?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Patients with midportion Achilles tendinopathy (AT) are thought to experience a gradual symptomatic improvement over time. The aim of this study was to prospectively investigate if patients with midportion AT have symptoms at 10-year follow-up. Patients withmidportion AT were invited to complete an online questionnaire 10 years after inclusion in an intervention trial. The primary outcomewas the presence of AT symptoms. Secondary outcomes were: the Victorian Institute of Sports Assessment-Achilles tendinopathy (VISA-A, 0–100) score and sports activity level. Of the 54 patientsincluded, 43 (80%) completed the questionnaire at an average follow-up of 10.4 years. Persisting symptoms were reported by 19%. The mean (standard deviation-SD) VISA-A score improved from 52 (17) at baseline to 79 (21) at 10-years follow-up with a mean change of 27 points (95% confidence interval: 21; 35, p < 0.001). Of the 38 active patients, 16 (42%) returned to their pre-injury level sports,of whom 14 (37%) performed them pain free. One-fifth of patients with conservatively treated midportion AT still have symptoms after 10years. One-third of patients were able to perform sports pain-free atpre-injury level. Patients should be adequately counselled to giverealistic expectations. Trial registration number: clinicaltrials.gov (identifier: NCT00761423).
Introduction
We often refer to long-standing Achilles tendinopathy (AT) as being chronic, yet we know little about the actual chronicity of symptoms on the long term. Symptoms normally improve quickly during the first year of treatment, but recovery is usually only partial (De Jonge et al., Citation2010, Citation2011; Silbernagel et al., Citation2007). After 1 year there seems to be a relative stagnation in improvement (Paavola et al., Citation2000; Silbernagel et al., Citation2011; Van der Plas et al., Citation2012). It seems that a subgroup of patients experience persisting symptoms.
Other follow-up studies of AT have heterogeneous study designs, populations, types of AT, follow-up durations and treatments (Johannsen et al., Citation2018; De Jonge et al., Citation2010, Citation2011; Paavola et al., Citation2000; Roos et al., Citation2004; Silbernagel et al., Citation2011, Citation2007; Van der Plas et al., Citation2012). This results in large variation of recovery rates with subjective excellent or asymptomatic recovery rates varying from 40% to 77% between 2 and 10 years follow-up (Johannsen et al., Citation2018; Paavola et al., Citation2000; Paoloni & Murrell, Citation2007; Silbernagel et al., Citation2011; Taylor et al., Citation2016; Van der Plas et al., Citation2012; Yeo et al., Citation2016). Participation in sports activity is scarcely described in the long-term follow-up studies, while this is important information from a patient perspective (Johannsen et al., Citation2018)
Additional items that are very interesting from a clinician and healthcare perspective are the course of symptoms, and prognostic factors. The course of symptoms at a very long-term follow-up has not been described sufficiently for this patient group and needs further investigation. Prognostic factors can help to identify important subgroups that develop longstanding symptoms. The subsequent development of specific interventions for this subgroup could have a large impact. Four studies have analysed possible prognostic factors associated with persisting symptoms, but found no significant associations (Johannsen et al., Citation2018; Silbernagel et al., Citation2011; Taylor et al., Citation2016; Van der Plas et al., Citation2012). There is a scarcity of accurate prospectively collected long-term follow-up data in this specific patient population with chronic midportion AT.
The primary aim of our study was to analyse which proportion of patients with chronic midportion AT have persisting symptoms at 10-year follow-up. Our secondary aims were to evaluate (1) symptom severity, (2) sports activity level, (3) course of symptoms, and (4) prognostic factors associated with persisting symptoms.
Methods
Original study design
This prospective cohort study is the 10-year follow-up of a previously published, double-blind, randomized placebo-controlled trial which compared the effects of an intratendinous platelet-rich plasma (PRP) injection for chronic midportion AT with a saline placebo injection (De Vos et al., Citation2010). Inclusion criteria were (1) a painful and thickened tendon in relation to activity and on palpation, (2) location of tendon pain was 2–7 cm proximal to the insertion of the calcaneus and (3) symptoms had to be present for at least 2 months. Patients were recruited at a large district general hospital (The Hague Medical Center, Leidschendam, the Netherlands) between August 2008 and January 2009. A total of 54 patients were included in the original study. After injection, patients in the intervention and placebo group followed a rehabilitation programme consisting of 1 week stretching and 12 weeks heavy-load eccentric calf muscle exercises (Alfredson & Lorentzon, Citation2000). Patients were advised not to undergo additional treatments before completing the 24-week follow-up. Detailed methods have been described in the trial register (clinicaltrials.gov, identifier: NCT00761423) and the original publication (De Vos et al., Citation2010). A PRP injection did not lead to a larger improvement in symptoms compared to the placebo injection at 24 or 52 weeks follow-up (De Jonge et al., Citation2011; De Vos et al., Citation2010). For this reason, we can regard this patient group as one large cohort.
Prognostic factor analysis was performed with data collected at baseline or a change in parameters from baseline to 1-year follow-up. We pre-defined a number of potentially relevant prognostic factors for long-term follow-up outcome. These were demographic variables at baseline (sex, age and body mass index (BMI)), symptom duration at baseline, difference in VISA-A score from baseline to 1-year follow-up and ultrasonographic parameters.
Ultrasonographic parameters were tendon diameter and degree of neovascularization, as described by de Vos et al. (De Vos et al., Citation2012). The degree of neovascularization was measured in longitudinal and transverse planes with colour Doppler ultrasonography (MyLab30, Esaote Piemedical, Maastricht, the Netherlands) and assessed using the modified Öhberg score (0–4+; Ohberg et al., Citation2002)
10-year follow-up
Our 10-year follow-up of the trial was approved by Medical Research Ethics Committee Southwest Holland, Voorburg, the Netherlands (MEC-18-114). We invited all patients who participated in the RCT (n = 54) by email to complete an online questionnaire between March and September 2019. Non-responding patients received two email reminders within 4 weeks and were contacted by phone. Consent was acquired from all patients who participated in this 10-year follow-up study.
The online questionnaire was divided into five sections: (1) current AT symptoms, (2) Victorian Institute of Sports assessment-Achilles tendinopathy (VISA-A) questionnaire, (3) current sports activity, (4) course of AT symptoms and (5) health care consumption (). The VISA-A score represents symptom severity of AT, where 100 points represents no pain during functional tasks and full pain-free sports participation (Robinson et al., Citation2001). This validated and disease-specific questionnaire has been translated and validated in Dutch language (Sierevelt, van Sterkenburg, Tol et al., Citation2018). Patients who did not do and did not wish to do any sports activities could score a maximum 60 points on the VISA-A scale (Sierevelt et al., Citation2018). Patients could fill in “not applicable” if they could not safely perform single leg hops, lowering their maximum VISA-A score by 10 points. As the maximum achievable VISA-A score is lower for patients who did not participate in sports activity and for patients who could not safely perform leg hops, we calculated the percentage of maximal achievable VISA-A score per patient per time point. The maximum achievable VISA-A score was calculated by dividing the VISA-A score at one time point with the maximum achievable VISA-A score at that time point. Formula:
Outcome measures
The primary outcome measure was the patient reported AT symptoms at 10 years follow-up. Secondary outcome measures were (1) the symptom severity using the VISA-A questionnaire (analysed using the validated VISA-A score ranging from 0 to 100 or using the maximum achievable VISA-A score ranging from 0 to 60 for sedentary patients as previously explained), (2) sports activity, (3) course of symptoms, and (4) (5) identification of potential prognostic factors for having persisting symptoms at 10-years follow-up. We pre-defined the following potential risk factors prior to the initiation this follow-up study: sex, age at baseline, body mass index at baseline, duration of symptoms at baseline, difference in mean VISA-A score between baseline and 52 weeks, ultrasonographically measured tendon diameter at baseline and degree of Doppler flow at baseline.
Statistical analysis
Data were checked for normal distribution using the Shapiro Wilk test. Normally distributed data are presented as mean with standard deviation (SD) and in case of non-normal distribution as median with interquartile range (IQR). Baseline characteristics of patients who completed and who did not complete the 10-year follow-up questionnaire were compared using an independent t-test (normal distribution), Mann-Whitney U test (non-normal distribution) or chi-square test (categorical variables). With this method, we were able to check for differences between responders and non-responders to the 10-year follow-up questionnaire. Patients were excluded from further analysis if they did not respond to the 10-year follow-up questionnaire.
The proportion of symptomatic patients was calculated by dividing symptomatic patients by the total of patients who filled in the 10-year follow-up questionnaire. The symptom severity of patients who filled in the 10-year follow-up questionnaire was reported using the mean VISA-A score. The change in mean VISA-A score was calculated with an independent samples t-test. Both mean and percentage of maximum achievable VISA-A score at 10-year follow-up were compared to the VISA-A scores at baseline, 6 weeks, 12 weeks, 24 weeks and 52 weeks.
We used descriptive statistics to report sports activity participation, and course of symptoms. Potential prognostic factors for the presence of persisting symptoms at 10-years follow-up were identified using an univariable binary logistic regression analysis. If more than 1 significant association was found with the univariable analysis, we performed a multivariable binary logistic regression analysis [ENTER model] with those specific variables. SPSS software (V.24.0.0.1; SPSS, Chicago, Illinois, USA) was used for statistical analysis.
Results
Patient population
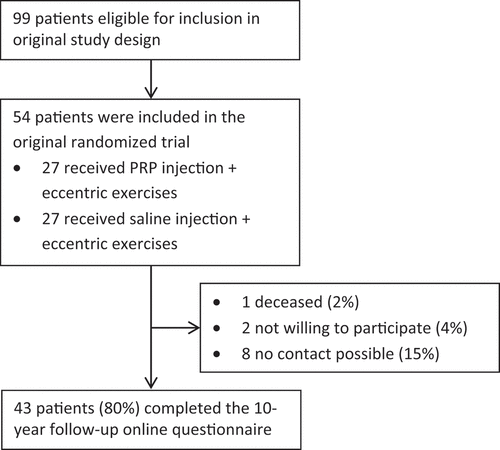
Of the 54 patients who were included in the original RCT, 43 (80%) agreed to participate in the 10-year follow-up study and filled in the follow-up questionnaire. The flow of patients in the study is shown in . The average follow-up time was 10.4 years.
Besides a statistically significant lower body mass index (BMI) in the group of patients who completed the 10-year follow-up (25.9 versus 28.6 kg/m2, p = 0.029), there were no differences between the groups of patients who did and did not participate in the 10-year follow-up study (). Of all patients who completed the 10-year follow-up, 12 patients (28%) underwent no other treatment for AT symptoms between the 1- and 10-year follow-up. Treatments undergone between the 1- and 10-year follow-up were: rest or temporary adjustments in sports activities (44%), stretching and/or strengthening exercises (58%), orthotics such as adjusted shoes, brace, bandage and/or insoles (42%), medication (26%) and passive treatment such as compression socks, tape, ultrasound and/or shockwave (28%). No patients had injections or underwent surgery.
Table 1. Baseline characteristics at the start of the study of patients who completed the 10-year follow-up questionnaire.
Primary outcome
Persisting AT symptoms
Persisting symptoms on the same side as baseline were reported by eight patients (19%) at a 10-year follow-up. Of these eight patients, one patient who had symptoms on the right Achilles tendon at baseline had bilateral symptoms at 10-year follow-up. Two other patients had symptoms on their left Achilles tendon at baseline and reported right-sided symptoms after 10 years.
Secondary outcomes
Symptom severity
Symptom severity improved significantly from baseline to 1-year follow-up with the VISA-A score (SD) changing from 52 (17) to 79 (21) points with a mean improvement of 27 (SD 21, 95% CI: 21; 34, p < 0.001) points (; De Jonge et al., Citation2011; De Vos et al., Citation2010). The symptom severity did not improve significantly from 1 year to 10-years follow-up with the VISA-A remaining 80 (17) points and a mean change of 1 (SD 27, 95% CI: −7; 9, p = 0.820) points.
Figure 3. Symptom severity over time, expressed in VISA-A score, of patients who completed the 10-year follow-up. Error bars denote standard deviations. A) Mean VISA-A score per time point. B) Percentage of maximal achievable VISA-A score per time point.
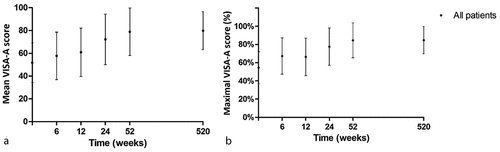
When analysing the VISA-A score expressed as a percentage of the maximum achievable VISA-A score, patients improved significantly from 55% (SD 18) at baseline to 85% (19) at 1-year follow-up with a mean change of 30% (SD 22, 95% CI: 23; 37, p < 0.001; ). The maximal achievable VISA-A score did not improve further between 1-year and 10-years follow-up with a mean change of 0% (SD 25, 95% CI: −7; 8, p = 0.980).
Sports activity
Sixteen of 38 patients (42%) returned to the preferred sports activity on their pre-injury level (), with 14 patients (37%) being pain free during sports activities of their preference at their pre-injury level. Three patients (8%) who participated in sports before their injury did not return to sports at 10-year follow-up. One patient who was sedentary at baseline took up sports during the follow-up period. In 52% of the cases, patients adjusted their sports activities because of their AT (). The mean duration that patients adjusted their sports was 24 (IQR; 114) months and most frequently they switched to another type of sports (18%).
Table 2. Sports activity and adjustments at 10-year follow-up.
Course of symptoms
The patients described the course of their AT symptoms most frequently as a gradually decreasing pain (74% of the whole group, 82% of patients who recovered and 50% of patients reporting persisting symptoms; ). Within the group of patients, different patterns were described. Patients who subjectively recovered used pain flares without pain in between as second most frequent description (15% of the patients who subjectively recovered compared to 10% of the patients with persisting symptoms). Persisting pain with slight fluctuations were reported by 40% of the patients with persisting symptoms, compared to none of the patients who recovered.
Table 3. Course of AT symptoms of the included patients.
Prognostic factors
All pre-defined potential prognostic factors (demographics at baseline, symptom duration at baseline, change in VISA-A score within 1 year and ultrasonographic parameters) were not associated with an increased risk of having persisting symptoms at 10-years follow-up (Supplementary file 1).
Discussion
Our prospective 10-year follow-up study shows that one-fifth of patients have Achilles tendon symptoms. Symptom improvement tails off after 1 year. Only one-third have returned to sports at their previous level without pain. More than 50% had adjusted their sports activities, and half of this subgroup made these adjustments because of AT symptoms. We did not identify prognostic factors for an increased risk of having symptoms at 10 years follow-up.
Symptoms at 10 year follow up
In our current study, 19% of patients had symptoms after 10 years. One other prospective study with a follow-up duration of 10 years in 77 patients with insertional or midportion AT reported that 37% experienced some form of physical limitation in the past 10 years (Johannsen et al., Citation2018). Unfortunately, this global assessment of function only addresses physical limitation, and it is not specified whether patients experienced AT symptoms at 10-years follow-up. One prospective study with a follow-up duration of 5 years reported that 35% of 34 patients with midportion AT were symptomatic (Silbernagel et al., Citation2011). Another prospective study with a follow-up duration of 5 years reported that 61% of 58 patients with chronic midportion AT experienced some degree of pain (Van der Plas et al., Citation2012). The results of these long-term follow-up trials suggest that 23–61% of the patients remain symptomatic between 5 and 10 years follow-up. The wide range in percentage of persisting symptoms can be explained by the heterogeneity of the studies: we suggest describing outcomes separately per specific type of pathology (e.g., insertional versus midportion AT). Another explanation might be the fact that this is not a validated outcome measure and therefore not assessed in a similar way across studies and cultures. We feel this simple dichotomous outcome is relevant, as it is easy to interpret in the clinical setting and aids in counselling patients to create realistic expectations.
Symptom severity
After 10 years, symptom severity in our study as measured with the VISA-A score was around 80 points. This is similar to the 10-year follow-up study of Johannsen et al.(Johannsen et al., Citation2018) who reported a VISA-A score of 84, with their primary study researching the effect of exercises in combination with glucocorticosteroid injections when necessary (Wetke, Johannsen, Langberg et al., Citation2015). Although there is currently no gradation in symptom severity based on the VISA-A score, Iversen et al.(Iversen, Bartels, Langberg et al., Citation2012) suggested that a VISA-A score of 96 or higher represents a healthy tendon. Only 9 out of 43 patients (21%) in our study had a VISA-A score of >96 points. An average VISA-A score of >96 was not achieved by patients in our study or the study of Johannsen et al. (Johannsen et al., Citation2018). This might be due to limitations of the VISA-A score. The VISA-A score is based on pain, function and sporting activity (Robinson et al., Citation2001). If patients are sedentary, their maximum VISA-A score is reduced by 40 points. In long-term follow-up studies, there can be other reasons why patients become less active resulting in a lower VISA-A score, while the tendon would actually be able to cope with higher loads. Furthermore, if patients cannot perform single legs hops due to poor balance or other reasons, their maximum VISA-A score is lowered by another 10 points. Give the above mentioned reasons, we also calculated the symptom severity as a percentage of maximal achievable VISA-A score. Even then, patients achieved around 85% of their maximal achievable score. These results suggest most patient with do not usually recover fully.
Sports activity
Only a third of patients were able to perform their preferred level of sports activity without pain. In total, 8% did not return to sports and 51% did not return to the sports of their preference or at their previous level. One 5-year follow-up study reported that 9% of the included active patients did not return to sports (Van der Plas et al., Citation2012). They did not report if patients returned to their preferred sport and whether this was at pre-injury levels. Additionally, they did not report whether patients adjusted their sports activity because of AT symptoms. In long-term follow-up studies, there might be other reasons for decreasing or stopping sports activities. Asking whether patients did not return to sports because of AT symptoms are relevant. In our study, half of the patients adjusted their sports activity because of symptoms and half due to other reasons. This implies that AT is an important reason for adjustments in sports activity and this impacts general health and quality of life (Ceravolo, Gaida, Keegan et al., Citation2018)
Course of symptoms
The course of symptoms in the subgroup of patients with symptoms at 10 years shows a characteristic profile of fluctuating pain over time in most. In practice, it could be helpful to inform patients about this typical course. This expectation management can help patients with persisting symptoms to understand that there will be periods with less or no symptoms and periods with increased pain levels.
Potential prognostic factors
Establishing prognostic factors for chronicity of disease is important for developing targeted intervention strategies and providing individualized prognosis. We were not able to identify prognostic factors that were associated with an increased risk of having symptoms at 10-years follow-up. Our findings are in accordance with previous literature, where sex, BMI, duration of symptoms at baseline, Doppler flow and tendon thickness also did not influence the severity of AT symptoms after 5 years of follow-up (Silbernagel et al., Citation2011; Van der Plas et al., Citation2012). Prognostic factors associated with an increased risk of developing persisting symptoms remain unknown; based on currently available evidence, it is unknown which factors facilitate recovery or trigger the development of persisting symptoms. Van der Vlist et al. published a systematic review that identified nine clinical risk factors which increase the risk of developing Achilles tendinopathy (Van der Vlist, Breda, Oei, Verhaar, de Vos et al., Citation2019). Future studies should include at least one of the risk factors in the study and analyse the risk of having persisting AT symptoms.
Strengths and limitations
A strength of our study is the adequate prospective methodology, based on the robust study protocol of a randomized controlled trial. We were able to contact a high percentage of patients for long-term follow-up. We found no clinically relevant differences between responders and non-responders, making it more likely that the group of responders were representative for this patient group. A potential limitation of this study is the low power when trying to identify potential prognostic factors. With the relatively small number of cases, we were only able to detect strong associations (Bahr, Citation2003). For our main research question and other secondary research questions, our number of included patients are similar to other studies that researched long-term follow-up of chronic midportion AT. It is also possible that treatments undergone in the past 9 years might have influenced prognosis. We did not adjust for this, as recall bias could be present, as we asked our patients about treatments and course of symptoms in the past 9 years. A solution for this in future long-term follow-up studies would be to administer questionnaires more frequently. A potential limitation could be the lack of clinical examination by a physician at follow-up. However, all patients had the clinical diagnosis which was ultrasonographically confirmed at baseline. It is unlikely that the diagnosis changed, as the patients recognized their injury pain. A possible limitation is that our primary outcome measure (presence of symptoms as dichotomous outcome) is not validated for its use in patients with Achilles tendinopathy. We decided to select this outcome measure based on the fact that interpretation for clinical practice is easy and also because the only validated alternative also has limitations. The VISA-A score is developed and validated for athletes but not for sedentary individuals. During the validation process of the Dutch VISA-A questionnaire, it was suggested to complete part of the questionnaire (pain and function questions, maximum score 60 points) by sedentary patients (Sierevelt, van Sterkenburg, Tol et al., Citation2018). The VISA-A score is also limited by the use of single leg hops: as we included older patients, many could not safely perform these which reduced their VISA-A score by 10 points. We handled these data by calculating the percentage of maximum obtainable VISA-A score per time point per patient, thereby overcoming this potential limitation. For these mentioned limitations of VISA-A assessment at long-term follow-up, we decided to use this outcome measure as secondary outcome and presence of current symptoms as dichotomous and easy-interpretable outcome measure for daily practice.
Recommendations for future research
We were not able to detect prognostic factors. This emphasizes the need for better identification and treatment of patients experiencing persisting symptoms. Large cohort studies with long-term follow-up or data pooling from existing studies are needed to identify the subgroup of patients with persisting symptoms. Innovative research with measuring prognostic factors will enhance our understanding of the chronicity of symptoms and this will enable the development of prevention strategies and better treatments for this subgroup.
Conclusion
One-fifth of patients with chronic AT report symptoms at 10 years of follow-up. There was no improvement in symptom severity after 1 year. Only one-third of all patients returned to their preferred sports activities at their pre-injury level without pain. Half of the patients were forced to adjust their sports activities. No prognostic factors were identified that were associated with the persistence of symptoms. Patients should be adequately counselled about the longstanding nature of AT, to create realistic expectations.
Source of Funding
This follow-up study received no financial support of a funding organization. The original study was funded by Biomet Biologics LLC, Warsaw, Indiana. The funder had no role in the design and conduction of the original or follow-up study. The views expressed in the submitted article are our own and not an official position of the institution or funder.
Ethical disclosure and trial registration
Exemption for comprehensive application of this follow-up study protocol was approved by the Medical Research Ethics Committee Southwest Holland, Voorburg, the Netherlands (MEC-18-114). The study protocol of the original study was approved by the Medical Research Ethics Committee Southwest Holland, Voorburg, The Netherlands (MEC 08-041). Trial registration number (The Netherlands Trial Register): ID number: NL5843.
Supplemental Material
Download MS Word (15 KB)Acknowledgments
We thank the patients of the PRICT study for participating in this 10-year follow-up study.
Disclosure statement
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data for this article can be accessed online https://doi.org/10.1080/02640414.2022.2163537
Additional information
Funding
References
- Alfredson, H., & Lorentzon, R. (2000, February). Chronic Achilles tendinosis: Recommendations for treatment and prevention. Sports Med, 29(2), 135–146. https://doi.org/10.2165/00007256-200029020-00005
- Bahr, R. (2003). Risk factors for sports injuries – a methodological approach. British Journal of Sports Medicine, 37(5), 384–392. https://doi.org/10.1136/bjsm.37.5.384
- Ceravolo, M. L., Gaida, J. E., & Keegan, R. J. (2018, August 13). Quality-of-life in achilles tendinopathy: An exploratory study. Clinical Journal of Sport Medicine, Publish Ahead of Print. https://doi.org/10.1097/JSM.0000000000000636
- de Jonge, S., de Vos, R. J., Van Schie, H. T., Verhaar, J. A. N., Weir, A., & Tol, J. L. (2010, July). One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion achilles tendinopathy. Br J Sports Med, 44(9), 673–677. https://doi.org/10.1136/bjsm.2008.052142
- de Jonge, S., de Vos, R. J., Weir, A., van Schie, H. T. M., Bierma-Zeinstra, S. M. A., Verhaar, J. A. N., Weinans, H., & Tol, J. L. (2011, August). One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: A double-blind randomized placebo-controlled trial. The American Journal of Sports Medicine, 39(8), 1623–1629. https://doi.org/10.1177/0363546511404877
- de Vos, R. J., Heijboer, M. P., Weinans, H., Verhaar, J. A. N., & van Schie, H. T. M. (2012, February). Tendon structure’s lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J Sport Rehabil, 21(1), 34–43. https://doi.org/10.1123/jsr.21.1.34
- de Vos, R. J., Weir, A., van Schie, H. T., Bierma-Zeinstra, S. M. A., Verhaar, J. A. N., Weinans, H., & Tol, J. L. (2010, January 13). Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA, 303(2), 144–149. https://doi.org/10.1001/jama.2009.1986
- Iversen, J. V., Bartels, E. M., & Langberg, H. (2012, February). The Victorian institute of sports assessment - achilles questionnaire (visa-a) - a reliable tool for measuring achilles tendinopathy. International Journal of Sports Physical Therapy, 7(1), 76–84. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3273883/
- Johannsen, F., Jensen, S., & Wetke, E. (2018). 10-year follow-up after standardised treatment for Achilles tendinopathy. BMJ Open Sport & Exercise Medicine, 4(1), e000415. https://doi.org/10.1136/bmjsem-2018-000415
- Ohberg, L., & Alfredson, H. (2002, June). Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: Pilot study of a new treatment. Br J Sports Med, 36(3), 173–175. https://doi.org/10.1136/bjsm.36.3.173
- Paavola, M., Kannus, P., Paakkala, T., Pasanen, M., & Järvinen, M. (2000, September-October). Long-term prognosis of patients with achilles tendinopathy. The American Journal of Sports Medicine, 28(5), 634–642. https://doi.org/10.1177/03635465000280050301
- Paoloni, J. A., & Murrell, G. A. (2007, October). Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot & Ankle International, 28(10), 1064–1068. https://doi.org/10.3113/FAI.2007.1064
- Robinson, J. M., Cook, J. L., Purdam, C., Visentini, P. J., Ross, J., Maffulli, N., Taunton, J.E., & Khan, K. M. (2001, October). The VISA-A questionnaire: A valid and reliable index of the clinical severity of Achilles tendinopathy. British Journal of Sports Medicine, 35 (5), 335–341. https://doi.org/10.1136/bjsm.35.5.335
- Roos, E. M., Engstrom, M., Lagerquist, A., & Soderberg, B. (2004, October). Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy - a randomized trial with 1-year follow-up. Scandinavian Journal of Medicine and Science in Sports, 14(5), 286–295. https://doi.org/10.1111/j.1600-0838.2004.378.x
- Sierevelt, I., van Sterkenburg, M., Tol, H., Dalen, B. V., Dijk, N. V., & Haverkamp, D. (2018, January 18). Dutch version of the Victorian institute of sports assessment-achilles questionnaire for achilles tendinopathy: Reliability, validity and applicability to non-athletes. World Journal of Orthopedics, 9(1), 1–6. https://doi.org/10.5312/wjo.v9.i1.1
- Silbernagel, K. G., Brorsson, A., & Lundberg, M. (2011, March). The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: A 5-year follow-up. The American Journal of Sports Medicine, 39(3), 607–613. https://doi.org/10.1177/0363546510384789
- Silbernagel, K. G., Thomee, R., Eriksson, B. I., & Karlsson, J. (2007, June). Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: A randomized controlled study. The American Journal of Sports Medicine, 35(6), 897–906. https://doi.org/10.1177/0363546506298279
- Taylor, J., Dunkerley, S., Silver, D., Redfern, A., Talbot, N., Sharpe, I., & Guyver, P. (2016, March). Extracorporeal shockwave therapy (ESWT) for refractory Achilles tendinopathy: A prospective audit with 2-year follow up. Foot (Edinb), 26, 23–29. https://doi.org/10.1016/j.foot.2015.08.007
- van der Plas, A., de Jonge, S., de Vos, R. J., van der Heide, H. J. L., Verhaar, J. A. N., Weir, A., & Tol, J. L. (2012, March). A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. British Journal of Sports Medicine, 46(3), 214–218. https://doi.org/10.1136/bjsports-2011-090035
- van der Vlist, A. C., Breda, S. J., Oei, E. H. G., Verhaar, J. A. N., & de Vos, R.-J. (2019, February 4). Clinical risk factors for Achilles tendinopathy: A systematic review. British Journal of Sports Medicine, 53(21), 1352–1361. https://doi.org/10.1136/bjsports-2018-099991
- Wetke, E., Johannsen, F., & Langberg, H. (2015, August). Achilles tendinopathy: A prospective study on the effect of active rehabilitation and steroid injections in a clinical setting. Scandinavian Journal of Medicine & Science in Sports, 25(4), e392–399. https://doi.org/10.1111/sms.12326
- Yeo, A., Kendall, N., & Jayaraman, S. (2016, July). Ultrasound-guided dry needling with percutaneous paratenon decompression for chronic Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc, 24(7), 2112–2118. https://doi.org/10.1007/s00167-014-3458-7