ABSTRACT
The aim was to provide a meta-analysis of studies investigating the effects of physical activity interventions on cognitive outcomes and academic performance in adolescents or young adults. A systematic review with meta-analysis was performed using the following databases: Embase, ERIC, MEDLINE, PsycINFO and Web of Science. Studies had to meet the following criteria: controlled study design, investigating the effects of physical activity interventions on cognitive outcomes and academic performance in healthy adolescents or young adults (12–30 years). Results showed that acute interventions (n=44) significantly improved processing speed (ES=0.39), attention (ES=0.34) and, inhibition (ES=0.32). In a subsequent meta-regression, shorter duration of intervention was significantly associated with greater improvements in attention (β=−0.02) and cognitive flexibility (β=−0.04), whereas age, percentage of boys, intensity and dose were not. Chronic interventions (n=27) significantly improved processing speed (ES=0.30), attention (ES=0.50), cognitive flexibility (ES=0.19), working memory (ES=0.59) and language skills (ES=0.31). In the meta-regression, higher percentage of boys was significantly associated with greater improvements in attention (β=0.02) and working memory (β=0.01) whereas age, duration, frequency, dose and load were not. In conclusion, acute and chronic physical activity interventions might be a promising way to improve several cognitive outcomes and language skills in adolescents and young adults.
Introduction
Physical activity confers strong positive health benefits, including lower risks of cardiovascular diseases (Janssen & LeBlanc, Citation2010; Li & Siegrist, Citation2012), adiposity (Haynos & O’Donohue, Citation2012) and type 2 diabetes (Stanford & Goodyear, Citation2014). Recent research has also linked physical activity to cognitive and academic benefits in both children and older adults (Esteban-Cornejo et al., Citation2015; Hillman et al., Citation2008; Northey et al., Citation2017). Despite the benefits of physical activity, nowadays more than 80% of adolescents do not adhere to the recommended physical activity guidelines (Piercy et al., Citation2018). The lack of physical activity is seen as a major threat for physical and cognitive health.
Physical activity is especially important during adolescence and young adulthood. During this stage of development, higher cortical functions, the so-called executive functions, which are located in the prefrontal cortex show rapid development (Lebel et al., Citation2008; Lenroot & Giedd, Citation2006). A growing number of studies have supported the idea that physical activity boosts these executive functions (Li et al., Citation2017; Verburgh et al., Citation2013; Xue et al., Citation2019). Furthermore, physical activity might also have a positive effect on attention and processing speed. These are two basic neurocognitive functions that act as prerequisite for executive functions to emerge. Furthermore, neurocognitive functions are an important prerequisite for successful learning (Brown & Blanton, Citation2002; Diamond, Citation2013), and executive functions are indispensable for success throughout life (Diamond & Lee, Citation2011). In terms of brain development, grey and white matter have been found to continue developing up to 30 years of age, with the most prominent changes taking place in the prefrontal cortex (Lebel et al., Citation2008; Lenroot & Giedd, Citation2006; Tamnes et al., Citation2017; Whitford et al., Citation2007). Therefore, cognitive outcomes and academic performance might not be ideally optimized and supported since these functions strongly rely on frontal lobe functioning.
There are some physiological mechanisms supposed to underlie the supposed beneficial effects of physical activity on cognitive outcomes and academic performance. A single bout of physical activity (also referred to as acute physical activity) increases cerebral blood flow and neurotransmitter secretion levels, resulting in increased levels of arousal, attention and effort, which in turn positively influence the performance on cognitive tasks shortly after performing physical activity (Best, Citation2010; Kashihara et al., Citation2009; Tomporowski, Citation2003). Repeated bouts of physical activity (also referred to as chronic physical activity or chronic exercise) has been shown to lead to angiogenesis (Best, Citation2010), synaptogenesis and neurogenesis (Hillman et al., Citation2015; Ross et al., Citation2015). Such morphological changes in brain structure may lead to enhanced cognitive outcomes and academic performance (Best, Citation2010). Due to the resulting structural changes, effects of a chronic intervention may persist longer.
The existing literature mostly focuses on acute physical activity interventions in preadolescent children (Fedewa & Ahn, Citation2011; De Greeff et al., Citation2018; Lees & Hopkins, Citation2013) or elderly populations (Angevaren et al., Citation2007; Northey et al., Citation2017). Relatively little is known about the age group of adolescents and young adults, and about the effects of chronic physical activity (Li et al., Citation2017; Verburgh et al., Citation2013; Xue et al., Citation2019). Furthermore, most studies focus on cognitive outcomes, while few studies describe effects of physical activity interventions on academic performance (Haapala, Citation2012). However, in the last decade new research is emerging in these topics. Therefore, it is important to get a full overview of the effects of acute- and chronic physical activity interventions on cognitive outcomes and academic performance in adolescents and young adults.
The aim of this paper is to provide a meta-analysis of studies investigating the effects of physical activity interventions on cognitive outcomes and academic performance in adolescents or young adults. We distinguished between studies addressing effects of acute and chronic physical activity. If possible, we distinguished between subdomains of cognitive outcomes and subdomains of academic performance. Moderator analyses were conducted to provide insight into the possible moderating effects of the nature of the sample studied, study and intervention characteristics and study quality.
Method
The protocol of this meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42019122030). This review was conducted in accordance with the guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Moher et al., Citation2009).
Inclusion and exclusion criteria
This meta-analysis included all studies examining the effects of physical activity interventions on cognitive outcomes and academic performance published in peer-reviewed English language journals. The search included all studies indexed till the 27th of March 2020. The selection of studies was based on multiple inclusion criteria. First, the physical activity intervention had to contain a sports or exercise component. Both acute physical activity interventions (one bout of physical activity) and chronic physical activity interventions (an intervention programme that contains continuous physical activity over several days) were selected. Second, the mean age of the participants in the sample had to be between 12 and 30 years (Rindfuss, Citation1991). This age range includes adolescents from later-puberty till early adulthood (American College of Sports Medicine, Citation2013). If no mean age was given then the age range of the sample had to be between 10 and 32 years old. Third, the studies had to use a controlled design, with or without random allocation. If no random allocation was used, adequate adjustment for baseline differences between groups was required (e.g., analyses of covariance). Fourth, the study had to report on outcomes that were of interest in the present article (e.g., processing speed, attention, executive function or academic performance) before and after the intervention. The following studies were excluded from the current meta-analysis: 1. studies targeting special populations, as generalisation of findings of these studies would be limited (e.g., adolescents or young adults with mental or cognitive disorders, nervous system diseases or brain injuries), 2. reviews, and 3. studies that combined a physical activity intervention with another intervention such as nutrition.
Search strategy
The electronic databases Embase, ERIC, MEDLINE, PsycINFO and Web of Science were searched for relevant studies. The search string combined four elements including terms describing 1. the physical activity intervention, 2. the age group studied, 3. the neurocognitive or academic outcome studied and 4. the study design used. The complete search string is provided in Appendix A.
After removal of duplicates, the first author BH and a trained research assistant independently screened the titles and abstracts to assess eligibility criteria. Following this, full-text articles were assessed independently by the first author BH and another trained research assistant. Disagreements were discussed and consensus was reached together. If multiple articles included the same participants and reported on the same outcome measure, the article with the largest sample size was selected. Reference lists of included studies were further examined as complementary sources.
Data extraction
Sample descriptives, study design, outcome measures and characteristics of the intervention were independently extracted by two review authors (BH, RW). Mean and accompanying standard deviations (SDs) were extracted for all outcome measures. If a study collected data at multiple time points, only baseline scores and scores closest to the end of the intervention were used. If a study reported the outcome of interest in multiple subgroups (e.g., fit vs. unfit or high vs. low social economic status), the mean of the subgroups weighted by their sample sizes was used in the analyses (Moher et al., Citation2009). If p values were reported as p < 0.05, we assumed a p value of 0.05 in order to calculate the standard error and the accompanying 95% CI. If p values were reported as p > 0.05, we assumed a p-value of 0.53 (i.e. the average of 0.05 and 1.00) in order to calculate the standard error and 95% CI (Bland, Citation2013). If multiple intervention groups were compared to the same control group, the sample size of the control group was divided by the number of intervention groups. If data required to calculate effect sizes were missing, authors were contacted to request the missing information. The effects sizes (ESs) were corrected for study design by the Comprehensive Meta-Analysis software (version 3). Given that all outcome measures were recorded as continuous measures, the standardized mean difference (SMD) was used to quantify the effect size.
Risk of bias assessment
Two authors (BH, RW) independently assessed the risk of bias of the included studies following the Cochrane Collaboration tool for assessing Risk of Bias (Higgins et al., Citation2011). This tool addressed six domains: selection bias, performance bias, detection bias, attribution bias, reporting bias and other bias. For each of these domains risk of bias was judged distinguishing between low risk of bias, high risk of bias or unclear risk of bias. Discrepancies regarding risk of bias were discussed until consensus was reached.
Statistical analysis
Statistical analysis was performed using Comprehensive Meta-Analysis. First, separate meta-analyses were conducted for acute and chronic physical activity interventions, thereby distinguishing between cognitive outcomes and academic performance. Second, based on the literature, a subgroup analysis was conducted for several subdomains. Subdomains of cognitive outcomes included: processing speed, attention and executive function. Executive functions were further separated into cognitive flexibility, working memory, and inhibition. The subdomains of academic performance were grade point average, language and mathematics. Third, to control for dependency between multiple outcome variables (e.g., accuracy and reaction time) within a subdomain (e.g., processing speed), a mean effect size was calculated across the related outcome measures. Finally, heterogeneity was assessed using the X2 test and the I2 statistic. If study results were heterogeneous (I2 > 50% or X2 test p value <0.05), the calculated effect size cannot be treated as estimate of a common effect size. In that case, a moderator analysis was conducted to study possible sources of the heterogeneity. Moderator analyses tested the effects of study, sample and/or intervention characteristics. If possible, moderator analyses were performed for type of intervention, type of control condition, study design and way of testing in academic performance. For each meta-analyses, moderator analyses were only performed if each category of the potential moderator was filled with at least three studies (Borenstein et al., Citation2011). If there were ten or more than ten studies, and the moderator was a continuous variable, a meta-regression was performed (Borenstein et al., Citation2011). This was the case in acute interventions for age, percentage of boys, duration (min), intensity and dose (duration x intensity). For chronic interventions, meta-regression analyses were performed for: age, percentage of boys, duration (weeks), frequency (times per week), dose (min per week), load (min per intervention) and difference in physical fitness levels. The effect sizes were corrected for small sample bias (Hedges’ g) and reported with 95% confidence intervals (CIs). The magnitude of Hedges’ g was interpreted using Cohen’s guidelines, distinguishing between small (<0.2), moderate (0.5), and large (>0.8) effect sizes (Cohen, Citation1988). Positive effect sizes favoured the exercise group while negative effect sizes favoured the rest/control group. A random effects approach was used to compute effect sizes. The presence of publication bias was assessed by using a funnel plot and performing the Egger’s linear regression method. Statistical significance was adopted for all tests when p < 0.05.
Results
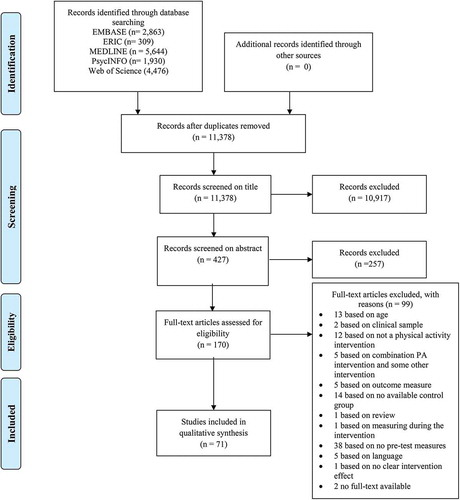
The initial search in the databases netted 15,222 records. After removing duplications and screening the titles, 427 papers were eligible for further screening (see ). One hundred and seventy papers were read full text and a further 99 papers not meeting the inclusion criteria were excluded. In total 71 papers were eligible for this review and meta-analysis. Overall, 44 studies (Aguirre-Loaiza et al., Citation2019; Akatsuka et al., Citation2015; Basso et al., Citation2015; Benzing et al., Citation2016; Browne et al., Citation2016; Budde et al., Citation2010, Citation2008; Chang & Etnier, Citation2009; Chang et al., Citation2011; Coles & Tomporowski, Citation2008; Cooper et al., Citation2016, Citation2012; ÉW et al., Citation2011; Guzmán & López-García, Citation2016; Hwang et al., Citation2016; Hwang & Lu, Citation2018; Jaffery et al., Citation2018; Lambourne, Citation2012; Lambourne et al., Citation2010; Ludyga, Pühse, et al., Citation2019; Mezcua-Hidalgo et al., Citation2019; Moore et al., Citation2012; Murray & Russoniello, Citation2012; Palmiere et al., Citation2018; Peruyero et al., Citation2017; Pontifex et al., Citation2009; Prashanth, Citation2020; Du Rietz et al., Citation2019; Schwarck et al., Citation2019; Sipavičienė et al., Citation2012; Sperlich et al., Citation2018; Takahashi et al., Citation2019; Tine, Citation2014; Tine & Butler, Citation2012; Tsai et al., Citation2014, Citation2016; Tsorbatzoudis et al., Citation1998; Tsukamoto et al., Citation2017; Van den Berg et al., Citation2018; Vonk et al., Citation2019; Wang et al., Citation2015; Whyte et al., Citation2014; Zhou & Qin, Citation2019; Zimmer et al., Citation2016) investigated the effects of acute physical activity interventions, and 27 studies (Ardoy et al., Citation2014; Butzer et al., Citation2015; Costigan et al., Citation2016; Duarte et al., Citation2020; Hagins & Rundle, Citation2016; Heisz et al., Citation2017; Jeon & Ha, Citation2017; Johann et al., Citation2016; Kauts & Sharma, Citation2009; Lennemann et al., Citation2013; Ludyga, Gerber, Herrmann, et al., Citation2018; Ludyga, Gerber, Kamijo, et al., Citation2018; Ludyga, Köchli, et al., Citation2019; Matthews et al., Citation2016; Pinto-Escalona & Martínez-de-Quel, Citation2019; Purohit & Pradhan, Citation2017; Sharma et al., Citation2015; Sjöwall et al., Citation2019; Spitzer & Hollmann, Citation2013; Stroth et al., Citation2009, Citation2010; Subramanian et al., Citation2015; Tarp et al., Citation2016; Torbeyns et al., Citation2017; Venckunas et al., Citation2016; Woost et al., Citation2018; Zheng et al., Citation2015) investigated the effects of chronic physical activity interventions.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram of each stage of the study selection
Study characteristics
Taken together, the studies included 3544 adolescents and 2397 young adults. Academic performance was only assessed in adolescents and not in young adults. Appendix B1 and B2 show the extracted details from the studies incorporated in this meta-analysis, including: mean age, sample size, outcome (measurement), in- and exclusion criteria and characteristics of the tested intervention: type, dose, frequency, duration and intensity.
Effects of physical activity interventions
The calculated effect sizes and heterogeneity statistics are shown in and . Meta-analytic effects were calculated separately for the acute and chronic intervention studies. Furthermore, meta-analytic effects were calculated for different subdomains of cognitive outcomes and academic performance. As heterogeneity was present in executive functions, this subdomain was further subdivided into cognitive flexibility, working memory and inhibition.
Table 1. Meta-analytic results of the effects for physical activity interventions on cognitive outcomes and academic performance
Effects of acute physical activity interventions
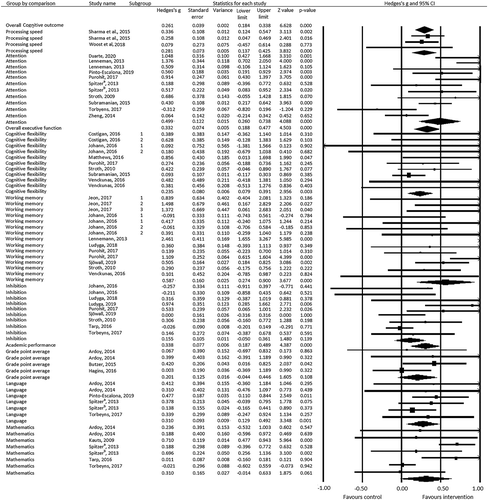
Fourty-four studies (k = 91) examined the effects of acute physical activity interventions on cognitive outcomes or academic performance. Only one study focused on academic performance, while the other 43 studies focused on cognitive outcomes. No meta-analysis was conducted for academic performance. Overall, acute physical activity resulted in a moderate effect on cognitive outcomes (Hedges’s g = 0.31; 95% CI 0.22 to 0.41). Heterogeneity in effect sizes was large (I2 = 64.9%, p < 0.001). Further analyses distinguishing between core domains of cognitive outcomes, showed a significant moderately sized effect for processing speed (Hedges’s g = 0.39; 95% CI 0.07 to 0.72), a significant moderately sized effect for attention (Hedges’s g = 0.34; 95% CI 0.13 to 0.55) and, a significant moderately sized effect for executive functions (Hedges’s g = 0.29; 95% CI 0.19 to 0.40). Heterogeneity was large for all three effects (see ). A further distinction between executive functions yielded a significant moderately sized effect for inhibition (Hedges’s g = 0.32; 95% CI 0.20 to 0.44), but no effects for cognitive flexibility (Hedges’s g = 0.37; 95% CI −0.00 to 0.75), and working memory (Hedges’s g = 0.14; 95% CI −0.11 to 0.39).
Moderator analysis
Moderator analyses were performed for attention, cognitive flexibility, working memory and inhibition because heterogeneity was present in these domains. summarises the results of these analyses.
Table 2. Results of moderator analyses and meta-regression analyses
Analyses with categorical moderators showed that the effects of acute physical activity interventions was not significantly different between RCT and cross-over studies in working memory and inhibition. Furthermore, there was no significant difference between studies contrasting the effects of acute physical activity to a rest condition or a condition involving some physical activity as a control condition. Other moderator analyses could not be performed due to the small number of studies available in the subgroups.
In the meta-regressions, longer duration of the intervention was inversely related to the effects on attention (β = −0.021, 95% CI −0.04 to −0.01, p = 0.006) and cognitive flexibility (β = −0.036, 95% CI −0.06 to −0.01, p = 0.012). Duration of the intervention was not significantly related to the effects for working memory and inhibition. Furthermore, higher dose of the intervention was inversely related to the effects of attention (β = −0.00; 95% CI −0.00 to 0.00, p = 0.028). Dose of the intervention was not significantly related to effects for working memory and inhibition. Age, percentage of boys and intensity were not significantly related with the studies’ effect sizes for any of the cognitive domains or there were too few to perform the meta regression analyses.
Effects of chronic physical activity interventions
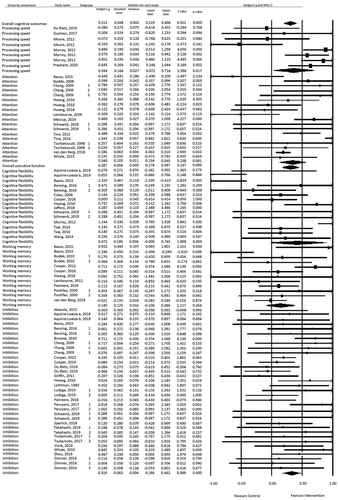
Twenty-seven studies examined the effects of chronic physical activity interventions on cognitive outcomes (k = 52) or academic performance (k = 15). Overall, chronic physical activity interventions had a moderate effect on cognitive outcomes (Hedges’s g = 0.36; 95% CI 0.25 to 0.47). There was moderate heterogeneity in the individual studies’ effect sizes (I2 = 62%, p < 0.001). Further analyses distinguishing between core domains of cognitive outcomes showed three significant moderately sized effects for: processing speed (Hedges’s g = 0.30; 95% CI 0.15 to 0.45), attention (Hedges’s g = 0.50; 95% CI 0.26 to 0.74), and executive functions (Hedges’s g = 0.35; 95% CI 0.21 to 0.50). Heterogeneity was large for all three effects (see ). A further distinction between executive functions yielded a significant small sized effect for cognitive flexibility (Hedges’s g = 0.19; 95% CI 0.05 to 0.33) and a significant large sized effect for working memory (Hedges’s g = 0.59; 95% CI 0.27 to 0.90), but no effect for inhibition (Hedges’s g = 0.16; 95% CI −0.05 to 0.36).
Furthermore, chronic physical activity interventions had a significant moderately sized effect on academic performance (Hedges’s g = 0.34; 95% CI 0.19 to 0.49). Heterogeneity in effect sizes was moderate (I2 = 63%, p = 0.001). Further analyses distinguishing between core domains of academic performance showed a significant moderately sized effect for language (Hedges’s g = 0.31; 95% CI 0.13 to 0.49), but no effects for grade point average (Hedges’s g = 0.20; 95% CI −0.04 to 0.45) and mathematics (Hedges’s g = 0.31; 95% CI −0.01 to 0.63).
Moderator analysis
Moderator analyses were performed for attention, working memory, and mathematics, since heterogeneity was present in these domains. summarises the results of the subgroup analysis and the meta-regressions. None of the domains had a sufficient number of studies to perform a subgroup analysis (design, type of intervention, control condition or way of testing in academic performance).
In the meta-regressions, percentage of boys was significantly and inversely related to the effects of attention (β = 0.02; 95% CI 0.00 to 0.03, p = 0.021) and working memory (β = 0.01; 95% CI 0.00 to 0.03, p = 0.033). Age, duration and frequency were not significantly related with the studies’ effect sizes in attention. There were not enough studies reporting dose, load and difference physical fitness to calculate their relation to the effect on attention. For working memory, age, duration, frequency, dose and load were not significantly related with the studies’ effect sizes. There were not enough studies reporting difference in physical fitness to calculate the relation to working memory’s effect size. Finally, meta-regression analysis could not be performed for mathematics due to the small number of studies available.
Risk of bias
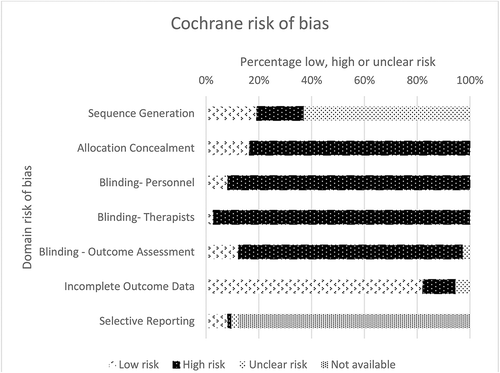
Risk of bias was assessed using the Cochrane risk of bias tool and the results are summarized in . First, selection bias was assessed by sequence generation and allocation concealment. Most of the included studies (63.0%) did not provide a sufficient description of the process of sequence generation which made it impossible to assess the risk of bias. Therefore, these studies were judged as having an unclear risk of bias for sequence generation. In addition, the majority of the studies (65.8%) did not describe the allocation concealment and were consequently judged as having high risk of bias. Second, performance bias was assessed by blinding the researchers and blinding those who delivered the intervention. In most studies it was not possible to blind researchers and those involved in delivering the intervention (91.8% and 97.3% respectively), therefore most studies were scored as having a high risk of bias. Third, detection bias was assessed by blinding the researchers during the measurements. In most studies this was not reported and scored as high risk of bias (84.9%). Fourth, the attrition bias was assessed by risk of bias due to incomplete data and was scored as low risk in most studies (82.2%). Fifth, reporting bias was assessed by the risk of bias due to selective reporting and this was judged as not available in most studies (87.7%). Sixth, no other sources that could have caused bias were observed.
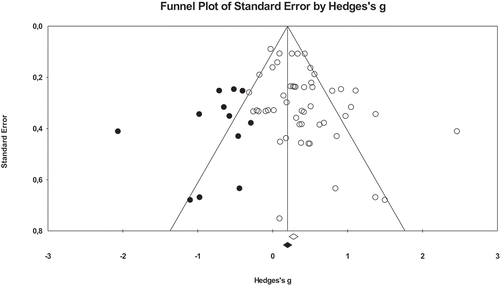
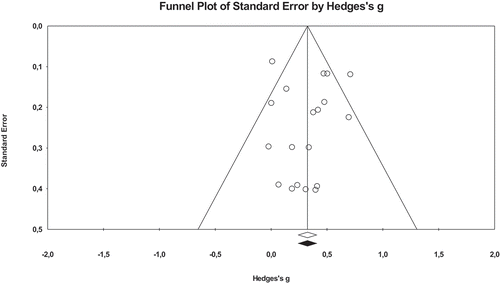
Publication bias
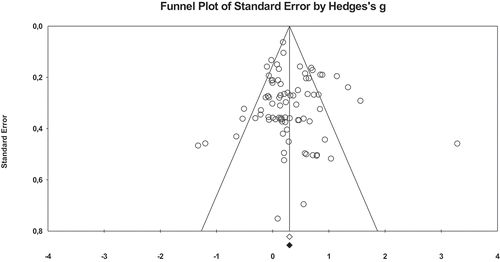
The funnel plot and the Egger’s regression procedure indicated that there was neither evidence for publication bias for any of the outcome measures in the acute intervention studies (p = 0.653) nor for any of the academic performance in the chronic intervention studies (p = 0.926). However, the funnel plot and the Egger’s regression procedure indicated that there were indications for the presence of publication bias for the cognitive outcome measures in the chronic physical activity intervention studies (p = 0.007), see Appendix C. Therefore, the observed associations between the chronic physical activity interventions and cognitive outcomes may be overestimated.
Discussion
This meta-analysis investigated the effects of acute and chronic physical activity interventions on cognitive outcomes and academic performance in adolescents and young adults. Results of the meta-analysis showed that acute physical activity interventions have a positive effect on attention, processing speed and inhibition. Furthermore, the meta-analysis showed that chronic physical activity interventions have a positive effect on processing speed, attention, cognitive flexibility, working memory and language, where the largest effect was found on working memory.
Interpretation
Most of the conducted meta-analyses showed large or medium heterogeneity between the individual studies’ effect sizes. Therefore, the findings should be interpreted with caution. We investigated potential sources of heterogeneity by performing several meta-regression analyses and subgroup analyses. Some remarkable findings and possible sources of heterogeneity will be discussed.
An interesting finding of this meta-analysis is the beneficial effect of chronic physical activity interventions on cognitive outcomes and especially on the core domains working memory and cognitive flexibility. Our findings confirm the results of Rathore & Lom (Citation2017) who also found positive significant effect sizes for working memory and those of De Greeff et al. (Citation2018) who found positive results for working memory and cognitive flexibility, but not in inhibition. However, our findings differ from the meta-analytic results of Xue et al. (Citation2019), who showed positive effects on inhibition, but not on working memory or cognitive flexibility and of Alvarez-Bueno et al. (Citation2017), who showed positive effects on inhibition, but not on working memory or cognitive flexibility. Overall, the previous meta-analytic studies were inconsistent in their findings. Probably, these divergent findings have emerged because these meta-analyses included mostly pre-adolescent children or older adults and almost no adolescents or early adulthood subjects. The transition from childhood to adulthood starts with physical changes and hormonal changes. These changes start in late childhood or in early adolescence, also referred to as early puberty, and continue into early adulthood (Malina et al., Citation2004). In the later-puberty, rapid development of the prefrontal cortex takes place which may make this structure especially sensitive to the effects of interventions, like physical activity interventions, compared to other developmental stages (Lebel et al., Citation2008). Moreover, the transition from childhood into adulthood is characterised by a marked decrease in physical activity and an increase in sedentary behaviour (Corder et al., Citation2017; Hardy et al., Citation2007; Kimm et al., Citation2000). The low levels of physical activity in adolescents might make physical activity interventions result in relatively large increases in physical activity in later-puberty than in pre-adolescent children. Concluding the current meta-analysis is the first to include a substantial number of studies investigating the effects of chronic physical activity interventions in adolescents and young adults which might explain the different conclusion reached as compared to previous reviews.
Another interesting finding was that chronic physical activity interventions were found to have a moderately sized beneficial effect on academic performance. Further analyses showed that a chronic intervention can positively affect language, but not grade point average or mathematics. This is slightly different from a previous meta-analysis, which showed positive effects on science, including mathematics, but not on language or grade point average (Spruit et al., Citation2016). In another meta-analysis, positive effects on language as well as on mathematics were found (Álvarez-Bueno, Pesce, Cavero-Redondo, Sánchez-López, Garrido-Miguel et al., Citation2017). However, in that meta-analysis mainly preadolescent children were included. Only 3 out of 25 studies were conducted in similar age ranges as included in the current meta-analysis (12–30 years). While in the age range of 12–18 years a lot of behavioural changes take place that stay present in adults (Cluskey & Grobe, Citation2009). If more studies are conducted in adolescence a more clear effect with possibly less heterogeneity will be visible in this age. Heterogeneous results between studies in this meta-analysis can possibly be explained by the fact that mostly school grades were used as outcome measure instead of standardized tests. School grades are less objective and comparable with other studies than standardized tests, because they depend on the topic discussed in that period and sometimes the grade is awarded subjectively by the teacher. Furthermore, it should be noted that academic performance was only measured in adolescents and not in young adults, so findings cannot be generalized to young adulthood. Moreover, the oldest mean age was 15.3 years. In sum, findings for the effects of chronic physical activity interventions on the subdomains of academic performance are inconsistent and more research is warranted, especially with standardized tests.
This meta-analysis showed significant effect sizes after a chronic intervention but not after an acute intervention on cognitive flexibility and working memory. While chronic interventions showed a large effect size, acute interventions yielded small or non-significant effects, suggesting that only chronic physical activity interventions may affect cognitive flexibility and working memory. One possible explanation for this difference in effect sizes is that different underlying mechanisms operate in acute and chronic interventions. It is suggested that physical activity, for example, facilitates working memory by increasing the efficiency of evaluating the stimulus (Chang et al., Citation2013). Underlying processes that could increase this efficiency are neurogenesis and synaptogenesis, which are related to chronic interventions (Hillman et al., Citation2015). In the review of Gomez-Pinilla & Hillman (Citation2013) brain-derived neurotrophic factor (BDNF) is suggested to be an important initiator of synaptic plasticity. Exercise influences the production of BDNF in the area critical for learning and memory. After acute interventions, BDNF levels are increased for a short time period and could explain increased levels of arousal, attention and effort, which positively influences the performance on cognitive tasks (Kashihara et al., Citation2009; Tomporowski, Citation2003). However, after a chronic intervention BDNF levels stay increased and therefore have a structural effect on neurons, like neurogenesis and synaptic plasticity. It seems that the underlying process like synaptogenesis, neurogenesis and synaptic plasticity are the underlying mechanisms of improved cognitive flexibility and working memory.
A remarkably large difference between acute and chronic physical activity interventions was found on working memory. Sample and study characteristics might explain the discrepant findings for this difference in effect size. Sample characteristics sex and age were tested as possible moderators. Sex did differ between the two types of studies: in chronic interventions, effects were stronger for studies with a high percentage of boys compared to low percentage of boys, while no difference in the effects for high and low percentage of boys was found in acute studies. This may have been due to differences in compliance (boys may have been more active in chronic studies) or stage of maturation. Moreover, during puberty physical activity patterns change, earlier in girls than in boys (Bacil et al., Citation2015). Due to the difference in time onset of physical activity changes and biological differences, boys have probably a better maximum oxygen uptake and are more active during and outside the intervention time, which will lead to a more pronounced effect on cognition in boys compared to girls. However, information about adherence and compliance in the intervention and the maturation was not always reported in the studies and therefore we cannot say whether this is the case. Therefore, it is suggested for future studies to measure compliance, adherence and maturation of boys and girls when conducting an intervention study. Next to sex, age was also tested as a moderator, however age had similar effects in acute versus chronic studies, and could therefore not explain the difference between chronic and acute studies. The used measurement instrument is an example of a study characteristic that could explain the discrepant findings. However, the conflicting findings do not seem to be related to differences in the measures used to assess working memory: for both acute as well as chronic intervention studies, similar instruments were used to measure working memory in the included studies. The majority of the studies used the Digit Span test or the N-back test. Therefore, it seems unlikely that the type of measurement instrument has contributed to the different results.
Furthermore, the type of interventions that were delivered seem to differ between acute and chronic studies. In acute intervention studies only aerobic or resistance exercises were given, but in chronic studies also yoga and cognitively challenging exercises were given besides or instead of the aerobic exercises. In cognitively challenging interventions the motor-cognition network might play a role in the improvement of the executive functions. This network is supported by the recruitment of neural regions during performance of motor tasks, which are typically associated with cognitive operations such as the dorsolateral prefrontal cortex and the neo-cerebellum (Ludyga et al., Citation2016). During physical activity, cognition may or may not be challenged by performing (increasingly) difficult movements or by increasing rules or the number of objects to be handled in an exercise (Tomporowski et al., Citation2015). We assume that in particular these characteristics of exercises challenge the motor-cognition network and this might explain the different results found in working memory. There were too few studies to examine whether cognitively challenging interventions have a different effect on executive functions compared to other types of physical activity interventions, but results of meta-analyses in preadolescent children support this hypothesis (De Greeff et al., Citation2018; Vazou et al., Citation2019). In sum, chronic intervention studies seem more promising to improve working memory than acute interventions in adolescents, however, it is not clear what causes this difference.
Findings in our meta-regression revealed that, in acute intervention studies, intervention effects were smaller with longer duration for attention and cognitive flexibility. Between studies, duration of interventions varied between 5 and 60 minutes. This finding suggests that a short bout of physical activity is more effective than longer bouts of physical activity to improve cognitive functioning. However, studies did not investigate the optimal intervention length, nor the persistence of the observed beneficial effects on cognitive functions. In the meta-analysis of Ludyga et al. (Citation2016) it was suggested that short aerobic exercise bouts of moderate intensity restore cognitive resources, a finding that is in line with our results. Furthermore, it could be that with increasing intervention duration, participants get more exhausted, which in turn would have a negative effect on task performance after a long intervention session. In sum, it seems that a short acute physical activity intervention is more efficient than a long bout of acute physical activity intervention to improve cognitive functions. Further research is needed to investigate the optimal duration of an acute physical activity intervention and to investigate how long positive effects remain after an acute bout of physical activity.
Strengths and limitations
One strength of this meta-analysis is the inclusion of both adolescents and young adults, since these age periods reflect a period of rapid maturation of the brain, with most prominent changes in the prefrontal cortex (Lebel et al., Citation2008). Moreover, the transition to adulthood often concurs with life changes, that influence health related behaviours, like behaviour changes in a new environment, with changing social contacts and support (Cluskey & Grobe, Citation2009; Horn et al., Citation2008). Another strength is the inclusion of studies with a RCT or cross-over design only and the exclusion of observational and cross-sectional studies. This strict inclusion increases the reliability of the causal effects of physical activity interventions on cognitive functions and academic performance. Finally, we conducted a risk of bias assessment to get more insight in the methodological quality of the included studies. A limitation of this review was that we could not perform all subgroup-analyses and meta-regression analyses due to the small number of studies available. Furthermore, for chronic intervention studies, we were neither able to perform meta-regression analyses to study the effects of total load (intensity × dose) nor to study the effects of improvement on physical fitness, because most studies did not report intensity levels. Another limitation is the possibility of publication bias in the studies investigating chronic physical activity interventions. Lastly, cognitive outcomes were measured across studies using a wide variety of measurement instruments, which could have contributed to the heterogeneity in the meta-analyses.
Conclusion
In conclusion, this meta-analysis demonstrates that there are positive effects of physical activity interventions on cognitive outcomes in both acute and chronic studies in adolescents and young adults. Furthermore, this meta-analysis demonstrates a positive effect of physical activity on academic performance within the language domain, but not on grade point average or mathematics, in adolescents. Additionally, this study shows that in acute interventions shorter bouts of exercise appear to be more effective than longer bouts of exercise for improving cognitive outcomes. Finally, both acute and chronic interventions seem promising to improve cognitive outcomes and academic performance in adolescents and young adults. However, chronic physical activity interventions seem to have larger effect sizes on a broader range of cognitive outcomes than acute physical activity interventions. So in practice it is recommended to implement chronic physical activity interventions instead of acute physical activity interventions when possible.
Acknowledgdements
This study was supported by a grant from the Netherlands Initiative for Education Research (405-16-411) and the Dutch Brain Foundation. The authors would like to thank Aveline Hylkema, Annemieke Wargers, Lonneke Snoeren and Renée van Dinter for their work during the search process.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Aguirre-Loaiza, H., Arenas, J., Arias, I., Franco-Jímenez, A., Barbosa-Granados, S., Ramos-Bermúdez, S., Ayala-Zuluaga, F., Núñez, C., & García-Mas, A. (2019). Effect of acute physical exercise on executive functions and emotional recognition: Analysis of moderate to high intensity in young adults. Frontiers in Psychology, 10(2774). https://doi.org/10.3389/fpsyg.2019.02774
- Akatsuka, K., Yamashiro, K., Nakazawa, S., Mitsuzono, R., & Maruyama, A. (2015). Acute aerobic exercise influences the inhibitory process in the go/no-go task in humans. Neuroscience Letters, 600, 80–84. https://doi.org/10.1016/j.neulet.2015.06.004
- Álvarez-Bueno, C., Pesce, C., Cavero-Redondo, I., Sánchez-López, M., Garrido-Miguel, M., & Martínez-Vizcaíno, V. (2017). Academic achievement and physical activity: A meta-analysis. Pediatrics, 140(6), e20171498. https://doi.org/10.1542/peds.2017-1498
- Álvarez-Bueno, C., Pesce, C., Cavero-Redondo, I., Sánchez-López, M., Martínez-Hortelano, J. A., & Martínez-Vizcaíno, V. (2017). The effect of physical activity interventions on children’s cognition and metacognition: A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(9), 729–738. https://doi.org/10.1016/j.jaac.2017.06.012
- American College of Sports Medicine. (2013). ACSM’s certification review. Lippincott Williams & Wilkins.
- Angevaren, M., Vanhees, L., Wendel-Vos, W., Verhaar, H. J. J., Aufdemkampe, G., Aleman, A., & Verschuren, W. M. M. (2007). Intensity, but not duration, of physical activities is related to cognitive function. European Journal of Cardiovascular Prevention & Rehabilitation, 14(6), 825–830. https://doi.org/10.1097/HJR.0b013e3282ef995b
- Ardoy, D. N., Fernández‐Rodríguez, J. M., Jiménez‐Pavón, D., Castillo, R., Ruiz, J. R., & Ortega, F. B. (2014). A physical education trial improves adolescents’ cognitive performance and academic achievement: The EDUFIT study. Scandinavian Journal of Medicine & Science in Sports, 24(1), 1. https://doi.org/10.1111/sms.12093
- Bacil, E. D. A., Júnior, O. M., Rech, C., Legnani, R. F. D. S., & de Campos, W. (2015). R., dos Santos Legnani, R. F., & de Campos, W. Physical activity and biological maturation: A systematic review. Revista Paulista De Pediatria (English Edition), 33(1), 114–121. https://doi.org/10.1016/S2359-3482(15)30037-3
- Basso, J. C., Shang, A., Elman, M., Karmouta, R., & Suzuki, W. A. (2015). Acute exercise improves prefrontal cortex but not hippocampal function in healthy adults. Journal of the International Neuropsychological Society, 21(10), 791–801. https://doi.org/10.1017/S135561771500106X
- Benzing, V., Heinks, T., Eggenberger, N., Schmidt, M., & Verdejo-García, A. (2016). Acute cognitively engaging exergame-based physical activity enhances executive functions in adolescents. PloS One, 11(12), e0167501. https://doi.org/10.1371/journal.pone.0167501
- Best, J. R. (2010). Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Developmental Review, 30(4), 331–351. https://doi.org/10.1016/j.dr.2010.08.001
- Bland, M. (2013). Do baseline p-values follow a uniform distribution in randomised trials? PloS One, 8(10), e76010. https://doi.org/10.1371/journal.pone.0076010
- Borenstein, M., Hedges, L. V., Higgins, J. P., & Rothstein, H. R. (2011). Introduction to meta-analysis. John Wiley & Sons.
- Brown, D. R., & Blanton, C. J. (2002). Physical activity, sports participation, and suicidal behavior among college students. Medicine and Science in Sports and Exercise, 34(7), 1087–1096. https://doi.org/10.1097/00005768-200207000-00006
- Browne, R. A. V., Costa, E. C., Sales, M. M., Fonteles, A. I., & Moraes, F. (2016). Acute effect of vigorous aerobic exercise on the inhibitory control in adolescents. Revista Paulista De Pediatria, 34(2), 154–161. https://doi.org/10.1016/j.rpped.2015.08.004
- Budde, H., Voelcker-Rehage, C., Pietrassyk-Kendziorra, S., Machado, S., Ribeiro, P., & Arafat, A. M. (2010). Steroid hormones in the saliva of adolescents after different exercise intensities and their influence on working memory in a school setting. Psychoneuroendocrinology, 35(3), 382–391. https://doi.org/10.1016/j.psyneuen.2009.07.015
- Budde, H., Voelcker-Rehage, C., Pietrassyk-Kendziorra, S., Ribeiro, P., & Tidow, G. (2008). Acute coordinative exercise improves attentional performance in adolescents. Neuroscience Letters, 441(2), 219–223. https://doi.org/10.1016/j.neulet.2008.06.024
- Butzer, B., van Over, M., Noggle Taylor, J. J., & Khalsa, S. B. S. (2015). Yoga may mitigate decreases in high school grades. Evidence-Based Complementary and Alternative Medicine, 2015 (259814), 1–8. https://doi.org/10.1155/2015/259814
- Chang, Y., & Etnier, J. L. (2009). Exploring the dose-response relationship between resistance exercise intensity and cognitive function. Journal of Sport & Exercise Psychology, 31(5), 640–656. https://doi.org/10.1123/jsep.31.5.640
- Chang, Y., Huang, C., Chen, K., & Hung, T. (2013). Physical activity and working memory in healthy older adults: An ERP study. Psychophysiology, 50(11), 1174–1182. https://doi.org/10.1111/psyp.12089
- Chang, Y., Tsai, C., Hung, T., So, E. C., Chen, F., & Etnier, J. L. (2011). Effects of acute exercise on executive function: A study with a tower of london task. Journal of Sport & Exercise Psychology, 33(6), 847–865. https://doi.org/10.1123/jsep.33.6.847
- Cluskey, M., & Grobe, D. (2009). College weight gain and behavior transitions: Male and female differences. Journal of the American Dietetic Association, 109(2), 325–329. https://doi.org/10.1016/j.jada.2008.10.045
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.) Lawrence Erlbaum Associates, Hillsdale NJ.
- Coles, K., & Tomporowski, P. D. (2008). Effects of acute exercise on executive processing, short-term and long-term memory. Journal of Sports Sciences, 26(3), 333–344. https://doi.org/10.1080/02640410701591417
- Cooper, S. B., Bandelow, S., Nute, M. L., Dring, K. J., Stannard, R. L., Morris, J. G., & Nevill, M. E. (2016). Sprint-based exercise and cognitive function in adolescents. Preventive Medicine Reports, 4, 155–161. https://doi.org/10.1016/j.pmedr.2016.06.004
- Cooper, S. B., Bandelow, S., Nute, M. L., Morris, J. G., & Nevill, M. E. (2012). The effects of a mid-morning bout of exercise on adolescents’ cognitive function. Mental Health and Physical Activity, 5(2), 183–190. https://doi.org/10.1016/j.mhpa.2012.10.002
- Corder, K., Winpenny, E., Love, R., Brown, H. E., White, M., & van Sluijs, E. (2017). Change in physical activity from adolescence to early adulthood: A systematic review and meta-analysis of longitudinal cohort studies. British Journal of Sports Medicine, 53(8), 496-503.http://dx.doi.org/10.1136/bjsports-2016-097330.
- Costigan, S. A., Eather, N., Plotnikoff, R. C., Hillman, C. H., & Lubans, D. R. (2016). High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Medicine & Science in Sports & Exercise, 48(10), 1985–1993. https://doi.org/10.1249/MSS.0000000000000993
- De Greeff, J. W., Bosker, R. J., Oosterlaan, J., Visscher, C., & Hartman, E. (2018). Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. Journal of Science and Medicine in Sport, 21(5), 501–507. https://doi.org/10.1016/j.jsams.2017.09.595
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
- Diamond, A., & Lee, K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333(6045), 959–964. https://doi.org/10.1126/science.1204529
- Du Rietz, E., Barker, A. R., Michelini, G., Rommel, A.-S., Vainieri, I., Asherson, P., & Kuntsi, J. (2019). Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men. Behavioural Brain Research, 359, 474–484. https://doi.org/10.1016/j.bbr.2018.11.024
- Duarte, L., Gonçalves, M., Mendes, P., Matos, L. C., Greten, H. J., & Machado, J. (2020). Can Qigong improve attention in adolescents? A prospective randomised controlled trial. Journal of Bodywork and Movement Therapies, 24(1), 175–181. https://doi.org/10.1016/j.jbmt.2019.05.005
- Esteban-Cornejo, I., Tejero-Gonzalez, C. M., Sallis, J. F., & Veiga, O. L. (2015). Physical activity and cognition in adolescents: A systematic review. Journal of Science and Medicine in Sport, 18(5), 534–539. https://doi.org/10.1016/j.jsams.2014.07.007
- ÉW, G., Mullally, S., Foley, C., Warmington, S. A., O’Mara, S. M., & Kelly, Á. M. (2011). Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behavior, 104(5), 934–941. https://doi.org/10.1016/j.physbeh.2011.06.005
- Fedewa, A. L., & Ahn, S. (2011). The effects of physical activity and physical fitness on children’s achievement and cognitive outcomes. Research Quarterly for Exercise and Sport, 82(3), 521–535. https://doi.org/10.1080/02701367.2011.10599785
- Gomez-Pinilla, F., & Hillman, C. (2013). The influence of exercise on cognitive abilities. Comprehensive Physiology, 3(1), 403–428. https://doi.org/10.1002/cphy.c110063
- Griffin É.W., Mullally S., Foley C., Warmington S.A., O'Mara S.M., Kelly Á.M. (2011). Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behavior 104(5), 934–941. https://doi.org/10.1016/j.physbeh.2011.06.005
- Guzmán, J. F., & López-García, J. (2016). Acute effects of exercise and active video games on adults’ reaction time and perceived exertion. European Journal of Sport Science, 16(8), 1197–1203. https://doi.org/10.1080/17461391.2016.1186744
- Haapala, E. (2012). Physical activity, academic performance and cognition in children and adolescents. A systematic review. Baltic Journal of Health and Physical Activity, 4(1), 53. https://doi.org/10.2478/v10131-012-0007-y
- Hagins, M., & Rundle, A. (2016). Yoga improves academic performance in urban high school students compared to physical education: A randomized controlled trial. Mind, Brain, and Education, 10(2), 105–116. https://doi.org/10.1111/mbe.12107
- Hardy, L. L., Bass, S. L., & Booth, M. L. (2007). Changes in sedentary behavior among adolescent girls: A 2.5-year prospective cohort study. Journal of Adolescent Health, 40(2), 158–165. https://doi.org/10.1016/j.jadohealth.2006.09.009
- Haynos, A. F., & O’Donohue, W. T. (2012). Universal childhood and adolescent obesity prevention programs: Review and critical analysis. Clinical Psychology Review, 32(5), 383–399. https://doi.org/10.1016/j.cpr.2011.09.006
- Heisz, J. J., Clark, I. B., Bonin, K., Paolucci, E. M., Michalski, B., Becker, S., & Fahnestock, M. (2017). The effects of physical exercise and cognitive training on memory and neurotrophic factors. Journal of Cognitive Neuroscience, 29(11), 1895–1907. https://doi.org/10.1162/jocn_a_01164
- Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Juni, P., Moher, D., Oxman, A. D., Savovic, J., Schulz, K. F., Weeks, L., & Sterne, J. A. C. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343(oct18 2), d5928. https://doi.org/10.1136/bmj.d5928
- Hillman, C. H., Erickson, K. I., & Kramer, A. F. (2008). Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews. Neuroscience, 9(1), 58. https://doi.org/10.1038/nrn2298
- Hillman, C. H., Khan, N. A., & Kao, S. (2015). The relationship of health behaviors to childhood cognition and brain health. Annals of Nutrition & Metabolism, 66(Suppl. 3), 1–4. https://doi.org/10.1159/000381237
- Horn, D. B., O’Neill, J. R., Pfeiffer, K. A., Dowda, M., & Pate, R. R. (2008). Predictors of physical activity in the transition after high school among young women. Journal of Physical Activity & Health, 5(2), 275–285. https://doi.org/10.1123/jpah.5.2.275
- Hwang, J., Brothers, R. M., Castelli, D. M., Glowacki, E. M., Chen, Y. T., Salinas, M. M., Kim, J., Jung, Y., & Calvert, H. G. (2016). Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neuroscience Letters, 630, 247–253. https://doi.org/10.1016/j.neulet.2016.07.033
- Hwang, J., & Lu, A. S. (2018). Narrative and active video game in separate and additive effects of physical activity and cognitive function among young adults. Scientific Reports, 8(1), 11020. https://doi.org/10.1038/s41598-018-29274-0
- Jaffery, A., Edwards, M. K., & Loprinzi, P. D. (2018). The effects of acute exercise on cognitive function: Solomon experimental design. The Journal of Primary Prevention, 39(1), 37–46. https://doi.org/10.1007/s10935-017-0498-z
- Janssen, I., & LeBlanc, A. G. (2010). Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. International Journal of Behavioral Nutrition and Physical Activity, 7(1), 40. https://doi.org/10.1186/1479-5868-7-40
- Jeon, Y. K., & Ha, C. H. (2017). The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environmental Health and Preventive Medicine, 22(1), 27. https://doi.org/10.1186/s12199-017-0643-6
- Johann, V. E., Stenger, K., Kersten, S., & Karbach, J. (2016). Effects of motor-cognitive coordination training and cardiovascular training on motor coordination and cognitive functions. Psychology of Sport and Exercise, 24, 118–127. https://doi.org/10.1016/j.psychsport.2016.01.008
- Kashihara, K., Maruyama, T., Murota, M., & Nakahara, Y. (2009). Positive effects of acute and moderate physical exercise on cognitive function. Journal of Physiological Anthropology, 28(4), 155–164. https://doi.org/10.2114/jpa2.28.155
- Kauts, A., & Sharma, N. (2009). Effect of yoga on academic performance in relation to stress. International Journal of Yoga, 2(1), 39. https://doi.org/10.4103/0973-6131.53860
- Kimm, S. Y., Glynn, N. W., Kriska, A. M., FITZGERALD, S. L., AARON, D. J., SIMILO, S. L., McMAHON, R. P., & BARTON, B. A. (2000). Longitudinal changes in physical activity in a biracial cohort during adolescence. Medicine & Science in Sports & Exercise, 32(8), 1445–1454. https://doi.org/10.1097/00005768-200008000-00013
- Lambourne, K. (2012). The effects of acute exercise on temporal generalization. Quarterly Journal of Experimental Psychology, 65(3), 526–540. https://doi.org/10.1080/17470218.2011.605959
- Lambourne, K., Audiffren, M., & Tomporowski, P. D. (2010). Effects of acute exercise on sensory and executive processing tasks. Medicine & Science in Sports & Exercise, 42(7), 1396–1402. https://doi.org/10.1249/MSS.0b013e3181cbee11
- Lebel, C., Walker, L., Leemans, A., Phillips, L., & Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage, 40(3), 1044–1055. https://doi.org/10.1016/j.neuroimage.2007.12.053
- Lees, C., & Hopkins, J. (2013). Peer reviewed: Effect of aerobic exercise on cognition, academic achievement, and psychosocial function in children: A systematic review of randomized control trials. Preventing Chronic Disease, 10, E174. https://doi.org/10.5888/pcd10.130010
- Lennemann, L. M., Sidrow, K. M., Johnson, E. M., Harrison, C. R., Vojta, C. N., & Walker, T. B. (2013). The influence of agility training on physiological and cognitive performance. The Journal of Strength & Conditioning Research, 27(12), 3300–3309. https://doi.org/10.1519/JSC.0b013e31828ddf06
- Lenroot, R. K., & Giedd, J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews, 30(6), 718–729. https://doi.org/10.1016/j.neubiorev.2006.06.001
- Li, J., & Siegrist, J. (2012). Physical activity and risk of cardiovascular disease—a meta-analysis of prospective cohort studies. International Journal of Environmental Research and Public Health, 9(2), 391–407. https://doi.org/10.3390/ijerph9020391
- Li, J. W., O’Connor, H., O’Dwyer, N., & Orr, R. (2017). The effect of acute and chronic exercise on cognitive function and academic performance in adolescents: A systematic review. Journal of Science and Medicine in Sport, 20(9), 841–848. https://doi.org/10.1016/j.jsams.2016.11.025
- Ludyga, S., Gerber, M., Brand, S., Holsboer‐Trachsler, E., & Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta‐analysis. Psychophysiology, 53(11), 1611–1626. https://doi.org/10.1111/psyp.12736
- Ludyga, S., Gerber, M., Herrmann, C., Brand, S., & Pühse, U. (2018). Chronic effects of exercise implemented during school-break time on neurophysiological indices of inhibitory control in adolescents. Trends in Neuroscience and Education, 10, 1–7. https://doi.org/10.1016/j.tine.2017.11.001
- Ludyga, S., Gerber, M., Kamijo, K., Brand, S., & Pühse, U. (2018). The effects of a school-based exercise program on neurophysiological indices of working memory operations in adolescents. Journal of Science and Medicine in Sport, 21(8), 833–838. https://doi.org/10.1016/j.jsams.2018.01.001
- Ludyga, S., Köchli, S., Pühse, U., Gerber, M., & Hanssen, H. (2019). Effects of a school-based physical activity program on retinal microcirculation and cognitive function in adolescents. Journal of Science and Medicine in Sport, 22(6), 672–676. https://doi.org/10.1016/j.jsams.2018.11.029
- Ludyga, S., Pühse, U., Lucchi, S., Marti, J., & Gerber, M. (2019). (2019). Immediate and sustained effects of intermittent exercise on inhibitory control and task-related heart rate variability in adolescents. Journal of Science and Medicine in Sport, 22(1), 96–100. https://doi.org/10.1016/j.jsams.2018.05.027
- Malina, R. M., Bouchard, C., & Bar-Or, O. (2004). Growth, maturation and physical activity (2nd ed.). Human Kinetics.
- Matthews, M. J., Yusuf, M., Doyle, C., & Thompson, C. (2016). Quadrupedal movement training improves markers of cognition and joint repositioning. Human Movement Science, 47, 70–80. https://doi.org/10.1016/j.humov.2016.02.002
- Mezcua-Hidalgo, A., Ruiz-Ariza, A., Suárez-Manzano, S., & Martínez-López, E. J. (2019). 48-hour effects of monitored cooperative high-intensity interval training on adolescent cognitive functioning. Perceptual and Motor Skills, 126(2), 202–222. https://doi.org/10.1177/0031512518825197
- Moher, D., Liberati, A., Tetzlaff, J., & DG, A., & Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097
- Moore, R. D., Romine, M. W., O’connor, P. J., & Tomporowski, P. D. (2012). The influence of exercise-induced fatigue on cognitive function. Journal of Sports Sciences, 30(9), 841–850. https://doi.org/10.1080/02640414.2012.675083
- Murray, N. P., & Russoniello, C. (2012). Acute physical activity on cognitive function: A heart rate variability examination. Applied Psychophysiology and Biofeedback, 37(4), 219–227. https://doi.org/10.1007/s10484-012-9196-z
- Northey, J. M., Cherbuin, N., Pumpa, K. L., Smee, D. J., & Rattray, B. (2017). Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. British Journal of Sports Medicine, 52(3), 154–160. http://dx.doi.org/10.1136/bjsports-2016-096587.
- Palmiere, S., Wade, M., DeBlois, J. P., Lefferts, W. K., & Heffernan, K. S. (2018). Aortic stiffness, central pulse pressure and cognitive function following acute resistance exercise. European Journal of Applied Physiology, 118(10), 2203–2211. https://doi.org/10.1007/s00421-018-3948-2
- Peruyero, F., Zapata, J., Pastor, D., & Cervelló, E. (2017). The acute effects of exercise intensity on inhibitory cognitive control in adolescents. Frontiers in Psychology, 8, 921. https://doi.org/10.3389/fpsyg.2017.00921
- Piercy, K. L., Troiano, R. P., Ballard, R. M., Carlson, S. A., Fulton, J. E., Galuska, D. A., George, S. M., & Olson, R. D. (2018). The physical activity guidelines for Americans. JAMA, 320(19), 2020–2028. https://doi.org/10.1001/jama.2018.14854
- Pinto-Escalona, T., & Martínez-de-Quel, Ó. (2019). Ten Minutes of Interdisciplinary Physical Activity Improve Academic Performance. Apunts: Educació Física I Esports, 138, 82–94.https://doi.org/10.5672/apunts.2014-0983.es.(2019/4).138.07
- Pontifex, M. B., Hillman, C. H., Fernhall, B. O., Thompson, K. M., & Valentini, T. A. (2009). The effect of acute aerobic and resistance exercise on working memory. Medicine and Science in Sports and Exercise, 41(4), 927–934. https://doi.org/10.1249/MSS.0b013e3181907d69
- Prashanth, P. (2020). Effect of cardiovascular fitness on cognitive functions among male medical students of South Kerala. National Journal of Physiology, Pharmacy and Pharmacology, 10(1), 37–41. http://dx.doi.org/10.5455/njppp.2020.10.1034006112019
- Purohit, S. P., & Pradhan, B. (2017). Effect of yoga program on executive functions of adolescents dwelling in an orphan home: A randomized controlled study. Journal of Traditional and Complementary Medicine, 7(1), 99–105. https://doi.org/10.1016/j.jtcme.2016.03.001
- Rathore, A., & Lom, B. (2017). The effects of chronic and acute physical activity on working memory performance in healthy participants: A systematic review with meta-analysis of randomized controlled trials. Systematic Reviews, 6(1), 124. https://doi.org/10.1186/s13643-017-0514-7
- Rindfuss, R. R. (1991). The young adult years: Diversity, structural change, and fertility. Demography, 28(4), 493–512. https://doi.org/10.2307/2061419
- Ross, N., Yau, P. L., & Convit, A. (2015). Obesity, fitness, and brain integrity in adolescence. Appetite, 93, 44–50. https://doi.org/10.1016/j.appet.2015.03.033
- Schwarck, S., Schmicker, M., Dordevic, M., Rehfeld, K., Müller, N., & Müller, P. (2019). Inter-individual differences in cognitive response to a single bout of physical exercise—A randomized controlled cross-over study. Journal of Clinical Medicine, 8(8), 1101. https://doi.org/10.3390/jcm8081101
- Sharma, V. K., Subramanian, S. K., Arunachalam, V., Radhakrishnan, K., Ramamurthy, S., & Ravindran, B. S. (2015). Auditory and visual reaction times in school going adolescents: Effect of structured and unstructured physical training–a randomized control trial. International Journal of Adolescent Medicine and Health, 29(4), 1–9. https://doi.org/10.1515/ijamh-2015-0060
- Sipavičienė, S., Dumčienė, A., Ramanauskienė, I., & Skurvydas, A. (2012). Effect of single physical load of different duration and intensity on cognitive function. Medicina, 48(4), 31. https://doi.org/10.3390/medicina48040031
- Sjöwall, D., Thorell, L. B., Mandic, M., & Westerståhl, M. (2019). No effects of a long-term physical activity intervention on executive functioning among adolescents. SAGE Open Medicine, 7, 2050312119880734. https://doi.org/10.1177/2050312119880734
- Sperlich, B., De Clerck, I., Zinner, C., Holmberg, H., & Wallmann-Sperlich, B. (2018). Prolonged sitting interrupted by 6-min of high-intensity exercise: Circulatory, metabolic, hormonal, thermal, cognitive, and perceptual responses. Frontiers in Physiology, 9, 1279. https://doi.org/10.3389/fphys.2018.01279
- Spitzer, U. S., & Hollmann, W. (2013). Experimental observations of the effects of physical exercise on attention, academic and prosocial performance in school settings. Trends in Neuroscience and Education, 2(1), 1–6. https://doi.org/10.1016/j.tine.2013.03.002
- Spruit, A., Assink, M., van Vugt, E., van der Put, C., & Stams, G. J. (2016). The effects of physical activity interventions on psychosocial outcomes in adolescents: A meta-analytic review. Clinical Psychology Review, 45, 56–71. https://doi.org/10.1016/j.cpr.2016.03.006
- Stanford, K. I., & Goodyear, L. J. (2014). Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Advances in Physiology Education, 38(4), 308–314. https://doi.org/10.1152/advan.00080.2014
- Stroth, S., Hille, K., Spitzer, M., & Reinhardt, R. (2009). Aerobic endurance exercise benefits memory and affect in young adults. Neuropsychological Rehabilitation, 19(2), 223–243. https://doi.org/10.1080/09602010802091183
- Stroth, S., Reinhardt, R. K., Thöne, J., Hille, K., Schneider, M., Härtel, S., Weidemann, W., Bös, K., & Spitzer, M. (2010). Impact of aerobic exercise training on cognitive functions and affect associated to the COMT polymorphism in young adults. Neurobiology of Learning and Memory, 94(3), 364–372. https://doi.org/10.1016/j.nlm.2010.08.003
- Subramanian, S. K., Sharma, V. K., Arunachalam, V., Radhakrishnan, K., & Ramamurthy, S. (2015). Effect of structured and unstructured physical activity training on cognitive functions in adolescents–a randomized control trial. Journal of Clinical and Diagnostic Research: JCDR, 9(11), CC04. https://doi.org/10.7860/JCDR/2015/13028.5790
- Takahashi, S., Grove, P. M., & Kotozaki, Y. (2019). Comparison of the effects of running and badminton on executive function: A within-subjects design. PloS One, 14(9), e0216842. https://doi.org/10.1371/journal.pone.0216842
- Tamnes, C. K., Herting, M. M., Goddings, A., Meuwese R., Blakemore S., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. (2017). Development of the cerebral cortex across adolescence: A multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. Journal of Neuroscience, 37(12) 3302–3316. https://doi.org/10.1523/JNEUROSCI.3302-16.2017
- Tarp, J., Domazet, S. L., Froberg, K., Hillman, C. H., Andersen, L. B., & Bugge, A. (2016). Effectiveness of a school-based physical activity intervention on cognitive performance in danish adolescents: Lcomotion—learning, cognition and motion–a cluster randomized controlled trial. PLoS One, 11(6), e0158087. https://doi.org/10.1371/journal.pone.0158087
- Tine, M. (2014). Acute aerobic exercise: An intervention for the selective visual attention and reading comprehension of low-income adolescents. Frontiers in Psychology, 5, 575. https://doi.org/10.3389/fpsyg.2014.00575
- Tine, M. T., & Butler, A. G. (2012). Acute aerobic exercise impacts selective attention: An exceptional boost in lower-income children. Educational Psychology, 32(7), 821–834. https://doi.org/10.1080/01443410.2012.723612
- Tomporowski, P. D. (2003). Effects of acute bouts of exercise on cognition. Acta Psychologica, 112(3), 297–324. https://doi.org/10.1016/S0001-6918(02)00134-8
- Tomporowski, P. D., McCullick, B., Pendleton, D. M., & Pesce, C. (2015). Exercise and children’s cognition: The role of exercise characteristics and a place for metacognition. Journal of Sport and Health Science, 4(1), 47–55. https://doi.org/10.1016/j.jshs.2014.09.003
- Torbeyns, T., de Geus, B., Bailey, S., Decroix, L., Van Cutsem, J., De Pauw, K., & Meeusen, R. (2017). Bike desks in the classroom: Energy expenditure, physical health, cognitive performance, brain functioning, and academic performance. Journal of Physical Activity & Health, 14(6), 429–439. https://doi.org/10.1123/jpah.2016-0224
- Tsai, C., Chen, F., Pan, C., Wang, C., Huang, T., & Chen, T. (2014). Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology, 41, 121–131. https://doi.org/10.1016/j.psyneuen.2013.12.014
- Tsai, C., Pan, C., Chen, F., Wang, C., & Chou, F. (2016). Effects of acute aerobic exercise on a task‐switching protocol and brain‐derived neurotrophic factor concentrations in young adults with different levels of cardiorespiratory fitness. Experimental Physiology, 101(7), 836–850. https://doi.org/10.1113/EP085682
- Tsorbatzoudis, H., Barkoukis, V., Danis, A., & Grouios, G. (1998). Physical exertion in simple reaction time and continuous attention of sport participants. Perceptual and Motor Skills, 86(2), 571–576. https://doi.org/10.2466/pms.1998.86.2.571
- Tsukamoto, H., Suga, T., Takenaka, S., Takeuchi, T., Tanaka, D., Hamaoka, T., Hashimoto, T., & Isaka, T. (2017). An acute bout of localized resistance exercise can rapidly improve inhibitory control. PloS One, 12(9), e0184075. https://doi.org/10.1371/journal.pone.0184075
- Van den Berg, V., Saliasi, E., Jolles, J., de Groot, R. H., Chinapaw, M. J., & Singh, A. S. (2018). Exercise of varying durations: No acute effects on cognitive performance in adolescents. Frontiers in Neuroscience, 12, 672. https://doi.org/10.3389/fnins.2018.00672
- Vazou, S., Pesce, C., Lakes, K., & Smiley-Oyen, A. (2019). More than one road leads to Rome: A narrative review and meta-analysis of physical activity intervention effects on cognition in youth. International Journal of Sport and Exercise Psychology, 17(2), 153–178. https://doi.org/10.1080/1612197X.2016.1223423
- Venckunas, T., Snieckus, A., Trinkunas, E., Baranauskiene, N., Solianik, R., Juodsnukis, A., Streckis, V., & Kamandulis, S. (2016). Interval running training improves cognitive flexibility and aerobic power of young healthy adults. Journal of Strength and Conditioning Research, 30(8), 2114–2121. https://doi.org/10.1519/JSC.0000000000001322
- Verburgh, L., Knigs, M., Scherder, E. J., & Oosterlaan, J. (2013). Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. British Journal of Sports Medicine, 48(12), 973–979. http://dx.doi.org/10.1136/bjsports-2012-091441.
- Vonk, M., Wikkerink, S., Regan, K., Middleton, L. E., & Mierau, A. (2019). Similar changes in executive function after moderate resistance training and loadless movement. PloS One, 14(2), 2. https://doi.org/10.1371/journal.pone.0212122
- Wang, C., Shih, C., Pesce, C., Song, T., Hung, T., & Chang, Y. (2015). Failure to identify an acute exercise effect on executive function assessed by the wisconsin card sorting test. Journal of Sport and Health Science, 4(1), 64–72. https://doi.org/10.1016/j.jshs.2014.10.003
- Whitford, T. J., Rennie, C. J., Grieve, S. M., Clark, C. R., Gordon, E., & Williams, L. M. (2007). Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Human Brain Mapping, 28(3), 228–237. https://doi.org/10.1002/hbm.20273
- Whyte, E. F., Gibbons, N., Kerr, G., & Moran, K. A. (2014). The effect of a high intensity, intermittent exercise protocol on neurocognitive function in healthy adults: Implications for return to play management following sport related concussion. Journal of Sport Rehabilitation, 24(4), 1-5. https://doi.org/http://dx.doi.10.1123/jsr.2014-0201
- Woost, L., Bazin, P.-L., Taubert, M., Trampel, R., Tardif, C. L., Garthe, A., Renner, U., Stalla, G., Ott, D. V. M., Rjosk, V., Obrig, H., Villringer, A., Roggenhofer, E., Klein, T. A., & Kempermann, G. (2018). Physical exercise and spatial training: A longitudinal study of effects on cognition, growth factors, and hippocampal plasticity. Scientific Reports, 8(1), 1–13. https://doi.org/10.1038/s41598-018-19993-9
- Xue, Y., Yang, Y., & Huang, T. (2019). Effects of chronic exercise interventions on executive function among children and adolescents: A systematic review with meta-analysis. British Journal of Sports Medicine, 53(22), 1397–1404. http://dx.doi.org/10.1136/bjsports-2018-099825.
- Zheng, G., Lan, X., Li, M., Ling, K., Lin, H., Chen, L., Tao, J., Li, J., Zheng, X., Chen, B., & Fang, Q. (2015). Effectiveness of tai chi on physical and psychological health of college students: Results of a randomized controlled trial. PLoS One, 10(7), e0132605. https://doi.org/10.1371/journal.pone.0132605
- Zhou, F., & Qin, C. (2019). Acute moderate-intensity exercise generally enhances attentional resources related to perceptual processing. Frontiers in Psychology, 10, 2547. https://doi.org/10.3389/fpsyg.2019.02547
- Zimmer, P., Stritt, C., Bloch, W., Schmidt, F.-P., Hübner, S. T., Binnebößel, S., Schenk, A., & Oberste, M. (2016). The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with stroop task performance: A randomized controlled trial. European Journal of Applied Physiology, 116(10), 2025–2034. https://doi.org/10.1007/s00421-016-3456-1
Appendices
Appendix A.
Search Strategy MEDLINE
(“Young Adult”[Mesh] OR “Adolescent”[Mesh] OR young adult*[tiab] OR adolescen*[tiab] OR youth[tiab] OR teens[tiab] OR teenager*[tiab] OR girl*[tiab] OR boy*[tiab] OR pubert*[tiab] OR ((high[tiab] OR middle[tiab] OR secondary[tiab]) AND school*[tiab]) OR (young*[tiab] AND (people[tiab] OR person*[tiab])))
AND
(“Physical Education and Training”[Mesh] OR “Motor Activity”[Mesh:NoExp] OR “Exercise”[Mesh] OR “Athletic Performance”[Mesh] OR “Exercise Therapy”[Mesh] OR “Exercise Movement Techniques” [Mesh] OR physical activ*[tiab] OR yoga [tiab] OR ((physical[tiab] OR aerobic[tiab] OR chronic[tiab] OR acute[tiab]) AND exercis*[tiab]) OR ((exercis*[tiab] OR fitness[tiab]) AND (training[tiab] OR intervention*[tiab] OR program*[tiab])) OR movement intervent*[tiab] OR movement program*[tiab] OR strength training[tiab] OR circuit training[tiab])
AND
(“Executive Function”[Mesh] OR “Attention”[Mesh] OR “Cognition”[Mesh:NoExp] OR “Problem Solving”[Mesh] OR “Inhibition (Psychology)”[Mesh] OR “Memory”[Mesh] OR “Academic Performance”[Mesh] OR executive function*[tiab] OR executive control*[tiab] OR attention[tiab] OR cognit*[tiab] OR inhibition[tiab] OR working memory[tiab] OR planning[tiab] OR set shift*[tiab] OR processing speed[tiab] OR processing time[tiab] OR ((academic[tiab] OR school[tiab]) AND (achiev*[tiab] OR perform*[tiab] OR skill*[tiab] OR competen*[tiab] OR behavio*[tiab] OR engagement[tiab])) OR academic outcome*[tiab] OR learning outcome*[tiab] OR language[tiab] OR spelling[tiab] OR reading comprehen*[tiab] OR math*[tiab] OR science[tiab] OR grade point*[tiab])
AND
(“Controlled Clinical Trial” [Publication Type] OR randomi*[tiab] OR randomly[tiab] OR control group*[tiab] OR groups[tiab] OR trial[ti] OR control condition*[tiab])