?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Athletes physically overload to improve performance. Unbalanced stress/recovery may induce overtraining, which is difficult to diagnosis as no diagnostic marker exists. Hormonal responses to a 55/80 cycle (30-min of alternating blocks of 1-min at 55% and 4-min at 80% maximum work rate) may highlight early-stage overtraining (overreaching), as blunted cortisol and testosterone responses to 55/80 follows intensified training. However, the reliability of hormonal responses to 55/80 when not overreached is unknown. Therefore, reported blunted hormonal responses could be due to inconsistent cortisol and testosterone responses to 55/80. Participants (n = 23) completed three 55/80 bouts, >7 days apart, with no exercise 24 h pre-trials. Pre-exercise urine osmolality and stress questionnaire responses were measured. Pre, post, and 30-min post-exercise saliva samples were collected for cortisol and testosterone assessment. Salivary cortisol and testosterone responses, osmolality and well-being were not different between trials. Salivary cortisol and testosterone elevated from pre- to post-exercise [by 4.2 nmol.L−1 (cortisol) and 307 pmol.L−1 (testosterone)], and 30 min post-exercise [by 160 pmol.L−1 (testosterone) only]. Intraclass correlation coefficients for pre to peak post-exercise cortisol (0.89; good) and testosterone (0.53; moderate) were calculated. This demonstrates that 55/80 induces reliable elevations of salivary cortisol and testosterone when in a healthy state.
Introduction
Individuals in high-demand occupations (e.g., athletes) push the limits of their physical abilities. Athletes overload the body physically by intensifying training stress, combining an elevation of volume, duration and intensity of exercise (Kraemer & Newton, Citation2000; Wenger & Bell, Citation2012). This can lead to a physical performance decrement for a limited period but following sufficient recovery of days to weeks a “supercompensatory” effect may occur, with the athlete exhibiting an enhanced performance when compared to baseline levels termed “functional overreaching” (Meeusen et al., Citation2013). Continued intensified training can move the athlete into a state of non-functional overreaching (NFOR) or the overtraining syndrome (OTS), reducing physical performance, which may not recover for several weeks to years (Meeusen et al., Citation2013). Signs of overreaching have been reported to occur within a period as short as 7 days of intensified training with limited recovery (Halson et al., Citation2002).
Retrospective diagnosis of NFOR/OTS is common, given that a valid and reliable protocol or a definitive diagnostic criterion are currently not available for use during the OTS progression (Meeusen et al., Citation2013). Therefore, an appropriate diagnostic marker and/or protocol to warn practitioners that NFOR/OTS may occur without a modification of training/competition, would be of benefit in practice. Especially, given rates of NFOR can be considered high in some circumstances, with 30–60% prevalence reported in elite athletes, elite runners, non-elite runners and adolescent swimmers (Birrer et al., Citation2013; Matos et al., Citation2011; Morgan et al., Citation1987).
Hormones associated with the hypothalamus and pituitary gland are suggested as possible markers of NFOR/OTS, as hypothalamic pituitary disturbances are reported following periods of intensified training (Meeusen et al., Citation2010, Citation2004; Urhausen et al., Citation1998). Indeed, a short duration (30 min) cycling exercise bout, referred to as the ‘55/80ʹ, has been developed, where a continuous 30 min cycle of alternating blocks of 1 min at 55% maximum work rate and 4 min at 80% maximum work rate are completed (Hough et al., Citation2011). The 55/80 demonstrated robust elevations in salivary cortisol (by ~7 nmol.L−1 from pre to post 55/80) and salivary testosterone (by ~400 pmol.L−1 from pre to post 55/80) concentrations in athletes not in a state of NFOR/OTS (i.e. healthy) (Hough et al., Citation2013, Citation2015; Hough et al., Citation2011). Blunted salivary cortisol (by ~70%) and salivary testosterone (by ~30%) responses to the 55/80 have been reported in physically active males and male elite triathletes following intensified training periods (i.e. possible suffering NFOR/OTS) (Hough et al., Citation2013, Citation2015). These blunted hormonal responses to the 55/80 were found in unison with increased fatigue and burnout scores measured by a psychological stress and recovery questionnaire (Hough et al., Citation2013, Citation2015). Consequently, the 55/80 was proposed as a useful tool to survey exercise-induced maladaptive cortisol and testosterone responses when in an overreached state (Hough et al., Citation2013, Citation2015). At rest, it is known that the intra-individual variability can be high for salivary cortisol (up to 51%) and testosterone (up to 30%) (Hough et al., Citation2015). Therefore, it is important to examine the simple reliability of the 55/80 hormone responses without the presence of NFOR/OTS pathologies as it is currently unknown. Knowing this reliability is important as the previously reported blunted cortisol and testosterone responses to the 55/80 following intensified training, could simply be due to an unreliable response of these hormones to the 55/80 bout. In measuring the reliability of the hormonal responses to the 55/80, it is important to understand that these responses can be influenced by physiological and psychological stress (Koolhaas et al., Citation2011). Therefore, a measure of psychological stress is important while completing this measure of hormonal reliability to the 55/80. The Recovery-Stress Questionnaire for Athletes (RESTQ) is a validated self-report of stress and recovery events that provides information on the individual’s state of well-being and predisposition to undertake physical activity (Kellmann & Kallus, Citation2001; Tibbert et al., Citation2009).
Therefore, the current study aimed to establish the reliability of the responses of salivary cortisol and testosterone concentrations to repeated 55/80 bouts across several days. A secondary aim was to examine the physiological and perceptual strain experienced to repeated exposure to the 55/80 bout. It is hypothesised that salivary hormone concentrations and physiological and perceptual strain experienced to the 55/80 will be similar across repeated trials, within an experimental design constructed to avoid NFOR/OTS.
Materials and methods
Participants
Twenty-three healthy, regularly active males (means ± SD; age: 21 ± 3 years; body mass: 80.7 ± 8.7 kg; height: 1.78 ± 0.07 m; peak oxygen uptake (): 50.9 ± 7.6 ml·kg−1·min−1; maximum work rate: 304 ± 49 W) volunteered for this study. This study was completed on two different laboratory sites and therefore study procedures were approved by the two local ethical advisory committees in line with the Helsinki Declaration.
Experimental design
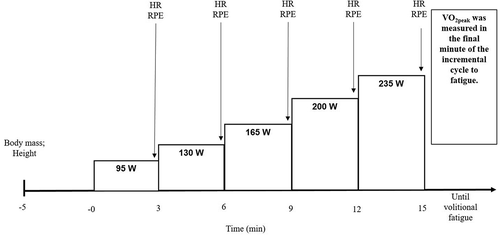
A repeated measures study design was conducted. Each participant visited the laboratory on four separate occasions. The protocol is presented in and in briefly consists of an incremental, continuous test in visit 1 with the remaining trials consisting of the completion of a 55/80 cycle. A written and verbal study explanation was provided and written informed consent to take part was obtained from each participant before testing began.
Methodology
During the first laboratory visit a continuous, incremental test was completed on a mechanically braked (Monark Ergomedic 894E, Vansbro, Sweden) or an electronically braked (Lode, Groningen, the Netherlands) cycle ergometer depending on the laboratory site visited (). Once allocated an ergometer, the same cycle ergometer was used on each visit. Maximum work rate (
max) was determined using the equation;
max =
+ (t/T).
where
= power output during the final stage completed, t = time (s) achieved in uncompleted stage, T = duration of each stage (180 s), and
= work rate increment (35 W). Power outputs equivalent to 55% and 80% of maximum work rate for each participant were calculated and used during the main experimental trials.
On all remaining laboratory visits, participants consumed a standard breakfast before 09:00 and at least 500 ml of water on the trial mornings to help ensure they were in an euhydrated state (Sawka et al., Citation2007). When measuring the reliability of the hormonal response to exercise there is a requirement to control for hydration status and time of day of sample collection. Hypohydration is known to elevate cortisol concentrations when compared to an euhydrated state (Judelson et al., Citation2008). The daily pattern of cortisol and testosterone concentration release into circulation is an elevation in the morning leading to a plateau in the circulation concentrations a few hours after awakening (Crofford et al., Citation1997; Walton et al., Citation2007). Therefore, all testing sessions took place at the same time of day (11:3013:00) to control for circadian rhythms in the hormones under examination. Participants remained fasted from 09:00 until the end of each trial at ~13:00. All participants abstained from exercise, caffeine and alcohol intake 24 h before each main trial and completed a food record diary. A similar diet was consumed 24 h before each main trial. Mean energy intake prior to each trial was 10.3 ± 3.1 MJ with 53 ± 13% (carbohydrate), 28 ± 12% (fat) and 18 ± 6% (protein). The participants drank water ad libitum during the trials except 10 min before the collection of all saliva samples reducing risk of saliva dilution.
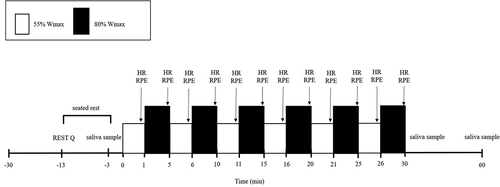
Each participant completed three main trials (Trial 1, Trial 2 and Trial 3) separated by at least 7 days (). The participant in each trial completed a 55/80 bout at 12:00. The 55/80 is a continuous 30 min cycle composed of alternating blocks of 1 min at 55% max and 4 min at 70%
max. Heart rate was collected in the final 30 s of each minute via short-range radio telemetry (Polar F2, Polar Electro Oy, Kempele, Finland) and ratings of perceived exertion (RPE) using a 6–20 Borg scale were recorded in the final 30 s of each alternating block. A 52-item RESTQ was completed at the beginning of each main trial. The RESTQ records the frequency of stress and recovery events over a period of 3 days and nights and presents the participant’s state of well-being and predisposition to undertake physical activity (Kellmann & Kallus, Citation2001). A saliva sample was collected at pre-exercise, post-exercise and 30 min post-exercise (). Saliva samples were unstimulated and collected by passive drool into a 7 mL Bijou vial (Sterilin, Newport, UK) while seated with eyes open, head tilted slightly forward and making minimal orofacial movement. The sample was collected for 2 min to allow for collection of sufficient sample volume. The pre-exercise and 30 min post-exercise samples were collected following a 10 min seated rest. The post-exercise samples were collected immediately following the 55/80. All samples were chilled immediately after collection and were divided into aliquots within 30 min and stored at −80°C until further analysis. For further detail on the 55/80 procedure please refer to Hough et al. (Citation2011) or Hough et al. (Citation2013).
The salivary cortisol and testosterone concentrations were determined using commercially available Enzyme Linked Immunosorbent Assay kits (Salimetrics, PA 16,803, USA). Samples from each participant were analysed on the same plate and went through one freeze thaw cycle only. Each sample was measured in duplicate with the mean salivary cortisol and testosterone concentrations reported. The mean inter-assay CVs were 5.1% and 6.8% for cortisol and testosterone, respectively. The mean intra-assay CVs were 4.8% and 4.4% for cortisol and testosterone, respectively.
Statistical analyses
All data in the text, tables and figures are presented as mean values ± standard deviation and/or range (minimum to maximum). Data were analysed using IBM® SPSS® 24.0 (IBM Corporation, Armonk NY USA). All data were checked for normality using quantile-quantile plots. Where data was not normally distributed, it was log transformed and re-examined. Salivary cortisol and testosterone data were log transformed and deemed to be normally distributed after transformation. For clarity, we have presented whole salivary cortisol and testosterone concentrations in the figures. All other data analyses were deemed to be normally distributed.
Linear mixed models were used to determine if there were any differences between trials (Trial 1; Trial 2 and Trial 3), time (Pre; Post and 30 min Post-Exercise) and any interactions between trial and time for absolute salivary hormone data. For clarity, figures presenting salivary hormone data sets were collapsed when no significant trial effects were found. Delta hormone values from pre-exercise to peak post-exercise were analysed with linear mixed models to determine if there were differences between trials. Differences were determined between trials for urine osmolality, averaged heart rate, ratings of perceived exertion, and RESTQ responses during each main trial visit. Fixed and random effects for the linear mixed models were fit for each dependent variable (West et al., Citation2014). Statistical significance was set at p < 0.05. Cohen’s d effect sizes are provided to supplement significant effects between trials or time. The magnitude of effect size was defined as trivial (d < 0.2), small (d ≥ 0.2, <0.5), medium (d ≥ 0.5, <0.8), and large (d ≥ 0.8) (Cohen, Citation1988).
Reliability was analysed using intra-individual coefficient of variations (CVi) for the salivary cortisol and testosterone concentrations at each timepoint. The intra-individual mean concentrations (meani) and standard deviations (SDi) were used to calculate the intra-individual CV (CV = (SDi/meani)*100). In addition, intraclass correlation coefficients (ICC) were calculated for the delta pre-exercise to peak post-exercise concentrations. These were calculated by hand using the ICC (2,1) model to measure relative reliability (Vincent & Weir, Citation2012). ICC values of less than 0.50 indicate poor reliability, 0.50–0.74 indicate moderate reliability, 0.75–0.90 good reliability, greater than 0.90 indicates excellent reliability (Koo & Li, Citation2016).
Results
Hydration status, recovery-stress questionnaires
There was no difference in urine osmolality or REST-Q scores for all trials ().
Table 1. The osmolality, RESTQ, mean heart rate and rating of perceived exertion, cortisol and testosterone responses for each main trial
Physiological and perceptual responses to exercise
No differences were found in the average heart rate and ratings of perceived exertion responses during the 55/80 (). ICC values for heart rate and ratings of perceived exertion responses to the 55/80 were 0.83 and 0.75, respectively.
Salivary cortisol
The response of the salivary cortisol concentration to the 55/80 bouts was similar over the 3 trial days (F2, 21.271 = 0.307, P = 0.739). A time effect was found with an elevation of salivary cortisol in response to the 55/80 bouts (F2, 22.011 = 13.949, P < 0.001) (). Acute increases in the salivary cortisol concentrations were found from Pre 55/80 to Post 55/80 (P = 0.01; d = 0.8) with a return to baseline at 30 min Post 55/80 (P = 0.79). There was no interaction between trial and timepoint (F4, 22.196 = 0.587, P = 0.675).
Figure 3. The collapsed (a) salivary cortisol and (b) salivary testosterone concentration responses to the 55/80 cycles in all trials
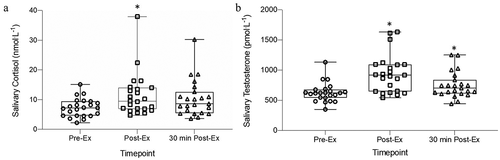
Similarly, delta salivary cortisol pre to peak post-exercise were similar over the 3 trial days (F2, 22 = 0.680, P = 0.518). A good reliability in the responses of the salivary cortisol to the exercise was found with an ICC value 0.89 calculated for the responses to the exercise bout. The CVi calculations of the salivary cortisol concentrations at each time point fall in line with that expected and are presented in .
Table 2. The mean intra-individual CVs (%) of the salivary cortisol and salivary testosterone concentrations at each timepoint in all main exercise trials
Salivary testosterone
The responses of the salivary testosterone concentrations to the 55/80 bouts were similar across the 3 trials (F2, 22.039 = 2.123 P = 0.144) (). A time effect was found (F2, 21.328 = 70.914, P < 0.001) with acute increases in the salivary testosterone concentrations found from Pre 55/80 to Post 55/80 (P < 0.001; d = 1.3) and 30 min Post 55/80 (P < 0.001;d = 0.7) (). There was no interaction between trial and timepoint (F4, 21.698 = 1.474, P = 0.245).
Delta changes pre to peak post-exercise were similar over the 3 trial days (F2, 22 = 1.324, P = 0.286). A moderate ICC value for the pre to peak post-exercise delta change of 0.53 was calculated and the CVi calculations of the salivary testosterone concentrations at each time point are presented in .
Discussion
This study aimed to establish the reliability of salivary cortisol, salivary testosterone, heart rate and ratings of perceived exertion responses to a short duration, high-intensity cycle bout (55/80) to determine the usefulness of the 55/80 as an exercise test to highlight alterations in exercise induced salivary cortisol and testosterone responses that may occur during NFOR/OTS. No differences in the salivary cortisol and testosterone responses to the repeated 55/80 trials were found. Therefore, the hypothesis that the salivary hormone responses to the 55/80 are similar on repeated exposure can be accepted. A secondary aim of this study was to measure the physiological (measured via heart rate responses) and perceptual (measured via ratings of perceived exertion scores) strain of the 55/80. Similar strain across the trials were found which confirms that repeated exposure to the exercise bout did not alter the strain experienced by the participants. This is important if the 55/80 is to be used as a physical stress test to examine possible dysfunction in the responses of hypothalamic pituitary adrenal and hypothalamic pituitary gonadal axes during periods of heavy training stress (i.e. NFORS/OTS).
The findings of robust elevations of salivary cortisol and testosterone from pre- to post-55/80 in the current study corresponds with previous reports of cortisol and testosterone elevations to a 55/80 bout in a healthy state (i.e. not in a state of NFOR/OTS) (Hough et al., Citation2013, Citation2015). The magnitude of elevation seen from pre to peak post-exercise in the current study (~6 nmol.L−1 for cortisol and ~315 pmol.L−1 for testosterone) is in line with that previously reported (~7 nmol.L−1 for cortisol and ~400 pmol.L−1 for testosterone) (Hough et al., Citation2013, Citation2015;Hough et al., Citation2011). The effect sizes reported with these findings in the current study highlight a large effect (>0.8) of the 55/80 on both these hormones from pre- to post-exercise. Our analysis revealed no differences in the hormonal responses to repeated 55/80 bouts. It also suggests that the reliability of salivary cortisol in response to the 55/80 can be interpreted as good; however, the reliability of the salivary testosterone response to the 55/80 was moderate. This reliability indicates that any changes to these responses, for example, when in a state of NFOR/OTS, should be viewed cautiously. The blunted responses of salivary testosterone to the 55/80 previously reported, following periods of intensified training, may have been due to the moderate reliability in the responses of this hormone in saliva to the 55/80. Examining the responses further showed a hormonal variability within the individuals of ~27% (salivary cortisol) and ~14% (salivary testosterone). This variability corresponds to that seen in resting plasma samples previously reported (Maes et al., Citation1997; Walton et al., Citation2007). Keeping in mind that the 55/80 exercise bout has been reported to highlight a blunted response of cortisol (of ~70%) and testosterone (of ~30%) following an intensified training period (i.e. when the athlete is in a state of possible NFOR/OTS) (Hough et al., Citation2013, Citation2015). These blunted alterations are in excess of the intra-individual variability this current study reports. This suggests that the blunted hormonal responses to the 55/80 following intensified training, found previously, were not due to the intra-individual variability of the hormones measured and may be due to the elevated physical stress during a period of heavy training (i.e. possible NFOR/OTS). To conclude, the data suggests that salivary cortisol elevated in response to the 55/80 and this response has a good reliability. Therefore, this may be a useful surveillance measure to complete during training periods to help to highlight states of NFOR/OTS with the expectation that during these periods the salivary cortisol responses will be blunted as previously reported (Hough et al., Citation2013, Citation2015).
The heart rate and ratings of perceived exertion analysis in the current study show that the physiological strain and the perception of exertion to the 55/80 do not differ across trials. The reliability analysis indicates a good reliability for the responses in both measures. If using this exercise stress test as a tool to highlight hormonal changes, our results indicate that hormonal alterations found are not due to changes in physiological strain or perception of exertion to the exercise bout.
Strengths and limitations
In measuring hormonal reliability, specific controls are required to help to remove external influences on the hormones being analysed. The strength of the current study is the control of these important external influences. Firstly, the RESTQ scores reported no significant disparities in stress or recovery scores within individuals during the study. This confirms that participants completed the 55/80 bouts in a similar state of well-being and predisposition to undertake physical activity (Kellmann & Kallus, Citation2001). It can be concluded from this that the hormonal responses have not been influenced by a change in well-being in the participants. Additionally, hydration status also influences cortisol and testosterone concentrations. Specifically, hypohydration (loss of ~5% body mass) elevates circulating cortisol and decreases testosterone when compared to an euhydrated state (Judelson et al., Citation2008). An indicator of euhydration is a urine osmolality value of <700 mosmol.kg−1, with each participant demonstrating an acceptable value (274–382 mosmol.kg−1) prior to completing the 55/80 (Sawka et al., Citation2007). Therefore, hydration status likely did not influence the hormonal responses to the 55/80 reported in this study.
It should be noted that the measurement of salivary hormones, specifically testosterone, may be inflated if measured by immunoassay when compared with another measurement technique for salivary hormone analysis such as liquid chromatography tandem mass spectrometry (LC-MS/MS) (Welker et al., Citation2016). However, this inflation found in immunoassay results compared with LC-MS/MS is most evident at low concentrations (<35 pmol.L−1). The concentrations reported in this current study were in excess of this low concentration value. However, it is important to know that different salivary hormone analysis methods may report different concentrations from the same samples. The relatively small sample size in the current research study must also be addressed. It is important to examine the power of the analysis completed within this study. A post-hoc computation of achieved power on two of the main variables in this research study was completed. These variables were the delta salivary cortisol and testosterone pre to peak post-exercise measures. The analyses achieved a power of 0.72 (cortisol) and 0.90 (testosterone). This finding details a 28% and 10% risk of committing type II errors (i.e. missing an effect if it genuinely exists). It is commonly agreed a power level of 80% is credible to determine actual effects (Field, Citation2009). The reader should be aware of this higher risk of missing an effect found in the cortisol data presented in this research study.
Conclusion
This study confirms that the 55/80 induces reliable elevations of salivary cortisol. It also highlights a moderate reliability when measuring salivary testosterone in response to the 55/80. This supports the use of the 55/80 to survey responses of salivary cortisol (and perhaps highlight their utility within NFOR/OTS cascades). Caution must be implemented if using the 55/80 to highlight alterations in salivary testosterone concentrations. The hormonal variability within individuals found in the current study (~27% cortisol and ~14% testosterone) are lower than those reported adaptations that occur in exercise-induced salivary cortisol and testosterone responses (to the 55/80 bout) following periods of intensified training (reductions of ~72% in salivary cortisol and ~34% in salivary testosterone following periods of intensified training when compared to before the training period (Hough et al., Citation2013, Citation2015). This finding further supports the potential use of the 55/80 as a tool for the surveillance of hormonal adaptations which may occur during periods of heavy training (e.g., NFOR/OTS).
Acknowledgments
The authors would like to thank all participants who took part in this study. We would like to acknowledge the technical staff at the Department of Sport Science and Physical Activity, University of Bedfordshire for their support throughout, specifically Mr Warwick Riley, Mr Callum Mould, and Miss Roisin McBride.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Birrer, D., Lienhard, D., Williams, C. A., Rothlin, R., & Morgan, G. (2013). Prevalence of non-functional overreaching and the overtraining syndrome in swiss elite athletes. Schweizerische Zeitschrift Fur Medizin Und Traumatologie, 61(4), 23–29. https://doi.org/10.1249/MSS.0b013e318207f87b
- Cohen, J. (1988). Statistical Power Analysis for the Behavioural Sciences (Second Edition). Lawrence Erlbaum Associates.
- Crofford, L. J., Kalogeras, K. T., Mastorakos, G., Magiakou, M. A., Wells, J., Kanik, K. S., Gold, P. W., Chrousos, G. P., & Wilder, R. L. (1997). Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: Failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. Journal of Clinical Endocrinology & Metabolism, 82(4), 1279–1283. https://doi.org/10.1210/jcem.82.4.3852
- Field, A. (2009). Discovering Statistics Using SPSS (Third Edition). Sage Publications Ltd., London.
- Halson, S. L., Bridge, M. W., Meeusen, R., Busschaert, B., Gleeson, M., Jones, D. A., & Jeukendrup, A. E. (2002). Time course of performance changes and fatigue markers during intensified training in trained cyclists. Journal of Applied Physiology, 93(3), 947–956. https://doi.org/10.1152/japplphysiol.01164.2001
- Hough, J., Corney, R., Kouris, A., & Gleeson, M. (2013). Salivary cortisol and testosterone responses to high-intensity cycling before and after an 11-day intensified training period. Journal of Sports Sciences, 31(14), 1614–1623. https://doi.org/10.1080/02640414.2013.792952
- Hough, J., Robertson, C., & Gleeson, M. (2015). A 10-day training camp blunts exercise-induced salivary testosterone in elite level triathletes. International Journal of Sports Physiology and Performance, 10 (7), 935–938. https://doi.org/10.1123/ijspp.2014-0360
- Hough, J. P., Papacosta, E., Wraith, E., & Gleeson, M. (2011). Plasma and salivary steroid hormone responses of men to high-intensity cycling and resistance exercise. Journal of Strength and Conditioning Research, 25(1), 23–31. https://doi.org/10.1519/JSC.0b013e3181fef8e7
- Judelson, D. A., Maresh, C. M., Yammoto, L. M., Farrell, M. J., Armstrong, L. E., Kraemer, W. J., Volek, J. S., Spiering, B. A., Casa, D. J., & Anderson, J. M. (2008). Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism and metabolism. Journal of Applied Physiology, 105(3), 816–824. https://doi.org/10.1152/japplphysiol.01010.2007
- Kellmann, M., & Kallus, K. W. (2001). Recovery-Stress Questionnaire for Athletes: User manual. Human Kinetics.
- Koo, T. K., & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. https://doi.org/10.1016/j.jcm.2016.02.012
- Koolhaas, J. M., Bartolomucci, A., Buwalda, B. D., de Boer, S. F., Flügge, G., Korte, S. M., Meerlo, P., Murison, R., Olivier, B., Palanza, P., Richter-Levin, G., Sgoifo, A., Steimer, T., Stiedl, O., Van Dijk, G., Wöhr, M., & Fuchs, E. (2011). Stress revisited: A critical evaluation of the stress concept. Neuroscience Biobehavioral Reviews, 35(5), 1291–1301. https://doi.org/10.1016/j.neubiorev.2011.02.003
- Kraemer, W. J., & Newton, R. U. (2000). Training for muscular power. Physical Medicine and Rehabilitation Clinic of North America, 11(12), 341–368. https://doi.org/10.1016/S1047-9651(18)30133-5
- Maes, M., Mommen, K., Hendrickx, D., Peeters, D., D’Hondt, P., Ranjan, R., DeMeyer, F., & Scharpe, S. (1997). Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clinical Endocrinology, 46(5), 587–598. https://doi.org/10.1046/j.1365-2265.1997.1881002.x
- Matos, N. F., Winsley, R. J., & Williams, C. A. (2011). Prevalence of nonfunctional overreaching/overtraining in young English athletes. Medicine and Science in Sports and Exercise, 43(287–294), 1287–1294. https://doi.org/10.1249/MSS.0b013e318207f87b
- Meeusen, R., Duclos, M., Forster, C., Fry, A., Gleeson, M., Nieman, D., Raglin, J., Rietjens, G., Steinacker, J., & Urhausen, A. (2013). Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European college of sport science and the American college of sports medicine. Medicine and Science in Sports and Exercise, 45(1), 186–205. https://doi.org/10.1080/17461391.2012.730061
- Meeusen, R., Nederhof, E., Buyse, L., Roelands, B., deSchutter, G., & Piacentini, M. F. (2010). Diagnosing overtraining in athletes using the two-bout exercise protocol. British Journal of Sports Medicine, 44(9), 642–648. https://doi.org/10.1136/bjsm.2008.049981
- Meeusen, R., Piacentini, M. F., Busschaert, B., Buyse, L., de Schutter, G., & Stray-Gundersen, J. (2004). Hormonal responses in athletes: The use of a two bout exercise protocol to detect subtle differences in (over)training status. European Journal of Applied Physiology, 91(2–3), 140–146. https://doi.org/10.1007/s00421-003-0940-1
- Morgan, W., O’Connor, P., Sparling, P., & Pate, R. R. (1987). Psychological characterization of the elite female distance runner. International Journal of Sports Medicine, 8(2), S124–S131. https://doi.org/10.1055/s-2008-1025717
- Sawka, M. N., Burke, L. M., Eichner, E. R., Maughan, R. J., Montain, S. J., & Stachenfeld, N. S. (2007, February). American college of sports medicine position stand. exercise and fluid replacement. Medicine and Science in Sports and Exercise, 39(2), 377–390. https://doi.org/10.1249/mss.0b013e31802ca597
- Tibbert, S., Morris, T., & Andersen, M. (2009). Validity of the recovery-stress questionnaire. Journal of Science and Medicine in Sport, 12, S32–S33. https://doi.org/10.1016/j.jsams.2008.12.077
- Urhausen, A., Gabriel, H. H., & Kindermann, W. (1998). Impaired pituitary hormonal response to exhaustive exercise in overtrained endurance athletes. Medicine and Science in Sports and Exercise, 30(3), 407–414. https://doi.org/10.1097/00005768-199803000-00011
- Vincent, W. J., & Weir, J. P. (2012). Statistics in kinesiology (4th ed.). Human Kinetics.
- Walton, M. J., Anderson, R. A., Kicman, A. T., Elton, R. A., Ossowska, K., & Baird, D. T. (2007). A diurnal variation in testicular hormone production is maintained following gonadotrophin suppression in normal men. Clinical Endocrinology, 66(2),123–129. https://doi.org/10.1111/j.1365-2265.2006.02696.x
- Welker, K. M., Lassetter, B., Brandes, C. M., Prasad, S., Koop, D. R., & Mehta, P. H. (2016). A comparison of salivary testosterone measurement using immunoassays and tandem mass spectrometry. Psychoneuroendocrinology, Sep; 71, 180–188. https://doi.org/10.1016/j.psyneuen.2016.05.022
- Wenger, H. A., & Bell, G. J. (2012). The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Medicine, 3(5), 346–356. https://doi.org/10.2165/00007256-198603050-00004
- West, B. T., Welch, K. B., & Galecki, A. T. (2014). Linear mixed models: A Practical Guide Using Statistical Software (Second Edition). Taylor & Francis.