change towards displeasure as the participants entered the severe domain (i.e. from 1 min after the ventilatory threshold and until volitional exhaustion). Consistent with an affective opponent process, within only 1 min of completing the test, 97% of the participants reported improvements. Finally, variability gradually re-emerged as the recovery progressed.
Table II. Percentages of participants reporting an improvement, no change, or a decline in affective valence as measured by the Feeling Scale across the phases of an incremental treadmill protocol
In the second within-participant study (see ), we examined changes during three runs, all at intensities proximal to the ventilatory threshold (one just 20% [Vdot]O2max below, one at and one just 10% [Vdot]O2max above it). In this case, we did not expect clear patterns of homogeneity, as the three intensities were closely spaced (with only 20 beats · min−1 separating the average heart rate at the end of the lowest- and highest-intensity runs) and we did not have a stimulus at the early part of the moderate domain or the late part of the severe domain. Instead, we aimed to observe the shifting patterns of variability in response to the transition from aerobic metabolism to anaerobic supplementation. In the two runs that depended on substantial anaerobic contributions, namely the runs at and above the ventilatory threshold, we found that 76.7% and 80.0% of the participants, respectively, reported declines in affective valence. By comparison, in response to the run that started at an intensity slightly below the ventilatory threshold, the pattern was more variable, with 43.3% reporting declines, 50.0% reporting no change and 6.7% reporting improvements. In the same study, the changes in valence during the 5-min cool-down walks were increasingly less variable with higher intensities, with 56.7%, 66.7% and 86.7% of participants reporting improvements after the runs below, at and above the ventilatory threshold, respectively. The correlations between the changes in affective valence during the runs and those during the cool-downs were all highly significant (−0.83, − 0.82 and − 0.82), suggesting a possible functional link.
Table III. Percentages of participants reporting an improvement, no change, or a decline in affective valence as measured by the Feeling Scale during 15-min runs below the ventilatory threshold ( < VT), at the ventilatory threshold (@VT) and above the ventilatory threshold ( > VT) (n = 30)
These data enable us to issue some important caveats and make refinements to the model. First, some deviations from perfect homogeneity are to be expected given the role of powerful individual differences as well as response sets (e.g. self-presentational bias or response carry-overs in assessment protocols involving frequent sampling). Second, there are transition zones between the three intensity domains rather than immediate pattern shifts from homogeneity to variability and vice versa. Thus, some variability in affective responses may begin to develop near the high end of the moderate domain and a trend towards homogeneous displeasure may begin to appear within the heavy domain. Third, there are some indications of what Cacioppo, Gardner and Berntson (Citation1997) named a “negativity bias”, namely a stronger propensity towards displeasure than pleasure. This is supported by two observations: (a) the more homogeneous and larger-magnitude displeasure responses in the severe domain than the pleasure responses in the moderate domain, and (b) the higher percentage of participants reporting displeasure when the overall response pattern was variable (i.e. in the high end of the moderate domain and low end of the heavy domain). From an adaptational standpoint, this negativity bias can be explained by the fact that, in most cases, displeasure (and withdrawal) have a stronger and more direct connection to survival than pleasure (and approach).
Recapitulation and conclusions
In this paper, we propose a new conceptualization of the dose – response relationship between the intensity of physical activity and affective responses, as an alternative to traditional models postulating a single, global dose – response curve (e.g. an inverted-U). The proposal is based on a series of assumptions drawing primarily from the knowledge bases of evolutionary psychology and biology:
| a. | physical activity is an integral part of the human Environment of Evolutionary Adaptedness, the context in which human behaviour evolved; | ||||
| b. | affective responses to physical activity are the manifestations of psychological mechanisms that evolved due to their ability to provide effective solutions to recurrent adaptational problems, with pleasure signifying utility and displeasure signifying danger; | ||||
| c. | affective responses depend on a hierarchically organized control structure, with oligosynaptic, inflexible and obligatory responses at the bottom and complex, flexible and highly individualized, cortically mediated responses at the top; and | ||||
| d. | homogeneity of responses reflects adaptational significance and control by evolutionarily older mechanisms, whereas variability reflects lack of consistent adaptational significance and control by evolutionarily recent mechanisms. | ||||
This framework places the issue of the dose – response relationship between the intensity of physical activity and affective responses under a new light. The cornerstone of the new approach is the following question: What are the issues of adaptational significance associated with physical activity performed within the three domains of intensity (i.e. moderate, heavy and severe)? Based on a review of diverse literatures, we suggested that (a) physical activity within the moderate domain of intensity is consistently adaptive, (b) physical activity within the heavy domain of intensity is neither consistently adaptive nor consistently maladaptive, as it entails both potential benefits and potential risks, and (c) physical activity within the severe domain of intensity is consistently maladaptive. Consequently, the alternative dose – response formulation presented here consists of the following postulates. First, a trend towards homogeneous responses of pleasure is expected when physical activity is performed within the moderate domain of intensity. Second, variability of responses, with some individuals reporting changes towards pleasure and some reporting changes towards displeasure, is expected when the intensity of physical activity approximates the heavy domain. Third, a trend towards homogeneous responses of displeasure is expected when physical activity is performed within the severe domain of intensity. Finally, during the period immediately after intense activity that brings about changes towards displeasure, a rapid, homogeneous reversal from displeasure to pleasure is expected (but, as recovery from exercise progresses, variability similar to that at baseline should gradually re-emerge).
We submit that a model that (a) considers the intensity of physical activity in terms of domains with distinct metabolic significance rather than arbitrarily selected percentages of maximal capacity and (b) acknowledges and incorporates the element of inter-individual variability holds more promise than traditional (i.e. single-curve, global) dose – response models. Nevertheless, the supporting data presented here are preliminary and, as such, are limited in scope and merely descriptive. Future research should examine the reliability of the reported patterns across diverse populations, including different cultural groups (to establish that the postulated patterns constitute human universals). Importantly, extensive work remains to be done on elucidating the mechanisms underlying the shifting trends between homogeneity and variability. Our initial hypothesis is that homogeneity reflects primarily subcortical mechanisms of affect generation, and thus the relative absence of cognitive mediation. Conversely, variability is hypothesized to reflect primarily cortical mechanisms, and thus the strong influence of cognitive factors (see a review of relevant data in Ekkekakis, Citation2003).
From a practical, public health standpoint, the model suggests that a revision of the current exercise prescription practices may be in order. A prescribed intensity based on an arbitrary percentage of maximal capacity may result in an individual exercising in the heavy or severe domains, with negative affective and motivational consequences. Instead, more effort should be directed at understanding the self-selection of physical activity intensity. Some evidence suggests that individuals will intuitively adjust their pace to optimize pleasure (Cabanac, Citation1985, Citation1986; Cabanac & LeBlanc, Citation1983). If the intensity is prescribed, the decision regarding whether higher intensity (e.g. within the heavy domain) should be applied should depend on assessments of individual differences (e.g. in self-efficacy or in preference for, and tolerance of, such intensities and the associated somatosensory cues). Consequently, the present limited knowledge base on the role of individual-difference variables that account for the variability in affective responses must be expanded. Finally, practitioners should note that, although here we have assumed that the shifts in the patterns of variability are prompted mainly by the cardiorespiratory cues associated with exercise within the three domains of intensity, these may not be the determining factors in individuals suffering from activity-limiting conditions (e.g. knee osteoarthritis, asthma, angina, COPD, intermittent claudication). In such cases, it will be symptom thresholds specific to these conditions that will be of primary importance.
Acknowledgements
We wish to thank Professor Michel Cabanac (Département de Physiologie, Faculté de Médecine, Université Laval, Québec, Canada) for his pioneering insights into the adaptational bases of the affective responses to physical activity, his encouragement, and his numerous suggestions on two earlier drafts of this manuscript. We also wish to thank Dr Elizabeth Queathem (Department of Biology, Grinnell College), Dr Joey C. Eisenmann (Department of Health and Human Performance, Iowa State University), the psychology section editor Professor Nanette Mutrie and the three anonymous reviewers for their critical and very constructive comments.
References
References
- Acevedo , EO , Kraemer , RR , Haltom , RW and Tryniecki , JL . 2003 . Perceptual responses proximal to the onset of blood lactate accumulation . Journal of Sports Medicine and Physical Fitness , 43 : 267 – 273 .
- Acevedo , EO , Rinehardt , KF and Kraemer , RR . 1994 . Perceived exertion and affect at varying intensities of running . Research Quarterly for Exercise and Sport , 65 : 372 – 376 .
- Albert , CM , Mittleman , MA , Chae , CU , Lee , IM , Hennekens , CH and Manson , JE . 2000 . Triggering of sudden death from cardiac causes by vigorous exertion . New England Journal of Medicine , 343 : 1355 – 1361 .
- American College of Sports Medicine 1995 ACSM's guidelines for exercise testing and prescription (5th edn.), Baltimore, MD: Williams & Wilkins
- American College of Sports Medicine 2000 ACSM's guidelines for exercise testing and prescription (6th edn.), Philadelphia, PA: Lippincott, Williams & Wilkins
- Amit , Z and Galina , ZH . 1986 . Stress-induced analgesia: Adaptive pain suppression . Physiological Reviews , 66 : 1091 – 1120 .
- Arnold , SJ . 1983 . Morphology, performance, and fitness . American Zoologist , 23 : 347 – 361 .
- Åstrand PO 1986 Why exercise? An evolutionary approach. Acta Medica Scandinavica Supplementum 711 241 242
- Åstrand , PO . 1992a . Why exercise? . Medicine and Science in Sports and Exercise , 24 : 153 – 162 .
- Åstrand , PO . 1992b . Physical activity and fitness . American Journal of Clinical Nutrition , 55 : 1231S – 1236S .
- Åstrand PO 1994 Physical activity and fitness: Evolutionary perspective and trends for the future In C. Bouchard, R. J. Shephard, & T. Stephens (Eds.), Physical activity, fitness, and health: International proceedings and consensus statement (pp. 98 – 105) Champaign, IL: Human Kinetics
- Bacon , CG , Mittleman , MA , Kawachi , I , Giovannucci , E , Glasser , DB and Rimm , EB . 2003 . Sexual function in men older than 50 years of age: Results from the health professionals follow-up study . Annals of Internal Medicine , 139 : 161 – 168 .
- Balady , GJ . 2002 . Survival of the fittest: More evidence . New England Journal of Medicine , 346 : 852 – 854 .
- Bartley , SH . 1970 . The homeostatic and comfort perceptual systems . Journal of Psychology , 75 : 157 – 162 .
- Beatty , WW and Costello , KB . 1982 . Naloxone and play fighting in juvenile rats . Pharmacology, Biochemistry and Behavior , 17 : 905 – 907 .
- Bekoff , M . 1988 . Motor training and physical fitness: Possible short- and long-term influences on the development of individual differences in behavior . Development Psychobiology , 21 : 601 – 612 .
- Belke , TW . 1997 . Running and responding reinforced by the opportunity to run: Effect of reinforcer duration . Journal of the Experimental Analysis of Behavior , 67 : 337 – 351 .
- Bennett AF 1987 Interindividual variability: An underutilized resource In M. E. Feder, A. F. Bennett, W. W. Burggren, & R. B. Huey (Eds.), New directions in ecological physiology (pp. 147 – 169) New York: Cambridge University Press
- Bennett , AF . 1991 . The evolution of activity capacity . Journal of Experimental Biology , 160 : 1 – 23 .
- Bennett AF Huey RB 1990 Studying the evolution of physiological performance In D. Futuyma & J. Antonovics (Eds.), Oxford surveys in evolutionary biology 7 pp. 251 – 284 New York: Oxford University Press
- Bennett , AF and Ruben , JA . 1979 . Endothermy and activity in vertebrates . Science , 206 : 649 – 654 .
- Berger , BG and Motl , RW . 2000 . Exercise and mood: A selective review and synthesis of research employing the Profile of Mood States . Journal of Applied Sport Psychology , 12 : 69 – 92 .
- Berntson , GG , Boysen , ST and Cacioppo , JT . 1993 . Neurobehavioral organization and the cardinal principle of evaluative bivalence . Annals of the New York Academy of Sciences , 702 : 75 – 102 .
- Berntson , GG and Cacioppo , JT . 2000 . Psychobiology and social psychology: Past, present, and future . Personality and Social Psychology Review , 4 : 3 – 15 .
- Beunen , G and Thomis , M . 1999 . Genetic determinants of sports participation and daily physical activity . International Journal of Obesity , 23 ( suppl. 3 ) : S55 – S63 .
- Biddle SJH Mutrie N 2001 Psychology of physical activity: Determinants, well-being and interventions London: Routledge
- Bixby , WR , Spalding , TW and Hatfield , BD . 2001 . Temporal dynamics and dimensional specificity of the affective response to exercise of varying intensity: Differing pathways to a common outcome . Journal of Sport and Exercise Psychology , 23 : 171 – 190 .
- Boer , DP , Epling , WF , Pierce , WD and Russell , JC . 1990 . Suppression of food deprivation-induced high-rate wheel running in rats . Physiology and Behavior , 48 : 339 – 342 .
- Booth , FW , Chakravarthy , MV , Gordon , SE and Spangenburg , EE . 2002 . Waging war on physical inactivity: Using modern molecular ammunition against an ancient enemy . Journal of Applied Physiology , 93 : 3 – 30 .
- Bortz , WM . 1985 . Physical exercise as an evolutionary force . Journal of Human Evolution , 14 : 145 – 155 .
- Boucher , CA , Anderson , MD , Schneider , MS , Murphy , JH , Okada , RD and Kanarek , DJ . 1985 . Left ventricular function before and after reaching the anaerobic threshold . Chest , 87 : 145 – 150 .
- Bouffard , M . 1993 . The perils of averaging data in adapted physical activity research . Adapted Physical Activity Quarterly , 10 : 371 – 391 .
- Boyd , AE , Giamber , SR , Mager , M and Lebovitz , HE . 1974 . Lactate inhibition of lypolysis in exercising man . Metabolism , 23 : 531 – 542 .
- Brownlee , A . 1954 . Play in domestic cattle in Britain: An analysis of its nature . British Veterinary Journal , 110 : 48 – 68 .
- Burghardt GM 1984 On the origins of play In P.K. Smith (Ed.), Play in animals and humans (pp. 5 – 41) New York: Blackwell
- Burghardt GM 1998 The evolutionary origins of play revisited: Lessons from turtles In M. Bekoff & J. A. Byers (Eds.), Animal play: Evolutionary, comparative, and ecological perspectives (pp. 1 – 26) New York: Cambridge University Press
- Burnett , RE , Blumenthal , JA , Mark , DB , Leimberger , JD and Califf , RM . 1995 . Distinguishing between early and late responders to symptoms of acute myocardial infarction . American Journal of Cardiology , 75 : 1019 – 1022 .
- Byers JA 1984 Play in ungulates In P. K. Smith (Ed.), Play in animals and humans (pp. 43 – 65) New York: Blackwell
- Byers , JA and Walker , C . 1995 . Refining the motor training hypothesis for the evolution of play . American Naturalist , 146 : 25 – 40 .
- Cabanac , M . 1971 . Physiological role of pleasure . Science , 173 : 1103 – 1107 .
- Cabanac , M . 1979 . Sensory pleasure . Quarterly Review of Biology , 54 : 1 – 29 .
- Cabanac M 1985 Optimisation du comportement par la minimisation du déplaisir dans un espace sensoriel à deux dimensions Comptes Rendus de l'Académie des Sciences, Série III (Sciences de la Vie) 301 607 610
- Cabanac , M . 1986 . Performance and perception at various combinations of treadmill speed and slope . Physiology and Behavior , 38 : 839 – 843 .
- Cabanac M 1995 What is sensation? In R. Wong (Ed.), Biological perspectives on motivated activities (pp. 399 – 418) Norwood, NJ: Ablex
- Cabanac , M and LeBlanc , J . 1983 . Physiological conflict in humans: Fatigue vs. cold discomfort . American Journal of Physiology , 244 : R621 – R628 .
- Cacioppo JT Berntson GG Klein DJ 1992 What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral “illusions” Review of Personality and Social Psychology 14 63 98
- Cacioppo , JT , Gardner , WL and Berntson , GG . 1997 . Beyond bipolar conceptualizations and measures: The case of attitudes and evaluative space . Personality and Social Psychology Review , 1 : 3 – 25 .
- Cadroy , Y , Pillard , F , Sakariassen , KS , Thalamas , C , Boneu , B and Riviere , D . 2002 . Strenuous but not moderate exercise increases the thrombotic tendency in healthy sedentary male volunteers . Journal of Applied Physiology , 93 : 829 – 833 .
- Calcagnetti , DJ and Schechter , MD . 1992 . Place conditioning reveals the rewarding aspect of social interaction in juvenile rats . Physiology and Behavior , 51 : 667 – 672 .
- Carrier , DR . 1984 . The energetic paradox of human running and hominid evolution . Current Anthropology , 25 : 483 – 495 .
- Chambliss , HO , Van Hoomissen , JD , Holmes , PV , Bunnell , BN and Dishman , RK . 2004 . Effects of chronic activity wheel running and imipramine of masculine copulatory behavior after olfactory bulbectomy . Physiology and Behavior , 82 : 593 – 600 .
- Clobert , J , Oppliger , A , Sorci , G , Ernande , B , Swallow , JG and Garland , T . 2000 . Trade-offs in phenotypic traits: Endurance at birth, growth, survival, predation and susceptibility to parasitism in a lizard, Lacerta vivipara . Functional Ecology , 14 : 675 – 684 .
- Conley , KE , Kemper , WF and Crowther , GJ . 2001 . Limits to sustainable muscle performance: Interaction between glycolysis and oxidative phosphorylation . Journal of Experimental Biology , 204 : 3189 – 3194 .
- Cordain , L , Gotshall , RW and Eaton , SB . 1997 . Evolutionary aspects of exercise . World Review of Nutrition and Dietetics , 81 : 49 – 60 .
- Cordain , L , Gotshall , RW and Eaton , SB . 1998 . Physical activity, energy expenditure and fitness: An evolutionary perspective . International Journal of Sports Medicine , 19 : 328 – 335 .
- Cosgrove , KP , Hunter , RG and Carroll , ME . 2002 . Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences . Pharmacology, Biochemistry and Behavior , 73 : 663 – 671 .
- Cox , KL , Burke , V , Gorely , TJ , Beilin , LJ and Puddey , IB . 2003 . Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40 – 65 years: The S.W.E.A.T. study (Sedentary Women Exercise Adherence Trial) . Preventive Medicine , 36 : 17 – 29 .
- Craig , AD . 1996 . An ascending general homeostatic afferent pathway originating in lamina I . Progress in Brain Research , 107 : 225 – 242 .
- Craig AD 2002 How do you feel? Interoception: The sense of the physiological condition of the body Nature Reviews Neuroscience 3 655 666
- Craig , AD . 2003 . Interoception: The sense of the physiological condition of the body . Current Opinion in Neurobiology , 13 : 500 – 505 .
- Damasio , AR . 1995 . Toward a neurobiology of emotion and feeling: Operational concepts and hypotheses . Neuroscientist , 1 : 19 – 25 .
- Damasio AR 1999 The feeling of what happens: Body and emotion in the making of consciousness New York: Harcourt Brace
- Damasio AR 2000 A neurobiology for consciousness In T. Metzinger (Ed.), Neural correlates of consciousness: Empirical and conceptual questions (pp. 111 – 120) Cambridge, MA: MIT Press
- den Dulk , P , Heerebout , BT and Phaf , RH . 2003 . A computational study into the evolution of dual-route dynamics for affective processing . Journal of Cognitive Neuroscience , 15 : 194 – 208 .
- Derby , CA , Mohr , BA , Goldstein , I , Feldman , HA , Johannes , CB and McKinlay , JB . 2000 . Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? . Urology , 56 : 302 – 306 .
- DeSouza , MJ , Arce , JC , Pescatello , LS , Scherzer , HS and Luciano , AA . 1994 . Gonadal hormones and semen quality in male runners: A volume threshold of endurance training . International Journal of Sports Medicine , 15 : 383 – 391 .
- Dwyer , J and Bybee , R . 1983 . Heart rate indices of the anaerobic threshold . Medicine and Science in Sports and Exercise , 15 : 72 – 76 .
- Eaton SB Shostak M Konner M 1988 The paleolithic prescription: A program of diet and exercise and a design for living New York: Harper & Row
- Edwards , EB and Gleeson , TT . 2001 . Can energetic expenditure be minimized by performing activity intermittently? . Journal of Experimental Biology , 204 : 599 – 605 .
- Eikelboom , R . 1999 . Human parallel to voluntary wheel running: Exercise . Animal Behaviour , 57 : F11 – F12 .
- Ekkekakis , P . 2003 . Pleasure and displeasure from the body: Perspectives from exercise . Cognition and Emotion , 17 : 213 – 239 .
- Ekkekakis , P , Hall , EE , Van Landuyt , LM and Petruzzello , SJ . 2000 . Walking in (affective) circles: Can short walks enhance affect? . Journal of Behavioral Medicine , 23 : 245 – 275 .
- Ekkekakis , P , Hall , EE and Petruzzello , SJ . 2004 . Practical markers of the transition from aerobic to anaerobic metabolism during exercise: Rationale and a case for affect-based exercise prescription . Preventive Medicine , 38 : 149 – 159 .
- Ekkekakis P Kavouras SA Casa DJ Herrera JA Armstrong LE Maresh CM Petruzzello SJ 1997 Affective responses to a bout of exhaustive exercise in the heat in dehydrated and rehydrated states: In search of physiological correlates In R. Lidor & M. Bar-Eli (Eds.), Innovations in sport psychology: Linking theory and practice (Vol. 1, pp. 253 – 255), Netanya, Israel: International Society of Sport Psychology
- Ekkekakis , P and Petruzzello , SJ . 1999 . Acute aerobic exercise and affect: Current status, problems, and prospects regarding dose – response . Sports Medicine , 28 : 337 – 374 .
- Ekkekakis , P and Petruzzello , SJ . 2000 . Analysis of the affect measurement conundrum in exercise psychology: I. Fundamental issues . Psychology of Sport and Exercise , 1 : 71 – 88 .
- Ewart , CK , Stewart , KJ , Gillilan , RE , Keleman , MH , Valenti , SA , Manley , JD and Keleman , MD . 1986 . Usefulness of self-efficacy in predicting overexertion during programmed exercise in coronary artery disease . American Journal of Cardiology , 57 : 557 – 561 .
- Eysenck HJ 1983 A biometrical-genetical analysis of impulsive and sensation-seeking behavior In M. Zuckerman (Ed.), Biological bases of sensation seeking, impulsivity, and anxiety (pp. 1 – 27) Hillsdale, NJ: Laurence Erlbaum
- Fagen RM 1976 Exercise, play, and physical training in animals In P. P. G. Bateson & P. H. Klopfer (Eds.), Perspectives in ethology (pp. 189 – 219) New York: Plenum Press
- Fagen R 1981 Animal play behavior New York: Oxford University Press
- Favero , TG , Zable , AC , Bowman , MB , Thompson , A and Abramson , JJ . 1995 . Metabolic end products inhibit sarcoplasmic reticulum Ca2 + release and [3H]ryanodine binding . Journal of Applied Physiology , 78 : 1665 – 1672 .
- Fodor JA 1983 The modularity of mind Cambridge, MA: MIT Press
- Fulker , DW , Eysenck , SBG and Zuckerman , M . 1980 . A genetic and environmental analysis of sensation seeking . Journal of Research in Personality , 14 : 261 – 281 .
- Gaesser , GA and Poole , DC . 1996 . The slow component of oxygen uptake kinetics in humans . Exercise and Sport Sciences Reviews , 24 : 35 – 70 .
- Garland , T . 1999 . Laboratory endurance capacity predicts variation in field locomotor behaviour among lizard species . Animal Behaviour , 58 : 77 – 83 .
- Garland T Lossos JB 1994 Ecological morphology of locomotor performance in squamate reptiles In P. C. Wainwright & S. M. Reilly (Eds.), Ecological morphology: Integrative organismal biology (pp. 240 – 302) Chicago, IL: University of Chicago Press
- Garland , T , Morgan , MT , Swallow , JG , Rhodes , JS , Girard , I , Belter , JG and Carter , PA . 2002 . Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels . Evolution , 56 : 1267 – 1275 .
- Gauvin L Brawley LR 1993 Alternative psychological models and methodologies for the study of exercise and affect In P. Seraganian (Ed.), Exercise psychology: The influence of physical exercise on psychological processes (pp. 146 – 171) New York: Wiley
- Geary , DC and Huffman , KJ . 2002 . Brain and cognitive evolution: Forms of modularity and functions of mind . Psychological Bulletin , 128 : 667 – 698 .
- Gillman , MA and Katzeff , IE . 1988 . Does opioid uncoupling of adrenal responses mediate opioid anti-stress effects? . Medical Science Research , 16 : 207 – 209 .
- Girard , I , McAleer , MW , Rhodes , JS and Garland , T . 2001 . Selection for high voluntary wheel running increases speed and intermittency in house mice (Mus domesticus) . Journal of Experimental Biology , 204 : 4311 – 4320 .
- Goldsmith , SR , Iber , C , McArthur , CD and Davies , SF . 1990 . Influence of acid – base status on plasma catecholamines during exercise in normal humans . American Journal of Physiology , 258 : R1411 – R1416 .
- Gordon , NS , Kollack-Walker , S , Akil , H and Panksepp , J . 2002 . Expression of c-fos gene activation during rough and tumble play in juvenile rats . Brain Research Bulletin , 57 : 651 – 659 .
- Griffiths , PE . 1990 . Modularity and the psychoevolutionary theory of emotion . Biology and Philosophy , 5 : 175 – 196 .
- Hakim , AA , Petrovich , H , Burchfiel , CM , Ross , GW , Rodriguez , BL , White , LR , Yano , K , Curb , JD and Abbott , RD . 1998 . Effects of walking on mortality among nonsmoking retired men . New England Journal of Medicine , 338 : 94 – 99 .
- Hall , EE , Ekkekakis , P and Petruzzello , SJ . 2002 . The affective beneficence of vigorous exercise revisited . British Journal of Health Psychology , 7 : 47 – 66 .
- Hardy , CJ and Rejeski , WJ . 1989 . Not what, but how one feels: The measurement of affect during exercise . Journal of Sport and Exercise Psychology , 11 : 304 – 317 .
- Harris JA 1996 Descending antinociceptive mechanisms in the brainstem: Their role in the animal's defensive system Journal of Physiology (Paris) 90 15 25
- Haskell , WL . 1994 . Health consequences of physical activity: Understanding and challenges regarding dose – response . Medicine and Science in Sports and Exercise , 26 : 649 – 660 .
- Hatfield , BD and Landers , DM . 1987 . Psychophysiology in exercise and sport research: An overview . Exercise and Sport Sciences Reviews , 15 : 351 – 387 .
- Hayes , JP and O'Connor , CS . 1999 . Natural selection on thermogenic capacity of high-altitude deer mice . Evolution , 53 : 1280 – 1287 .
- Hill , K , Kaplan , H , Hawkes , K and Hurtado , AM . 1985 . Men's time allocation to subsistence work among the Ache of eastern Paraguay . Human Ecology , 13 : 29 – 47 .
- Hoffmann , P , Terenius , L and Thorén , P . 1990 . Cerebrospinal fluid immunoreactive β-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat . Regulatory Peptides , 28 : 233 – 239 .
- Holloszy , JO . 1993 . Exercise increases average longevity of female rats despite increased food intake and no growth retardation . Journal of Gerontology , 48 : B97 – B100 .
- Holloszy , JO and Smith , EK . 1987 . Effects of exercise on longevity of rats . Federation Proceedings , 46 : 1850 – 1853 .
- Hootman , JM , Macera , CA , Ainsworth , BE , Martin , M , Addy , CL and Blair , SN . 2001 . Association among physical activity level, cardiorespiratory fitness, and risk of musculoskeletal injury . American Journal of Epidemiology , 154 : 251 – 258 .
- Houle-Leroy , P , Garland , T , Swallow , JG and Guderley , H . 2000 . Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus . Journal of Applied Physiology , 89 : 1608 – 1616 .
- Houle-Leroy , P , Guderley , H , Swallow , JG and Garland , T . 2003 . Artificial selection for high activity favors mighty mini-muscles in house mice . American Journal of Physiology , 284 : R433 – R443 .
- Humphreys , AP and Einon , DF . 1981 . Play as a reinforcer for maze-learning in juvenile rats . Animal Behaviour , 29 : 259 – 270 .
- Hurtado , AM , Hawkes , K , Hill , K and Kaplan , H . 1985 . Female subsistence strategies among Ache hunter-gatherers of eastern Paraguay . Human Ecology , 13 : 1 – 28 .
- Irons , W . 1998 . Adaptively relevant environments versus the environment of evolutionary adaptedness . Evolutionary Anthropology , 6 : 194 – 204 .
- Irschick , DJ . 2002 . Evolutionary approaches for studying functional morphology: Examples from studies of performance capacity . Integrative and Comparative Biology , 42 : 278 – 290 .
- Irschick , DJ and Garland , T . 2001 . Integrating function and ecology in studies of adaptation: Investigations of locomotor capacity as a model system . Annual Review of Ecology and Systematics , 32 : 367 – 396 .
- Iversen , IH . 1993 . Techniques for establishing schedules with wheel running as reinforcement in rats . Journal of the Experimental Analysis of Behavior , 60 : 219 – 238 .
- Jasienska , G . 2003 . Energy metabolism and the evolution of reproductive suppression in the human female . Acta Biotheoretica , 51 : 1 – 18 .
- Jayne , BC and Bennett , AF . 1990 . Selection on locomotor performance capacity in a natural population of garter snakes . Evolution , 44 : 1204 – 1229 .
- Kanarek , RB , Marks-Kaufman , R , D'Anci , KE and Przypek , J . 1995 . Exercise attenuates oral intake of amphetamine in rats . Pharmacology, Biochemistry and Behavior , 51 : 725 – 729 .
- Kaplan , H and Hill , K . 1985 . Hunting ability and reproductive success among male Ache foragers: Preliminary results . Current Anthropology , 26 : 131 – 133 .
- Katch , V , Weltman , A , Sady , S and Freedson , P . 1978 . Validity of the relative percent concept for equating training intensity . European Journal of Applied Physiology , 39 : 219 – 227 .
- Keay , KA , Crawfoot , LJ , Floyd , NS , Henderson , LA , Christie , MJ and Bandler , R . 1997 . Cardiovascular effects of microinjections of opioid agonists into the “depressor region” of the ventrolateral periaqueductal gray region . Brain Research , 762 : 61 – 71 .
- Kirkcaldy , BC and Shephard , RJ . 1990 . Therapeutic implications of exercise . International Journal of Sport Psychology , 21 : 165 – 184 .
- Koike , A , Itoh , H , Taniguchi , K and Hiroe , M . 1989 . Detecting abnormalities in left ventricular function during exercise by respiratory measurement . Circulation , 80 : 1737 – 1746 .
- Koteja , P , Garland , T , Sax , JK , Swallow , JG and Carter , PA . 1999a . Behaviour of house mice artificially selected for high levels of voluntary wheel running . Animal Behaviour , 58 : 1307 – 1318 .
- Koteja , P , Swallow , JG , Carter , PA and Garland , T . 1999b . Energy cost of wheel running in house mice: Implications for coadaptation of locomotion and energy budgets . Physiological and Biochemical Zoology , 72 : 238 – 249 .
- Kramer , DL and McLaughlin , RL . 2001 . The behavioral ecology of intermittent locomotion . American Zoologist , 41 : 137 – 153 .
- Lauderdale , DS , Fabsitz , R , Meyer , JM , Sholinsky , P , Ramakrishnan , V and Goldberg , J . 1997 . Familial determinants of moderate and intense physical activity: A twin study . Medicine and Science in Sports and Exercise , 29 : 1062 – 1068 .
- LeDoux , JE . 1986 . Sensory systems and emotion: A model of affective processing . Integrative Psychiatry , 4 : 237 – 248 .
- Lee , JY , Jensen , BE , Oberman , A , Fletcher , GF , Fletcher , BJ and Raczynski , JM . 1996 . Adherence in the Training Levels Comparison Trial . Medicine and Science in Sports and Exercise , 28 : 47 – 52 .
- Leonard , WR and Robertson , ML . 1997 . Comparative primate energetics and hominid evolution . American Journal of Physical Anthropology , 102 : 265 – 281 .
- Leonard , WR and Ulijaszek , SJ . 2002 . Energetics and evolution: An emerging research domain . American Journal of Human Biology , 14 : 547 – 550 .
- Lett , BT , Grant , VL and Koh , MT . 2001 . Naloxone attenuates the conditioned place preference induced by wheel running in rats . Physiology and Behavior , 72 : 355 – 358 .
- Maia , JAR , Thomis , M and Beunen , G . 2002 . Genetic factors in physical activity levels: A twin study . American Journal of Preventive Medicine , 23 ( suppl. 2 ) : 87 – 91 .
- Malina RM 1991 Darwinian fitness, physical fitness and physical activity In C. G. N. Mascie-Taylor & G. W. Laster (Eds.), Applications of biological anthropology to human affairs (pp. 143 – 184) New York: Cambridge University Press
- Malina RM 1998 Physical activity, sport, social status and Darwinian fitness In S. S. Strickland & P. S. Shetty (Eds.), Human biology and social inequality (pp. 165 – 192) New York: Cambridge University Press
- Martin P Caro TM 1985 On the functions of play and its role in behavioral development In J. S. Rosenblatt, C. Beer, M. C. Busnel, & P. J. B. Slater (Eds.), Advances in the study of behavior 15 pp. 59 – 103 San Diego, CA: Academic Press
- Mather , JG . 1981 . Wheel-running activity: A new interpretation . Mammalian Review , 11 : 41 – 51 .
- McCann , DJ , Mollé , PA and Caton , JR . 1995 . Phosphocreatine kinetics in humans during exercise and recovery . Medicine and Science in Sports and Exercise , 27 : 278 – 387 .
- McMillan , DE , McClure , GYH and Hardwick , WC . 1995 . Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol . Drug and Alcohol Dependence , 40 : 1 – 7 .
- Meaney , MJ , Dodge , AM and Beatty , WW . 1981 . Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats . Physiology and Behavior , 26 : 467 – 472 .
- Meischke , H , Ho , MT , Eisenberg , MS , Schaeffer , SM and Larsen , MP . 1995 . Reasons patients with chest pain delay or do not call 911 . Annals of Emergency Medicine , 25 : 193 – 197 .
- Miller , MN and Byers , JA . 1991 . Energetic cost of locomotor play in pronghorn fawns . Animal Behaviour , 41 : 1007 – 1013 .
- Mogil , JS . 1999 . The genetic mediation of individual differences in sensitivity to pain and its inhibition . Proceedings of the National Academy of Sciences , 96 : 7744 – 7751 .
- Mogil , JS , Sternberg , WF , Balian , H , Liebeskind , JC and Sadowski , B . 1996 . Opioid and nonopioid swim stress-induced analgesia: A parametric analysis in mice . Physiology and Behavior , 59 : 123 – 132 .
- Morgan WP 1997 Methodological considerations In W. P. Morgan (Ed.), Physical activity and mental health (pp. 3 – 32) Washington, DC: Taylor & Francis
- Murphy , MR , MacLean , PD and Hamilton , SC . 1981 . Species-typical behavior of hamsters deprived from birth of the neocortex . Science , 213 : 459 – 461 .
- Myers , J , Prakash , M , Froelicher , V , Do , D , Partington , S and Atwood , JE . 2002 . Exercise capacity and mortality among men referred for exercise testing . New England Journal of Medicine , 346 : 793 – 801 .
- Nabokov P 1981 Indian running: Native American history and tradition Santa, Fe, NM: Ancient City Press
- Nakagawasai , O , Tadano , T , Tan-No , K , Niijima , F , Sakurada , S , Endo , Y and Kisara , K . 1999 . Changes in β-endorphin and stress-induced analgesia in mice after exposure to forced walking stress . Methods and Findings in Experimental and Clinical Pharmacology , 21 : 471 – 476 .
- Nesse , RM . 1990 . Evolutionary explanations of emotions . Human Nature , 1 : 261 – 289 .
- Nieman , DC . 1997 . Immune response to heavy exertion . Journal of Applied Physiology , 82 : 1385 – 1394 .
- Norgan , NG , Ferro-Luzzi , A and Durnin , JVGA . 1974 . The energy and nutrient intake and the energy expenditure of 204 New Guinean adults . Philosophical Transactions of the Royal Society of London B , 268 : 309 – 348 .
- Normansell , L and Panksepp , J . 1990 . Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats . Developmental Psychobiology , 23 : 75 – 83 .
- Ojanen , M . 1994 . Can the true effects of exercise on psychological variables be separated from placebo effects? . International Journal of Sport Psychology , 25 : 63 – 80 .
- Panksepp J 1998a Affective neuroscience: The foundations of human and animal emotions New York: Oxford University Press
- Panksepp , J . 1998b . The periconscious substrates of consciousness: Affective states and the evolutionary origins of the self . Journal of Consciousness Studies , 5 : 566 – 582 .
- Panksepp , J , Normansell , L , Cox , JF and Siviy , SM . 1994 . Effects of neonatal decortication on the social play of juvenile rats . Physiology and Behavior , 56 : 429 – 443 .
- Panksepp , J , Siviy , S and Normansell , L . 1984 . The psychobiology of play: Theoretical and methodological perspectives . Neuroscience and Biobehavioral Reviews , 8 : 465 – 492 .
- Panocka , I , Marek , P and Sadowski , B . 1986 . Inheritance of stress-induced analgesia in mice: Selective breeding study . Brain Research , 397 : 152 – 155 .
- Parfitt , G and Eston , R . 1995 . Changes in ratings of perceived exertion and psychological affect in the early stages of exercise . Perceptual and Motor Skills , 80 : 259 – 266 .
- Parfitt , G , Eston , R and Connolly , D . 1996 . Psychological affect at different ratings of perceived exertion in high- and low-active women: A study using a production protocol . Perceptual and Motor Skills , 82 : 1035 – 1042 .
- Parfitt , G , Markland , D and Holmes , C . 1994 . Responses to physical exertion in active and inactive males and females . Journal of Sport and Exercise Psychology , 16 : 178 – 186 .
- Pellegrini , AD and Smith , PK . 1998 . Physical activity play: The nature and function of a neglected aspect of play . Child Development , 69 : 577 – 598 .
- Pender , NJ , Bar-Or , O , Wilk , B and Mitchell , S . 2002 . Self-efficacy and perceived exertion of girls during exercise . Nursing Research , 51 : 86 – 91 .
- Perri , MG , Anton , SD , Durning , PE , Ketterson , TU , Sydeman , SJ , Berlant , NE , Kanasky , WF , Newton , RL , Limacher , MC and Martin , AD . 2002 . Adherence to exercise prescriptions: Effects of prescribing moderate versus higher levels of intensity and frequency . Health Psychology , 21 : 452 – 458 .
- Plaut , I . 2001 . Critical swimming speed: Its ecological relevance . Comparative Biochemistry and Physiology Part A , 131 : 41 – 50 .
- Pough , FH . 1989 . Organismal performance and Darwinian fitness: Approaches and interpretations . Physiological Zoology , 62 : 199 – 236 .
- Premack , D and Schaeffer , RW . 1963 . Some parameters affecting the distributional properties of operant-level running in rats . Journal of the Experimental Analysis of Behavior , 6 : 473 – 475 .
- Reber AS 1993 Implicit learning and tacit knowledge: An essay on the cognitive unconscious New York: Oxford University Press
- Rejeski WJ 1994 Dose – response issues from a psychosocial perspective In C. Bouchard, R. J. Shephard, & T. Stephens (Eds.), Physical activity, fitness, and health: International proceedings and consensus statement (pp. 1040 – 1055) Champaign, IL: Human Kinetics
- Rhodes , JS , Garland , T and Gammie , SC . 2003 . Patterns of brain activity associated with variation in voluntary wheel running behavior . Behavioral Neuroscience , 117 : 1243 – 1256 .
- Riley , M , Maehara , K , Pórszász , J , Engelen , MPKJ , Barstow , TJ , Tanaka , H and Wasserman , K . 1997 . Association between the anaerobic threshold and the break-point in the double product / work rate relationship . European Journal of Applied Physiology , 75 : 14 – 21 .
- Robson , MA and Miles , DB . 2000 . Locomotor performance and dominance in male tree lizards, Urosaurus ornatus . Functional Ecology , 14 : 338 – 344 .
- Rowland , TW . 1998 . The biological basis of physical activity . Medicine and Science in Sports and Exercise , 30 : 392 – 399 .
- Rudolph , DL and McAuley , E . 1996 . Self-efficacy and perceptions of effort: A reciprocal relationship . Journal of Sport and Exercise Psychology , 18 : 216 – 223 .
- Sabol , KE , Richards , JB and Freed , CR . 1990 . In vivo dialysis measurements of dopamine and DOPAC in rats trained to turn on a circular treadmill . Pharmacology, Biochemistry and Behavior , 36 : 21 – 28 .
- Saklofske , DH , Blomme , GC and Kelly , IW . 1992 . The effects of exercise and relaxation on energetic and tense arousal . Personality and Individual Differences , 13 : 623 – 625 .
- Schneirla TC 1959 An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal In M. R. Jones (Ed.), Nebraska symposium on motivation VII pp. 1 – 42 Lincoln, NE: University of Nebraska Press
- Schneirla TC 1965 Aspects of stimulation and organization in approach/withdrawal processes underlying vertebrate behavioral development In D. S. Lehrman, R. Hinde, & E. Shaw (Eds.), Advances in the study of behavior 1 pp. 1 – 71 New York: Academic Press
- Schulze G 1995 Homeostatic mechanisms may instigate and shape adaptive behaviors through the generation of hedonic states In R. Worg (Ed.), Biological perspectives on motivated activities (pp. 268 – 288) Norwood, NJ: Ablex
- Sgherza , AL , Axen , K , Fain , R , Hoffman , RS , Dunbar , CC and Haas , F . 2002 . Effect of naloxone on perceived exertion and exercise capacity during maximal cycle ergometry . Journal of Applied Physiology , 93 : 2023 – 2028 .
- Sher , L . 1998 . The endogenous euphoric reward system that reinforces physical training: A mechanism for mankind's survival . Medical Hypotheses , 51 : 449 – 450 .
- Sherwin , CM . 1998 . Voluntary wheel running: A review and novel interpretation . Animal Behaviour , 56 : 11 – 27 .
- Shinohara , M and Moritani , T . 1992 . Increase in neuromuscular activity and oxygen uptake during heavy exercise . Annals of Physiological Anthropology , 11 : 257 – 262 .
- Simonen , RL , Rankinen , T , Pérusse , L , Leon , AS , Skinner , JS , Wilmore , JH , Rao , DC and Bouchard , C . 2003 . A dopamine D2 receptor gene polymorphism and physical activity in two family studies . Physiology and Behavior , 78 : 751 – 757 .
- Sinervo , B , Miles , DB , Frankino , A , Klukowski , M and DeNardo , DF . 2000 . Testosterone, endurance, and Darwinian fitness: Natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards . Hormones and Behavior , 38 : 222 – 233 .
- Sisti , HM and Lewis , MJ . 2001 . Naloxone suppression and morphine enhancement of voluntary wheel-running activity in rats . Pharmacology, Biochemistry and Behavior , 70 : 359 – 365 .
- Siviy SM 1998 Neurobiological substrates of play behavior: Glimpses into the structure and function of mammalian playfulness In M. Bekoff & J. A. Byers (Eds.), Animal play: Evolutionary, comparative, and ecological perspectives (pp. 221 – 242) New York: Cambridge University Press
- Siviy , SM and Atrens , DM . 1992 . The energetic costs of rough-and-tumble play in the juvenile rat . Developmental Psychobiology , 25 : 137 – 148 .
- Siviy , MJ and Panksepp , J . 1985 . Dorsomedial diencephalic involvement in the juvenile play of rats . Behavioral Neuroscience , 99 : 1103 – 1113 .
- Siviy , MJ and Panksepp , J . 1987 . Juvenile play in the rat: Thalamic and brain stem involvement . Physiology and Behavior , 41 : 103 – 114 .
- Solomon , RL . 1980 . The opponent-process theory of acquired motivation: The costs of pleasure and the benefits of pain . American Psychologist , 35 : 691 – 712 .
- Solomon RL 1991 Acquired motivation and affective opponent-processes In J. Madden (Ed.), Neurobiology of learning, emotion, and affect (pp. 307 – 347) New York: Raven
- Solomon , RL and Corbit , JD . 1974 . An opponent- process theory of motivation: I. Temporal dynamics of affect . Psychological Review , 81 : 119 – 145 .
- Sorensen , MV and Leonard , WR . 2001 . Neandertal energetics and foraging efficiency . Journal of Human Evolution , 40 : 483 – 495 .
- Spriet , LL , Lindinger , MI , McKelvie , RS , Heigenhauser , GJF and Jones , NL . 1989 . Muscle glycogenolysis and H + concentration during maximal intermittent cycling . Journal of Applied Physiology , 66 : 8 – 13 .
- Swallow , JG , Garland , T , Carter , PA , Zhan , WZ and Sieck , GC . 1998 . Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus) . Journal of Applied Physiology , 84 : 69 – 76 .
- Tanaka , H , Kiyonaga , A , Terao , Y , Ide , K , Yamauchi , M , Tanaka , M and Shindo , M . 1997 . Double product response is accelerated above the blood lactate threshold . Medicine and Science in Sports and Exercise , 29 : 503 – 508 .
- Tanaka , K , Yoshimura , T , Sumida , S , Mitsuzono , R , Tanaka , S , Konishi , Y , Watanabe , H , Yamada , T and Maeda , K . 1986 . Transient responses in cardiac function below, at, and above anaerobic threshold . European Journal of Applied Physiology , 55 : 356 – 361 .
- Taylor , DV , Boyajian , JG , James , N , Woods , D , Chicz-Demet , A , Wilson , AF and Sandman , CA . 1994 . Acidosis stimulates β-endorphin release during exercise . Journal of Applied Physiology , 77 : 1913 – 1918 .
- Thayer , RE . 1987a . Problem perception, optimism, and related states as a function of time of day (diurnal rhythm) and moderate exercise: Two arousal systems in interaction . Motivation and Emotion , 11 : 19 – 36 .
- Thayer , RE . 1987b . Energy, tiredness, and tension effects of a sugar snack versus moderate exercise . Journal of Personality and Social Psychology , 52 : 119 – 125 .
- Thayer , RE , Peters , DP , Takahashi , PJ and Birkhead-Flight , AM . 1993 . Mood and behavior (smoking and sugar snacking) following moderate exercise: A partial test of self-regulation theory . Personality and Individual Differences , 14 : 97 – 104 .
- Thorburn , AW and Proietto , J . 2000 . Biological determinants of spontaneous physical activity . Obesity Reviews , 1 : 87 – 94 .
- Thorngate W 1986 The production, detection, and explanation of behavioral patterns In J. Valsiner (Ed.), The individual subject and scientific psychology (pp. 71 – 93) New York: Plenum Press
- Toates , F . 1998 . The interaction of cognitive and stimulus – response processes in the control of behavior . Neuroscience and Biobehavioral Reviews , 22 : 59 – 83 .
- Toates , F . 2002 . Application of a multilevel model of behavioural control to understanding emotion . Behavioural Processes , 60 : 99 – 114 .
- Tobach E 1970 Some guidelines to the study of the evolution and development of emotion In L. R. Aronson, E. Tobach, D. S. Lehrman, & J. S. Rosenblatt (Eds.), Development and evolution of behavior: Essays in memory of T. C. Schneirla pp. 238 – 253 San Francisco, CA: W. H. Freeman
- Tooby , J and Cosmides , L . 1990a . The past explains the present: Emotional adaptations and the structure of ancestral environments . Ethology and Sociobiology , 11 : 375 – 424 .
- Tooby , J and Cosmides , L . 1990b . On the universality of human nature and the uniqueness of the individual: The role of genetics and adaptation . Journal of Personality , 58 : 17 – 67 .
- Tuson KM Sinyor D 1993 On the affective benefits of acute aerobic exercise: Taking stock after twenty years of research In P. Seraganian (Ed.), Exercise psychology: The influence of physical exercise on psychological processes (pp. 80 – 121) New York: Wiley
- Urhausen , A , Weiler , B , Coen , B and Kindermann , W . 1994 . Plasma catecholamines during endurance exercise of different intensities as related to the individual anaerobic threshold . European Journal of Applied Physiology , 69 : 16 – 20 .
- US Department of Health and Human Services 2000 Healthy People 2010 (2nd edn. 2 Washington, DC: US Government Printing Office
- Vanderschuren , LJMJ , Niesink , RJM and Van Ree , JM . 1997 . The neurobiology of social play behavior in rats . Neuroscience and Biobehavioral Reviews , 21 : 309 – 326 .
- Van Landuyt , LM , Ekkekakis , P , Hall , EE and Petruzzello , SJ . 2000 . Throwing the mountains into the lakes: On the perils of nomothetic conceptions of the exercise – affect relationship . Journal of Sport and Exercise Psychology , 22 : 208 – 234 .
- Wasserman , K . 1978 . Breathing during exercise . New England Journal of Medicine , 298 : 780 – 785 .
- Wasserman K Hansen JE Sue DY Casaburi R Whipp BJ 1999 Principles of exercise testing and interpretation including pathophysiology and clinical applications (3rd edn.), Philadelphia, PA: Lippincott, Williams & Wilkins
- Weinstein , RB and Full , RJ . 1999 . Intermittent locomotion increases endurance in a gecko . Physiological and Biochemical Zoology , 72 : 732 – 739 .
- Wells , JG , Balke , B and Van Fossan , DD . 1957 . Lactic acid accumulation during work: A suggested standardization of work classification . Journal of Applied Physiology , 10 : 51 – 55 .
- Weltman , A , Wood , CM , Womack , CJ , Davis , SE , Blumer , JL , Alvarez , J , Sauer , K and Gaesser , GA . 1994 . Catecholamine and blood lactate responses to incremental rowing and running exercise . Journal of Applied Physiology , 76 : 1144 – 1149 .
- Werme , M , Messer , C , Olson , L , Gilden , L , Thorén , P , Nestler , EJ and Brené , S . 2002 . FosB regulates wheel running . Journal of Neuroscience , 22 : 8133 – 8138 .
- Westerterp , KR . 2001 . Pattern and intensity of physical activity: Keeping moderately active is the best way to boost total daily energy expenditure . Nature , 410 : 539
- White , JR , Case , DA , McWhirter , D and Mattison , AM . 1990 . Enhanced sexual behavior in exercising men . Archives of Sexual Behavior , 19 : 193 – 209 .
- Wilson , WM and Marsden , CA . 1995 . Extracellular dopamine in the nucleus accumbens of the rat during treadmill running . Acta Physiologica Scandinavica , 155 : 465 – 466 .
- Woakes , AJ . 1991 . Introduction . Journal of Experimental Biology , 160 : i – ii .
- Workman , BJ and Lumb , BM . 1997 . Inhibitory effects evoked from the anterior hypothalamus are selective for the nociceptive responses of dorsal horn neurons with high- and low-threshold inputs . Journal of Neurophysiology , 77 : 2831 – 2835 .
- Yamada , K and Nabeshima , T . 1995 . Stress-induced behavioral responses and multiple opioid systems in the brain . Behavioural Brain Research , 67 : 133 – 145 .
- Yeung , RR . 1996 . The acute effects of exercise on mood state . Journal of Psychosomatic Research , 40 : 123 – 141 .
- Yoo , HS , Tackett , RL , Crabbe , JB , Bunnell , BN and Dishman , RK . 2000 . Antidepressant-like effects of physical activity versus imipramine: Neonatal clomipramine model . Psychobiology , 28 : 540 – 549 .
- Zhan , WZ , Swallow , JG , Garland , T , Proctor , DN , Carter , PA and Sieck , GC . 1999 . Effects of genetic selection and voluntary activity on the medial gastrocnemius muscle in house mice . Journal of Applied Physiology , 87 : 2326 – 2333 .
- Zuckerman , M . 1990 . The psychophysiology of sensation seeking . Journal of Personality , 58 : 313 – 345 .