ABSTRACT
This systematic review and meta-analysis investigated the benefits, safety and adherence of exercise interventions delivered in inpatient mental health settings, quantified the number of exercise trials that provided support to maintain engagement in exercise post-discharge, and reported patient feedback towards exercise interventions. Major databases were searched from inception to 22.06.2022 for intervention studies investigating exercise in mental health inpatient settings. Study quality was assessed using Cochrane and ROBINS-1 checklists. Fifty-six papers were included from 47 trials (including 34 RCTs), bias was high. Exercise improved depression (Standardised mean difference = −0.416; 95% Confidence interval −0.787 to −0.045, N = 15) compared to non-exercise comparators amongst people with a range of mental illnesses, with further (albeit limited) evidence suggesting a role of exercise in cardiorespiratory fitness and various other physical health parameters and ameliorating psychiatric symptoms. No serious exercise-related adverse events were noted, attendance was ≥80% in most trials, and exercise was perceived as enjoyable and useful. Five trials offered patients post-discharge support to continue exercise, with varying success. In conclusion, exercise interventions may have therapeutic benefits in inpatient mental health settings. More high-quality trials are needed to determine optimal parameters, and future research should investigate systems to support patients to maintain exercise engagement once discharged.
Introduction
Patients with severe mental illnesses (SMI), including schizophrenia, bipolar disorder and major depressive disorder (MDD), have a 78% higher risk for developing cardiovascular disease (CVD) compared to healthy controls (Correll et al., Citation2017). Moreover, the risk of developing coronary heart disease is 26–41% higher in anxiety/stress disorders versus the general population (Emdin et al., Citation2016; Roest et al., Citation2010). Further, the occurrence of diabetes and metabolic syndrome is two-fold higher across those with SMI, anxiety/stress disorders, and alcohol and substance use disorders (AUD; SUD) (Correll et al., Citation2017; De Hert et al., Citation2009) (Whiteford et al., Citation2013). This increased risk of physical co-morbidity contributes to 10–25 years shortened life expectancy for SMI (Correll et al., Citation2015) and 7–20 years shortened life expectancy for AUDs, SUDs, anxiety and stress disorders (Denollet et al., Citation2009; Edward et al., Citation2014; Lohr et al., Citation2015) (Weitoft & Rosén, Citation2005).
Structured exercise can improve cardiorespiratory and cardiometabolic parameters in people with mental illnesses (Firth et al., Citation2015) (Vancampfort, Rosenbaum, et al., Citation2015). Exercise has benefits for cognitive functioning, depression severity, sleep quality, alcohol intake and psychiatric symptoms (Czosnek et al., Citation2019; Stubbs et al., Citation2018; Smith & Lynch, Citation2012). Despite the breadth in the evidence basis in terms of exercise modality, intervention duration, frequency and mental health diagnosis, the majority of exercise intervention studies have recruited patients with mental illnesses receiving care from community and outpatient settings. Less research has been conducted in inpatient settings, where patients are more acutely unwell, receive higher doses of psychotropic medication (Barbui et al., Citation2007) and polypharmacy (Weinmann et al., Citation2004).
People receiving psychiatric inpatient care show significantly greater levels of sedentary behaviour, reduced levels of exercise, and substantially reduced objectively measured moderate/vigorous physical activity levels than healthy controls (Kruisdijk et al., Citation2017). Reasons for inactivity and sedentary behaviour of people receiving inpatient care are likely to be multifactorial and associated with impact of acutely distressing symptoms, sedative effects of neuroleptics and reduced opportunity for physical activity due to lack of suitable facilities and restrictions on leave to access physical activity for those detained under mental health legislation (Firth et al., Citation2016; Gilburt et al., Citation2008). Moreover, people receiving psychiatric inpatient treatment may struggle to manage their physical health whilst acutely unwell and commonly experience acute mental health symptoms that may not fully remit with traditional first-line treatments (i.e., medication or therapies) alone (Kennedy et al., Citation2014). Inpatient exercise programmes may provide crucial opportunities to impact on both the physical and mental recovery of acutely distressed inpatients.
One 2014 review examined the effect of exercise interventions in people hospitalized with SMI and anxiety disorders (Stanton & Happell, Citation2014a), observing reductions in depression and improvements in quality-of-life following exercise interventions in those hospitalized with depression. There was preliminary evidence for improvements in psychiatric symptoms and aerobic fitness in inpatients with schizophrenia, bipolar disorder, and anxiety disorders. However, the review included only eight studies of low-to-moderate quality thus limiting generalizability (Stanton & Happell, Citation2014a). Moreover, a 2018 meta-analysis examined the anti-depressant effects of aerobic exercise in MDD and found large therapeutic effects (Morres et al., Citation2019). Since this time, many more inpatient exercise studies have been conducted, although there is a lack of clarity on the therapeutic effects of exercise across all mental health disorders, and post-discharge follow-up.
The aim of this systematic review and meta-analysis was to investigate the benefits, safety, adherence and description of exercise interventions for individuals receiving inpatient mental health treatment, using evidence from controlled and uncontrolled intervention studies. Secondary aims were to quantify and illustrate the form of any support to maintain engagement in exercise interventions once discharged from hospital. Moreover, we report patient feedback to the included exercise regimes, and whether exercise impacted length of hospital stay.
Methods
The systematic review was reported according to PRISMA guidelines following a pre-determined protocol (Prospero CRD42020183359).
Searches and study selection
Medline, PsychoInfo and Embase were searched from inception to 22.06.2022 for intervention studies investigating any form of exercise among people receiving inpatient treatment for a SMI (including schizophrenia spectrum disorders, bipolar disorder, MDD); anxiety and stress disorders; AUD and SUD’s; and eating disorders. All study designs were included (including RCTs, non-randomized controlled trials (NRCTs), pre- and post-test studies, naturalistic trials).
The search terms used are found in Supplementary Material 1. The reference lists of included articles were hand-searched.
Definition of exercise interventions
Aerobic exercise (walking, running, cycling or similar continuous activities) and/or non-aerobic exercise (free weight training, resistance training, body weight training) were included. Yoga, tai-chi and dance were excluded seen as these activities may benefit mental health through additional factors distinct from the physical activity itself (Stubbs et al., Citation2018).
Eligibility criteria
Two authors independently assessed articles based on the following criteria: 1) Intervention study investigating exercise, in a mental health inpatient setting, in any age. Where a control group was used, we considered any form of control group including no treatment, therapeutic and lifestyle interventions and pharmacotherapy; 2) Exercise interventions of any duration and frequency, excluding single exercise session studies; 3) a study population which included any forms of mental illness.
Non-English language articles, conference abstracts and dissertation theses were excluded. Exercise interventions that included therapeutic and lifestyle components and studies conducted in supported housing/residential facilities were excluded.
Outcomes
Primary outcomes
Changes in mental health parameters including psychiatric symptom scores, depression, anxiety, sleep quality and substance/alcohol cravings.
Secondary outcomes
Changes in cardiometabolic risk factors (e.g., BMI, systolic and diastolic blood pressure (SBP, DBP), heart rate (HR), lipid profiles), cardiorespiratory fitness (e.g., VO2max/VO2peak) and cognitive functioning. In the review planning stage (Prospero CRD42020183359) the decision was undertaken to report a wider range of outcomes including appetite/cravings, medication dose and sedentary behaviour. These outcomes have not been reported due to paucity of trials for each of these outcomes.
Further, we reported: 1) adverse events (AEs); 2) completion and drop-out rates; 3) referral to community exercise and support to exercise post discharge; 4) participant feedback/enjoyment; 5) length of inpatient stay.
Search results and data extraction
The title and abstract of all studies identified were reviewed, and relevant full-texts reviewed to determine eligibility. Eligibility was determined by three independent researchers (RM, BS, NK).
Data was extracted, from the papers selected for inclusion in the review, by one researcher (RM) and verified by a second researcher (BS). For each intervention study, we reported: study design, sample size, participant demographics, intervention descriptions (including control interventions), effect sizes (ES), adherence; and AEs.
Quality assessment
The quality of the included RCTs were assessed using The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials and The Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) for non-RCTs (J. A. C. Sterne et al., Citation2019; J. A. Sterne et al., Citation2016). Assessment was carried out by two independent researchers (RM and NK).
For each study, the intervention description was reported using the Consensus on Exercise Reporting Template (CERT) (Slade et al., Citation2016) (Supplementary Material 2).
Data synthesis
Primary and secondary outcomes were summarised narratively. We subdivided intervention studies based on mental health diagnosis and type of trial (RCTs, NRCTs, pre-post-test) and narratively summarised the number of studies that supported a significant improvement (p ≤ 0.05) in each outcome.
Additionally, we conducted between group meta-analyses for RCTs comparing exercise with non-exercise comparators for depression and BMI using data from all RCTs across all mental health diagnoses. Due to variation in HIIT protocols and participant characteristics, we used a random-effects model calculating the standardised mean difference (SMD) and 95% confidence intervals (95% CI) using the difference between the two groups’ pre-post change scores. The mean and standard deviation (SD) of pre-post scores was extracted for each study to calculate effect sizes. Heterogeneity was assessed using the I2 statistic. Publication bias was assessed with the Begg-Mazumdar Kendall’s tau. Comprehensive Meta-Analysis Software (CMA) was used for analysis. Meta-analyses could not be conducted for other primary and secondary outcomes due to heterogeneity/paucity of studies for each outcome and mental health diagnosis.
Results
Search results and included studies
Search results are in . Overall, 56 papers were included (from 47 trials) encompassing exercise interventions in depressive disorders (N = 27 from 20 trials, n = 892 participants including control groups) (Imboden et al., Citation2020; Kerling et al., Citation2017), schizophrenia spectrum disorders (N = 9 from 8 trials, n = 365) (Mazyarkin et al., Citation2019; Shimada et al., Citation2019), bipolar disorder (N = 1, n = 49) (Ng et al., Citation2007), SUDs and/or AUDs (N = 6, n = 207) (Schneider et al., Citation2016; McCartney et al., Citation2021), post-traumatic stress disorder (PTSD) (N = 1, n = 81) (Rosenbaum et al., Citation2015), anxiety (N = 2, n = 122) (Gaul-Aláčová et al., Citation2005; Martinsen, Hoffart, & Solberg, Citation1989), anorexia nervosa (N = 1, n = 41) (Kern et al., Citation2020), and inpatient samples with a range of mental health disorders (N = 9 from 8 trials, n = 591) (Kim et al., Citation2014; Philippot et al., Citation2022). Mean age of participants ranged from 15.5 to 68.7 years.
Figure 1. Prisma flow chart.
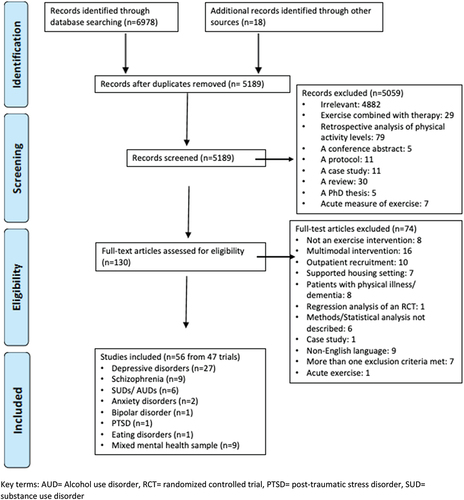
Thirty-four trials (across 42 papers) followed an RCT design (Imboden et al., Citation2020) (Haussleiter et al., Citation2020; Imboden et al., Citation2021) (Jae et al., Citation2014; Kerling et al., Citation2015, Citation2017; Manjunath et al., Citation2013; Roy et al., Citation2018) (Gary & Guthrie, Citation1972; Palmer et al., Citation1995; Schneider et al., Citation2016; Shimada et al., Citation2019) (Kim et al., Citation2014; Knapen et al., Citation2003, Citation2005; Lan et al., Citation2022; Martinsen, Hoffart, & Solberg, Citation1989; Philippot et al., Citation2022; Rosenbaum et al., Citation2015; Sexton et al., Citation1989). Other study designs included a semi-randomized controlled trial (N = 1 across 2 papers) (Wunram et al., Citation2018, Citation2021), NRCT (N = 5) (Dürmüş et al., Citation2020; Gaul-Aláčová et al., Citation2005; Heggelund et al., Citation2011; Mazyarkin et al., Citation2019; Song et al., Citation2018), a pre-post-test-controlled trial (N = 1) (Kerling et al., Citation2016), pre-post-test (N = 5) (Kern et al., Citation2020; Martinsen, Sandvik, et al., Citation1989; Stanley et al., Citation2019; Tsukue & Shohoji, Citation1981; Woodward et al., Citation2018) and a retrospective cohort study (N = 1) (Ng et al., Citation2007). Where a control group was utilised, activities included treatment as usual (TAU) (N = 18) (Kerling et al., Citation2018) (Bosscher, Citation1993; Haussleiter et al., Citation2020; Legrand & Neff, Citation2016; Roy et al., Citation2018; Schuch et al., Citation2014, Citation2015; Wunram et al., Citation2021) (Kerling et al., Citation2015, Citation2017) (Jae et al., Citation2014; Kurebayashi et al., Citation2022) (Dürmüş et al., Citation2020; Gaul-Aláčová et al., Citation2005; Kim et al., Citation2014; Lan et al., Citation2022; Ng et al., Citation2007; Rosenbaum et al., Citation2015), stretching/relaxation (N = 10) (Gerber et al., Citation2020; Imboden et al., Citation2020; Legrand & Neff, Citation2016) (CWH et al., Citation2014; Knapen et al., Citation2003, Citation2005; Knubben et al., Citation2007; McCartney et al., Citation2021; Philippot et al., Citation2022), yoga (N = 1) (Manjunath et al., Citation2013), occupational therapy (OT) (N = 1) (Buschert et al., Citation2019), computer games (N = 1) (Heggelund et al., Citation2011), association splitting (N = 1) (Schneider et al., Citation2016), passive muscular training (N = 1) (Wunram et al., Citation2018, Citation2021), and electroconvulsive therapy (N = 1) (Salehi et al., Citation2016).
respectively compile information on sample and intervention details, and primary and secondary outcomes.
Table 1. Basic characteristics of included RCTs including study design, population characteristics and details of exercise and control interventions.
Table 2. Basic characteristics of included non-RCTs including study design, population characteristics and details of exercise and control interventions.
Table 3. Mental health findings, additional outcomes, adherence and adverse events from included RCTs.
Table 4. Mental health findings, additional outcomes, adherence and adverse events from included Non-RCTs.
Quality of included studies and exercise reporting
A Cochrane risk of bias assessment table and ROBINS-1 tables for non-RCTs are provided in Supplementary Materials 3 and 4, respectively.
Briefly, 26/42 RCTs scored as low risk of bias for random sequence generation, the remaining RCTs did not divulge into randomisation procedure, 18/42 RCTs scored low bias for allocation concealment, 19/42 RCTs scored low bias for blinding of outcome assessment and 26/42 RCTs scored low risk of bias for incomplete outcome data.
In terms of non-RCTs, one study was rated low bias, four studies as moderate bias and nine studies as high risk of bias.
Supervision was noted in 40 trials (85.1%), seven trials (Dürmüş et al., Citation2020; Gary & Guthrie, Citation1972; Gaul-Aláčová et al., Citation2005; Jae et al., Citation2014; Schneider et al., Citation2016; Song et al., Citation2018; Stanley et al., Citation2019) did not provide supervision details, and no trial reported unsupervised exercise. Exercise was mainly supervised by an exercise instructor, physiotherapist, occupational therapist, a registered nurse or a researcher (Knubben et al., Citation2007; Kurebayashi et al., Citation2022; Legrand & Neff, Citation2016; Salehi et al., Citation2016; Schuch et al., Citation2014, Citation2015; Shachar-Malach et al., Citation2015; Wunram et al., Citation2018) (Lan et al., Citation2022; McCartney et al., Citation2021; Palmer et al., Citation1995; Philippot et al., Citation2022; Shimada et al., Citation2020). Twenty trials conducted exercise in group settings (Buschert et al., Citation2019; Haussleiter et al., Citation2020; Heissel et al., Citation2015; Martinsen, Hoffart, & Ø, Citation1989; Wunram et al., Citation2021) (Manjunath et al., Citation2013; Mazyarkin et al., Citation2019; Wunram et al., Citation2018) (Martinsen, Hoffart, & Solberg, Citation1989; Ng et al., Citation2007; Palmer et al., Citation1995; SY et al., Citation2016; Tsukue & Shohoji, Citation1981) (Kim et al., Citation2014; Knapen et al., Citation2003, Citation2005; Lan et al., Citation2022; Philippot et al., Citation2022; Sexton et al., Citation1989), 9 trials conducted 1:1 sessions (Kerling et al., Citation2016; Knubben et al., Citation2007; Legrand & Neff, Citation2016; McCartney et al., Citation2021; Rosenbaum et al., Citation2015; Salehi et al., Citation2016; Schuch et al., Citation2014, Citation2015; Shachar-Malach et al., Citation2015; Woodward et al., Citation2018) and 16 trials did not report delivery method (Imboden et al., Citation2020) (CWH et al., Citation2014; Dürmüş et al., Citation2020; Gary & Guthrie, Citation1972; Gaul-Aláčová et al., Citation2005; Gerber et al., Citation2018; Hanssen, Minghetti, Faude, et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Heggelund et al., Citation2011; Jae et al., Citation2014; Kahl et al., Citation2016; Kerling et al., Citation2015, Citation2017; Martinsen, Sandvik, et al., Citation1989; Minghetti et al., Citation2018; Schneider et al., Citation2016; Song et al., Citation2018; Stanley et al., Citation2019). In one trial, the first three sessions took part individually to establish optimal pace and subsequent sessions ran in groups (Bosscher, Citation1993), and in another trial a mixture of group and individual sessions were offered (Shimada et al., Citation2019, Citation2020). Forty trials tailored exercise intensity to each individual’s bodyweight/cardiorespiratory fitness/movement limitations.
Meta-analysis
Exercise resulted in a moderate reduction in depression severity compared to non-exercise comparators with high heterogeneity (SMD: −0.416; 95%CI: −0.787 to −0.045; I2 = 79.690%; N = 15) (Imboden et al., Citation2020; Legrand & Neff, Citation2016) (CWH et al., Citation2014; Haussleiter et al., Citation2020; Knubben et al., Citation2007; Salehi et al., Citation2016)– (Kerling et al., Citation2015; Manjunath et al., Citation2013; Philippot et al., Citation2022; Rosenbaum et al., Citation2015; Roy et al., Citation2018). There was no evidence of publication bias.
Following exercise, no change in BMI was observed compared to non-exercise comparators (SMD: −0.212; 95%CI: −0.507 to −0.084; I2 = 0%; N = 5) (Imboden et al., Citation2020; Jae et al., Citation2014; Kahl et al., Citation2016; Kim et al., Citation2014; Rosenbaum et al., Citation2015).
Depressive disorders
Twenty exercise trials (across 27 papers) were conducted in inpatients with MDD (562 = exercise and 330 = control groups) including 18 RCTs (Imboden et al., Citation2020)– (Kerling et al., Citation2015, Citation2017; Roy et al., Citation2018)], one semi-randomised trial[(Wunram et al., Citation2018)] and one pre-post-test [(Kerling et al., Citation2016)].
Primary Outcomes
Seven trials (six RCTs (CWH et al., Citation2014; Haussleiter et al., Citation2020; Kerling et al., Citation2015; Legrand & Neff, Citation2016; Roy et al., Citation2018; Schuch et al., Citation2015) and one semi-randomised trial (Wunram et al., Citation2018)) measured depression following exercise and non-active control, of which 6/7 studies (85.7%) (CWH et al., Citation2014; Haussleiter et al., Citation2020; Legrand & Neff, Citation2016; Roy et al., Citation2018; Schuch et al., Citation2015; Wunram et al., Citation2018) observed a significant reduction in depressive symptoms following exercise compared to non-active control. Nine trials (8 RCTs (Imboden et al., Citation2020; Legrand & Neff, Citation2016)– (Bosscher, Citation1993; Buschert et al., Citation2019; Knubben et al., Citation2007; Salehi et al., Citation2016; Shachar-Malach et al., Citation2015) and one semi-randomised trial (Wunram et al., Citation2018)) measured depression following exercise and an active control (stretching/relaxation = 6, OT = 1, passive muscular training = 1, ECT = 1), of which 3/9 studies (33.3%) (Heissel et al., Citation2015; Knubben et al., Citation2007; Shachar-Malach et al., Citation2015) observed a significant reduction in depressive symptoms following exercise compared to active control, although all of these trials (9/9, 100%) observed a significant reduction in depressive symptoms, following exercise, in within-group measures. Moreover, 5 RCTs compared the effects of differing exercise modalities on depression severity (Gerber et al., Citation2018; Hanssen, Minghetti, Faude, et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Martinsen, Hoffart, & Ø, Citation1989; Minghetti et al., Citation2018). One RCT observed a greater reduction in depression severity following high-intensity interval training (HIIT) compared to moderate-intensity continuous training (MICT) [(Hanssen, Minghetti, Faude, et al., Citation2018). Three RCTs observed no difference between HIIT and MICT (Gerber et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Minghetti et al., Citation2018)], and one observed no difference between aerobic and non-aerobic exercise (Martinsen, Hoffart, & Ø, Citation1989).
Secondary Outcomes
Two trials (one RCT (Kerling et al., Citation2015) and one semi-randomised trial (Wunram et al., Citation2018)) assessed VO2peak and BMI following exercise and non-active control conditions; both (2/2, 100%) observed significantly greater improvements in VO2peak following exercise compared to TAU but no change in BMI (0/2, 0%). One RCT (Kahl et al., Citation2016; Kerling et al., Citation2015, Citation2018) found a reduction in epicardial and subcutaneous adipose tissue, high-density lipoproteins (HDL), and a significant increase in muscle mass following exercise compared to TAU, and reductions in DBP, resting HR and fasting glucose in within-group measures. Three RCTs (Imboden et al., Citation2021; Salehi et al., Citation2016; Schuch et al., Citation2014) and one semi-randomised trial (Wunram et al., Citation2021) assessed brain-derived neurotrophic factor (BDNF). Plasma and serum BDNF levels increased in within group measures (4/4, 100%) and increased compared to non-active control in one study (1/2, 100%) (Wunram et al., Citation2021) but did not differ between exercise and active control groups (0/3, 0%) (Wunram et al., Citation2021) (Salehi et al., Citation2016). Moreover, one RCT assessed oxidative stress serum levels and observed a significant reduction in oxidative stress compared to non-active control (Schuch et al., Citation2014)], and one semi-randomized trial observed an increase in IGF – in within group measures (Wunram et al., Citation2021).
One RCT (Gerber et al., Citation2020; Imboden et al., Citation2020) found an increase in VO2max following both exercise and a stretching control group, with no changes in cortisol reactivity, blood pressure or resting HR. Three RCTs (Buschert et al., Citation2019; Heissel et al., Citation2015; Imboden et al., Citation2020) measured cognitive outcomes following exercise and active control (stretching or OT). Improvements in working memory were seen following exercise compared to active control in two RCTs (2/3, 66.7%) (Buschert et al., Citation2019; Imboden et al., Citation2020).
Adherence and adverse events
Eight trials reported ≥80% attendance at exercise sessions (Bosscher, Citation1993; Heissel et al., Citation2015; Kerling et al., Citation2018; Legrand & Neff, Citation2016; Salehi et al., Citation2016; Schuch et al., Citation2015; Wunram et al., Citation2018) (Kerling et al., Citation2017). Attendance did not differ from control conditions (Bosscher, Citation1993; Legrand & Neff, Citation2016; Wunram et al., Citation2018). Eleven trials (57.9%) did not report attendance (Shachar-Malach et al., Citation2015) (Buschert et al., Citation2019; Hanssen, Minghetti, Faude, et al., Citation2018; Haussleiter et al., Citation2020; Martinsen, Hoffart, & Ø, Citation1989; Minghetti et al., Citation2018; Roy et al., Citation2018).
Nineteen trials reported the rate of participant drop-out. No drop-outs were reported in four trials (Kerling et al., Citation2015, Citation2018; Salehi et al., Citation2016; Shachar-Malach et al., Citation2015) (Kerling et al., Citation2017). In 15 trials, the dropout rate for the exercise group was ≤27% (CWH et al., Citation2014; Gerber et al., Citation2020; Heissel et al., Citation2015; Imboden et al., Citation2020; Legrand & Neff, Citation2016) (Martinsen, Hoffart, & Ø, Citation1989; Minghetti et al., Citation2018) (Hanssen, Minghetti, Faude, et al., Citation2018; Schuch et al., Citation2015) (Wunram et al., Citation2018), and did not differ from control conditions (Gerber et al., Citation2018, Citation2020; Imboden et al., Citation2020; Legrand & Neff, Citation2016) (Bosscher, Citation1993; Hanssen, Minghetti, Faude, et al., Citation2018; Minghetti et al., Citation2018; Schuch et al., Citation2015) (Wunram et al., Citation2018).
Nine trials observed no exercise-related AEs (Gerber et al., Citation2020; Imboden et al., Citation2020; Shachar-Malach et al., Citation2015) (Minghetti et al., Citation2018; Schuch et al., Citation2015; Wunram et al., Citation2018). Two trials observed AEs including transient muscular/joint soreness, headache, fatigue and hiatus hernia (Legrand & Neff, Citation2016; Martinsen, Hoffart, & Ø, Citation1989). Nine trials (45%) did not report on AEs (Bosscher, Citation1993; Heissel et al., Citation2015; Kahl et al., Citation2016; Kerling et al., Citation2018) (Buschert et al., Citation2019; Haussleiter et al., Citation2020; Kerling et al., Citation2015; Roy et al., Citation2018; Salehi et al., Citation2016) (Kerling et al., Citation2017).
Schizophrenia spectrum disorders
Eight exercise trials (across nine papers) were conducted in inpatients with schizophrenia-spectrum disorders (201 = exercise and 164 = control groups) (Mazyarkin et al., Citation2019)– (Shimada et al., Citation2019) including five RCTs (Jae et al., Citation2014; Manjunath et al., Citation2013)– (Shimada et al., Citation2019), two NRCTs (Heggelund et al., Citation2011; Mazyarkin et al., Citation2019) and one pre-post-test (Woodward et al., Citation2018).
Primary Outcomes
Five out of seven trials (71.4%) observed an improvement in Positive and Negative Syndrome Scale scores (PANSS) PANSS total scores in within-group measures.
Two RCTs (Shimada et al., Citation2019; SY et al., Citation2016) and one NRCT (Mazyarkin et al., Citation2019) observed a significant improvement in PANSS total score (Mazyarkin et al., Citation2019; Shimada et al., Citation2019), negative symptoms (Mazyarkin et al., Citation2019; Shimada et al., Citation2019; SY et al., Citation2016), positive symptoms (Shimada et al., Citation2019; SY et al., Citation2016) and PANSS general score (Mazyarkin et al., Citation2019; SY et al., Citation2016) compared to non-active control. One RCTs (Kurebayashi et al., Citation2022) observed no between-group changes in PANSS scores compared to non-active control. Moreover, significant increases in cognitive domains (including attention, executive function, verbal fluency and processing speed) (Kurebayashi et al., Citation2022; Shimada et al., Citation2019) and global functioning (SY et al., Citation2016) were found following exercise compared to non-active control, whereas one RCT (Shimada et al., Citation2019, Citation2020) observed within group increases in global functioning but no change compared to non-active control.
One RCTs (Manjunath et al., Citation2013) and one NRCT (Heggelund et al., Citation2011) measured PANSS scores following exercise and active control conditions (yoga = 1, computer games = 1). One RCT observed a significant decrease in PANSS total score, PANSS general score, and depressive symptoms following both exercise and yoga, and a greater decrease in depressive symptoms following yoga compared to exercise (Manjunath et al., Citation2013). One NRCT (Heggelund et al., Citation2011) observed no change in either PANSS scores following exercise or computer games, and no change in depression severity following both exercise and computer games training.
One pre-post-test (Woodward et al., Citation2018) observed a significant decrease in PANSS total scores and an improvement in social functioning following exercise but no change in depression severity nor anxiety.
Secondary Outcomes
One NRCT found no change in BMI, fasting blood glucose and lipid profiles following up to 6 months of aerobic and resistance exercise versus a non-active control group (Mazyarkin et al., Citation2019)
Two controlled trials (one RCT (Jae et al., Citation2014) and one NRCT (Heggelund et al., Citation2011)) assessed a range of physical health measures following exercise and active control. One RCT observed improvements in BDNF, BMI, SBP, DBP and resting HR following exercise but not following stretching, dancing and recreation activities, and no change in HDL (Jae et al., Citation2014). One NRCT observed a significantly greater increase in VO2peak following HIIT compared to computer games training, a decrease in HDL following computer training but not following HIIT, and no change in BMI (Heggelund et al., Citation2011). Both trials (Heggelund et al., Citation2011; Jae et al., Citation2014) observed no change in total cholesterol, triglycerides, LDL, fasting blood glucose and total glycerol.
One pre-post-test (Woodward et al., Citation2018) observed no change in VO2max, BMI, total cholesterol, triglycerides, blood pressure and resting HR following aerobic and non-aerobic exercise. Increases in hippocampal total volume were observed.
Adherence and adverse events
Attendance to exercise sessions was reported in 2/8 trials (Heggelund et al., Citation2011; Woodward et al., Citation2018). Both observed ≥80% attendance, and attendance did not differ from control (Heggelund et al., Citation2011). Across seven trials the dropout rate for the exercise group ranged from 0% to 37% and did not differ from control (Heggelund et al., Citation2011; Jae et al., Citation2014; Manjunath et al., Citation2013) (Shimada et al., Citation2020). The occurrence of AEs was measured in 4/8 trials of which three experienced no exercise-related AEs (Kurebayashi et al., Citation2022; Manjunath et al., Citation2013; Shimada et al., Citation2020) and another experienced one account of ankle pain (Heggelund et al., Citation2011).
Substance and Alcohol Use Disorders
Six exercise trials were conducted in inpatients with SUD and/or AUD (144 = exercise and 63 = control groups) (Schneider et al., Citation2016) (McCartney et al., Citation2021) including four RCTs (Gary & Guthrie, Citation1972; McCartney et al., Citation2021; Palmer et al., Citation1995; Schneider et al., Citation2016), a NRCT (Dürmüş et al., Citation2020) and a pre-post-test (Tsukue & Shohoji, Citation1981).
Primary Outcomes
One RCT (Gary & Guthrie, Citation1972) assessed the number of drinking episodes and sleep outcomes in inpatients with AUD, following exercise and non-active control. One NRCT (Dürmüş et al., Citation2020) assessed substance craving, depression severity and anxiety in inpatients with opioid use disorder following exercise and non-active control. There was a significant reduction in sleep disturbances, substance craving scores, depression and anxiety following exercise compared to non-active control, and no change in the number of drinking episodes. One RCT (Schneider et al., Citation2016) observed a significant reduction in alcohol craving following exercise, in inpatients with AUD, but no difference versus association splitting therapy. In another RCT a significant reduction in depression was seen following bodybuilding but not following aerobic exercise nor circuit training (Palmer et al., Citation1995). Moreover, one RCT (McCartney et al., Citation2021) assessed sleep outcomes during inpatient admission for cannabis withdrawal and observed increased sleep duration and decreased wake bout following cycling compared to stretching.
Secondary Outcomes
One RCT found a significant increase in insulin-like growth factor (IGF-1) (Dürmüş et al., Citation2020), and a decrease in resting HR in exercise versus a non-active control (Gary & Guthrie, Citation1972).
Adherence and adverse events
Two trials reported attendance at individual exercise sessions (Dürmüş et al., Citation2020), 45% of participants attended all sessions in one trial[(Dürmüş et al., Citation2020) and in another 3% of sessions were missed (McCartney et al., Citation2021). Four trials provided participant drop-out, which ranged from 0% to 55%. The occurrence of exercise-related AEs was not reported.
Anxiety Disorders
Two exercise trials were conducted in inpatients with anxiety disorders (106 = exercise and 16 = control groups) including one RCT, which allocated patients to jogging or non-aerobic exercise (Martinsen, Hoffart, & Solberg, Citation1989), and one controlled trial comparing cycling combined with psychotherapy to psychotherapy alone (Gaul-Aláčová et al., Citation2005). Both trials observed a significant reduction in anxiety symptoms (2/2, 100%) and there was a greater decrease in anxiety symptoms following exercise compared to psychotherapy alone (Gaul-Aláčová et al., Citation2005). Changes in VO2max were measured in one RCT with significant improvements following jogging but not following non-aerobic exercise (Martinsen, Hoffart, & Solberg, Citation1989). One trial gave details regarding the rate of participant drop-out and AE’s (Martinsen, Hoffart, & Solberg, Citation1989). The drop-out rate was 11% and one participant experienced problems with a hiatus hernia.
Bipolar Disorder
One retrospective cohort study assessed the effects of a daily walking programme in people with bipolar disorder (Ng et al., Citation2007). Fourteen inpatients regularly attended the walking group until discharge whereas 35 patients did not engage. At discharge, people who regularly attended the walking group during hospitalization showed significantly lower Depression Anxiety Stress Scale (DASS) scores compared to those who did not engage. AEs were not reported.
Ptsd
A two-armed RCT assessed the effects of a 12-week exercise programme in inpatients with PTSD (Rosenbaum et al., Citation2015). Thirty-nine participants received the exercise programme and 42 received usual care. Following exercise, there was a significant improvement in PTSD symptoms, depression, stress, anxiety, sleep quality and body fat percentage compared to usual care. Mean attendance at supervised sessions was 58% and no exercise-related AEs were observed.
Eating Disorders
One pre-post-test intervention assessed the effects of an 8-week physical activity program, designed to reduce dependence towards unhealthy physical activity patterns, in 41 female adolescent inpatients with Anorexia Nervosa (Kern et al., Citation2020). A significant reduction in exercise dependence scores and eating disorders symptomology scores (eating, body shape, weight) were noted. BMI increased from pre-post-test; thus, exercise did not impede weight gain. The exercise programme was reported to be “safely and well adapted”, and 70.7% of patients completed the programme.
Combined Mental Illness Samples
Eight exercise trials (across nine papers) summarised the effects of exercise in pooled inpatient samples including a range of mental health diagnoses (e.g., schizophrenia, depression, bipolar disorder, anxiety, personality disorders and SUDs) (414 = exercise and 177 = control groups). This included five RCTs (Kim et al., Citation2014; Knapen et al., Citation2003, Citation2005; Lan et al., Citation2022; Philippot et al., Citation2022; Sexton et al., Citation1989), one NRCT (Song et al., Citation2018) and two pre-post-tests (Martinsen, Sandvik, et al., Citation1989; Stanley et al., Citation2019).
Primary Outcomes
Four RCTs measured anxiety and depression. One RCT (Lan et al., Citation2022) observed a significant decrease in depression and anxiety compared to non-active control, and one RCT (Philippot et al., Citation2022) observed a significant decrease in depression but no change in anxiety following exercise compared to relaxation. Moreover, two RCTs observed a significant decrease in within-group measures (2/2, 100%) but no differences in magnitude of change between exercise and an active control conducting progressive sports and relaxation (Knapen et al., Citation2005), and between walking and jogging (Sexton et al., Citation1989). There was a significant decrease in the Brief Psychiatric Rating Scale (BPRS) (Sexton et al., Citation1989) and mental health symptom rating scores (Martinsen, Sandvik, et al., Citation1989) in within-group measures.
Secondary Outcomes
One RCT (Kim et al., Citation2014) and one NRCT (Song et al., Citation2018) measured changes in BMI, blood pressure, resting HR and gastrointestinal motility (colonic transit time (CTT)). One trial (Kim et al., Citation2014)(1/2, 50%) observed a greater decrease in SBP following exercise, compared to non-active control, and both trials observed within-group decreases in DBP and resting HR. A greater reducation in total CTT was observed following exercise compared to non-active control in both trials (2/2, 100%).
Two RCTs (Knapen et al., Citation2003; Philippot et al., Citation2022) measured cardiorespiratory fitness by means of physical work capacity (Knapen et al., Citation2003) and VO2max (Philippot et al., Citation2022) and observed significantly greater gains in cardiorespiratory fitness following exercise compared to active controls conducting progressive sports and relaxation. One RCT (Sexton et al., Citation1989) measured cardiorespiratory fitness across two exercise groups. Significantly greater gains in VO2max were observed following jogging compared to walking. One pre-post-test (Stanley et al., Citation2019) observed a decrease in resting HR following exercise.
Adherence and adverse events
Two trials reported attendance (Knapen et al., Citation2003, Citation2005; Philippot et al., Citation2022), reporting 76–85.42% attendance in exercise, and no difference in attendance between exercise and control groups. Seven trials reported participant drop-out, which ranged from 16% to 44% and did not differ from control conditions (Kim et al., Citation2014; Philippot et al., Citation2022; Song et al., Citation2018). In a two-armed exercise intervention, a significantly higher drop-out rate was observed following jogging (39%) compared to walking (8%) (Sexton et al., Citation1989). Exercise-related AEs were not reported. A summary of meta-analysis findings are presented in .
Table 5. Summary of Meta-analysis Findings.
Support to Exercise Post-Discharge
Forty-one trials (87.2%) gave no support to exercise post-discharge or did not report support post-discharge (Imboden et al., Citation2020) (Imboden et al., Citation2021; Schuch et al., Citation2015) (Kerling et al., Citation2015, Citation2017) (Kurebayashi et al., Citation2022; Woodward et al., Citation2018) (Gaul-Aláčová et al., Citation2005; McCartney et al., Citation2021) (Knapen et al., Citation2003; Martinsen, Sandvik, et al., Citation1989) (Philippot et al., Citation2022), and in several instances, participants with early discharge were lost from the study as they were unable to continue participating in exercise or control conditions (Bosscher, Citation1993; Buschert et al., Citation2019; CWH et al., Citation2014; Gerber et al., Citation2020; Haussleiter et al., Citation2020; Heissel et al., Citation2015; Imboden et al., Citation2020; Martinsen, Hoffart, & Ø, Citation1989) (Heggelund et al., Citation2011; Kern et al., Citation2020; Knapen et al., Citation2003, Citation2005; Kurebayashi et al., Citation2022; Martinsen, Hoffart, & Solberg, Citation1989; Palmer et al., Citation1995; Philippot et al., Citation2022; Schneider et al., Citation2016; SY et al., Citation2016; Woodward et al., Citation2018; Wunram et al., Citation2018).
Five trials (10.6%) offered exercise post-discharge. In two trials, participants could continue with the exercise regime as outpatients (Kerling et al., Citation2016; Stanley et al., Citation2019), in one trial patients continued to exercise on their own post-discharge until completion of the 8-week intervention period with no support (Sexton et al., Citation1989), and in another trial, patients exercised at home with supervision of family members after discharge (Manjunath et al., Citation2013). In another trial, patients continued a less-intensive out-patient programme for several months post-discharge and home-based exercise sessions (Rosenbaum et al., Citation2015).
Feedback towards Exercise
Participant feedback was provided in four trials (8.5% of trials) (Kern et al., Citation2020; Martinsen, Sandvik, et al., Citation1989; Philippot et al., Citation2022; Wunram et al., Citation2018). In one trial (Wunram et al., Citation2018), ergometer training was rated as statistically more “fun” compared to an active control group conducting passive muscular training, and the item “would recommend it to others” was rated higher for the exercise group. In another trial (Martinsen, Sandvik, et al., Citation1989), patients were asked to rank the therapeutic elements of their inpatient treatment they found most useful. Patients retrospectively ranked physical exercise as the most important therapeutic element, followed by psychotherapy, education, and community meetings. In a further trial (Kern et al., Citation2020), follow-up qualitative interviews revealed patients appreciated the exercise programme, and perceived it as enjoyable and useful for muscular health and relaxation. In one trial (Philippot et al., Citation2022) enjoyment was rated using the VAS hedonia index, and did not differ between exercise and relaxation groups (exercise: 7.2 ± 1.9, control: 7.6 ± 1.5 on a 10-point scale).
Length of Hospital Stay
Across all patient groups, five trials reported mean length of inpatient stay and observed no significant difference between exercise and control groups (Knubben et al., Citation2007; Ng et al., Citation2007; Schuch et al., Citation2014, Citation2015; Shachar-Malach et al., Citation2015; Wunram et al., Citation2018).
Discussion
There has been a growing interest in the provision of exercise interventions among inpatient mental health services to assist the holistic recovery of people with mental illness, endorsed by numerous international and national guidelines (Lambert et al., Citation2017; National Institute for Health and Care Excellence NICE, Citation2014). The current systematic review is the most comprehensive examination of exercise interventions in inpatient psychiatry and seeks to understand and synthesise characteristics and outcomes of trials that may aid clinicians in the future translation of research into clinical practice.
Across all mental health diagnoses, exercise resulted in a moderate reduction in depression severity compared to non-exercise comparators but did not change BMI. In people hospitalised for MDD, the majority of trials (6/7, 85.7%) observed a reduction in depressive symptoms following exercise compared to non-active control, and evidence from a small number of trials suggested gains in cardiorespiratory fitness, adipose tissue, muscle mass, oxidative stress and cognition. In people hospitalised for schizophrenia spectrum disorders, 3/4 trials (75%) observed an improvement in psychiatric symptoms compared to non-active control, and there was a suggestion that exercise may improve depression and social functioning. In people receiving inpatient treatment for SUDs and AUDs, there was limited evidence that exercise may reduce craving scores, anxiety, depression and sleep disturbances. In anxiety disorders, evidence from two trials suggested a role of exercise in ameliorating anxiety. Exercise significantly reduced depression, stress and anxiety in those with bipolar disorder and PTSD and led to improvements in PTSD symptoms and sleep quality. In anorexia nervosa, exercise led to a significant reduction in exercise dependence scores and eating disorders symptomology scores but did not impede weight gain. In all categories of mental illness, there was inconclusive evidence regarding the effects of exercise on resting HR, blood pressure and lipid profiles. These narrative findings were hampered by variability of outcome measurement tools, differences in exercise regimes and population characteristics, and small sample size. Taken together, our findings suggest exercise to be a viable and effective intervention to improve some aspects of mental and physical health in inpatient mental health settings, although more research is needed to establish the full range of benefits.
Exercise interventions appear to be safe when offered in inpatient mental health settings. Eighteen intervention studies (38.3%) reported on AEs, of which 14 studies (77.8%) reported no AEs. Based on current evidence, exercise appeared to be safe in people with depression, schizophrenia, anxiety disorders, PTSD, and anorexia nervosa which confirms overall findings regarding the safety of exercise interventions in previous research in mental health outpatient and community services (Stanton & Happell, Citation2014b). Caveats to this are that safety was only observed from a single inpatient trial in PTSD and anorexia nervosa and safety was not reported at all in samples with SUDs and AUDs, or bipolar disorder. Further research on safety in these conditions is required, particularly as previous qualitative research in bipolar disorder has highlighted the potential for exercise to accelerate activation during mania (Malhi & Byrow, Citation2016; Wright et al., Citation2012). Interestingly, all studies that observed AE’s conducted jogging or treadmill-based exercise whereas the majority of those studies that did not observe AEs conducted bicycle-based exercise regimes, or bicycle regimes combined with other exercise modalities (n = 8, 57%) suggesting that bicycle-based exercise may be safer than treadmill exercise. There did not appear to be any association between exercise intensity and occurrence of AEs.
To add, 17 trials gave data regarding attendance, with the majority (12/17 trials, 70.6%) reporting≥80% attendance to exercise sessions. Attendance did not appear to differ from active control groups, suggesting that non-attendance may be attributable to the mental health symptoms of the participants, or other external factors, rather than the exercise per se. All five trials that reported lower attendance recruited patients subject to an acute inpatient stay, with mean length of stay ranging from 19 days to 6 weeks (Dürmüş et al., Citation2020; Imboden et al., Citation2020; Ng et al., Citation2007; Philippot et al., Citation2022; Rosenbaum et al., Citation2015). It may be that patient discharge in acute settings may be a contributing factor to declining attendance.
The rate of participant dropout was reported in 40 trials and was noted to be <30% in 36 trials. Similar to attendance, in those trials that reported highest dropout (44–55%) (Dürmüş et al., Citation2020; Knapen et al., Citation2003; Palmer et al., Citation1995), patients were subject to an acute inpatient stay and multiple instances of participant dropout were directly attributable to patient discharge. Six trials compared the rate of dropout between differing exercise modalities (Gerber et al., Citation2018; Hanssen, Minghetti, Faude, et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Martinsen, Hoffart, & Ø, Citation1989; Minghetti et al., Citation2018; Sexton et al., Citation1989). Additionally, MICT and jogging were found to have a higher rate of dropout when compared to non-aerobic exercise, HIIT and walking (Gerber et al., Citation2018; Hanssen, Minghetti, Faude, et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Martinsen, Hoffart, & Ø, Citation1989; Minghetti et al., Citation2018; Sexton et al., Citation1989).
Several controlled trials made comparisons between differing exercise regimes and associated therapeutic benefit. In one study, greater gains in PANSS scores were seen when exercise was conducted for 3–6 months compared to up to 3 months (Mazyarkin et al., Citation2019), suggesting that continuous long-term participation in exercise is needed before all benefits of exercise can be observed. When comparing aerobic and non-aerobic exercise, two trials observed greater gains in VO2max following aerobic exercise regimes (Martinsen, Hoffart, & Solberg, Citation1989; Martinsen, Hoffart, & Ø, Citation1989). Addressing comparisons between high- and low-intensity regimes, one RCT reported that HIIT may have a beneficial effect in lowering depression severity compared to MICT (Hanssen, Minghetti, Faude, et al., Citation2018) whereas three RCTs observed similar reductions in depression severity in both HIIT and MICT groups (Gerber et al., Citation2018; Hanssen, Minghetti, Faude, Schmidt-Trucksäss, et al., Citation2018; Minghetti et al., Citation2018). Another RCT observed greater gains in cardiorespiratory fitness following jogging compared to walking, but no difference in mental health parameters (Sexton et al., Citation1989). Research across community and inpatient settings has demonstrated greater improvements in depression severity following HIIT compared to MICT (Korman et al., Citation2020; Martland et al., Citation2020), however more high-quality research with a larger sample is needed before optimal modalities and intensities can be observed in mental health inpatients.
Overall, risk of bias was high; only 34 trials (72.3%) followed an RCT design of which only 45% blinded assessors. Moreover, only 8.5% of trials gave details regarding feedback or patient enjoyment which is a key factor for autonomous motivation to sustain exercise in people with SMI (Vancampfort, Stubbs, et al., Citation2015). Despite an expansion in the number of exercise interventions carried out in inpatient mental health settings in recent years, and indeed since the 2014 systematic review (Stanton & Happell, Citation2014a), more high-quality RCTs, with thorough reporting of safety, attendance, adherence and enjoyment, are needed to determine optimal parameters and aid translation into clinical practice. Consulting participants regarding their beliefs about the acceptability of exercise interventions underpins principles of “co-design” which is in turn associated with increased engagement in programmes. To this end, co-design in exercise intervention development should be explored in future (Wheeler et al., Citation2018).
Moreover, only 10.6% of studies offered patients the opportunity to continue the exercise regime post discharge, with varying levels of support. Future research should address the best ways to support exercise in both the inpatient environment and its continuation once discharged. Another avenue to explore in future work is the influence of exercise participation on length of hospital stay. Only five studies investigated differences in hospital stay and found no change, more research is needed before conclusions can be drawn.
Despite encouraging findings, it is important to note several limitations. Firstly, the heterogeneity in terms of study designs, outcomes, exercise regimes and population characteristics, and small sample size coupled with poor quality of many included studies and limited number of studies for each mental health diagnoses limits conclusive recommendations. A more thorough description of safety, adherence and enjoyment must be provided to draw definitive conclusions concerning the feasibility and acceptability of differing exercise regimes. Finally, we found only one study involving adolescents/youth inpatients, despite international endorsement of the importance of early intervention, highlighting the need for more work in this inpatient group (collab NICE guideline collab, year Citation2013year) (Shiers & Curtis, Citation2014).
Conclusion
Exercise regimes appear to be safe, viable and effective as therapeutic interventions in inpatient mental health settings. Evidence suggests exercise may improve depression, psychiatric symptoms, anxiety, cardiorespiratory fitness, cognition and muscle mass in inpatients with a range of mental health disorders. Additionally, exercise interventions appear to have good attendance and safety, with the caveat that no definitive conclusions can be drawn about effectiveness or safety of exercise in people with acute mania currently. The evidence base has expanded significantly in support of exercise interventions within inpatients settings over recent years though more research and thorough reporting is needed to determine optimal parameters and aid translation into clinical practice.
Declarations
Ethics approval and consent to participate – Not applicable
Consent for publication – Not applicable
Availability of data and material – All data analysed during this study is included in this published article and its supplementary information files.
Authors’ contributions
RM and BS designed the systematic review and search criteria. RM and BS determined study eligibility. RM and NK assessed study quality. Data extraction was performed by RM. The paper was drafted by RM and revised by BS. All authors contributed to protocol development and read and approved the final manuscript.
Supplemental Material
Download MS Word (75.7 KB)Disclosure statement
BS has received Honoria from ASICS. BS and JF have consulted for Parachutebh, for an unrelated project.
Supplementary Material
Supplemental data for this article can be accessed online https://doi.org/10.1080/02640414.2023.2207855.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Barbui, C., Biancosino, B., Esposito, E., Marmai, L., Donà, S., & Grassi, L. (2007). Factors associated with antipsychotic dosing in psychiatric inpatients: A prospective study. International Clinical Psychopharmacology, 22(4), 221–225. https://doi.org/10.1097/YIC.0b013e3281084ea8
- Bosscher, R. J. (1993). Running and mixed physical exercises with depressed psychiatric patients. International Journal of Sport Psychology, 24(2), 170–184.
- Buschert, V., Prochazka, D., Bartl, H., Diemer, J., Malchow, B., Zwanzger, P., & Brunnauer, A. (2019). Effects of physical activity on cognitive performance: A controlled clinical study in depressive patients. European Archives of Psychiatry and Clinical Neuroscience, 269(5), 555–563. https://doi.org/10.1007/s00406-018-0916-0
- Correll, C. U., Detraux, J., & De Lepeleire, J., De Hert M. (2015). Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry, 14(2), 119–136. https://doi.org/10.1002/wps.20204
- Correll, C. U., Solmi, M., & Veronese, N., Bortolato B, Rosson S, Santonastaso P, Thapa‐Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G. (2017). Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry, 16(2), 163–180. https://doi.org/10.1002/wps.20420
- CWH, H., Chan, S. C., Wong, J. S., Cheung, W. T., Chung, D. W. S., & Lau, T. F. O. (2014). Effect of Aerobic Exercise Training on Chinese Population with Mild to Moderate Depression in Hong Kong. Rehabilitation Research and Practice, 2014. https://doi.org/10.1155/2014/627376
- Czosnek, L., Lederman, O., Cormie, P., Zopf, E., Stubbs, B., & Rosenbaum, S. (2019). Health benefits, safety and cost of physical activity interventions for mental health conditions: A meta-review to inform translation efforts. Mental Health and Physical Activity, 16, 140–151. https://doi.org/10.1016/j.mhpa.2018.11.001
- De Hert, M., Dekker, J. M., Wood, D., Kahl, K. G., Holt, R. I. G., & Möller, H. -J. (2009). Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology. European Psychiatry, 24, 412–424. https://doi.org/10.1016/j.eurpsy.2009.01.005
- Denollet, J., Maas, K., Knottnerus, A., Keyzer, J. J., & Pop, V. J. (2009). Anxiety predicted premature all-cause and cardiovascular death in a 10-year follow-up of middle-aged women. Journal of Clinical Epidemiology, 62(4), 452–456. https://doi.org/10.1016/j.jclinepi.2008.08.006
- Dürmüş, P. T., Vardar, M. E., & Kaya, O., Tayfur P, Süt N, Vardar SA. (2020). Evaluation of the Effects of High Intensity Interval Training on Cytokine Levels and Clinical Course in Treatment of Opioid Use Disorder. Turk Psikiyatr Derg, 31(3), 151–158.
- Edward, C., Goodwind, G. M., & Fazel, S. (2014). Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry, 13(2), 153–160. https://doi.org/10.1002/wps.20128
- Emdin, C. A., Odutayo, A., Wong, C. X., Tran, J., Hsiao, A. J., & Hunn, B. H. M. (2016). Meta-Analysis of Anxiety as a Risk Factor for Cardiovascular Disease. The American Journal of Cardiology, 118(4), 511–519. https://doi.org/10.1016/j.amjcard.2016.05.041
- Firth, J., Cotter, J., Elliott, R., French, P., & Yung, A. R. (2015). A systematic review and meta-Analysis of exercise interventions in schizophrenia patients. Psychological Medicine, 45(7), 1343–1361. https://doi.org/10.1017/S0033291714003110
- Firth, J., Rosenbaum, S., Stubbs, B., Gorczynski, P., Yung, A. R., & Vancampfort, D. (2016). Motivating factors and barriers towards exercise in severe mental illness: A systematic review and meta-analysis. Psychological Medicine, 46(14), 2869–2881. https://doi.org/10.1017/S0033291716001732
- Gale, C. R., Batty, G. D., & Osborn, D. P. J., Tynelius P, Rasmussen F. (2014). Mental disorders across the adult life course and future coronary heart disease: Evidence for general susceptibility. Circulation, 129(2), 186–193. https://doi.org/10.1161/CIRCULATIONAHA.113.002065
- Gary, V., & Guthrie, D. (1972). The effect of jogging on physical fitness and self-concept in hospitalized alcoholics. Quarterly Journal of Studies on Alcohol, 33(4), 1073–1078. https://doi.org/10.15288/qjsa.1972.33.1073
- Gaul-Aláčová, P., Bouček, J., Stejskal, P., Kryl, M., Pastucha, P., & Pavlík, F. (2005). Assessment of the influence of exercise on heart rate variability in anxiety patients. Neuroendocrinol Lett, 26(6), 713–718.
- Gerber, M., Imboden, C., Beck, J., Brand, S., Colledge, F., Eckert, A., Holsboer-Trachsler, E., Pühse, U., & Hatzinger, M. (2020). Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(5), 1419. https://doi.org/10.3390/jcm9051419
- Gerber, M., Minghetti, A., Beck, J., Zahner, L., & Donath, L. (2018). Sprint Interval Training and Continuous Aerobic Exercise Training Have Similar Effects on Exercise Motivation and Affective Responses to Exercise in Patients with Major Depressive Disorders: A Randomized Controlled Trial. Frontiers in Psychiatry / Frontiers Research Foundation, 9. https://doi.org/10.3389/fpsyt.2018.00694
- Gilburt, H., Rose, D., & Slade, M. (2008). The importance of relationships in mental health care: A qualitative study of service users’ experiences of psychiatric hospital admission in the UK. BMC Health Services Research, 8(1), 92. https://doi.org/10.1186/1472-6963-8-92
- Hanssen, H., Minghetti, A., Faude, O., Schmidt-Trucksäss, A., Zahner, L., Beck, J., & Donath, L. (2018). Effects of different endurance exercise modalities on retinal vessel diameters in unipolar depression. Microvascular Research, 120, 111–116. https://doi.org/10.1016/j.mvr.2018.07.003
- Hanssen, H., Minghetti, A., & Faude, O., Schmidt-Trucksäss A, Zahner L, Beck J, Donath L. (2018). Effects of endurance exercise modalities on arterial stiffness in patients suffering from unipolar depression: A randomized controlled trial. Frontiers in Psychiatry / Frontiers Research Foundation. https://doi.org/10.3389/fpsyt.2017.00311
- Haussleiter, I., Bolsinger, B., Assion, H. -J., & Juckel, G. (2020). Adjuvant Guided Exercise Therapy versus Self-Organized Activity in Patients with Major Depression. The Journal of Nervous and Mental Disease, 208(12), 982–988. https://doi.org/10.1097/NMD.0000000000001240
- Heggelund, J., Nilsberg, G. E., Hoff, J., Morken, G., & Helgerud, J. (2011). Effects of high aerobic intensity training in patients with schizophrenia—A controlled trial. Nordic Journal of Psychiatry, 65(4), 269–275. https://doi.org/10.3109/08039488.2011.560278
- Heissel, A., Vesterling, A., & White, S. A., Kallies G, Behr D, Arafat AM, Reischies FM, Heinzel S, Budde H. (2015). Feasibility of an exercise program for older depressive inpatients: A pilot study. International Journal Of Geriatric Psychiatry, 28(4), 163–171. https://doi.org/10.1024/1662-9647/a000134
- Imboden, C., Gerber, M., & Beck, J., Eckert A, Lejri I, Pühse U, Holsboer-Trachsler E, Hatzinger M. (2021). Aerobic Exercise and Stretching as Add-On to Inpatient Treatment for Depression Have No Differential Effects on Sleep Measures. Brain Sciences, 11, 411. https://doi.org/10.3390/brainsci11040411
- Imboden, C., Gerber, M., Beck, J., Holsboer-Trachsler, E., Pühse, U., & Hatzinger, M. (2020). Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. Journal of Affective Disorders, 276, 866–876. https://doi.org/10.1016/j.jad.2020.07.052
- Inskip, H. M., Harris, E. C., & Barraclough, B. (1998). Lifetime risk of suicide for affective disorder, alcoholism and schizophrenia. The British Journal of Psychiatry: The Journal of Mental Science, 172(1), 35–37. https://doi.org/10.1192/bjp.172.1.35
- Jae, K. H., Kil, S. B., So, B., Lee, O., Song, W., & Kim, Y. (2014). Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry Research, 220(3), 792–796. https://doi.org/10.1016/j.psychres.2014.09.020
- Kahl, K. G., Kerling, A., Tegtbur, U., Gützlaff, E., Herrmann, J., Borchert, L., Ates, Z., Westhoff-Bleck, M., Hueper, K., & Hartung, D. (2016). Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: A randomized pilot study. Journal of Affective Disorders, 192, 91–97. https://doi.org/10.1016/j.jad.2015.12.015
- Kennedy, J. L., Altar, C. A., Taylor, D. L., Degtiar, I., & Hornberger, J. C. (2014). The social and economic burden of treatment-resistant schizophrenia. International Clinical Psychopharmacology, 29(2), 63–76. https://doi.org/10.1097/yic.0b013e32836508e6
- Kerling, A., Hartung, D., Stubbs, B., Kück, M., Tegtbur, U., Grams, L., Weber-Spickschen, T. S., & Kahl, K. G. (2018). Impact of aerobic exercise on muscle mass in patients with major depressive disorder: A randomized controlled trial. Neuropsychiatric Disease and treatment, Volume 14, 1969–1974. Published Online First: 2018. https://doi.org/10.2147/NDT.S167786
- Kerling, A., Kück, M., Tegtbur, U., Grams, L., Weber-Spickschen, S., Hanke, A., Stubbs, B., & Kahl, K. G. (2017). Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. Journal of Affective Disorders, 215, 152–155. https://doi.org/10.1016/j.jad.2017.03.034
- Kerling, A., Tegtbur, U., & Gützlaff, E., Kück M, Borchert L, Ates Z, von Bohlen A, Frieling H, Hüper K, Hartung D, Schweiger U. (2015). Effects of adjunctive exercise on physiological and psychological parameters in depression: A randomized pilot trial. Journal of Affective Disorders, 177, 1–6. https://doi.org/10.1016/j.jad.2015.01.006
- Kerling, A., von Bohlen, A., Kück, M., Tegtbur, U., Grams, L., Haufe, S., Gützlaff, E., & Kahl, K. G. (2016). Exercise therapy improves aerobic capacity of inpatients with major depressive disorder. Brain and Behavior, 6(6). https://doi.org/10.1002/brb3.469
- Kern, L., Morvan, Y., Mattar, L., Molina, E., Tailhardat, L., Peguet, A., De Tournemire, R., Hirot, F., Rizk, M., Godart, N., & Fautrelle, L. Development and evaluation of an adapted physical activity program in anorexia nervosa inpatients: A pilot study. (2020). European Eating Disorders Review: The Journal of the Eating Disorders Association, 28(6), 687–700. Published Online First: 2020. https://doi.org/10.1002/erv.2779
- Kim, Y. S., Song, B. K., & Oh, J. S., Woo SS. (2014). Aerobic exercise improves gastrointestinal motility in psychiatric inpatients. World Journal of Gastroenterology: WJG, 20(30), 10577–10584. https://doi.org/10.3748/wjg.v20.i30.10577
- Knapen, J., Van de Vliet, P., Van Coppenolle, H., David, A., Peuskens, J., Knapen, K., & Pieters, G. (2003). The effectiveness of two psychomotor therapy programmes on physical firness and physical self-concept in nonpsychotic psychiatric patients: A randomized controlled trial. Clinical Rehabilitation, 17)6(6), 637–647. https://doi.org/10.1191/0269215503cr659oa
- Knapen, J., Van De Vliet, P., Van Coppenolle, H., David, A., Peuskens, J., Pieters, G., & Knapen, K. (2005). Comparison of changes in physical self-concept, global self-esteem, depression and anxiety following two different psychomotor therapy programs in nonpsychotic psychiatric inpatients. Psychotherapy and Psychosomatics, 74(6), 353–361. https://doi.org/10.1159/000087782
- Knubben, K., Reischies, F. M., Adli, M., Schlattmann, P., Bauer, M., Dimeo, F., & Ansley, L. (2007). A randomised, controlled study on the effects of a short-term endurance training programme in patients with major depression. British Journal of Sports Medicine, 41(1), 29–33. https://doi.org/10.1136/bjsm.2006.030130
- Korman, N., Armour, M., Chapman, J., Rosenbaum, S., Kisely, S., Suetani, S., Firth, J., & Siskind, D. (2020). High Intensity Interval training (HIIT) for people with severe mental illness: A systematic review & meta-analysis of intervention studies– considering diverse approaches for mental and physical recovery. Psychiatry Research, 284, 112601. https://doi.org/10.1016/j.psychres.2019.112601
- Kruisdijk, F., Deenik, J., Tenback, D., Tak, E., Beekman, A. -J., van Harten, P., Hopman-Rock, M., & Hendriksen, I. (2017). Accelerometer-measured sedentary behaviour and physical activity of inpatients with severe mental illness. Psychiatry Research, 254, 67–74. https://doi.org/10.1016/j.psychres.2017.04.035
- Kurebayashi, Y., Mori, K., & Otaki, J. (2022). Effects of mild-intensity physical exercise on neurocognition in inpatients with schizophrenia: A pilot randomized controlled trial. Perspectives in Psychiatric Care, 2021(3), 1–11. https://doi.org/10.1111/ppc.12896
- Lambert, T. J. R., Reavley, N. J., Jorm, A. F., & Oakley Browne, M. A. Royal Australian and New Zealand College of Psychiatrists expert consensus statement for the treatment, management and monitoring of the physical health of people with an enduring psychotic illness. (2017). The Australian and New Zealand Journal of Psychiatry, 51(4), 322–337. Published Online First: 2017. https://doi.org/10.1177/0004867416686693
- Lan, Y. L., Ping, L. Y., Su, L. W., & Chen, C. C. (2022). The Impact of Health Promotion Activities on the Physiological, Psychological, and Social Functions of Inpatients with Chronic Mental Illness. Psychiatry Investigation, 19(3), 171–177. https://doi.org/10.30773/pi.2021.0128
- Legrand, F. D., & Neff, E. M. (2016). Efficacy of exercise as an adjunct treatment for clinically depressed inpatients during the initial stages of antidepressant pharmacotherapy: An open randomized controlled trial. Journal of Affective Disorders, 191, 139–144. https://doi.org/10.1016/j.jad.2015.11.047
- Lohr, J. B., Palmer, B. W., Eidt, C. A., Aailaboyina, S., Mausbach, B. T., Wolkowitz, O. M., Thorp, S. R., & Jeste, D. V. (2015). Is post-traumatic stress disorder associated with premature senescence? A review of the literature. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 23(7), 709–725. https://doi.org/10.1016/j.jagp.2015.04.001
- Malhi, G. S., & Byrow, Y. 2016 Advance online publication. Exercising control over bipolar disorder. Evidence-Based Mental Health, 19(4), 103–105, https://doi.org/10.1136/eb-2016-102430
- Manjunath, R. B., Varambally, S., Thirthalli, J., Basavaraddi, I. V., & Gangadhar, B. N. (2013). Efficacy of yoga as an add-on treatment for in-patients with functional psychotic disorder. Indian Journal of Psychiatry, 55(7), S374–378. https://doi.org/10.4103/0019-5545.116314
- Martinsen, E. W., Hoffart, A., & Ø, S. (1989). Comparing aerobic with nonaerobic forms of exercise in the treatment of clinical depression: A randomized trial. Comprehensive Psychiatry, 30(4), 324–331. https://doi.org/10.1016/0010-440X(89)90057-6
- Martinsen, E. W., Hoffart, A., & Solberg, Y. (1989). Aerobic and non‐aerobic forms of exercise in the treatment of anxiety disorders. Stress Medicine, 5(2), 115–120. https://doi.org/10.1002/smi.2460050209
- Martinsen, E. W., Sandvik, L., & Kolbjørnsrud, O. B. (1989). Aerobic exercise in the treatment of nonpsychotic mental disorders: An exploratory study. Nordic Journal of Psychiatry, 43(6), 521–529. https://doi.org/10.3109/08039488909103250
- Martland, R., Mondelli, V., Gaughran, F., & Stubbs, B. (2020). Can high intensity interval training improve health outcomes among people with mental illness? A systematic review and preliminary meta-analysis of intervention studies across a range of mental illnesses. Journal of Affective Disorders, 263, 629–660. https://doi.org/10.1016/j.jad.2019.11.039
- Mazyarkin, Z., Peleg, T., & Golani, I., Sharony L, Kremer I, Shamir A. (2019). Health benefits of a physical exercise program for inpatients with mental health; a pilot study. Journal of Psychiatric Research, 113, 10–16. https://doi.org/10.1016/j.jpsychires.2019.03.002
- McCartney, D., Isik, A. D., Rooney, K., Arnold, J. C., Bartlett, D. J., Murnion, B., Richards, E., Arkell, T. R., Lintzeris, N., & McGregor, I. S. (2021). The effect of daily aerobic cycling exercise on sleep quality during inpatient cannabis withdrawal: A randomised controlled trial. Journal of Sleep Research, 30(3), 1–12. https://doi.org/10.1111/jsr.13211
- Minghetti, A., Faude, O., & Hanssen, H., Zahner L, Gerber M, Donath L. (2018). Sprint interval training (SIT) substantially reduces depressive symptoms in major depressive disorder (MDD): A randomized controlled trial. Psychiatry Research, 265, 292–297. https://doi.org/10.1016/j.psychres.2018.04.053
- Morres, I. D., Hatzigeorgiadis, A., Stathi, A., Comoutos, N., Arpin-Cribbie, C., Krommidas, C., & Theodorakis, Y. (2019). Aerobic exercise for adult patients with major depressive disorder in mental health services: A systematic review and meta-analysis. Depression and Anxiety, 36(1), 39–53. https://doi.org/10.1002/da.22842
- National Institute for Health and Care Excellence (NICE). (2014). Psychosis and schizophrenia in adults: Prevention and management. https://www.nice.org.uk/guidance/cg178
- Ng, F., Dodd, S., & Berk, M. (2007). The effects of physical activity in the acute treatment of bipolar disorder: A pilot study. Journal of Affective Disorders, 101(1–3), 259–262. https://doi.org/10.1016/j.jad.2006.11.014
- NICE guideline.Psychosis and schizophrenia in children and young people. 2013.
- Palmer, J. A., Palmer, L. K., Michiels, K., & Thigpen, B. (1995). Effects of type of exercise on depression in recovering substance abusers. Perceptual and Motor Skills, 80(2), 523–530. https://doi.org/10.2466/pms.1995.80.2.523doi:10.2466/pms.1995.80.2.523
- Philippot, A., Dubois, V., Lambrechts, K., Grogna, D., Robert, A., Jonckheer, U., Chakib, W., Beine, A., Bleyenheuft, Y., & De Volder, A. G. (2022). Impact of physical exercise on depression and anxiety in adolescent inpatients: A randomized controlled trial. Journal of Affective Disorders, 301, 145–153. https://doi.org/10.1016/j.jad.2022.01.011
- Roest, A. M., Martens, E. J., de Jonge, P., & Denollet, J. (2010). Anxiety and Risk of Incident Coronary Heart Disease. A Meta-Analysis. Journal of the American College of Cardiology, 56(1), 38–46. https://doi.org/10.1016/j.jacc.2010.03.034
- Rosenbaum, S., Sherrington, C., & Tiedemann, A. Exercise augmentation compared with usual care for post-traumatic stress disorder: A randomized controlled trial. (2015). Acta Psychiatrica Scandinavica, 131(5), 350–359. Published Online First: 2015. https://doi.org/10.1111/acps.12371
- Roy, A., Govindan, R., & Muralidharan, K. (2018). The impact of an add-on video assisted structured aerobic exercise module on mood and somatic symptoms among women with depressive disorders: Study from a tertiary care centre in India. Asian Journal of Psychiatry, 32, 118–122. https://doi.org/10.1016/j.ajp.2017.12.004
- Salehi, I., Hosseini, S. M., Haghighi, M., Jahangard, L., Bajoghli, H., Gerber, M., Pühse, U., Holsboer-Trachsler, E., & Brand, S. (2016). Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. Journal of Psychiatric Research, 76, 1–8. https://doi.org/10.1016/j.jpsychires.2016.01.012
- Schneider, B. C., Moritz, S., Hottenrott, B., Reimer, J., Andreou, C., & Jelinek, L. (2016). Association Splitting: A randomized controlled trial of a new method to reduce craving among inpatients with alcohol dependence. Psychiatry Research, 238, 310–317. Published Online First: 2016. https://doi.org/10.1016/j.psychres.2016.02.051
- Schuch, F. B., Vasconcelos-Moreno, M. P., & Borowsky, C., Zimmermann AB, Wollenhaupt-Aguiar B, Ferrari P, de Almeida Fleck MP. (2014). The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. European Archives of Psychiatry and Clinical Neuroscience, 264(7), 605–613. https://doi.org/10.1007/s00406-014-0489-5
- Schuch, F. B., Vasconcelos-Moreno, M. P., Borowsky, C., Zimmermann, A. B., Rocha, N. S., & Fleck, M. P. (2015). Exercise and severe major depression: Effect on symptom severity and quality of life at discharge in an inpatient cohort. Journal of Psychiatric Research, 61, 25–32. https://doi.org/10.1016/j.jpsychires.2014.11.005
- Sexton, H., Mære, D., & Dahl, N. H. (1989). Exercise intensity and reduction in neurotic symptoms: A controlled follow‐up study. Acta Psychiatrica Scandinavica, 80(3), 231–235. https://doi.org/10.1111/j.1600-0447.1989.tb01332.x
- Shachar-Malach, T., Cooper Kazaz, R., Constantini, N., Lerer B. (2015). Effectiveness of Aerobic Exercise as an Augmentation Therapy for Inpatients with Major Depressive Disorder: A Preliminary Randomized Controlled Trial. The Israel Journal of Psychiatry and Related Sciences, 52(3), 65–70.
- Shiers, D., & Curtis, J. (2014). Cardiometabolic health in young people with psychosis. The Lancet Psychiatry, 1(7), 492–494. https://doi.org/10.1016/S2215-0366(14)00072-8
- Shimada, T., Ito, S., Makabe, A., Yamanushi, A., Takenaka, A., Kawano, K., & Kobayashi, M. (2020). Aerobic exercise and cognitive functioning in schizophrenia: Results of a 1-year follow-up from a randomized controlled trial. Psychiatry Research, 286, 112854. https://doi.org/10.1016/j.psychres.2020.112854
- Shimada, T., Ito, S., & Makabe, A., Yamanushi A, Takenaka A, Kobayashi M (2019). Aerobic exercise and cognitive functioning in schizophrenia: A pilot randomized controlled trial. Psychiatry Research, 282, 112638. https://doi.org/10.1016/j.psychres.2019.112638
- Slade, S. C., Dionne, C. E., Underwood, M., & Buchbinder, R. (2016). Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. British Journal of Sports Medicine, 50(23), 1428–1437. https://doi.org/10.1136/bjsports-2016-096651
- Smith, M. A., & Lynch, W. J. (2012). Exercise as a potential treatment for drug abuse: Evidence from preclinical studies. Frontiers in Psychiatry / Frontiers Research Foundation, 2. https://doi.org/10.3389/fpsyt.2011.00082
- Song, B. K., Kim, Y. S., Kim, H. S., Oh JW, Lee O, Kim JS. Combined exercise improves gastrointestinal motility in psychiatric in patients. World J Clin Cases. 2018. https://doi.org/10.12998/wjcc.v6.i8.207
- Stanley, S. H., Ng, S. M., & Laugharne, J. D. E. (2019). The ‘Fit for Life’ exercise programme: Improving the physical health of people with a mental illness. Psychol Heal Med, 24(2), 187–192. https://doi.org/10.1080/13548506.2018.1530366
- Stanton, R., & Happell, B. (2014a). Exercise for mental illness: A systematic review of inpatient studies. International Journal of Mental Health Nursing, 23(3), 232–242. https://doi.org/10.1111/inm.12045
- Stanton, R., & Happell, B. (2014b). A systematic review of the aerobic exercise program variables for people with schizophrenia. Current Sports Medicine Reports, 13(4), 260–266. https://doi.org/10.1249/JSR.0000000000000069
- Sterne, J. A., Hernán, M. A., & Reeves, B. C., Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. The BMJ, 355, 4–10. https://doi.org/10.1136/bmj.i4919
- Sterne, J. A. C., Savović, J., & Page, M. J., Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. The BMJ, 366, 1–8. https://doi.org/10.1136/bmj.l4898
- Stubbs, B., Rosenbaum, S., Vancampfort, D., Ward, P. B., & Schuch, F. B. (2016). Exercise improves cardiorespiratory fitness in people with depression: A meta-analysis of randomized control trials. Journal of Affective Disorders, 190, 249–253. https://doi.org/10.1016/j.jad.2015.10.010
- Stubbs, B., Vancampfort, D., Hallgren, M., Firth, J., Veronese, N., Solmi, M., Brand, S., Cordes, J., Malchow, B., Gerber, M., Schmitt, A., Correll, C. U., De Hert, M., Gaughran, F., Schneider, F., Kinnafick, F., Falkai, P., Möller, H. -J., & Kahl, K. G. (2018). EPA guidance on physical activity as a treatment for severe mental illness: A meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental. European Psychiatry, 54, 124–144. https://doi.org/10.1016/j.eurpsy.2018.07.004
- SY, L., Abdullah, A., Abu Bakar, A. K., Thambu, M., & Nik Jaafar, N. R. (2016). Structured Walking and Chronic Institutionalized Schizophrenia Inmates: A pilot RCT Study on Quality of Life. Global Journal of Health Science, 8(1), 238–248. https://doi.org/10.5539/gjhs.v8n1p238
- Tsukue, I., & Shohoji, T. Movement therapy for alcoholic patients. (1981). Journal of Studies on Alcohol, 42(1), 144–149. Published Online First: 1981. https://doi.org/10.15288/jsa.1981.42.144
- Vancampfort, D., Rosenbaum, S., & Probst, M., Soundy A, Mitchell AJ, De Hert M, Stubbs B. (2015). Promotion of cardiorespiratory fitness in schizophrenia: A clinical overview and meta-analysis. Acta Psychiatrica Scandinavica, 132(2), 131–143. https://doi.org/10.1111/acps.12407
- Vancampfort, D., Stubbs, B., Venigalla, S. K., & Probst, M. (2015). Adopting and maintaining physical activity behaviours in people with severe mental illness: The importance of autonomous motivation. Prev Med (Baltim), 81, 216–220. https://doi.org/10.1016/j.ypmed.2015.09.006
- Vogelzangs, N., Seldenrijk, A., Beekman, A. T. F., van Hout, H. P. J., de Jonge, P., & Penninx, B. W. J. H. (2010). Cardiovascular disease in persons with depressive and anxiety disorders. Journal of Affective Disorders, 125(1–3), 241–248. https://doi.org/10.1016/j.jad.2010.02.112
- Weinmann, S., Janssen, B., & Gaebel, W. (2004). Switching antipsychotics in inpatient schizophrenia care: Predictors and outcomes. The Journal of Clinical Psychiatry, 65(8), 1099–1105. https://doi.org/10.4088/JCP.v65n0812
- Weitoft, G. R., & Rosén, M. (2005). Is perceived nervousness and anxiety a predictor of premature mortality and severe morbidity? A longitudinal follow up of the Swedish survey of living conditions. Journal of Epidemiology and Community Health, 59, 794–798. https://doi.org/10.1136/jech.2005.033076
- Wheeler, A. J., Roennfeldt, H., Slattery, M., Krinks, R., & Stewart, V. (2018). Codesigned recommendations for increasing engagement in structured physical activity for people with serious mental health problems in Australia. Heal Soc Care Community, 26(6), 860–870. https://doi.org/10.1111/hsc.12597
- Whiteford, H. A., Degenhardt, L., Rehm, J., Baxter, A. J., Ferrari, A. J., Erskine, H. E., Charlson, F. J., Norman, R. E., Flaxman, A. D., Johns, N., Burstein, R., Murray, C. J., & Vos, T. (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. The Lancet, 382(9904), 1575–1586. https://doi.org/10.1016/S0140-6736(13)61611-6
- Woodward, M. L., Gicas, K. M., Warburton, D. E., White, R. F., Rauscher, A., Leonova, O., Su, W., Smith, G. N., Thornton, A. E., Vertinsky, A. T., Phillips, A. A., Goghari, V. M., Honer, W. G., & Lang, D. J. (2018). Hippocampal volume and vasculature before and after exercise in treatment-resistant schizophrenia. Schizophrenia Research, 202, 158–165. https://doi.org/10.1016/j.schres.2018.06.054
- Wright, K., Armstrong, T., Taylor, A., & Dean, S. ‘It’s a double edged sword’: A qualitative analysis of the experiences of exercise amongst people with Bipolar Disorder. (2012). Journal of Affective Disorders, 136(3), 634–642. Published Online First: 2012. https://doi.org/10.1016/j.jad.2011.10.017
- Wunram, H. L., Hamacher, S., Hellmich, M., Volk, M., Jänicke, F., Reinhard, F., Bloch, W., Zimmer, P., Graf, C., Schönau, E., Lehmkuhl, G., Bender, S., & Fricke, O. (2018). Whole body vibration added to treatment as usual is effective in adolescents with depression: A partly randomized, three-armed clinical trial in inpatients. European Child & Adolescent Psychiatry, 27(5), 645–662. https://doi.org/10.1007/s00787-017-1071-2
- Wunram, H. L., Oberste, M., Ziemendorff, A., Hamacher, S., Kapanci, T., Heller, R., Blick, S., Bloch, W., Clajus, T. C., Schönau, E., Bender, S., & Fricke, O. (2021). Differential effects of ergometer-cycling and Whole-Body-Vibration training on serological BDNF and IGF-1 in the treatment of adolescent depression - is there an impact of BDNFp.Val66Met variants? Physiology and Behavior, 241, 113596. https://doi.org/10.1016/j.physbeh.2021.113596
