ABSTRACT
Athlete Biological Passport (ABP) is an indirect approach, implemented by WADA, aimed at detecting blood manipulation based on abnormal changes in haematological markers. Cases report the use of hyperhydration as masking method during anti-doping urine sample collection which could potentially mask suspicious fluctuations on ABP profiles. This study investigated the hyperhydration effect on haemoglobin concentration, reticulocyte percentage and OFF-hr score (an algorithm based on haemoglobin concentration and reticulocyte percentage), with and without recombinant human erythropoietin (rHuEPO) administration. A five-week clinical study performed; Baseline and rHuEPO Phase. Water and a sports drink were used as hyperhydration agents. To examine the hyperhydration effect on the normal ABP profile per volunteer, hyperhydration was implemented at 0, 24 and 48 hours during the baseline. During the rHuEPO phase, volunteers received Epoetin beta (3000 IU) with hyperhydration to be implemented at 0, 24 and 48 hours after drug administration. Blood and urine samples were collected and analysed according to WADA guidelines. No significant effect on ABP markers was observed due to hyperhydration at any time during the study. Pre- and post-hyperhydration data were not statistically different compared to individual baseline data. In conclusion, hyperhydration does not affect the ABP haematological markers under the examined conditions.
1. Introduction
The haematological module of the Athlete Biological Passport (ABP) is an indirect approach implemented by the World Anti-Doping Agency (WADA) for the detection of blood doping, either blood transfusions or erythropoiesis-stimulating agents (ESAs) (World Anti-Doping Agency, Citation2019). The ABP World Anti-Doping Agency, (Citation2019a) is based on the longitudinal monitoring of seven haematological markers of which the haemoglobin (HGB) concentration, the reticulocyte percentage (RET%) and the calculated OFF-hr score (an algorithm based on [HGB] and RET%: HGB (g/L) − 60×√RET%) (Robinson et al., Citation2007; Sottas et al., Citation2006) are the most important. Analytical results are uploaded on the Anti-Doping Administration and Management System (ADAMS) and evaluated automatically by the adaptive model based on the intra-individual ranges of each athlete. The members of the Haematological module of the Athlete Passport Management Unit (APMU) (World Anti-Doping Agency, Citation2019b) and external experts are reviewing the results and give recommendations in regards of additional target testing (ESAs) or to get expert advice in case of abnormalities.
One of the most challenging parts of the passport is the interpretation of the concentration-based blood markers. Alterations on the athletes’ plasma volume due to longitudinal adaptations always need to be kept in mind when interpreting an individual ABP profile. Among the most important factors affecting plasma volume are reported changes in hydration state, heat exposure, training or altitude (Lombardi, Citation2013; Racinais et al., Citation2012; Siebenmann et al., Citation2013; Voss et al., Citation2014). Plasma volume expanders can be used to enhance athletic performance and to mask the effect of other prohibited methods or substances (Simoni et al., Citation2011) such as elevated HGB levels due to recombinant human erythropoietin (rHuEPO) administration. Therefore, these substances are prohibited according to the WADA Prohibited List (World Anti-Doping Agency, Citation2020a) under the class S5 of diuretics and masking agents.
Acute increase in plasma volume after the ingestion of glycerol, water or isotonic sodium solutions could dilute the blood and, therefore, decrease the ABP concentration-based blood markers (Bejder et al., Citation2016; Freund et al., Citation1995; Kimura et al., Citation1986). Unreported cases (VeloNation Press, Citation2020) give reason to believe that hyperhydration, i.e. the consumption of high volumes of water or sports drinks is being used by a number of athletes as a masking method based on the fact that dilution is not prohibited as per WADA`s current regulations (World Anti-Doping Agency, Citation2020a). Doped athletes are trying to consume high amounts of fluid(s) (hyperhydration) to lower their HGB concentrations and stay within the individual ranges calculated by the ABP adaptive model software. Therefore, the study of hyperhydration induced effect on the plasma volume as a confounding factor on ABP profiles is of utmost importance.
Athletes consume different types of sports drinks on a daily basis in a volume of at least 1 L per day. In case of lack of adequate fluid replacement (hypohydration), plasma volume decreases while sodium concentration and blood osmolality increase. On the other hand, decreased osmolality in the blood will result in decreased water reabsorption, highly diluted urine, and more concentrated blood plasma due to lower plasma volume.
The osmolality of a beverage is a marker that can influence the rate of gastric emptying and intestinal water absorption (Sadowska et al., Citation2017). Based on this marker, beverages are classified into groups depending on their tonicity and their ability to ensure proper body hydration. In general, an increase in total carbohydrate content leads to an increased osmolality which is also influenced by the proportion of monosaccharides, disaccharides and polysaccharides. Hypotonic drinks including water with an osmolality of less than 275 mOsm/kg H2O are rapidly absorbed into the body resulting in a fast body hydration. However, due to their low electrolyte concentration, they are unable to rapidly improve the body`s water-electrolyte balance. Hypertonic drinks with an osmolality higher than 295 mOsm/kg H2O are absorbed more slowly than water reducing the rate of water absorption.
In the present study, as part of a WADA research grant (10D21CG), we investigated the effect of hyperhydration on the haematological markers of ABP using two different hyperhydration agents (water and a commercial sports drink) with different osmolality, before and after rHuEPO administration, i.e. a single dose of Epoetin beta (3000 IU). The scope, and novelty, of the present work, was to examine whether the ingestion of high volume of fluids, as well as of their type (e.g. different osmolality), would influence the concentration-based blood markers of ABP compared to the values obtained under a normal hydration status due to alterations of plasma volume.
2. Experimental
The present study was a part of a generic WADA funded research project entitled “Influence of athletes hyperhydration on sample collection procedure in terms of plasma and urine pharmacokinetics of representative prohibited substances”. The study was approved by the Ethics Committee of the Anti-Doping Lab Qatar, Doha, Qatar (F2013000006) and performed in accordance with the Declaration of Helsinki.
2.1 Study population
Seven healthy, recreationally active, non-smoking Caucasian male volunteers were recruited for the present study. The volunteer`s demographic and clinical characteristics are summarized age 38.4 ± 8.2 years, body weight (BW) 88 ± 17 kg, height 178 ± 6 cm, 2.1 ± 0.3 m2 body surface area and creatinine clearance 136 ± 19 mL/min. A complete medical history was obtained by interview with each volunteer to verify the initial inclusion criteria as part of the clinical trial. A complete medical screening procedure including physical and haematological examinations was performed to ensure the healthy state of each volunteer. All the volunteers were found eligible to participate in the present study. Written consent was provided prior to participation and volunteers were free to withdraw from the study at any time. The study was approved by the Ethics Committee of the Anti-Doping Lab Qatar, Doha, Qatar (F2013000006) and performed in accordance with the Declaration of Helsinki.
2.2 Study design
As described previously Athanasiadou et al. (Citation2019), a 31-day clinical study comprised a baseline (Days 0, 1‒3, 8‒10) and a drug phase (Days 15‒17, 22‒24, 29‒31) was performed. Water and a commercial sports drink were used as hyperhydration agents at a volume of 20 mL per kg BW (i.e. 1400 mL for a 70-kg heavy person). The sports drink consisted of a non-carbonated balanced glucose-electrolyte solution (6.0% w/v carbohydrate, 104.6 kJ, Na 0.05% w/v, K 0.012% w/v, pH 3.6) with an osmolality of 438 mOsm/kg H2O. The osmolality of water was equal to 54 mOsm/kg H2O. Both hyperhydration agents were kept at room temperature.
Each volunteer was requested to maintain his normal training and dietary protocol throughout the study period. However, no food and drinks, which may interact with circulatory, gastrointestinal, hepatic or renal function (i.e. alcohol, caffeine-containing beverages, grapefruit juice and diuretic substances) were allowed for at least 48 hours before inclusion and throughout the study. Furthermore, all the volunteers were asked to record their daily fluid consumption.
2.2.1. Baseline phase
Prior to hyperhydration (phases A and B), blood samples were collected at four different time points to establish the baseline blood profile per volunteer (Day 0). Volunteers were asked to perform three identical experimental days (Days 1‒3 and 8‒10) according to the study protocol ()). A four-day wash-out was introduced between the phases A and B to avoid any carry-over effect.
Figure 1. Experimental study protocol. (a) Baseline phase: Days 0 (no hyperhydration), 1‒3 (Phase A: water) and 8‒10 (Phase B: sports drink). Blood samples were collected at four different time points to establish the baseline blood profile per volunteer (Day 0). Hyperhydration was applied in the morning (20 mL/kg body weight water or sports drink) at 0, 24 and 48 hours as shown. Blood samples were collected before and after fluid ingestion and exercise as shown in schematic diagram. Urine samples were collected at each voluntary micturition. (b) Drug phase: Days 15‒17 (rHuEPO & no hyperhydration), 22‒24 (Phase C: rHuEPO & water) and 29‒31 (Phase D: rHuEPO & sports drink). rHuEPO was injected subcutaneously as a single dose of 3000 IU on Days 15, 22 and 29. A seven-day wash-out period was followed between the phases. Hyperhydration was induced (20 mL/kg body weight water or sports drink) three times in the morning of a 72-hour time interval as shown. Blood samples were collected pre-dose, 30 minutes, 1, 2, 4, 6, 8, 12, 24,48 and 72 hours after drug administration (TAD). Blood collection scheme for the phases C and D included two more samples after fluid ingestion as shown. Urine samples were collected at each voluntary micturition.
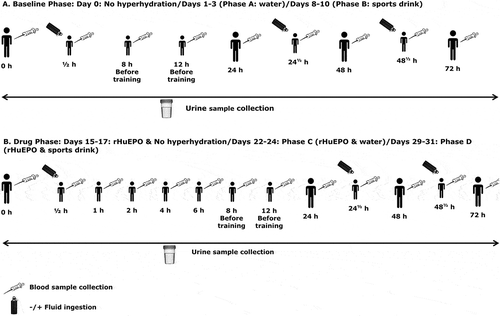
Volunteers were instructed to proceed to the testing venue between 7:00 to 8:00 am following an overnight fast (8‒12 hours). Upon arrival, urine was collected and vital signs (BW, blood pressure, heart rate, respiration rate) were measured. Volunteers` euhydrated state was confirmed when specific gravity (SG) of the first collected urine sample was between 1.005 and 1.020 (Voss et al., Citation2014). After urine collection, a venous blood sample (pre-hyperhydration sample) was drawn in a sitting posture (World Anti-Doping Agency, Citation2016).
Hyperhydration was induced within 30 minutes by ingesting either water (Phase A: Days 1‒3) or a commercial sports drink (Phase B: Days 8‒10). After remaining seated for 15 minutes without ingesting any sort of fluids, a second blood (post-hyperhydration) and urine sample were collected. Vital signs were measured after completion of the blood sample collection. Then, volunteers were released and instructed to collect their urine samples at each voluntary micturition until a 24-hour collection scheme was completed. To monitor the volunteers` hydration state, SG was measured for all the urine samples.
Hyperhydration was applied in the morning on three consecutive days (Phase A: Days 1‒3 and Phase B: Days 8‒10), i.e. 0, 24 and 48 hours after first fluid ingestion. Both hyperhydration agents were examined by all the volunteers.
2.2.2. Drug phase
Recombinant human Epoetin beta (NeoRecormon, Roche Diagnostics, Welwyn Garden City, United Kingdom) (Neorecormon® DS 180625, Citation2018) was administered subcutaneously (SC) at a single dose of 3000 IU (Days 15, 22 and 29) according to the study protocol (). A seven-day wash-out period was introduced between drug administrations to ensure complete rHuEPO elimination (Neorecormon® DS 180625, Citation2018; Salmonson et al., Citation1990).
As in the Baseline phase, a urine sample was collected and vital signs were measured upon arrival at the testing venue (7:00 to 8:00 am) after an overnight fast. Immediately after first blood collection (pre-dose sample), Epoetin beta was administered SC and a second blood sample was taken 30 minutes later. No hyperhydration was applied during the third week of the study (Days 15‒17) and blood sampling was performed at the following time points after drug administration (TAD): pre-dose, 30 minutes, 1, 2, 4, 6, 8, 12, 24, 48- and 72-hours TAD. Urine sampling was performed at each voluntary micturition throughout the whole phase. These samples were used to establish the baseline blood and urinary profile of each volunteer after rHuEPO administration. Hyperhydration was implemented in the morning on Days 22‒24 (Phase C: rHuEPO & water) and Days 29‒31 (Phase D: rHuEPO & sports drink), as mentioned previously. Urine sample collection was performed as described above. Blood collection scheme for the phases C and D was as follows: pre-dose, 30 minutes TAD, 1-hour TAD and after completion of hyperhydration, 2, 4, 6, 8, 12 hours TAD, 24 hours TAD (pre and post-hyperhydration), 48 hours TAD (pre and post-hyperhydration) and 72 hours TAD. Vital signs were measured before and after drug administration, hyperhydration and exercise.
2.3. Sample analysis
2.3.1. Blood
Blood samples were collected in 3.0 mL K2 EDTA vacuum tubes and 4.5 mL serum separator tubes (SST 2 Advance) from Becton-Dickinson (BD, Franklin Lakes, USA) according to WADA’s blood collection guidelines (World Anti-Doping Agency, Citation2016).
Complete blood count and reticulocyte analysis was performed within four hours maximum after collection using a Sysmex XT2000i haematology analyser (Sysmex, Kobe, Japan) which is subjected to monthly CSCQ proficiency tests (Quality Control Centre Switzerland) organized by WADA. In compliance to the criteria of the WADA’s operating guidelines (World Anti-Doping Agency, Citation2019) and TD2017 BAR (World Anti-Doping Agency., Citation2017), a fresh blood sample and quality control samples (XT-checks levels 1, 2 and 3) were analysed together with the samples to confirm the appropriate precision and accuracy of the analyser on each analysis day. All the samples were homogenized for at least 15 minutes on a roller mixer prior to analysis. Each sample was analysed in duplicate. Absolute differences between the two consecutive analyses should be equal or less of 0.1 g/dL for HGB, 0.15 absolute difference for RET% analysis (0.25 absolute difference if either the first or second measurement is lower or equal to 1.00%). Only the first injection data was used if the absolute differences were within the criteria above otherwise the analysis was repeated.
Osmolality (Osmol/kg) was measured to all the serum samples via freezing point depression with an osmometer (OSMOMAT 030, Gonotec, Berlin, Germany). Standard sodium chloride (NaCl) solutions with an osmolality of 0.300 and 0.850 Osmol/kg were used for the calibration of the instrument before analysis. A zero calibration was also performed using distilled water.
2.3.2. Urine
Urine samples were collected into 200 mL sterile containers at each voluntary micturition according to the study protocol. SG was determined using an Atago 3464 Refractometer (Atago, Tokyo, Japan).
2.4 Statistical analysis
Data analysis was performed using SPSS version 25.0 (IBM SPSS Statistics for Windows, Version 25.0, IBM Corporation, Armonk, NY, USA) software package.
Results are presented as mean ± standard deviation (SD). Normal distribution was evaluated with the Shapiro-Wilk (S-W) test for the examined variables HGB (g/dL), RET% and OFF-hr score. Significance was set at P < 0.05 level and all tests were two-tailed with 95% confidence intervals (CI). Outlier detection on the individual baseline data occurred applying the interquartile range (IQR) using a step of 1.5 x IQR. Box and whisker plots were also established to investigate possible outliers. No outliers were found.
Prior to inclusion in the study, HBG (g/dL), RET% and OFF-hr score data were collected to establish the baseline profile of each volunteer with and without rHuEPO administration. Percent coefficient of variation (%CV) was employed as a measure of variability. Inter-day variability was assessed for all the variables by comparing the data before and after hyperhydration. The S-W test results revealed that the data followed a non-normal distribution even after a logarithmic transformation. The non-parametric Mann-Whitney U test was used to examine possible significant differences between the different groups (i.e. different phases) and, therefore, to confirm any dilution effect on the ABP markers. Comparisons of pre- and post-hyperhydration HBG (g/dL), RET% and OFF-hr score data at specific time points were performed by Wilcoxon signed-rank test to evaluate the dilution effect on the ABP markers.
3. Results
All the volunteers followed the clinical study protocol without any deviations and received the scheduled volume of each hyperhydration agent with no discomfort being reported at any time during the study.
3.1. Influence of hyperhydration on HGB (g/dL), RET% and OFF-hr score
The hydration state of the volunteers at the different phases of the study was evaluated through the SG measurements of the collected urine samples. Upon arrival, all volunteers were adequately euhydrated in all phases with a SG above 1.005 and less than 1.020 (World Anti-Doping Agency, Citation2020b).
3.1.1. Baseline phase
Comparison of HGB (g/dL), RET% and OFF-hr values before and after hyperhydration at 0, 24 and 48 hours showed no statistically significant difference (P range 0.10‒0.85, 95% CI) individually and for the population study. Pre- and post-average values of the examined ABP markers are presented in in the form of box and whisker plots for both hyperhydration agents at the different time points.
Figure 2. Haematological changes in haemoglobin concentration (HGB, g/dL), reticulocyte percentage (RET%) and OFF-hr score during the 48-hour study period for both hyperhydration agents (water and sports drink). Statistical differences pre- and post-hyperhydration were non-significant at 0, 24 and 48 hours (P > 0.05, 95% confidence interval).
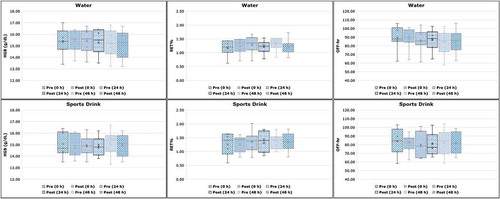
At 0, 24 and 48 hours prior to water hyperhydration (Phase A), average HGB concentrations were 15.40 ± 1.15 g/dL, 15.41 ± 1.10 g/dL, and 15.23 ± 1.24 g/dL, respectively. Average post HGB values remained at a similar level (15.40 ± 1.01 g/dL) at 0 hours while a non-significant decrease of 0.14 g/dL and 0.19 g/dL was observed at 24 and 48 hours, respectively. Similarly, average post HGB values showed non-significant decrease of 0.16, 0.03 and 0.18 g/dL at 0, 24 and 48 hours after excessive fluid intake during sports drink hyperhydration (phase B). The same pattern was also followed for RET% without any significant deviations to be observed pre- and post-hyperhydration for both phases A and B. No statistically significant difference was also reported for OFF-hr, despite the clear trend for lower values especially in Phase A (water hyperhydration), probably revealing also a slight change in HGB and RET% that is masked by the observed variability. Results are presented in , expressed as mean ± SD and in .
Table 1. Haematological changes in the examined ABP (HGB: haemoglobin concentration g/dL, RET%: reticulocytes percentage, OFF-hr) parameters per study phase expressed as mean ± SD.
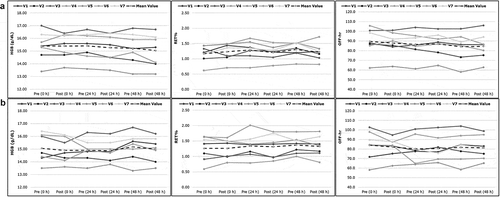
Individual changes in HGB (g/dL), RET% and OFF-hr score are illustrated in for both phases A and B. Non-significant decrease was reported by the majority of the volunteers for all ABP markers. Three of the volunteers showed a slight increase in post-hydration values for both phases A and B. However, the reported increase was much lower than the inter-day variability of the corresponding baseline values for each of these volunteers. In all other cases, a non-significant decrease (P range 0.10‒0.65, 95% CI) was reported which was again less than the inter-day variability of the baseline values.
Figure 3. Individual profiles in haemoglobin concentration (HGB, g/dL), reticulocyte percentage (RET%) and OFF-hr score during intake of 20 mL/kg body weight of water (A) or sports drink (B) within 30 minutes. The study population mean is represented by the bold dashed line. V1-V7: volunteers 1 to 7.
3.1.2. Drug phase
After Epoetin beta administration at Day 15 (no hyperhydration), the ABP markers were measured within a 72-hour time period and the results are presented in in the form of box and whiskers plots. For all the evaluated ABP markers, no statistically significant difference (P range 0.08‒0.98, 95% CI) was observed between the measured values within the 72-hour study period. More specifically, at 0, 24 and 48 hours, average HGB values, expressed as mean ± SD, were 14.86 ± 0.68 g/dL, 14.84 ± 0.98 g/dL, and 14.94 ± 0.93 g/dL, respectively.
Figure 4. Comparison of haemoglobin concentration (HGB, g/dL), reticulocyte percentage (RET%) and OFF-hr score after a single subcutaneous dose of recombinant human erythropoietin (3000 IU) under normal hydration conditions at 0, 24 and 48 hours after administration.

Similarly, average RET% and OFF-hr values were also found non-statistically significant different compared to the baseline phase (no drug administration, no hyperhydration). However, consistently lower OFF-hr values were observed after Epoetin beta administration (). The observed trend for lower OFF-hr values is consistent with the results of Bedjer et al. (Bejder et al., Citation2016) after multiple dose administration of rHuEPO. Furthermore, the results obtained from rHuEPO administration phase after hyperhydration (Phases C and D) for up to 72 hours TAD, also showed the same trend for lower ΟFF-hr values (although non-statistically significant, P > 0.05) compared to those observed at phases A and B without drug administration, as well as at the drug phase before hyperhydration (, ). The small number of volunteers included in the study and the observed variability may probably mask the effect of the administered 3000 IU single dose of rHuEPO both in absence and presence of hyperhydration, on the haematological profile of the volunteers ().
Figure 5. Haematological changes in haemoglobin concentration (HGB, g/dL), reticulocyte percentage (RET%) and OFF-hr score during the 72-hour study period for both hyperhydration agents (water and sports drink) after drug administration. Statistical differences pre- and post-hyperhydration were non-significant at 0, 24 and 48 hours (P > 0.05, 95% confidence interval).
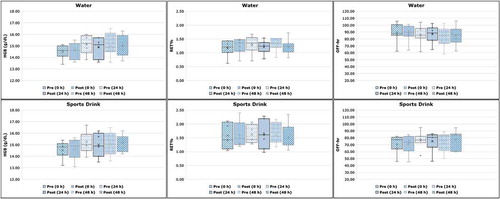
To further evaluate the possible effect of excessive fluid intake, pre- and post-hyperhydration values per each marker within the phases C and D were compared. More specifically, comparison of pre- and post-values at ½, 1 ½, 3 ½, 5 ½, 7 ½, 9 ½ hours after hyperhydration by either water (Phase C) or sports drink (Phase D) was performed. As shown in , no statistically significant difference (Phase C: P range 0.09‒0.80, Phase D: P range 0.07‒0.92, 95% CI) was observed between Phases C and D at any time point for any of the examined ABP markers. Accordingly, since no effect was observed per time point, the average values per ABP marker (as pooled data), expressed as mean ± SD, were used for further comparison with the respective values of rHuEPO phase without hyperhydration. Again, in this case, no effect was reported due to hyperhydration (Phase C: P range 0.06‒0.51, Phase D: P range 0.12‒0.61).
4. Discussion
Analytical standardization procedures have been set up by WADA (World Anti-Doping Agency, Citation2019) in order to reduce the biological variation observed due to fluctuations occurring on the athletes’ plasma volume that could affect the ABP markers.
Among the most important factors affecting plasma volume are reported changes in hydration state, heat exposure, training and altitude or. For example, large interindividual variation (−10 to 20%) on the plasma volume was observed after heat exposure, (Racinais et al., Citation2012) while a few days of exposure to high altitude, leaded to decreased plasma and total blood volume and increased arterial HGB (Rasmussen et al., Citation0000).
Most importantly, an acute intake of a high volume of water or sports drink is discussed to result in plasma volume expansion that, might affects ABP markers. An acute increase in plasma volume is expected to dilute the blood and, therefore, decrease the concentration-based blood markers and ABP sensitivity. Alterations on hydration state could result in possible atypical ABP profiles due to changes on the haematological markers. According to previous studies (Freund et al., Citation1995; Kimura et al., Citation1986), a decrease of 0.6 g/dL for HGB was reported in conjunction with an increase in plasma volume of 4–5% within 60 minutes after water ingestion of approximately 1700 mL. Recently, Bejder et al. (Citation2016) reported an acute hyperhydration effect on OFF-hr score after ingestion of a 1000 mL bolus of water in volunteers following a three-week administration period with rHuEPO. All volunteers underwent a boosting period with the administration of Epoetin beta for 3 weeks, receiving 30 IU/kg BW SC three times per week. After a 10-day washout period of rHuEPO, the subjects received an intravenous micro-dose injection of approximately 900 IU Epoetin alpha. Results showed that the OFF-hr score was reduced by ~ 4%, ~ 3% and ~ 2% at 40, 60 and 80 min after hyperhydration and, therefore, ABP sensitivity was also reduced. The decrease in OFF-hr during hyperhydration treatment was related to a decrease in HGB, since no deviation was observed for RET%. The unchanged fraction of reticulocytes was expected since RET% per definition should be independent of plasma volume fluctuations.
In the present study, the findings did not reveal any statistically significant fluctuations in plasma volume and, therefore, in blood variables.
During the baseline phase, pre- and post-average values of the examined ABP markers showed no significant difference individually and for the study population at 0, 24 and 48 hours. Any observed increase or decrease of the blood markers after excessive fluid intake was non-significant and lower than the inter-day variability of the corresponding baseline values for each of these volunteers at any time point. However, it is worthy to mention, that a clear trend for lower OFF-hr values was observed after excessive fluid ingestion, especially in Phase A (water hyperhydration), probably revealing that HGB and RET% may also be affected by hyperhydration but alterations are masked by the observed variability.
After Epoetin beta administration, average HGB, RET% and OFF-hr values were again not statistically different compared to the baseline values without any drug treatment (P > 0.05). These results revealed that a single rHuEPO dose of 3000 IU may not affect the haematological profile of the volunteers. Induced hyperhydration at 0, 24 and 48 hours combined with rHuEPO administration also showed non-significant alterations on the ABP markers. It should be noted however, that a clear trend for lower OFF-hr values was observed after Epoetin beta administration both without and with hyperhydration (, ).
With respect to the nature of the hyperhydration agent, sodium containing drinks have a higher fluid retention which results in significant increases in plasma volume and, therefore, lower urinary flow rates. In this case, we would expect the ingestion of the sports drink to result in significant changes in the ABP markers compared to the water. However, no statistically significant difference was observed between the two hyperhydration agents. Both water and sports drink act similarly without statistically significant effect on any of the ABP markers.
Furthermore, it would be expected that excessive fluid intake would cause plasma volume expansion and, therefore, lower serum osmolality values due to dilution after hyperhydration. As shown in , serum osmolality was not affected by the hyperhydration for both water and sports drink phases with or without rHuEPO administration. Comparison of the values before and after hyperhydration showed no significant difference (P range 0.11‒0.93).
Figure 6. Statistical comparison of serum osmolality (Osmol/kg) between the baseline and the drug phase under normal hydration conditions and after excessive fluid intake. Significance was set at P < 0.05 level (95% confidence interval).
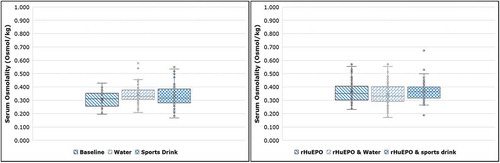
None of the individual HGB, RET% and OFF-hr values collected in the present study were considered statistically different under normal and hydration conditions with and without rHuEPO treatment. However, a clear trend for lower OFF-hr values was observed in all cases after hyperhydration and Epoetin beta administration.
Limitations explaining the absence of statistically significant differences of the values obtained after drug administration (with or without hyperhydration) may be attributed to the dose scheme (dosage used, number of injections), the route of administration (intravenous or subcutaneous) and the limited number of individuals enrolled in the study. For example, in the study of Bedjer (Bejder et al., Citation2016), a clear effect on the ABP sensitivity was observed, that could probably be attributed to the applied multiple dose protocol, compared to a single dose administration performed in our study.
However, the herein presented results are in compliance with those of studies investigating the hydration influence on the detection sensitivity of rHuEPO in blood and urine applying the current analytical methodology (IEF, SDS-PAGE, SAR-PAGE) (World Anti-Doping Agency., Citation2014).
Recent studies showed that the consumption of a large bolus of fluids (e.g. water or sports drink) did not reduce the detection sensitivity of rHuEPO in blood or urine matrices. Martin et al., (Citation2016) investigated the hyperhydration effect on the identification of rHuEPO, after intravenous micro-doses, in plasma and urine samples by IEF and SDS-PAGE analysis following the same study protocol of Bedjer et al. The detection sensitivity of a single intravenous micro-dose of Eprex (Epoetin alpha) with and without a boosting period (30 IU Epoetin beta/kg BW three times/week for three weeks) was examined under different hydration protocols. Results showed no effect on the detection rate after water ingestion. Recently, Athanasiadou et al., (Citation2019) examined the reliability and robustness of SAR-PAGE analysis of rHuEPO in urine and serum samples under normal and hydration conditions. Hyperhydration had no effect on the detection sensitivity of endogenous EPO and rHuEPO either in serum or urine samples after a single SC dose of 3000 IU Epoetin beta with a detection window up to 72 h TAD. Based on the above-mentioned results, the reliability and robustness of the routine anti-doping analysis of rHuEPO was verified. The use of hyperhydration as a masking method to alter rHuEPO profiles is not effective supporting the reliability of the routine anti-doping analysis methodology.
The outcomes from this study suggest that hyperhydration does not affect the ABP haematological markers under the present examined conditions. However, additional studies with larger number of volunteers and application of different hyperhydration schemes, would give further inside on the possible use of excessive fluid intake as a doping masking method.
5. Conclusion
In the present study, hyperhydration induced by two different agents (water and a commercial sports-drink) showed no statistically significant changes on the haematological markers of the ABP at any time during the study, although consistently lower OFF-hr values were observed after excessive fluid ingestion and after Epoetin beta administration with or without hyperhydration. Under the conditions of this study, the magnitude of change between pre- and post-hydration values was too small and non-significant different independently of the hyperhydration agent.
Acknowledgments
The authors sincerely thank the World Anti-Doping Agency for the financial support of the project (contract 10D21CG). Furthermore, the authors would like to acknowledge the medical staff of Al-Hayat Medical Center, Doha, Qatar for their help during the clinical part of the project performed under the supervision of Dr. Khaled Youssef, MD and Dr. Fawaz Amin Saad, MD. Finally, Mrs. Noor Al-Motawa, ADLQ Education and Research Office Director, is sincerely acknowledged for the constant support on the research projects.
Disclosure statement
The authors have no conflict of interest to declare.
Additional information
Funding
References
- Athanasiadou, I., Dokoumetzidis, A., Voss, C. S., El Saftawy, W., Al Maadheed, M., Valsami, G., & Georgakopoulos, C. (2019). Hyperhydration effect on pharmacokinetic parameters and detection sensitivity of recombinant human erythropoietin in urine and serum doping control analysis of males. Journal of Pharmaceutical Sciences, 108(6), 2162–2172. https://doi.org/10.1016/j.xphs.2019.01.017
- Bejder, J., Hoffmann, M. F., Ashenden, M., Nordsborg, N. B., Karstoft, K., & Mørkeberg, J. (2016). Acute hyperhydration reduces athlete biological passport OFF-hr score. Scandinavian Journal of Medicine & Science in Sports, 26(3), 338–347. https://doi.org/10.1111/sms.12438
- Freund, B. J., Montain, S. J., Young, A. J., Sawka, M. N., DeLuca, J. P., Pandolf, K. B., & Valeri, C. R. (1995). Glycerol hyperhydration: Hormonal, renal, and vascular fluid responses. Journal of Applied Physiology, 79(6), 2069–2077. https://doi.org/10.1152/jappl.1995.79.6.2069
- Kimura, T., Abe, K., Ota, K., Omata, K., Shoji, M., Kudo, K., Matsui, K., Inoue, M., Yasujima, M., & Yoshinaga, K. (1986). Effects of acute water load, hypertonic saline infusion, and furosemide administration on atrial natriuretic peptide and vasopressin release in humans. The Journal of Clinical Endocrinology & Metabolism, 62(5), 1003–1010. https://doi.org/10.1210/jcem-62-5-1003
- Lombardi, G. (2013). Reply to Gore et al.: Plasma volume shift during multiday racing. Clinical Chemistry and Laboratory Medicine, 51(6), 111–112. https://doi.org/10.1515/cclm-2012-0887
- Martin, L., Ashenden, M., Bejder, J., Hoffmann, M., Nordsborg, N., Karstoft, K., Morkeberg, J., Sharpe, K., Lasne, F., & Marchand, A. (2016). New insights for identification of doping with rHuEPO microdoses after high hydration. Drug Testing and Analysis, 8(11–12), 1119‒1130. https://doi.org/10.1002/dta.2004
- Neorecormon® DS 180625. (2018). Roche products limited. Retrieved October 11, 2018, from. http://medsafe.govt.nz/profs/Datasheet/n/Neorecormoninj.pdf.
- Racinais, S., Mohr, M., Buchheit, M., Voss, S. C., Gaoua, N., Grantham, J., & Nybo, L. (2012). Individual responses to short-term heat acclimatisation as predictors of football performance in a hot, dry environment. British Journal of Sports Medicine, 46(11), 810–815. https://doi.org/10.1136/bjsports-2012-091227
- Rasmussen, P., Siebenmann, C., Diaz, V., & Lundby, C. (0000). Red cell volume expansion at altitude: A meta-analysis and monte carlo simulation. Medicine & Science in Sports & Exercise, 45(9), 1767–1772. https://doi.org/10.1249/MSS.0b013e31829047e5
- Robinson, N., Sottas, P. E., Mangin, P., & Saugy, M. (2007). Bayesian detection of abnormal hematological values to introduce a no-start rule for heterogeneous populations of athletes. Haematologica, 92(8), 1143–1144. https://doi.org/10.3324/haematol.11182
- Sadowska, A., Świderski, F., Rakowska, R., Waszkiewicz-Robak, B., Żebrowska-Krasuska, M., & Dybkowska, E. (2017). Beverage osmolality as a marker for maintaining appropriate body hydration. Roczniki Panstwowego Zakladu Higieny, 68(2), 167–173. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28646834
- Salmonson, T., Danielson, B. G., & Wikström, B. (1990). The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. British Journal of Clinical Pharmacology, 29(6), 709‒713. https://doi.org/10.1111/j.1365-2125.1990.tb03692.x
- Siebenmann, C., Hug, M., Keiser, S., Müller, A., van Lieshout, J., Rasmussen, P., & Lundby, C. (2013). Hypovolemia explains the reduced stroke volume at altitude. Physiological Reports, 1(5), 1–9. https://doi.org/10.1002/phy2.94
- Simoni, R. E., Scalco, F. B., De Oliveira, M. L. C., & Neto, F. R. A. (2011). Plasma volume expanders: Use in medicine and detecting misuse in sports. Bioanalysis, 3(2), 215–226. https://doi.org/10.4155/bio.10.181
- Sottas, P. E., Robinson, N., Giraud, S., Taroni, F., Kamber, M., Mangin, P., & Saugy, M. (2006). Statistical classification of abnormal blood profiles in athletes. The International Journal of Biostatistics, 2(1), 1–21. https://doi.org/10.2202/1557-4679.1011
- VeloNation Press. Retrieved February 1, 2020, from. http://www.velonation.com/News/ID/3948/Thomas-Frei-used-micro-doses-and-water-to-avoid-EPO-detection.aspx
- Voss, S. C., Alsayrafi, M., Bourdon, P. C., Klodt, F., Nonis, D., Hopkins, W. G., & Schumacher, Y. O. (2014). Variability of serum markers of erythropoiesis during 6 days of racing in highly trained cyclists. International Journal of Sports medicine, 35(2), 89–94. https://doi.org/10.1055/s-0033-1345177
- World Anti-Doping Agency. (2016). ISTI blood sample collection guidelines. Version 5.0. Retrieved February 1, 2020, from. https://www.wada-ama.org/sites/default/files/resources/files/guidelines_blood_sample_collection_v5_sept_2016.pdf
- World Anti-Doping Agency. (2019). ISTI, ISL athlete biological passport operating guidelines. Version 7.1. Retrieved February 1, 2020, from. https://www.wada-ama.org/sites/default/files/resources/files/guidelines_abp_v71.pdf
- World Anti-Doping Agency. World anti-doping code: The 2020 prohibited list. Available at: https://www.wada-ama.org/sites/default/files/wada_2020_english_prohibited_list_0.pdf (accessed February 1st, 2020a)
- World Anti-Doping Agency. ISTI Urine Sample Collection Guidelines (ver. 6.0). Available at: https://www.wada-ama.org/sites/default/files/resources/files/wada_guidelines_urine_sample_collection_2014_v1.0_en.pdf (accessed February 1st, 2020b).
- World Anti-Doping Agency. WADA Technical Document - TD2014EPO (ver. 1.0): Harmonization of analysis and reporting of erythropoiesis stimulating agents (ESAs) by electrophoretic techniques, 2014. Available at https://www.wada-ama.org/sites/default/files/resources/files/WADA-TD2014EPO-v1-Harmonization-of-Analysis-and-Reporting-of-ESAs-by-Electrophoretic-Techniques-EN.pdf. (accessed February 1st, 2020).
- World Anti-Doping Agency. WADA Technical Document – TD2017BAR (ver. 6.0): Blood Analytical Requirements for the Athlete Biological Passport, 2017. Available at: https://www.wada-ama.org/sites/default/files/resources/files/wada_td2017bar_blood_analysis_requirements_en.pdf (accessed February 1st, 2020).
- World Anti-Doping Agency. WADA Technical Document – TD2019BAR (ver. 1.0): Blood Analytical Requirements for the Athlete Biological Passport, 2019a. Available at: https://www.wada-ama.org/sites/default/files/resources/files/td2019bar_0.pdf (accessed February 1 2020).
- World Anti-Doping Agency. WADA Technical Document – TD2019APMU (ver. 1.0): Athlete Passport Management Unit. Requirements and Procedures, 2019b. Available at: https://www.wada-ama.org/sites/default/files/resources/files/td2019apmu_final2.pdf (accessed February 1st, 2020).
