Abstract
High-intensity exercise training contributes to the production and accumulation of blood lactate, which is cleared by active recovery. However, there is no commonly agreed intensity or mode for clearing accumulated blood lactate. We studied clearance of accumulated blood lactate during recovery at various exercise intensities at or below the lactate threshold after high-intensity interval runs that prompted lactate accumulation. Ten males repeated 5-min running bouts at 90% of maximal oxygen uptake ([Vdot]O2max), which increased blood lactate concentration from 1.0 ± 0.1 to 3.9 ± 0.3 mmol · l−1. This was followed by recovery exercises ranging from 0 to 100% of lactate threshold. Repeated blood lactate measurements showed faster clearance of lactate during active versus passive recovery, and that the decrease in lactate was more rapid during higher (60–100% of lactate threshold) than lower (0–40% of lactate threshold) (P < 0.05) intensities. The more detailed curve and rate analyses showed that active recovery at 80–100% of lactate threshold had shorter time constants for 67% lactate clearance and higher peak clearance rates than 40% of lactate threshold or passive recovery (P < 0.05). Finally, examination of self-regulated intensities showed enhanced lactate clearance during higher versus lower intensities, further validating the intensity dependence of clearance of accumulated blood lactate. Therefore, active recovery after strenuous exercise clears accumulated blood lactate faster than passive recovery in an intensity-dependent manner. Maximum clearance occurred at active recovery close to the lactate threshold.
Introduction
Experimental (Haram et al., Citation2009; Kemi et al., Citation2005), clinical (Helgerud et al., Citation2007; Tjonna et al., Citation2008), and epidemiological (Lee, Sesso, Oguma, & Paffenbarger, Citation2003; Moholdt, Wisloff, Nilsen, & Slordahl, Citation2008) trials in both health and disease show that the beneficial effects of exercise training depend on the intensity at which the exercise training is performed, with high intensities superior to moderate-to-low intensities. Since high-intensity exercise is performed above the lactate threshold – that is, the intensity at which lactate starts to accumulate in skeletal muscle – the exercise is normally carried out in repeated bouts that are interspersed with recovery periods, as in an interval training regime. The reason for lactate accumulation is that more of the pyruvate is converted to lactate by lactate dehydrogenase, primarily as a result of changes in the intramuscular redox state, and because oxidation of the excess lactate relies on redistribution by the blood flow to other muscles and the heart and liver (Gladden, Citation2004; Wasserman, Beaver, & Whipp, Citation1986). Thus, muscle lactate is mirrored by blood lactate.
Since most of the lactate is oxidized by skeletal muscles working at a lower intensity, and since the lactate redistribution occurs via the blood flow (Gladden, Citation2004), active rather than passive recovery after lactate-accumulating exercise appears to be more effective at clearing accumulated lactate (Belcastro & Bonen, Citation1975; Boileau, Misner, Dykstra, & Spitzer, Citation1983; Bonen, Campbell, Kirby, & Belcastro, Citation1979; Hermansen & Stensvold, Citation1972). However, no commonly agreed strategy or optimal intensity of active recovery for clearing accumulated lactate has yet been identified. Previous studies have suggested active recovery intensities in the range 25–63% of maximal oxygen uptake ([Vdot]O2max) (Boileau et al., Citation1983; Bonen & Belcastro, Citation1976; Dodd, Powers, Callender, & Brooks, Citation1984; Hermansen & Stensvold, Citation1972), but these studies quantified the intensity of the active recovery to maximal aerobic capacity ([Vdot]O2max), where lactate production has a non-linear relationship to workload. Only recently have investigators related active recovery intensities to lactate threshold (Baldari, Videira, Madeira, Sergio, & Guidetti, Citation2004, Citation2005; Greenwood, Moses, Bernardino, Gaesser, & Weltman, Citation2008), which may more directly link it to the workload at which production exceeds removal. However, these studies have not studied the intensity dependence of active recovery in detail, or the temporal characteristics of lactate clearance. It also remains unclear whether active recovery should be enforced by a set exercise intensity, or whether voluntary control by the individual would be optimal (Bonen & Belcastro, Citation1976).
In the present study, after an interval run used in high-intensity interval training regimes, we determined the intensity at which blood lactate started to accumulate exponentially (lactate threshold), relating recovery intensities to this marker, and then studied the patterns of blood lactate clearance during passive and active recovery over a range of exercise intensities. We also studied the effect of self-regulated active recovery periods, by allowing the participants to control the active recovery exercise intensity.
Methods
The study was approved by the Institutional Review Board and all participants signed a consent form before inclusion in the study. Exclusion criteria were regular smoking, medication, and cardiovascular or metabolic disease or other dysfunction/disease that would impair exercise.
Participants
Ten moderately trained adult, healthy males volunteered for this study; their characteristics are presented in . Participants were asked to refrain from exhaustive exercise for 48 h and avoid food and fluids except water for 2 h before all laboratory visits.
Table I. Participant characteristics at the start of the study (n = 10)
Lactate threshold and maximal oxygen uptake
After a 10-min warm-up by treadmill running (0% grade), below the lactate threshold at 8 km · h−1, the lactate threshold was assessed by an incremental ramp test protocol. While the treadmill remained at a 0% grade, velocity was increased by 0.5 km · h−1 every 4 min until the intensity surpassed the lactate threshold, and blood lactate concentration increased exponentially. Lactate threshold was identified by the deflection point at which blood lactate concentration started to increase, as observed by plotting blood lactate concentration over intensity with algorithms developed for this purpose (Newell et al., Citation2007) and by visual curve inspection. Blood lactate concentration was measured by analysing capillary blood samples taken from finger pricks after each increment (Analox GM7 Lactate Analyser, Analox, Hammersmith, UK). At each point, two samples were averaged. The lactate analyser was calibrated by standard solutions before and after each test. Oxygen uptake ([Vdot]O2) (Servomex 4100 Gas Analyser, Servomex, Sussex, UK) and heart rate (Polar Heart Rate Monitor FS1, Kempele, Finland) were measured simultaneously throughout the protocol. On a subsequent day, [Vdot]O2max was assessed by an exhaustive treadmill running test protocol, during which intensity was increased every 2 min until volitional exhaustion. Oxygen uptake and heart rate were measured throughout the test, and [Vdot]O2max was defined as meeting at least three of the following criteria: (1) [Vdot]O2 reached a plateau despite increased intensity; (2) respiratory exchange ratio >1.15; (3) post-exercise blood lactate concentration >8 mmol · l−1; and (4) heart rate within 10 beats · min−1 of age-predicted maximum, according to published guidelines (Duncan, Howley, & Johnson, Citation1997).
Active and passive recovery trials
Each recovery trial started with a 10-min warm-up followed by a 5-min high-intensity run at 90% of [Vdot]O2max. Immediately thereafter, participants continued with the recovery bouts, at 100%, 80%, 60%, 40% or 0% (complete rest for passive recovery) of the lactate threshold, by setting the 0% grade treadmill to the velocity that corresponded to the designated intensity in relation to the speed at which lactate threshold was defined. In addition, the participants also undertook a self-regulated active recovery session, with the intensity controlled by the participant selecting the treadmill velocity. No guidelines were provided as to exercise intensity, apart from the participants being informed that they were to exercise at an intensity of their own choice. Recovery trials were separated by at least 48 h, with the order of the trials being randomized. Each trial continued until blood lactate concentration returned to resting values. Capillary blood samples obtained by finger prick were sampled for analysis of blood lactate before and after the warm-up, at the end of the 5-min high-intensity interval, and every 4 min thereafter during the active or passive recovery until return to baseline. Heart rate was also recorded at the same time points as blood lactate concentration.
Computational and statistical analysis
Lactate recordings during the recovery trials were first normalized to a relative scale such that the resting and peak blood lactate concentrations were set to a value of 0 and 1, respectively, thus standardizing the amplitude, whereupon the first derivative of the lactate clearance was computed to identify the maximal rate of clearance during each recovery session. Next, an exponential decay curve was fitted for each individual trial to assess the time constant for 67% (2/3) clearance of the accumulated blood lactate. The fitted curves were compared with the raw curves by regression analysis.
Data are presented as means ± standard errors of the mean (sx ). A repeated-measures general linear model with a Scheffépost-hoc test was used to assess differences in the repeated measurements between the active and passive recovery trials, whereas one-way analysis of variance (ANOVA) with a Scheffépost-hoc test was used to assess the time constants and maximal rates of lactate clearance between the active and passive recovery trials. Statistical significance was set at P < 0.05.
Results
Physical and physiological characteristics, including [Vdot]O2max and lactate threshold, are shown in . These measurements indicate a moderate level of fitness.
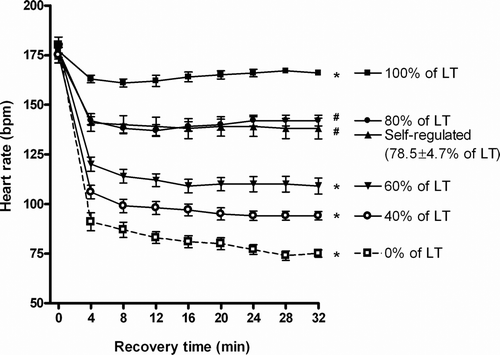
To monitor the intensity of the recovery sessions, heart rates were recorded continuously. These recordings confirm that the participants exercised at the intended recovery intensity (). For instance, the heart rates recorded during the 100% lactate threshold active recovery trial are in close agreement with the heart rates at lactate threshold, as determined from the lactate threshold test (). A closer inspection of the recordings revealed that the individual variation was linked between the 100% lactate threshold recovery trial and the lactate threshold test. Thus, those that deviated from the mean value did so on both records. The self-regulated active recovery trials were on average performed at 79 ± 5% of lactate threshold (range 55–102%). This was confirmed by comparing the running velocities and heart rates to those observed during active recovery at set intensities ().
Figure 1. Heart rates during active and passive recovery sessions. *Significantly different from other intensities (P < 0.01). #Significantly different from other intensities apart from self-regulated and 80% of lactate threshold intensities (P < 0.01).
The 5-min run at 90% of [Vdot]O2max resulted in blood lactate concentration rising from a baseline value of 1.0 ± 0.1 mmol · l−1 to 3.9 ± 0.3 mmol · l−1 (range 2.1–6.7) measured immediately after the run (). Blood lactate concentration returned to baseline values within 32 min of active or passive recovery, but more quickly after active than passive recovery. Furthermore, exercise intensities approaching lactate threshold cleared accumulated blood lactate faster than low exercise intensities, as active recovery intensities at 60–100% of lactate threshold were more effective at clearing accumulated lactate than active recovery at 40% of lactate threshold (P < 0.05) or passive recovery at 0% of lactate threshold (P < 0.01). Active recovery at 40% of lactate threshold was not different from passive recovery (P > 0.05). The measured blood lactate concentrations are shown in , whereas the normalized lactate is shown in .
Figure 2. (A) Blood lactate concentration ([La−]) at baseline (warm-up), after a 5-min run at 90% of [Vdot]O2max (exercise, 0 min on x-axis), and during active or passive recovery at exercise intensities of 0–100% of the individual lactate threshold (LT) and a self-regulated active recovery exercise intensity (79 ± 5% of lactate threshold). (B) Normalized blood lactate clearance during active and passive recovery. The baseline is marked with a dashed line. *Significantly different from passive recovery (0% of lactate threshold; P < 0.01) and 40% of lactate threshold (p < 0.05). Note that 40% of lactate threshold and passive recovery were not different from one another.
![Figure 2. (A) Blood lactate concentration ([La−]) at baseline (warm-up), after a 5-min run at 90% of [Vdot]O2max (exercise, 0 min on x-axis), and during active or passive recovery at exercise intensities of 0–100% of the individual lactate threshold (LT) and a self-regulated active recovery exercise intensity (79 ± 5% of lactate threshold). (B) Normalized blood lactate clearance during active and passive recovery. The baseline is marked with a dashed line. *Significantly different from passive recovery (0% of lactate threshold; P < 0.01) and 40% of lactate threshold (p < 0.05). Note that 40% of lactate threshold and passive recovery were not different from one another.](/cms/asset/928fe195-ed45-4217-a404-e2160971983a/rjsp_a_481721_o_f0002g.gif)
Next, we computed the first derivative of each individual lactate clearance curve (see example traces in ). This allowed us to analyse the peak rate of clearance at each of the active recovery intensities as well as with passive recovery. Active recovery at 80% and 100% of lactate threshold was equal, but occurred with a higher peak rate than during recovery intensities of 60% and 40% of lactate threshold, or passive recovery at 0% of lactate threshold. Moreover, active recovery at 60% and 40% of lactate threshold was also associated with a higher peak rate of lactate clearance than passive recovery. The self-regulated intensity, which occurred at 79 ± 4% of lactate threshold, confirmed these results ().
Figure 3. (A) Example graph of lactate clearance during active recovery after a 5-min bout of high-intensity exercise at 90% of [Vdot]O2max; the active recovery intensity in this example is 60% of the lactate threshold (LT). The first derivative of the lactate clearance curve is plotted on the same graph on the y2-axis, illustrating the peak rate of clearance during the recovery. (B) Peak rates of lactate clearance during active and passive recovery. #Significantly different from 60% and 40% of lactate threshold (P < 0.05). *Significantly different from passive recovery (0% of lactate threshold; P < 0.01). •Significantly different from passive recovery (0% of lactate threshold; P < 0.05).
![Figure 3. (A) Example graph of lactate clearance during active recovery after a 5-min bout of high-intensity exercise at 90% of [Vdot]O2max; the active recovery intensity in this example is 60% of the lactate threshold (LT). The first derivative of the lactate clearance curve is plotted on the same graph on the y2-axis, illustrating the peak rate of clearance during the recovery. (B) Peak rates of lactate clearance during active and passive recovery. #Significantly different from 60% and 40% of lactate threshold (P < 0.05). *Significantly different from passive recovery (0% of lactate threshold; P < 0.01). •Significantly different from passive recovery (0% of lactate threshold; P < 0.05).](/cms/asset/a07e1217-4d42-49fc-8b9d-1df3e28d8085/rjsp_a_481721_o_f0003g.gif)
We also fitted the exponential decay on the basis of each lactate clearance curve in order to analyse the time constant for lactate clearance (see example trace in ). First, R 2 values ranging from 0.971 to 0.995 (P < 0.01) and a coefficient of variation of 4% show a close relationship between the measured lactate clearance curves and the fitted exponential decay curves. This analysis showed that the time constants for 67% (2/3) lactate clearance were similar between active recovery intensities of 80% and 100% of lactate threshold, and that these were smaller (i.e. faster clearance of lactate) than active recovery at 60%, 40%, and passive (0%) recovery intensities. Furthermore, active recovery at 60% of lactate threshold cleared lactate faster than active recovery at 40% of lactate threshold or passive recovery, whereas there was no difference between 40% of lactate threshold and passive recovery. The self-regulated active recovery intensity again confirmed these results (), and these results parallel those of the rate analysis by the first derivatives of the lactate clearance curves ().
Figure 4. (A) Example graph of lactate clearance and its fitted exponential decay curve during active recovery after a 5-min bout of high-intensity exercise at 90% of [Vdot]O2max; the active recovery intensity in this example is 60% of the lactate threshold (LT). Inset is the correlation between the two curves and the 67% (2/3) time constant. (B) The 67% (2/3) time constants of lactate clearance during each intensity of active or passive recovery. #Significantly different from 60% of lactate threshold (P < 0.05). *Significantly different from passive recovery (0% of lactate threshold; P < 0.01). •Significantly different from passive recovery (0% of lactate threshold) and 40% of lactate threshold (P < 0.05). Note that 40% of lactate threshold and passive recovery were not different from one another.
![Figure 4. (A) Example graph of lactate clearance and its fitted exponential decay curve during active recovery after a 5-min bout of high-intensity exercise at 90% of [Vdot]O2max; the active recovery intensity in this example is 60% of the lactate threshold (LT). Inset is the correlation between the two curves and the 67% (2/3) time constant. (B) The 67% (2/3) time constants of lactate clearance during each intensity of active or passive recovery. #Significantly different from 60% of lactate threshold (P < 0.05). *Significantly different from passive recovery (0% of lactate threshold; P < 0.01). •Significantly different from passive recovery (0% of lactate threshold) and 40% of lactate threshold (P < 0.05). Note that 40% of lactate threshold and passive recovery were not different from one another.](/cms/asset/454c6714-6849-4950-8f2b-c3f489ae2060/rjsp_a_481721_o_f0004g.gif)
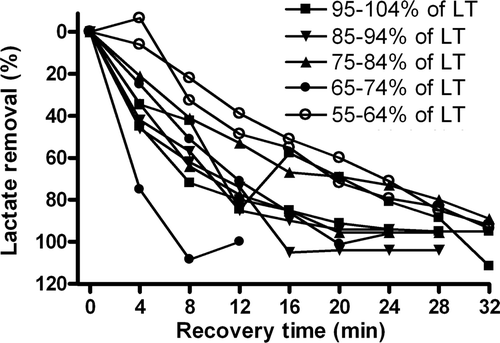
Because the self-regulated active recovery trials contained a wide intensity range (55–102% of lactate threshold), this allowed us to investigate further whether the intensity dependence of lactate clearance during active recovery was true also under these conditions. As displayed in , normalized lactate clearance during the individual self-regulated active recovery trials show a relationship that largely confirms the intensity dependence of active recovery. The active recovery trials at low intensities cleared lactate slower than trials at higher intensities. It is also noteworthy that the two highest exercise intensities displayed relatively slow lactate clearance patterns, but both were above the lactate threshold (102% and 104% of lactate threshold), which suggests that the active recovery was performed under conditions in which excess lactate was still being produced by the exercising skeletal muscles, which may slow the clearance of accumulated blood lactate.
Figure 5. Normalized individual blood lactate clearance curves during active recovery at self-recovery exercise intensities. Individual curves are displayed, but labelled into 10-percentile blocks with respect to relative intensity of the lactate threshold (LT). This resulted in each block containing two trials. Note that the lowest exercise intensities resulted in the slowest clearance of blood lactate, whereas the higher intensities tended to clear blood lactate faster.
Discussion
Our results show that the decrease in accumulated blood lactate after treadmill running at 90% of [Vdot]O2max is more effective when followed by active rather than passive recovery, and that active recovery at 80–100% of the individual lactate threshold (i.e. at or just below the lactate threshold) is more effective than active recovery at lower exercise intensities. Active recovery at 60% of lactate threshold was also more effective than at 40% of lactate threshold. Thus, blood lactate clearance during active recovery displays a dose–response relationship between lactate threshold and passive recovery, and the active recovery intensities at or close to lactate threshold are preferable for blood lactate clearance. This confirms previous studies showing that active recovery clears blood lactate faster than passive recovery, although the intensity dependence of the active recovery as a function of lactate threshold had not been established (Belcastro & Bonen, Citation1975; Boileau et al., Citation1983; Bonen & Belcastro, Citation1976; Bonen et al., Citation1979; Dodd et al., Citation1984; Gupta, Goswami, Sadkukhan, & Mathur, Citation1996; Hermansen & Stensvold, Citation1972; Mondero & Donne, Citation2000). These studies have shown that active recovery for clearing lactate may be most effective in the range 25–63% of [Vdot]O2max, where the top end of that range approaches 80–100% of lactate threshold. Critically, in contrast to our study, the previous studies quantified the intensity of the active recovery relative to [Vdot]O2max. This approach may confound the results because blood lactate accumulates non-linearly at intensities above the lactate threshold, and because lactate threshold may vary widely between individuals with respect to its relationship to [Vdot]O2max. Only a few studies have related the active recovery intensity to lactate threshold (Greenwood et al., Citation2008) or ventilatory threshold (Baldari et al., Citation2004, Citation2005), which serves as a correlate to lactate threshold. However, although these studies also indicated that active recovery depends on the exercise intensity, they did not investigate the temporal or dose–response characteristics of the intensity dependence of the active recovery.
This study also suggests that the exercise intensity to optimize lactate clearance during active recovery does not need to be fixed, but rather that the participant may be able to control the intensity such that it clears lactate with equal effectiveness as active recovery fixed at high intensities relative to lactate threshold. This was demonstrated by the use of self-regulated intensities, in which the participants were given no instructions as to exercise intensity. Thus, the exercise intensity was measured but not controlled by the investigators. The reason for the equal effectiveness was that the participants chose to run at an active recovery intensity close to 80% of lactate threshold, and in fact those who deviated from this intensity experienced slower lactate clearance. The biological feedback system that led the participants to choose the most effective lactate recovery intensities remains unknown.
The importance of clearance of accumulated blood lactate is a matter of debate, but it has been recognized that elevated concentrations of skeletal muscle and blood lactate are associated with impaired muscle function and exercise performance (Andrews, Godt, & Nosek, Citation1996; Hogan, Gladden, Kurdak, & Poole, Citation1995; Minshull, Gleeson, Walters-Edwards, Eston, & Rees, Citation2007; Sahlin & Ren, Citation1989; Westerblad & Allen, Citation1992). Although the cause–effect relationship between lactate and fatigue remains unclear (Gladden, Citation2004), it is clear that accumulation of lactate may at least indirectly contribute to reduced performance, because conversion of lactic acid to lactate releases H+ that leads to a metabolic acidosis with subsequent inhibition of glycolytic rate-limiting enzymes, lipolysis, and contractility of the skeletal muscles (Brooks, Citation2002; Gladden, Citation2000; Gollnick, Bayly, & Hodgson, Citation1986; Mainwood & Renaud, Citation1985). Whether lactate production causes or reflects fatigue, it may be used as a marker of fatigue because of its correlation with muscle fatigue and performance. Thus, it becomes relevant to design strategies that clear blood lactate after high-intensity exercise bouts, as this enables a faster recovery and may support subsequent high-intensity exercise, leading to greater overload and consequently enhanced training adaptation. This study contributes towards this end.
The mechanisms by which accumulated lactate is cleared include oxidation by working skeletal muscles. This mainly occurs by oxidative type I fibres, whereas the bulk production is confined to the glycolytic type II fibres, as well as myocardial oxidation and gluconeogenesis via the Cori cycle (Gladden, Citation2000, Citation2004). These are rate-limited by the monocarboxylate transporter-facilitated lactate shuttle to the blood and by the blood flow itself (Bonen et al., Citation2000). Thus, although measuring blood lactate only indirectly assesses the intramuscular environment, it opens a window of opportunity that can be repeatedly accessed with a high temporal resolution, which direct intramuscular measurements cannot match.
By definition, the lactate threshold occurs at the highest exercise intensity where lactate production and removal are balanced. However, our results suggest that the threshold intensity may increase when blood lactate concentration increases to above resting levels. If it had remained constant, one would have expected blood lactate concentration to remain stable during the active recovery trials at 100% of lactate threshold and the self-regulated trials where the intensity exceeded the lactate threshold. This did not happen, despite careful determination of the lactate threshold, which included re-testing where doubts occurred as to its accuracy. In fact, active recovery at 100% of lactate threshold very effectively cleared accumulated blood lactate. It is conceivable that under conditions of accumulated lactate, oxidation of lactate is increased and/or production is reduced.
The high-intensity exercise bout at 90% of [Vdot]O2max that induced lactate accumulation was designed to reflect the high-intensity bouts frequently utilized by aerobic interval training programmes (Haram et al., Citation2009; Helgerud et al., Citation2007; Kemi et al., Citation2005; Tjonna et al., Citation2008). These intervals are typically interspersed by 2–4 min active recovery periods that are aimed at clearing lactate and reducing fatigue to enable more intervals. Our results suggest that 2–4 min may not be sufficient for this purpose, although this may be improved by active recovery at an exercise intensity close to the lactate threshold. This may therefore have implications for improving exercise training programmes or performance during intermittent-type or repeated sports events. In swimming, this concept has already been demonstrated (Greenwood et al., Citation2008).
Conclusion
Our results demonstrate that active recovery after strenuous aerobic exercise leads to a faster clearance of accumulated blood lactate than passive recovery, and that the rate of blood lactate clearance depends on the exercise intensity of the active recovery, with peak lactate clearance rates occurring at intensities close to lactate threshold. This was observed by measuring blood lactate clearance during active and passive recovery ranging from 0 to 100% of the individual lactate threshold. Active recovery intensities at 80–100% of lactate threshold were more beneficial than exercise intensities at 60% of lactate threshold or below, and this dose–response relationship also existed during active recovery at 60%, 40%, and 0% (passive recovery) of lactate threshold. Thus, after a strenuous high-intensity aerobic exercise bout at an intensity close to [Vdot]O2max, the fastest lactate clearance is achieved by active recovery at an exercise intensity close to or just below the individual lactate threshold.
Acknowledgements
The authors are indebted to the participants for agreeing to take part in this study. The study was supported by funding from the University of Glasgow.
References
- Andrews , M. A. , Godt , R. E. and Nosek , T. M. 1996 . Influence of physiological lactate concentrations on contractility of skinned striated muscle fibres of rabbit . Journal of Applied Physiology , 80 : 2060 – 2065 .
- Baldari , C. , Videira , M. , Madeira , F. , Sergio , J. and Guidetti , L. 2004 . Lactate removal during active recovery related to the individual anaerobic and ventilatory thresholds in soccer players . European Journal of Applied Physiology , 93 : 224 – 230 .
- Baldari , C. , Videira , M. , Madeira , F. , Sergio , J. and Guidetti , L. 2005 . Blood lactate removal during recovery at various intensities below the individual anaerobic threshold in triathletes . Journal of Sports Medicine and Physical Fitness , 45 : 460 – 466 .
- Belcastro , A. and Bonen , A. 1975 . Lactic acid removal rates during controlled and uncontrolled recovery exercise . Journal of Applied Physiology , 39 : 932 – 936 .
- Boileau , R. A. , Misner , J. E. , Dykstra , G. L. and Spitzer , T. A. 1983 . Blood lactic acid removal during treadmill and bicycle exercise at various intensities . Journal of Sports Medicine and Physical Fitness , 2 : 159 – 167 .
- Bonen , A. and Belcastro , A. N. 1976 . Comparison of self-selected recovery methods on lactic acid removal rates . Medicine and Science in Sports , 8 : 176 – 178 .
- Bonen , A. , Campbell , C. J. , Kirby , R. L. and Belcastro , A. N. 1979 . A multiple regression model for blood lactate removal in man . Pflugers Archives , 380 : 205 – 210 .
- Bonen , A. , Tonouchi , M. , Miskovic , D. , Heddle , C. , Heikkila , J. J. and Halestrap , A. P. 2000 . Isoform-specific regulation of lactate transporters MCT1 and MCT4 by contractile activity . American Journal of Physiology: Endocrinology and Metabolism , 79 : E1131 – E1138 .
- Brooks , G. A. 2002 . Lactate shuttles in nature . Biochemical Society Transactions , 30 : 258 – 264 .
- Dodd , S. , Powers , S. K. , Callender , T. and Brooks , E. 1984 . Blood lactate disappearance at various intensities of recovery exercise . Journal of Applied Physiology , 57 : 1462 – 1465 .
- Duncan , G. E. , Howley , E. T. and Johnson , B. N. 1997 . Applicability of [Vdot]O2max criteria: Discontinuous versus continuous protocols . Medicine and Science in Sports and Exercise , 29 : 273 – 278 .
- Gladden , L. B. 2000 . Muscle as a consumer of lactate . Medicine and Science in Sports and Exercise , 32 : 764 – 771 .
- Gladden , L. B. 2004 . Lactate metabolism: A new paradigm for the third millennium . Journal of Physiology , 558 : 5 – 30 .
- Gollnick , P. D. , Bayly , W. M. and Hodgson , D. R. 1986 . Exercise intensity, training, diet and lactate concentration in muscle and blood . Medicine and Science in Sports and Exercise , 18 : 334 – 340 .
- Greenwood , J. D. , Moses , G. E. , Bernardino , F. M. , Gaesser , G. A. and Weltman , A. 2008 . Intensity of exercise recovery, blood lactate disappearance, and subsequent swimming performance . Journal of Sports Sciences , 26 : 29 – 34 .
- Gupta , S. , Goswami , A. , Sadkukhan , A. K. and Mathur , D. N. 1996 . Comparative study of lactate removal in short term massage of extremities, active recovery and a passive recovery period after supramaximal exercise sessions . International Journal of Sports Medicine , 17 : 106 – 110 .
- Haram , P. M. , Kemi , O. J. , Lee , S. J. , Bendheim , M. O. , Al-Share , Q. Y. Waldum , H. L. 2009 . Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity . Cardiovascular Research , 81 : 723 – 732 .
- Helgerud , J. , Hoydal , K. , Wang , E. , Karlsen , T. , Berg , P. Bjerkaas , M. 2007 . Aerobic high-intensity intervals improve [Vdot]O2max more than moderate training . Medicine and Science in Sports and Exercise , 39 : 665 – 671 .
- Hermansen , L. and Stensvold , I. 1972 . Production and removal of lactate during exercise in man . Acta Physiologica Scandinavica , 86 : 191 – 201 .
- Hogan , M. C. , Gladden , L. B. , Kurdak , S. S. and Poole , D. C. 1995 . Increased [lactate] in working dog muscle reduces tension development independent of pH . Medicine and Science in Sports and Exercise , 27 : 371 – 377 .
- Kemi , O. J. , Haram , P. M. , Loennechen , J. P. , Osnes , J. B. , Skomedal , T. Wisloff , U. 2005 . Moderate vs. high intensity: Differential effects on aerobic fitness, cardiomyocyte contractility and endothelial function . Cardiovascular Research , 67 : 161 – 172 .
- Lee , I. M. , Sesso , H. D. , Oguma , Y. and Paffenbarger , R. S. Jr. 2003 . Relative intensity of physical activity and risk of coronary heart disease . Circulation , 107 : 1110 – 1116 .
- Mainwood , G. W. and Renaud , J. M. 1985 . The effect of acid–base on fatigue of skeletal muscle . Canadian Journal of Physiology and Pharmacology , 63 : 416
- Minshull , C. , Gleeson , N. , Walters-Edwards , M. , Eston , R. and Rees , D. 2007 . Effects of acute fatigue on the volitional and magnetically-evoked electromechanical delay of the flexors in males and females . European Journal of Applied Physiology , 100 : 469 – 478 .
- Moholdt , T. , Wisloff , U. , Nilsen , T. I. and Slordahl , S. A. 2008 . Physical activity and mortality in men and women with coronary heart disease: A prospective population-based cohort study in Norway (the HUNT study) . European Journal of Cardiovascular Prevention and Rehabilitation , 15 : 639 – 645 .
- Mondero , J. and Donne , B. 2000 . Effect of recovery interventions on lactate removal and subsequent performance . International Journal of Sports Medicine , 21 : 593 – 597 .
- Newell , J. , Higgins , D. , Madden , N. , Cruickshank , J. , Einbeck , J. and McDonald , R. 2007 . Software for calculating blood lactate endurance markers . Journal of Sports Sciences , 25 : 1403 – 1409 .
- Sahlin , K. and Ren , J. M. 1989 . Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction . Journal of Applied Physiology , 67 : 648 – 654 .
- Tjonna , A. E. , Lee , S. J. , Rognmo , O. , Stolen , T. O. , Bye , A. Haram , P. M. 2008 . Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study . Circulation , 118 : 346 – 354 .
- Wasserman , K. , Beaver , W. L. and Whipp , B. J. 1986 . Mechanisms and patterns of blood lactate increase during exercise in man . Medicine and Science in Sports and Exercise , 18 : 344 – 352 .
- Westerblad , H. and Allen , D. G. 1992 . Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle . Journal of Physiology , 453 : 413 – 434 .