 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Although it is generally assumed that face recognition relies on holistic processing, whether face recognition deficits observed in Developmental Prosopagnosics (DPs) can be explained by impaired holistic processing is currently under debate. The mixed findings from past studies could be the consequence of DP’s heterogeneous deficit nature and the use of different measures of holistic processing—the inversion, part-whole, and composite tasks—which showed a poor association among each other. The present study aimed to gain further insight into the role of holistic processing in DPs. Groups of DPs and neurotypicals completed three tests measuring holistic face processing and non-face objects (i.e., Navon task). At a group level, DPs showed (1) diminished, but not absent, inversion and part-whole effects, (2) comparable magnitudes of the composite face effect and (3) global precedence effect in the Navon task. However, single-case analyses showed that these holistic processing deficits in DPs are heterogeneous.
Introduction
Face processing is fundamental to human social interaction, in which many different types of information, such as emotions, gender and identity, are conveyed through faces (Bruce & Young, Citation1986; Estudillo, Citation2012; Little et al., Citation2011). Despite face processing being typical during human interactions, some individuals suffer a lifelong neurodevelopmental condition with severe deficits in face recognition called Developmental Prosopagnosia (DP; Duchaine & Nakayama, Citation2006). Developmental Prosopagnosics (DPs) fail to develop face recognition skills despite having normal intelligence, visual acuity, and memory, along with no obvious brain damage (Cook & Biotti, Citation2016; Susilo & Duchaine, Citation2013). Multiple studies have proposed that face processing impairments in DPs extend from personally familiar faces to their own faces, and cannot be treated or cured (Bowles et al., Citation2009; Kennerknecht et al., Citation2006, Citation2008). In addition, people with developmental prosopagnosia are more likely to suffer social and psychological dysfunctions, including increased levels of anxiety in social situations, depression, lack of interest in social activities, and difficulties creating and maintaining personal relationships (Yardley et al., Citation2008).
Holistic processing is a pivotal concept in the face processing literature (Boutet et al., Citation2021; Rossion, Citation2013; Yovel et al., Citation2014). According to the holistic account, face recognition does not rely on the isolated processing of individual facial features, but instead, all the features are integrated into a whole (Diamond & Carey, Citation1986; Leder & Bruce, Citation2000; Piepers & Robbins, Citation2012). Three different experimental paradigms are generally considered the gold-standard measures of holistic face processing: the inversion task (Maurer et al., Citation2002; Rossion, Citation2008; Rossion & Gauthier, Citation2002; Yin, Citation1969), the composite face task (Rossion, Citation2013; Young et al., Citation1987) and the part-whole task (Tanaka & Farah, Citation1993; Tanaka & Simonyi, Citation2016). The inversion task shows that face recognition is impaired when the faces are seen inverted along the fronto-parallel plane compared to upright faces (see Rossion & Gauthier, Citation2002; Yin, Citation1969). The composite task involves using face stimuli created by combining complementary top and bottom halves of two different face identities, split at the horizontal meridian. Aligning the top half of one identity with the bottom half of another identity creates the illusion of a new identity, making it hard to attend to one half of the face while ignoring the other. However, misaligning both halves would eliminate the effect (Rossion, Citation2013; Young et al., Citation1987). Finally, the part-whole task shows that the identification of a specific facial feature, such as the eyes, is more accurate when it is presented in the context of a whole face, compared to when it is presented in isolation (Tanaka & Farah, Citation1993; Tanaka & Simonyi, Citation2016).
If holistic processing is important for face recognition, one would expect to see holistic processing impairments in DPs (e.g., Avidan et al., Citation2011; Behrmann et al., Citation2005; DeGutis et al., Citation2012; Liu & Behrmann, Citation2014; Susilo & Duchaine, Citation2013; Towler et al., Citation2018). Consistent with this interpretation, a study by Avidan et al. (Citation2011) showed reduced inversion and composite effects in DP patients, suggesting impairments in holistic processing. Similarly, a different study using the part-whole task showed holistic processing impairments of the eye region in DPs (DeGutis et al., Citation2012). Other research has shown that holistic processing deficits in DPs also extend to non-face objects (Bentin et al., Citation2007; Gerlach et al., Citation2017; Gerlach & Starrfelt, Citation2018, Citation2021; but see Duchaine et al., Citation2007b), such as recognition of Navon compound stimuli (e.g., a global H formed with local S; see Navon, Citation1977). For instance, Gerlach et al. (Citation2017) found that DPs were not only slower in the processing of global letters, but also showed a diminished global precedence effect (i.e., more reliance on featural processing). Their findings suggest that holistic deficits seen in DPs may be extended to several object categories (i.e., not face-specific).
However, other studies have reported normal holistic processing in DPs (Biotti et al., Citation2017; Le Grand et al., Citation2006; Palermo et al., Citation2011; Susilo et al., Citation2010; Ulrich et al., Citation2017). For example, Susilo et al. (Citation2010) presented the case of a DP, who despite being severely impaired in face recognition, demonstrated normal composite and inversion effects. Similarly, Le Grand et al. (Citation2006) found that seven out of eight of their DPs showed typical composite effects, suggesting some individual differences in holistic processing among DPs. More recently, Biotti et al. (Citation2017) also found normal holistic processing in a large group of DPs compared to neurotypicals (NTs), as measured with the composite task. Nonetheless, we must be cautious in interpreting face composite effects as its functional significance has been questioned in some studies with NTs showing little to no association between this task and face recognition abilities (Konar et al., Citation2010; Rezlescu et al., Citation2017; Verhallen et al., Citation2017; but see Richler et al., Citation2011). In addition, some other studies report that the composite effect may reflect other aspects of face processing that are not strongly associated with holistic processing per se, such as attention and working memory (see Fitousi, Citation2015, Citation2020).
Given the mixed findings, whether DPs’ face identification deficits can be explained by holistic processing impairments is still an open question. One major weakness of studies that have attempted to address this question in the past is the assumption that the three conventional measures of holistic processing (i.e., inversion, part-whole and composite effects) reflect the same underlying cognitive mechanism(s). However, in a recent study, Rezlescu et al. (Citation2017) found that the inversion effect and the part-whole effect were only weakly correlated with each other, and the composite effect did not correlate with either of those two (see also Lee et al., Citation2022; Wang et al., Citation2012). These findings suggest that holistic processing is a multifaceted construct and there may not be a common mechanism explaining the three putative effects of these holistic processing tasks (see review by Boutet et al., Citation2021). Furthermore, Rezlescu et al. (Citation2017) also found that each of the three measures had distinct relationships with face identification measured through the Cambridge Face Perception Test (CFPT; Duchaine et al., Citation2007a). Specifically, while the inversion and the part-whole effects moderately correlated with face identification, the composite effect did not (Rezlescu et al., Citation2017). This suggests that the individual differences seen in holistic face recognition are task-dependent. Consequently, impaired holistic processing indexed with conventional holistic measures may also be dissociable between different DPs (Biotti et al., Citation2017).
The fact that the inversion, the part-whole and the composite tasks reflect different cognitive mechanisms is perhaps unsurprising, as there are notable differences between these tasks (Boutet et al., Citation2021; Li et al., Citation2017, Citation2019; Rezlescu et al., Citation2017). For example, in the part-whole task, holistic processing is generally demonstrated by the magnitude of facilitation in encoding and/or integration of featural information into a whole (Rezlescu et al., Citation2017). However, in the composite task, holistic advantage is based on the magnitude of holistic interference, reflected by the failure to selectively attend to the top half of the face (Richler & Gauthier, Citation2014). Neural studies have also shown differences in the activation patterns of the face fusiform area (FFA) between the two tasks. For instance, Li et al. (Citation2017) found that dissociation of hemispheric neural activity in the FFA between the PWE and CFE. Specifically, PWE was associated with face selectivity in the right FFA and the CFE was associated with face selectivity in the left FFA. Importantly, while the correlation for the PWE was found to be driven by responses to faces, the CFE was driven by suppression of non-face objects.
In contrast, holistic processing measured by the inversion task is considered an index for the sensitivity towards facial configuration (Rossion, Citation2008; Carbon & Leder, Citation2005). For example, configural manipulations impaired the recognition of upright faces more strongly than inverted faces (Carbon & Leder, 2005). Additionally, McKone et al. (Citation2013) demonstrated that inverting faces also significantly affects the magnitude of both the part-whole and composite effects. This argues that the FIE taps into an overlapping mechanism encompassing all three tasks (Boutet et al., Citation2021; Gerlach & Mogensen, Citation2024).
Individuals with DP often display varying degrees of impairment, ranging from mild to severe, and these impairments may impact different aspects of face processing (e.g., holistic processing). Thus, analyzing data on an individual level allows us to discern distinct patterns of impairment, thereby facilitating a more nuanced comprehension of the condition. The above leads to three possibilities. First, all three paradigms discussed above do not measure the same aspect of holistic processing (i.e., the same underlying cognitive mechanism) and therefore, all DPs may be impaired in some but not other aspects of holistic processing (e.g., all DPs may show relatively reduced susceptibility towards the part-whole effect but not the composite or inversion effects). We call this account the universal holistic processing deficit hypothesis. Second, different DPs may present qualitatively different holistic processing impairments (e.g., case A might show reduced susceptibility only to the part-whole effect, while case B may present reduced susceptibility only to the composite face effect). We call this the heterogeneous holistic processing deficit hypothesis. Third, DP’s deficits in face identification might not be explained by holistic processing impairments.
To explore the first and third possibilities, this study employed the three gold-standard measures of holistic face processing on the same group of DPs and compared their performance with those of a control group of NTs. In addition, to examine potential holistic processing deficits for non-face stimuli, our participants also performed a Navon task. To explore the second possibility, besides classic group-level comparisons between DPs and a group of NTs, we compared each DP’s performance individually to their corresponding age-matched NT group, as this approach could provide further insight into whether holistic deficits are universal or heterogeneous across DPs (Corrow et al., Citation2016; Le Grand et al., Citation2006; Susilo & Duchaine, Citation2013).
Methods
Participants
All participants were recruited through online social media platforms (i.e., prosopagnosia social support groups) and word of mouth. The initial recruitment of DPs was based on self-reports of their severe difficulties in recognizing faces (Burns et al., Citation2023) and these participants’ face recognition deficits were confirmed by impaired performance in the CFMT (score < 42; see below) and normal performance in the Cambridge Car Memory Test (CCMT; Dennett et al., Citation2012). Initially, we recruited 27 Caucasian suspected DPs, but only data from 17 suspected DPs were included in the analysis.Footnote1 Seven of the suspected DPs were excluded as their face recognition abilities score was in the normal range (not less than two SD from the mean scores of NTs) for the CFMT (Duchaine & Nakayama, Citation2006). In addition, these excluded suspected DPs also performed similarly or better with the recognition of faces than cars (i.e., scores in CCMT), which suggests that their self-reported recognition difficulties are not face-specific. Another three suspected DPs did not complete the experiment. All suspected DPs indicated no previous brain damage and other known neurological or psychiatric disorders. We also recruited 45 Caucasian NTs based on self-reports of normal face recognition ability, and this confirmed by their CFMT scores which were above the specific cut-off (i.e., more than 42). Our final sample comprised 17 Caucasian suspected DPs (4 males) and 45 Caucasian NTs (21 males) participants. The age of our DPs ranged from 19 to 69 (M = 46.88 years, SD = 17.69 years), while that of NTs ranged from 20 to 70 (M = 46.36 years, SD = 17.47 years).
Participants were included in a lucky draw that gifted every 1 in 10 participants an Amazon eGift card valued at £30, as compensation for their time and effort. A digital informed consent was obtained prior to participation. All experimental procedures were approved by the Science and Engineering Research Ethics Committee of the University of Nottingham Malaysia (approval code: BLQZ250920).
Apparatus
This study was conducted fully online using the experimental platform Testable (www.testable.org; Rezlescu et al., Citation2020) and all tasks were completed on participants’ own computers (laptops or desktops). To minimise differences in displayed stimuli size across different computer screens, participants were required to adjust the length of a yellow line that appeared on their screens to match the width of a debit/credit card they had in possession. This allowed Testable to calculate how many screen pixels were mapped onto 1 centimetre (cm) and scale all stimuli based on this mapping. Adobe Photoshop CS6 and Matlab R2019b (Mathworks, Version 9.7.0.1247435) were used to edit stimuli where necessary (refer to Stimuli and Procedure).
Stimuli and procedure
Each participant was first briefed about the experiment and was informed that they had to complete two different stages: the “evaluation” stage and the “experimental” stage, over two different days (i.e., one stage per day). The “evaluation” stage, which was always completed first, included the CFMT, the CFPT and the CCMT (see below for abbreviations). This was followed by the “experimental” stage, which included the part-whole task, the composite task, the face inversion task and the Navon’s task. The order of the face holistic measures was counterbalanced across all participants. However, the Navon’s task was always completed last as some research has shown that this task could bias responses in subsequent face processing tasks (see Estudillo et al., Citation2022; Lewis et al., Citation2009; Macrae & Lewis, Citation2002; Wong et al., Citation2021). Accuracy and reaction time (Navon’s task only) were measured and recorded.
Evaluation stage
This stage comprised the basic evaluation tasks: the CFMT, CFPT and CCMT. The CFMT was used as a measure of face recognition abilities and the CFPT was used to examine whether suspected DPs’ impairment is also characterized by a deficit in the mere perception of faces, while the CCMT was to control for potential object recognition deficits in participants.
Cambridge Face Memory Test (CFMT)
We used the original version of the CFMT. The stimuli and procedures of the CFMT were from Duchaine and Nakayama (Citation2006). In this task, six unique target face identities and 46 unique distractor face identities (all Caucasian men) were used. For each identity, there are three face images taken from different viewpoints (1 left 1/3 profile, 1 full-frontal and 1 right 1/3 profile). All faces were cropped so that no hair, clothing, or facial blemishes were visible. The faces were embedded in the centre of a uniformly black background (195 × 222 pixels (px); 3.9 × 4.44 cm: width × height). The test contained a total of 72 trials from three different stages (e.g., 18 Learning, 30 Novel and 24 Noise). All trials consisted of three faces (one target and two distractors) and participants were required to select which of the three matched a learned face by pressing the allocated key. All images in the trials were presented in a fixed order. The maximum score on the CFMT is 72. A score below 42 suggests face identification deficits (Bowles et al., Citation2009; Dalrymple & Palermo, Citation2016; Estudillo et al., Citation2020; Estudillo & Wong, Citation2021).
Cambridge Face Perception Test (CFPT)
The stimuli and procedures of CFPT were from Duchaine et al. (Citation2007a). The CFPT is a computerized sorting task in which participants arrange six morphed images of faces (i.e., test faces) based on their similarity to a target face. A total of eight male faces in ¾ profile views are used as target faces. “Test” faces are morphs of the target faces, whereby any single target face in its frontal view was morphed with one of the other targets in their frontal view. The morphed images are 88%, 76%, 64%, 52%, 40%, or 28% similar to the target face. External face features are covered by a black seamed cap. Both test and target faces were cropped similarly (e.g., from above the eyebrows) and embedded on a 190 × 190 px (3.8 × 3.8 cm) grey (e.g., morphed faces) and white (e.g., target faces) background.
Eight different sort trials were created, and each sort was presented once in upright and once in inverted orientation (total 16 trials). Participants reordered the test faces (e.g., select a test face and then click on the column they want it to be repositioned) in terms of resemblance to a target face (e.g., most similar at the very left to least similar at the very right). Participants had one minute to complete each sorting trial. Scores for each item were computed by summing the deviations (i.e., errors) from the correct position for each face. For example, if a face was one position away from its correct position, that was counted as an error of one. If it were two positions away, that would be an error of two. Thus, a higher score in the CFPT represents poorer performance. Scores for the eight upright items and the eight inverted items were averaged. Performance at chance in the CFPT is 93.3 errors (Duchaine et al., Citation2007a).
Cambridge Car Memory Test (CCMT)
The CCMT (based on the stimuli and procedures from Dennett et al., Citation2012) follows an identical format as the CFMT, with the exception that the stimuli were modified computer-generated images of actual car models (instead of faces), created using 3D Studio Max. To minimise matching based on easily noticeable visual features, all cars are of the same colour, and no identifying badges, logos, or insignias are visible. Car stimuli for the CCMT were sized approximately 465 × 215 px (9.3 × 4.3 cm) (average across cars and viewpoints). Similar to the CFMT, the CCMT also comprises three stages: learning (18 trials), novel (30 trials), and noise (24 trials). The maximum possible score is 72. Any score above 40 denotes normal recognition ability for non-face objects (Dennett et al., Citation2012).
Experimental stage
Face inversion task
A total of 30 male face identities in three different viewpoints (taken from Rezlescu et al., Citation2012) were used for this task. The face stimuli were all male faces, with their hair completely covered by a standard black cap. This was done to ensure that recognition judgements were based on internal facial features alone. The faces were also in greyscale and were embedded in a 300 × 300 px (6 × 6 cm) white background.
On any given experimental trial, participants were asked to match one of three test faces (i.e., mid-profile view) shown to the identity of a target face (i.e., frontal view). The orientation of the target and test faces are always consistent within each trial. Target identities in one trial were also used as test faces (i.e., distractor faces) in other trials that had a different target identity. Similar to the original design (Rezlescu et al., Citation2012), participants first saw the target face flashed for 400 ms, followed by the three test images simultaneously presented for 2000 ms, and a blank screen that was presented until the participant responded. Participants were required to press the key “1” if the test face on the left matched the target, “2” for the face in the middle and “3” for the face on the right. The task had a total of 60 trials (30 upright and 30 inverted), presented in a randomized order. Participants were instructed not to tilt their heads when they see inverted faces. Across all trials, each target identity was presented twice—once upright and once inverted.
Part-whole task
Face images for this task were taken from Wong et al. (Citation2021; see also Estudillo et al., Citation2022) and procedures were similar to those used in DeGutis et al. (Citation2012) and Estudillo et al. (Citation2022). These images were modified to create new faces with unique combinations of internal features using Photoshop. Target faces were created using either a male or female face template that included the hair and the face outline only. For each (gender) template, six target faces were created by adding internal features such as distinct noses, mouths, and eyes, from six different identities. These six target faces did not share any similar internal feature.
Two types of test stimuli were also created. One of these types of test stimuli consisted of isolated features (mouth, nose, or eyes only) taken from the target faces. The other type comprised full faces (“whole foils”) that were created by switching only one of the distinct features of a target face (eyes, nose, or mouth) with that of a different target face. All faces were in greyscale and embedded in a 370 × 500 px (7.4 × 10 cm) grey background. All isolated features were also cropped (e.g., eyes: 234 × 80 px; 4.68 × 1.6 cm, nose: 97 × 77 px; 1.94 × 1.54 cm, mouth: 138 × 71 px; 2.76 × 1.42 cm) from the original face stimuli and the size was kept constant (i.e., the same size as the features in full faces) in the experiment (see ).
Figure 1. An example of the stimuli used in the part-whole task.
Note. A target face is shown on the left-hand side and 4 test stimuli are shown on the right-hand side: the whole condition (top row) and the part condition (bottom row).
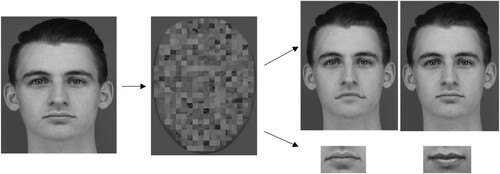
In each experimental trial, one target image of a whole face was presented for 1000 ms, immediately followed by a mask (i.e., a scrambled face created by dividing the face into tiles and then shuffled the position of the tiles) shown for 500 ms. Two test images were presented side-by-side until participants responded. The test images were either two whole faces (whole conditions) or two isolated features (e.g., two eyes), one from each face (part conditions). Participants had to indicate which of the test stimuli matched the target, by pressing one of two allocated keys. There were 144 trials: 2 conditions (whole and part) × 3 features (eyes, mouth and nose) × 24 trials per feature. The trials had an equal number of male and female targets presented in a randomized order.
Face composite task
Stimuli were obtained from Retter and Rossion (Citation2015) and were made from 15 faces (seven females). All faces were in greyscale with neutral expressions. Composite faces have their top and bottom halves separated horizontally by a white gap of three pixels. The separation between halves is achieved by splitting the face at the bridge of the nose (5% of the length of the face above the nostrils). Initially, five composites were created, wherein one of them had a combination of the same identity for the top and bottom halves. The other four were a combination of the top half of one identity with the bottom half of one of the other remaining identities, chosen to match for gender and face width as closely as possible. These composites were duplicated to create “misaligned composites” where the bottom half of the composite was translated to the right by 25% of its width. These aligned and misaligned composites were used as “target” stimuli. All of the composites used as targets (227 × 325 px; 4.54 × 6.5 cm) were enlarged by 5% of their original size to create the “test” stimuli (238 × 350 px; 4.76 × 7 cm). This was done to minimise matching based on low-level features alone (Rossion, Citation2013).
The bottom halves were always different between the test and target composites, while the top halves were the “same” in half of the trials and “different” in the remaining trials. Participants were asked to ignore the bottom halves and decide whether the top halves of the two composites are the same or different. The participants were required to press the key “Q” for same and “P” for different. The procedures for this task were adopted from Susilo et al. (Citation2010). The test had 120 randomized trials (40 same-aligned, 40 same-misaligned, 20 different-aligned, and 20 different-misaligned). Each trial consisted of two composite faces that were presented sequentially (e.g., the first composite for 400 ms and the second composite for 400 ms) and separated by a grey blank screen for 500 ms. In each trial, both composite faces presented were either aligned or misaligned. This procedure followed the standard version of the composite task (Rossion, Citation2013).
Navon’s task
Participants were presented with large letters, either “H” or “S”, that were made of either smaller “H”s or “S”s. Congruent stimuli had the same alphabetical character for the large and small letters, whereas incongruent stimuli did not (see ). The large letters were 278 × 162 px (5.56 × 3.24 cm) in size, and the small letters were 37 × 22 px (0.74 × 0.44 cm) in size. All stimuli were in white and were centred on a 6 × 6 cm black background.
Figure 2. Examples of the stimuli used in the Navon’s task.
Note. (From left to right) S-congruent, S-incongruent, H-congruent, and H-incongruent.

The procedure was adopted from Gerlach et al. (Citation2017). Each participant was presented with four experimental blocks. In two blocks (“A”), participants were required to report the identity of the global letter (e.g., press the key “H” if the global letter “H” is presented). In the remaining two blocks (“B”), participants were to report the identity of the local letters. The blocks were always presented in an ABAB order. Across the four blocks, participants performed a total of 48 trials, where 24 trials consisted of congruent stimuli (e.g., the same identity of local and global letters) and 24 trials consisted of incongruent stimuli. An equal number of stimulus types were presented within each block. Congruent and incongruent trials were randomized within each block. In all blocks, each trial began with a fixation cross (22 × 22 px; 0.44 × 0.44 cm) presented in the middle of the screen for 1000 ms, followed by the test stimulus shown for 180 ms and a blank screen which remained until a response was recorded. The participants were also required to perform 16 practice trials (equal amount of all 4 trial types) at the beginning of the experiment.
Data analysis
To test for internal consistency and/or reliability of our tasks, we calculated Guttman’s λ2 and Cronbach’s α with the raw scores for each task, separated by group (i.e., DPs and NTs) and conditions. We separated the data by groups because DPs impairment in face recognition would affect the observed reliability of these tasks. Previous studies that used the three evaluation tasks—CFMT, CFPT, CCMT—have consistently shown that they have high reliabilities (Bowles et al., Citation2009; Dennett et al., Citation2012; Estudillo et al., Citation2020; Kho et al., Citation2023; Murray & Bate, Citation2020; Rezlescu et al., Citation2017). The reliability of the Navon task (Dale & Arnell, Citation2013; Hedge et al., Citation2018) and the global precedence index (Gerlach et al., Citation2017) have also been recently examined in detail. For this reason, we only assessed the reliabilities of our four holistic tasks (face inversion, part-whole, composite face, and Navon’s tasks). The analyses were done using the R package psych (Revelle, Citation2023). Additionally, using Guttman’s λ2, we also calculated the reliability of our tasks in computing holistic advantage using the subtraction (for Navon’s task) and regression (for face inversion, part-whole, composite face task) approach (Malgady & Colon-Malgady, Citation1991), following the method of calculation in DeGutis et al. (Citation2013). We used Guttman’s λ2 due to its robustness in measuring reliability when dealing with measures that include multiple factors (Callender & Osburn, Citation1979).
We ran three types of analyses. First, we wanted to confirm that our different measures of holistic processing performed similarly as in other studies. For this, we ran multiple repeated-measures analyses of variance (ANOVA) to compare participant’s performances in each condition of interest (i.e., upright, whole, and same-aligned trials in the inversion, part-whole and composite tasks, respectively) to their respective control conditions (i.e., inverted, part, same-misaligned trials). Second, to examine group differences in holistic processing for each holistic task, we used the control-based regression approach (DeGutis et al., Citation2013) in which the variances of the control conditions are regressed from the condition of interest (see DeGutis et al., Citation2013; Rezlescu et al., Citation2017) of the NT group. We only included the data of NTs to ensure that the regression lines were based on normative performances (DeGutis et al., Citation2012). Then, we applied this equation to calculate the residuals for each of the DPs, as seen in EquationEquation (1)(1)
(1) (Berger et al., Citation2022).
(1)
(1) We then compared residual scores across both groups using independent t-tests. A higher residual score represents stronger holistic processing.
For the Navon’s task, we calculated the global precedence index for correct trials as the standardized mean difference (Cohen’s d) between RTs of Local congruent and Global congruent trials. Compared to other Navon indexes, this index offers a purer precedence index as it is not confounded with interference effects (see Gerlach & Krumborg, Citation2014; Gerlach & Starrfelt, Citation2018). These standardized differences were then compared between DPs and NTs (see Gerlach & Krumborg, Citation2014). A higher standardized difference represents a stronger holistic advantage.
Moreover, we also examined whether holistic processing deficits in DPs (if any) are universal or heterogeneous across different DPs (e.g., case A is only impaired in the inversion task, but case B is impaired only in the part-whole task) for our third analysis. Although holistic processing tends to be maintained with age (Boutet & Meinhardt-Injac, Citation2019), aging has a significant negative impact on both face matching (i.e., face perception) and memory-based recognition of faces (Boutet & Meinhardt-Injac, Citation2021). Previous studies have shown that face recognition ability peaks at the age of 35, remains stable and/or declines from 36 years onwards, and falls below the initial threshold after 60 years of age (see Germine et al., Citation2011; Jaworska et al., Citation2020; Meinhardt et al., Citation2016). To account for such age-related differences, we first separated DPs and NTs into three different age groups: 18–35 years old, 36–59 years old, and 60 years and above. Mean age of NTs for each group was 25.1 (SD = 5 years), 48.6 (SD = 6 years) and 65.4 years (SD = 3 years), respectively. Then, we calculated the holistic face advantage using the regression approach for all participants in each of the three age groups, separately. As for the Navon’s task, the effect size was calculated similarly as previously specified. We ran modified t-tests designed for single-case analyses (Crawford et al., Citation2010; Crawford & Garthwaite, Citation2002) comparing the residual scores (e.g., inversion, part-whole and composite effect) and standardized differences (Navon’s task) between each suspected DP’s performance and their age-matched NT group (N = 15). This statistical approach is an appropriate choice for the study of DP, wherein it allows for the comparison of an individual’s performance against a control group, addressing the challenge of variability within DP individuals. To examine if these heterogeneous impairments also met the criteria of strong or classical dissociation (e.g., DPs are impaired on one task but performed normally on another), we ran additional post-hoc tests using a revised difference test (i.e., dissocs.exe; Crawford & Garthwaite, Citation2005; see Supplementary Table S2).
Results
A summary of our suspected DPs’ performance in the evaluation stage is shown in . Our selected sample of suspected DPs (N = 17) and NT controls (N = 45) performed in accordance with our predictions in the evaluation tests (i.e., CFMT, CFPT, and CCMT, see ). At a group-level, suspected DPs had significantly poorer performance than NTs in face perception as measured by the CFPT. The single-case analyses of the CFPT showed that seven out of 17 suspected DPs showed significantly higher errors than their age-matched control group (e.g., NC: t = 1.946, p = .036; MM: t = 3.179, p = .003; BC: t = 1.918, p = .038; LM: t = 3.523, p = .002; DM: t = 2.720, p = .008; DG: t = 1.839, p = .044; RP: t = 2.849, p = .006). However, suspected DPs and NTs were comparable in the CCMT. The single-case analyses of the CCMT also revealed that none of the suspected DPs had a significantly poorer performance than their age-matched control group.
Table 1. Suspected DPs demographics, followed by scores on the CFMT, CFPT and CCMT.
Face inversion task
The internal consistencies of the inversion effect using the regression approach in NTs (λ2 = .692) and suspected DPs (λ2 = .189) were moderate and weak, respectively. A Levene’s test confirmed that, for the inversion effect scores, the variance in DPs did not differ from that of NTs (p = .726). Our ANOVA revealed a significant main effect of condition, where participants were more accurate with upright compared to inverted trials, F(1,60) = 62.897, p < .001, = .512. This pattern replicates the classical inversion effect reported by previous studies (e.g., Rossion, Citation2008; Yin, Citation1969). Furthermore, there was a significant main effect of group, F(1,60) = 18.798, p < .001,
= .239, showing that suspected DPs performed significantly poorer than NTs in the inversion task. In addition, there was a significant interaction between condition and group, F(1,60) = 17.081, p < .001,
= .222. Holm Bonferroni-corrected paired samples t-tests revealed that suspected DPs were significantly poorer than NTs in the upright (t(60) = −5.783, p < .001), but not the inverted (t(60) = −1.836, p = .139) conditions. Further, although NT performed better with upright compared to inverted faces (t(44) = 11.519, p < .001), such a difference was not found in DPs (t(16) = 2.229, p = .089), suggesting impaired or negligible inversion effects in this group. This was further supported by the analysis of residuals that revealed a smaller inversion effect in suspected DPs (M = −0.201, SD = 0.131) compared to NTs (M = 2.289e−5, SD = 0.116), t(60) = −5.890, p < .001, d = −1.677 (see ). However, despite these group differences, our single-case analyses of the residual scores showed that only nine out of 17 suspected DPs had a significantly smaller inversion effect than their age-matched control groups (refer to ).
Figure 3. The magnitude of holistic advantage (residuals and Cohen’s d) between suspected DPs and NTs in the four holistic measures.
Note. Error bar represents the standard error of the mean and grey dots represent individual residuals or effect size.
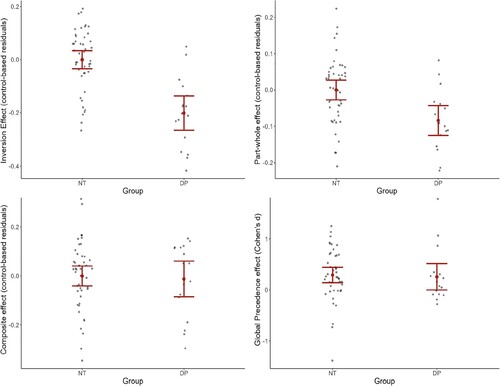
Table 2. Accuracy (inversion, part-whole and composite task) and reaction time (Navon’s task) performances between suspected DPs and NTs.
Table 3. Single-case analyses of each suspected DPs and their age-matched control groups in the four holistic measures.
Part-whole task
The internal consistencies of the part-whole effect using the regression approach in NTs (λ2 = .699) and suspected DPs (λ2 = .635) were modest. The variance in the part-whole effect scores did not differ between the two participant groups, as confirmed by a Levene’s test (p = .530). We found a significant main effect of condition, where all participants in general were more accurate with whole compared to part trials, F(1,60) = 17.228, p < .001, = .223, replicating previous results using this task (DeGutis et al., Citation2013; Estudillo et al., Citation2022). Furthermore, there was a significant main effect of group, F(1,60) = 18.515, p < .001,
= .236, showing that suspected DPs performed significantly poorer than NTs in the part-whole task. In addition, there was a significant interaction effect between condition and group, F(1,60) = 4.982, p = .029,
= .077. Holm Bonferroni-corrected paired samples t-tests showed that suspected DPs were significantly poorer than NTs in both whole (t(60) = −4.854, p < .001) and part (t(60) = −2.676, p = .026) conditions. Further, while NTs performed better with whole compared to part faces (t(44) = 11.519, p < .001), such a difference was not found in suspected DPs (t(16) = 1.126, p = .265), suggesting impaired part-whole effect in suspected DPs. This was further supported by the analysis of residuals that revealed a smaller part-whole effect in DPs (M = −0.084, SD = 0.083) compared to NTs (M = −3.796e−5, SD = 0.093), t(60) = −3.279, p = .002, d = -.933 (see ). Our single-case analyses showed that only two DPs had a significantly smaller part-whole effect than their age-matched control groups (refer to ).
To examine if suspected DPs have impaired holistic processing only for specific features, we also compared the residuals between suspected DP and NTs for each feature (see DeGutis et al., Citation2012). For detailed description of the analyses, refer to Supplementary Materials (Table S1).
Face composite task
The internal consistencies of the composite face effect using the regression approach in NTs (λ2 = .774) and suspected DPs (λ2 = .730) were moderate. A Levene’s test confirmed that the variance in the composite effect scores did not differ between the two groups (p = .416). Our ANOVA revealed a significant main effect of condition, where all participants in general were more accurate with same-misaligned compared to same-aligned trials, F(1,60) = 9.787, p = .003, = .140, replicating previous results (Hole, Citation1994; Rossion, Citation2013). However, there was no significant main effect of group, F(1,60) = 1.945, p = .168,
= .031, showing that suspected DPs and NTs were comparable in the composite task, irrespective of conditions. In addition, there was also no significant interaction between condition and group, F(1,60) = .429, p = .515,
= .007, suggesting normal composite effects in suspected DPs. This was further supported by the analysis of residuals that revealed comparable composite effects between suspected DPs (M = −0.012, SD = 0.149) and NTs (M = −4.056e−5, SD = 0.138), t(60) = −0.301, p = .765, d = −.086 (see ). The single-case analyses showed that one of the suspected DPs had a significantly smaller composite effect than their age-matched control group (refer to ).
Navon’s task
The internal consistencies of the global precedence effect using the subtraction approach in NTs (λ2 = .274) and suspected DPs (λ2 = .769) were weak and moderate, respectively. Levene’s test confirmed that the global precedence effect in DPs and NTs had equal variances (p = .896). We found a significant main effect of condition, participants were faster with global-congruent compared to local-congruent trials, F(1,60) = 4.556, p = .037, = .071, replicating previous results (Navon, Citation1977). Furthermore, there was a significant main effect of group, F(1,60) = 18.994, p < .001,
= 0.240, showing that suspected DPs performed significantly slower than NTs in the Navon’s task, irrespective of conditions. However, there was no significant interaction effect between condition and group, F(1,60) = .719, p = .400,
= .012, suggesting comparable global precedence effect in NTs and suspected DPs. The standardized difference (i.e., Cohen’s d) of the global precedence effect in suspected DPs (M = 0.259, SD = 0.528) was comparable to that of the NTs (M = 0.293, SD = 0.508), t(60) = −0.231, p = .818, d = −.066 (see ). Our single-case analyses revealed that none of the suspected DPs showed a significantly smaller global precedence effect than their age-matched control groups (refer to ).
Discussion
This study aimed to determine whether deficits in holistic processing can explain the impairment in recognizing faces in individuals with Developmental Prosopagnosia. Additionally, we wanted to examine whether these potential holistic processing impairments (if present) are universal or heterogeneous across DPs. In the inversion, part-whole, composite and Navon’s tasks, our analyses revealed that our participants in general (NTs and suspected DPs combined) replicated previous effects with these tasks (Hole, Citation1994; Navon, Citation1977; Rossion, Citation2008, Citation2013; Tanaka & Farah, Citation1993; Tanaka & Simonyi, Citation2016; Yin, Citation1969). Interestingly, at a group level, suspected DPs were less susceptible to the inversion and part-whole effects compared to NTs but were comparable in the composite and Navon tasks. In other words, among the three conventional measures of holistic processing of faces, NTs showed stronger holistic face processing compared to suspected DPs only in the inversion and part-whole effects (Avidan et al., Citation2011; Behrmann et al., Citation2005; DeGutis et al., Citation2012; Duchaine et al., Citation2007b; Klargaard et al., Citation2018).
Interestingly, results from our single-case analyses revealed that holistic processing deficits in DPs, rather than being universal, are heterogeneous. This is because, out of the 17 suspected DPs, only one (Case CN) was impaired for both the inversion and composite tasks, another (Case VG) was impaired in both the inversion and part-whole tasks, and eight were impaired only in the inversion or part-whole tasks (see ). Interestingly, seven suspected DPs showed no evidence of impaired holistic processing, despite clear impairments in the CFMT (Case DI, TM, CL, EM, and DJ), and/or in both the CFMT and the CFPT (Case MT, RP). More importantly, this heterogeneity in holistic impairments was confirmed by our dissociation analyses (refer to Supplementary Table S2). Together, these findings suggest that holistic processing, although impaired at a group level, is not totally absent in DPs (DeGutis et al., Citation2012). These findings suggest that holistic processing impairments in DPs, rather than being consistent, present both quantitative and qualitative differences across distinct individuals (see Corrow et al., Citation2016; Le Grand et al., Citation2006; Tardif et al., Citation2019). This also further supports the idea that holistic processing is not a unitary process, and that no common mechanism explains these three distinct effects of holistic processing (Boutet et al., Citation2021; Rezlescu et al., Citation2017). Additionally, the single-case analyses of the Navon’s task provide further evidence that DPs’ impairments in holistic processing, if any, are specific to faces (Duchaine et al., Citation2007b; Fry et al., Citation2020; Wang et al., Citation2012).
The group analysis revealed normal performance of our suspected DPs in the CFE, with only one suspected DP significantly impaired in this task. This is in line with previous studies showing that DPs have normal CFE (e.g., Biotti et al., Citation2017; Le Grand et al., Citation2006; Susilo et al., Citation2010; Ulrich et al., Citation2017), suggesting that the CFE might be measuring an aspect of holistic processing that is preserved in DPs. Alternatively, it is possible that the CFE may tap into other underlying cognitive mechanisms that involve general perceptual abilities (Fitousi, Citation2015, Citation2020). Another possibility is related to the version of the composite task used. Some studies have proposed that the complete version or full design of the composite task is a more reliable (e.g., reduced susceptibility to response biases) and robust method for measuring holistic interference (Richler & Gauthier, Citation2014). In view of this, we did not include the complete CFE in this study as it has been argued that the complete version does not capture face-specific holistic mechanisms (Bukach et al., Citation2010: McKone et al., Citation2013; Rezlescu et al., Citation2017; Wong et al., Citation2011).
It has recently been suggested that the standard (or partial) composite task, which we utilized in this study, reflects the interference from the to-be-ignored face part (Biotti et al., Citation2017; Jin et al., Citation2024). On the contrary, the complete composite task captures both facilitation (i.e., both top and bottom halves are identical, or both are different) and interference (i.e., both faces have identical top parts but different bottom halves, or vice versa) effects (Jin et al., Citation2024). In view of this, it is possible that separating interference and facilitation effects could reveal differences that are not captured in our current study. Jin et al. (Citation2024) also found that these two effects are asymmetrical. For instance, interference effects significantly reduced for incongruent bottom-composite faces (instead of incongruent top halves), but facilitation effects appear consistent across varying conditions. In view of this, it is possible that separating interference and facilitation effects could reveal differences that are not captured in our current study. Accordingly, if judgments were to be made about the bottom half of the composite face, it might have yielded different results in our study. Since bottom-composite faces rely on holistic facilitation, and DPs are impaired in holistic facilitation (e.g., part-whole effect), we expect to observe impaired bottom-composite effect in DPs compared to NTs here. Future research should explore these possibilities to better understand the composite effect in DPs. It is possible that the standard composite task we used may not be as sensitive to holistic processing deficits in DPs, considering task-specific factors and/or differences in how DPs process facial information.
Our findings above also lend support to an alternative possibility—DPs’ impairment extends to both holistic and featural processing (Bennetts et al., Citation2022; Esins et al., Citation2016; Verfaillie et al., Citation2014; Yovel & Duchaine, Citation2006). Despite being poorer in the conditions of interest (e.g., whole conditions) of the part-whole task, our suspected DPs were also poorer in the control conditions (e.g., part conditions), which often reflects featural processing of faces (see ). Recently, Bennetts et al. (Citation2022) found that some DPs showed typical inversion effects, while some DPs had reduced or abolished inversion effects. However, they found that these DPs with typical inversion effects were also significantly poorer at perceiving and/or recognizing inverted faces, arguing that some DPs’ face recognition difficulties are the result of impaired featural processing. Similarly, a study by Tsantani et al. (Citation2020) also found that DPs were poorer than NTs when faces were viewed as a whole or through an aperture (i.e., holistic processing is disrupted; see Murphy & Cook, Citation2017). They argued that the perceptual difficulties seen in DPs arise from imprecise recognition of facial features, not impaired holistic processing. If DPs are impaired or underdeveloped in strategic perceptual encoding (Dalrymple & Palermo, Citation2016; Towler et al., Citation2018), facial information encoded may be less accurate and/or less differentiated at recognition (Shah et al., Citation2015; Stumps et al., Citation2020). Consequently, impaired face encoding might introduce both poorer holistic and featural perceptual representations in DPs (McKone & Yovel, Citation2009). Overall, this suggests that individual differences in face recognition abilities may also reflect variations in the ability to process the featural aspects of faces, and not only holistic processing expertise. Particularly, DPs’ heterogeneity might even extend to different “information-gathering” processes.
Nevertheless, the current study is not without limitations. Some studies have shown that prosopagnosia often co-occurs with other neurodevelopmental disorders, such as autism spectrum disorder (ASD) or dyslexia (co-occurrence hypothesis; Cook et al., Citation2015; Cook & Biotti, Citation2016; Minio-Paluello et al., Citation2020). Cook et al. (Citation2015) suggested that despite being independent of each other, individuals with prosopagnosia reported higher autistic traits than controls, while individuals with ASD reported higher prosopagnosic traits than controls. Consequently, domain-general holistic processing deficits (i.e., weak central coherence; Happé, Citation1996; Nakahachi et al., Citation2008) seen in ASD are more likely to also present themselves in DPs, which would then explain why some DPs also present impaired holistic processing across all visual perceptual domains (Gerlach et al., Citation2017; Gerlach & Starrfelt, Citation2018). While none of our suspected DPs were impaired in the Navon’s task or reported other neurodevelopmental disorders, our study did not include any measure of autistic traits (Baron-Cohen et al., Citation2001). Hence, we cannot entirely rule out the possibility that the face recognition difficulties in some suspected DPs can be explained by high autistic traits. In brief, future studies involving DPs would benefit from the use of ASD screening in participants (e.g., Nijhof et al., Citation2024).
Our single-case analyses provide interesting insights about the heterogeneity of the deficits observed in developmental prosopagnosia, however, some caution is needed due to the reliability of the holistic processing tasks. While compared to other studies (e.g., Bennetts et al., Citation2022; Rezlescu et al., Citation2017), the reliabilities of the part-whole and inversion tasks in the NTs were considerably high (approximately 0.69), these reliabilities are only in the acceptable range (Nunnally & Bernstein, Citation2007). Additionally, our findings also revealed that the reliability of the FIE in suspected DPs was notably weak. This is not surprising given that suspected DPs’ performance in the inverted conditions was close to chance, therefore restricting observable variance in their scores. One possible reason for this could be due to the nature of the FIE. Recent studies have argued that the FIE does not solely captures holistic processing of upright faces, but also holistic processing of inverted faces (Gerlach & Mogensen, Citation2024; Murphy & Cook, Citation2017). For instance, disrupting holistic processing by limiting participants to view faces through an aperture was shown to affect recognition performance of both upright and inverted faces alike (Murphy & Cook, Citation2017). Since upright faces retain most, if not all, of the holistic information contained in a face, holistic processing deficits would therefore be more prominent here. In contrast, inverted faces only retain some holistic information, and therefore DPs’ deficits are less obvious. The possibility that holistic information is preserved for inverted faces, together with the notion that DPs have deficits in holistic face processing, would explain the floor performance in the inverted conditions. Overall, findings involving holistic processing, measured with FIE, should be interpreted cautiously.
In conclusion, our results suggest that at a group level, suspected DPs have a specific, yet reduced susceptibility for holistic effects (as reflected by the inversion and part-whole, but not the composite effect). However, not all the suspected DPs showed holistic processing deficits and none of them were impaired in all three holistic face measures. This suggests that holistic processing deficits in DPs are heterogeneous, highlighting the importance of single-case analyses in neuropsychological and neurodevelopmental disorders (Cubelli & Della Sala, Citation2017). Recognizing and appreciating individual differences not only enriches our understanding of these conditions but also advances the field towards more personalized and effective approaches to diagnosis, treatment, and support.
Prosopagnosia_Supplementary information_BL_final.docx
Download MS Word (68.5 KB)Acknowledgements
Bryan Leong: Conceptualization; Methodology; Software; Validation; Formal analysis; Investigation; Data curation; Roles/Writing—Original Draft; Visualization, Hussain Ismail: Conceptualization; Software; Resources; Supervision; Roles/Writing—Review & Editing; Project administration, Hoo Keat Wong: Conceptualization; Resources; Supervision; Roles/Writing—Reviewing & Editing; Visualization, Alejandro Estudillo: Conceptualization; Methodology; Formal analysis; Data curation; Resources; Supervision; Roles/Writing—Original Draft; Project administration.
Data availability statement
The datasets generated during and/or analyzed during the current study are available in the Open Science Framework repository, https://osf.io/q32nk/?view_only=27331e519b0f448c871f689608176e8a
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 Although the CFMT is the main task used to diagnose prosopagnosia, some authors suggest that a reliable diagnosis requires impairment in at least two different face identification tasks (DeGutis et al., Citation2023). This approach is important because relying on a single measure may not always provide a reliable basis for making a diagnosis (Sachdev et al., Citation2014). Of these 17 DPs, seven of the DPs meet this diagnosis criteria, confirmed by their impaired performance in both CFMT and CFPT. However, the other 10 DPs were only impaired in the CFMT, but not CFPT. Nonetheless, previous studies have shown that face perceptual abilities can be preserved in DPs (Klargaard et al., Citation2018; Pertzov et al., Citation2020), and according to some authors (Burns et al., Citation2023) incorporating a subjective questionnaire and another objective measures of non-face objects (e.g., CCMT) to confirm face-specific difficulties is sufficient. In any case, we refer to our prosopagnosic participants as suspected developmental prosopagnosics.
References
- Avidan, G., Tanzer, M., & Behrmann, M. (2011). Impaired holistic processing in congenital prosopagnosia. Neuropsychologia, 49(9), 2541–2552. https://doi.org/10.1016/j.neuropsychologia.2011.05.002
- Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., & Clubley, E. (2001). The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. https://doi.org/10.1023/A:1005653411471
- Behrmann, M., Avidan, G., Marotta, J. J., & Kimchi, R. (2005). Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. Journal of Cognitive Neuroscience, 17(7), 1130–1149. https://doi.org/10.1162/0898929054475154
- Bennetts, R., Gregory, N., Tree, J., Luft, C., Banissy, M., Murray, E., Penton, T., & Bate, S. (2022). Face specific inversion effects provide evidence for two subtypes of developmental prosopagnosia. Neuropsychologia, 174, 108332. https://doi.org/10.1016/j.neuropsychologia.2022.108332
- Bentin, S., DeGutis, J. M., D’Esposito, M., & Robertson, L. C. (2007). Too many trees to see the forest: Performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. Journal of Cognitive Neuroscience, 19(1), 132–146. https://doi.org/10.1162/jocn.2007.19.1.132
- Berger, A., Fry, R., Bobak, A. K., Juliano, A., & DeGutis, J. (2022). Distinct abilities associated with matching same identity faces versus discriminating different faces: Evidence from individual differences in prosopagnosics and controls. Quarterly Journal of Experimental Psychology, 75(12), 2256–2271. https://doi.org/10.1177/17470218221076817
- Biotti, F., Wu, E., Yang, H., Jiahui, G., Duchaine, B., & Cook, R. (2017). Normal composite face effects in developmental prosopagnosia. Cortex, 95, 63–76. https://doi.org/10.1016/j.cortex.2017.07.018
- Boutet, I., & Meinhardt-Injac, B. (2019). Age differences in face processing: The role of perceptual degradation and holistic processing. The Journals of Gerontology: Series B, 74(6), 933–942. https://doi.org/10.1093/geronb/gbx172
- Boutet, I., & Meinhardt-Injac, B. (2021). Measurement of individual differences in face-identity processing abilities in older adults. Cognitive research: principles and implications, 6(1), 1–11. https://doi.org/10.1186/s41235-021-00310-4
- Boutet, I., Nelson, E. A., Watier, N., Cousineau, D., Béland, S., & Collin, C. A. (2021). Different measures of holistic face processing tap into distinct but partially overlapping mechanisms. Attention, Perception, & Psychophysics, 83(7), 2905–2923. https://doi.org/10.3758/s13414-021-02337-7
- Bowles, D. C., McKone, E., Dawel, A., Duchaine, B., Palermo, R., Schmalzl, L., Rivolta, D., Wilson, C. E., & Yovel, G. (2009). Diagnosing prosopagnosia: Effects of ageing, sex, and participant–stimulus ethnic match on the Cambridge Face Memory Test and Cambridge Face Perception Test. Cognitive Neuropsychology, 26(5), 423–455. https://doi.org/10.1080/02643290903343149
- Bruce, V., & Young, A. (1986). Understanding face recognition. British Journal of Psychology, 77(3), 305–327. https://doi.org/10.1111/j.2044-8295.1986.tb02199.x
- Bukach, C. M., Phillips, W. S., & Gauthier, I. (2010). Limits of generalization between categories and implications for theories of category specificity. Attention, Perception & Psychophysics, 72(7), 1865–1874. https://doi.org/10.3758/APP.72.7.1865
- Burns, E. J., Gaunt, E., Kidane, B., Hunter, L., & Pulford, J. (2023). A new approach to diagnosing and researching developmental prosopagnosia: Excluded cases are impaired too. Behavior Research Methods, 55(8), 4291–4314. https://doi.org/10.3758/s13428-022-02017-w
- Callender, J. C., & Osburn, H. G. (1979). An empirical comparison of coefficient alpha, Guttman’s lambda-2, and MSPLIT maximized split-half reliability estimates. Journal of Educational Measurement, 16(2), 89–99. https://doi.org/10.1111/j.1745-3984.1979.tb00090.x
- Carbon, C.-C., & Leder, H. (2005). When feature information comes first! early processing of inverted faces. Perception, 34(9), 1117–1134. https://doi.org/10.1068/p5192
- Cook, R., & Biotti, F. (2016). Developmental prosopagnosia. Current Biology, 26(8), R312–R313. https://doi.org/10.1016/j.cub.2016.01.008
- Cook, R., Shah, P., Gaule, A., Brewer, R., & Bird, G. (2015). Autism and developmental prosopagnosia: A cross-disorder study. Journal of Vision, 15(12), 1211–1211. https://doi.org/10.1167/15.12.1211
- Corrow, S. L., Dalrymple, K. A., & Barton, J. J. (2016).Prosopagnosia: Current perspectives. Eye and Brain, 8, 1655–1175. https://doi.org/10.2147/EB.S92838
- Crawford, J. R., & Garthwaite, P. H. (2002). Investigation of the single case in neuropsychology: Confidence limits on the abnormality of test scores and test score differences. Neuropsychologia, 40(8), 1196–1208. https://doi.org/10.1016/S0028-3932(01)00224-X
- Crawford, J. R., & Garthwaite, P. H. (2005). Testing for suspected impairments and dissociations in single-case studies in neuropsychology: Evaluation of alternatives using monte carlo simulations and revised tests for dissociations. Neuropsychology, 19(3), 318–331. https://doi.org/10.1037/0894-4105.19.3.318
- Crawford, J. R., Garthwaite, P. H., & Porter, S. (2010). Point and interval estimates of effect sizes for the case-controls design in neuropsychology: Rationale, methods, implementations, and proposed reporting standards. Cognitive Neuropsychology, 27(3), 245–260. https://doi.org/10.1080/02643294.2010.513967
- Cubelli, R., & Della Sala, S. (2017). Looking back to go forward: Promoting single case studies. Cortex, 97, A1–A3. https://doi.org/10.1016/j.cortex.2017.09.023
- Dale, G., & Arnell, K. M. (2013). Investigating the stability of and relationships among global/local processing measures. Attention, Perception, & Psychophysics, 75(3), 394–406. https://doi.org/10.3758/s13414-012-0416-7
- Dalrymple, K. A., & Palermo, R. (2016). Guidelines for studying developmental prosopagnosia in adults and children. WIREs Cognitive Science, 7(1), 73–87. https://doi.org/10.1002/wcs.1374
- DeGutis, J., Bahierathan, K., Barahona, K., Lee, E., Evans, T. C., Shin, H. M., Mishra, M., Likitlersuang, J., & Wilmer, J. B. (2023). What is the prevalence of developmental prosopagnosia? An empirical assessment of different diagnostic cutoffs. Cortex, 161, 51–64. https://doi.org/10.1016/j.cortex.2022.12.014
- DeGutis, J., Cohan, S., Mercado, R. J., Wilmer, J., & Nakayama, K. (2012). Holistic processing of the mouth but not the eyes in developmental prosopagnosia. Cognitive Neuropsychology, 29(5-6), 419–446. https://doi.org/10.1080/02643294.2012.754745
- DeGutis, J., Wilmer, J., Mercado, R. J., & Cohan, S. (2013). Using regression to measure holistic face processing reveals a strong link with face recognition ability. Cognition, 126(1), 87–100. https://doi.org/10.1016/j.cognition.2012.09.004
- Dennett, H. W., McKone, E., Tavashmi, R., Hall, A., Pidcock, M., Edwards, M., & Duchaine, B. (2012). The Cambridge Car Memory Test: A task matched in format to the Cambridge Face Memory Test, with norms, reliability, sex differences, dissociations from face memory, and expertise effects. Behavior Research Methods, 44(2), 587–605. https://doi.org/10.3758/s13428-011-0160-2
- Diamond, R., & Carey, S. (1986). Why faces are and are not special: An effect of expertise. Journal of experimental psychology: general, 115(2), 107–117. https://doi.org/10.1037/0096-3445.115.2.107
- Duchaine, B., Germine, L., & Nakayama, K. (2007a). Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cognitive Neuropsychology, 24(4), 419–430. https://doi.org/10.1080/02643290701380491
- Duchaine, B., & Nakayama, K. (2006). The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia, 44(4), 576–585. https://doi.org/10.1016/j.neuropsychologia.2005.07.001
- Duchaine, B., Yovel, G., & Nakayama, K. (2007b). No global processing deficit in the Navon task in 14 developmental prosopagnosics. Social Cognitive and Affective Neuroscience, 2(2), 104–113. https://doi.org/10.1093/scan/nsm003
- Esins, J., Schultz, J., Stemper, C., Kennerknecht, I., & Bülthoff, I. (2016). Face perception and test reliabilities in congenital prosopagnosia in seven tests. i-Perception, 7(1), 204166951562579. https://doi.org/10.1177/2041669515625797
- Estudillo, A. J. (2012). Facial memory: The role of the pre-existing knowledge in face processing and recognition. Europe’s Journal of Psychology, 8(2), 231–244. https://doi.org/10.5964/ejop.v8i2.455
- Estudillo, A. J., Lee, J. K. W., Mennie, N., & Burns, E. (2020). No evidence of other-race effect for Chinese faces in Malaysian non-Chinese population. Applied Cognitive Psychology, 34(1), 270–276. https://doi.org/10.1002/acp.3609
- Estudillo, A. J., Leong, B. Q. Z., & Wong, H. K. (2022). Navon processing biases fail to affect the recognition of whole faces and isolated facial features. Journal of Cognitive Psychology, 34(6), 744–754. https://doi.org/10.1080/20445911.2022.2105341
- Estudillo, A. J., & Wong, H. K. (2021). Associations between self-reported and objective face recognition abilities are only evident in above- and below-average recognisers. PeerJ, 9, e10629. https://doi.org/10.7717/peerj.10629
- Fitousi, D. (2015). Composite faces are not processed holistically: Evidence from the Garner and redundant target paradigms. Attention, Perception, & Psychophysics, 77(6), 2037–2060. https://doi.org/10.3758/s13414-015-0887-4
- Fitousi, D. (2020). Decomposing the composite face effect: Evidence for non-holistic processing based on the ex-Gaussian distribution. Quarterly Journal of Experimental Psychology, 73(6), 819–840. https://doi.org/10.1177/1747021820904222
- Fry, R., Wilmer, J., Xie, I., Verfaellie, M., & DeGutis, J. (2020). Evidence for normal novel object recognition abilities in developmental prosopagnosia. Royal Society Open Science, 7(9), 200988. https://doi.org/10.1098/rsos.200988
- Gerlach, C., Klargaard, S. K., Petersen, A., & Starrfelt, R. (2017). Delayed processing of global shape information in developmental prosopagnosia. PLoS One, 12(12), e0189253. https://doi.org/10.1371/journal.pone.0189253
- Gerlach, C., & Krumborg, J. R. (2014). Same, same — but different: On the use of Navon derived measures of global/local processing in studies of face processing. Acta Psychologica, 153, 28–38. https://doi.org/10.1016/j.actpsy.2014.09.004
- Gerlach, C., & Mogensen, E. (2024). The face inversion effect does not provide a pure measure of holistic face processing. Behavior Research Methods, 56(1), 330–341. https://doi.org/10.3758/s13428-022-02054-5
- Gerlach, C., & Starrfelt, R. (2018). Delayed processing of global shape information is associated with weaker top-down effects in developmental prosopagnosia. Cognitive Neuropsychology, 35(8), 471–478. https://doi.org/10.1080/02643294.2018.1519505
- Gerlach, C., & Starrfelt, R. (2021). Patterns of perceptual performance in developmental prosopagnosia: An in-depth case series. Cognitive Neuropsychology, 38(1), 27–49. https://doi.org/10.1080/02643294.2020.1869709
- Germine, L. T., Duchaine, B., & Nakayama, K. (2011). Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition, 118(2), 201–210. https://doi.org/10.1016/j.cognition.2010.11.002
- Happé, F. G. (1996). Studying weak central coherence at low levels: Children with autism do not succumb to visual illusions. A research note. Journal of Child Psychology and Psychiatry, 37(7), 873–877. https://doi.org/10.1111/j.1469-7610.1996.tb01483.x
- Hedge, C., Powell, G., & Sumner, P. (2018). The reliability paradox: Why robust cognitive tasks do not produce reliable individual differences. Behavior Research Methods, 50(3), 1166–1186. https://doi.org/10.3758/s13428-017-0935-1
- Hole, G. J. (1994). Configurational factors in the perception of unfamiliar faces. Perception, 23(1), 65–74. https://doi.org/10.1068/p230065
- Jaworska, K., Yi, F., Ince, R. A., Van Rijsbergen, N. J., Schyns, P. G., & Rousselet, G. A. (2020). Healthy aging delays the neural processing of face features relevant for behavior by 40 ms. Human Brain Mapping, 41(5), 1212–1225. https://doi.org/10.1002/hbm.24869
- Jin, H., Ji, L., Cheung, O. S., & Hayward, W. G. (2024). Facilitation and interference are asymmetric in holistic face processing. Psychonomic Bulletin & Review, 1–12. https://doi.org/10.3758/s13423-024-02481-9
- Kennerknecht, I., Grueter, T., Welling, B., Wentzek, S., Horst, J., Edwards, S., & Grueter, M. (2006). First report of prevalence of non-syndromic hereditary prosopagnosia (HPA). American Journal of Medical Genetics Part A, 140A(15), 1617–1622. https://doi.org/10.1002/ajmg.a.31343
- Kennerknecht, I., Ho, N. Y., & Wong, V. C. (2008). Prevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese population. American Journal of Medical Genetics Part A, 146A(22), 2863–2870. https://doi.org/10.1002/ajmg.a.32552
- Kho, S. K., Leong, B. Q. Z., Keeble, D. R., Wong, H. K., & Estudillo, A. J. (2023). A new Asian version of the CFMT: The Cambridge Face Memory Test–Chinese Malaysian (CFMT-MY). Behavior Research Methods, 56(3), 1192–1206. https://doi.org/10.3758/s13428-023-02085-6
- Klargaard, S. K., Starrfelt, R., & Gerlach, C. (2018). Inversion effects for faces and objects in developmental prosopagnosia: A case series analysis. Neuropsychologia, 113, 52–60. https://doi.org/10.1016/j.neuropsychologia.2018.03.026
- Konar, Y., Bennett, P. J., & Sekuler, A. B. (2010). Holistic processing is not correlated with face-identification accuracy. Psychological Science, 21(1), 38–43. https://doi.org/10.1177/0956797609356508
- Leder, H., & Bruce, V. (2000). When inverted faces are recognised: The role of configural information in face recognition. The quarterly journal of experimental psychology Section A, 53(2), 513–536. https://doi.org/10.1080/713755889
- Lee, J. K. W., Janssen, S. M., & Estudillo, A. J. (2022). A featural account for own-face processing? Looking for support from face inversion, composite face, and part-whole tasks. i-Perception, 13(4), 20416695221111409.
- Le Grand, R., Cooper, P. A., Mondloch, C. J., Lewis, T. L., Sagiv, N., de Gelder, B., & Maurer, D. (2006). What aspects of face processing are impaired in developmental prosopagnosia? Brain and Cognition, 61(2), 139–158. https://doi.org/10.1016/j.bandc.2005.11.005
- Lewis, M. B., Mills, C., Hills, P. J., & Weston, N. (2009). Navon letters affect face learning and face retrieval. Experimental Psychology, 56(4), 258–264. https://doi.org/10.1027/1618-3169.56.4.258
- Li, J., Huang, L., Song, Y., & Liu, J. (2017). Dissociated neural basis of two behavioral hallmarks of holistic face processing: The whole-part effect and composite-face effect. Neuropsychologia, 102, 52–60. https://doi.org/10.1016/j.neuropsychologia.2017.05.026
- Li, J., Song, Y., & Liu, J. (2019). Functional connectivity pattern in the core face network reflects different mechanisms of holistic face processing measured by the whole-part effect and composite-face effect. Neuroscience, 408, 248–258. https://doi.org/10.1016/j.neuroscience.2019.04.017
- Little, A. C., Jones, B. C., & DeBruine, L. M. (2011). The many faces of research on face perception. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1571), 1634–1637. https://doi.org/10.1098/rstb.2010.0386
- Liu, T. T., & Behrmann, M. (2014). Impaired holistic processing of left-right composite faces in congenital prosopagnosia. Frontiers in Human Neuroscience, 8, 750.
- Macrae, C. N., & Lewis, H. L. (2002). Do I know you? Processing orientation and face recognition. Psychological Science, 13(2), 194–196. https://doi.org/10.1111/1467-9280.00436
- Malgady, R. G., & Colon-Malgady, G. (1991). Comparing the reliability of difference scores and residuals in analysis of covariance. Educational and Psychological Measurement, 51(4), 803–807. https://doi.org/10.1177/001316449105100401
- Maurer, D., Grand, R. L., & Mondloch, C. J. (2002). The many faces of configural processing. Trends in Cognitive Sciences, 6(6), 255–260. https://doi.org/10.1016/S1364-6613(02)01903-4
- McKone, E., Davies, A. A., Darke, H., Crookes, K., Wickramariyaratne, T., Zappia, S., Fiorentini, C., Favelle, S., Broughton, M., & Fernando, D. (2013). Importance of the inverted control in measuring holistic face processing with the composite effect and part-whole effect. Frontiers in Psychology, 4, 33. https://doi.org/10.3389/fpsyg.2013.00033
- McKone, E., & Yovel, G. (2009). Why does picture-plane inversion sometimes dissociate perception of features and spacing in faces, and sometimes not? Toward a new theory of holistic processing. Psychonomic Bulletin & Review, 16(5), 778–797. https://doi.org/10.3758/PBR.16.5.778
- Meinhardt, G., Persike, M., & Meinhardt-Injac, B. (2016). The composite effect is face-specific in young but not older adults. Frontiers in Aging Neuroscience, 8, 187. https://doi.org/10.3389/fnagi.2016.00187
- Minio-Paluello, I., Porciello, G., Pascual-Leone, A., & Baron-Cohen, S. (2020). Face individual identity recognition: a potential endophenotype in autism. Molecular Autism, 11(1), 1–16. https://doi.org/10.1186/s13229-020-00371-0
- Murphy, J., & Cook, R. (2017). Revealing the mechanisms of human face perception using dynamic apertures. Cognition, 169, 25–35. https://doi.org/10.1016/j.cognition.2017.08.001
- Murray, E., & Bate, S. (2020). Diagnosing developmental prosopagnosia: Repeat assessment using the Cambridge Face Memory Test. Royal Society Open Science, 7(9), 200884. https://doi.org/10.1098/rsos.200884
- Nakahachi, T., Yamashita, K., Iwase, M., Ishigami, W., Tanaka, C., Toyonaga, K., Maeda, S., Hirotsune, H., Tei, Y., Yokoi, K., Okajima, S., Shimizu, A., & Takeda, M. (2008). Disturbed holistic processing in autism spectrum disorders verified by two cognitive tasks requiring perception of complex visual stimuli. Psychiatry Research, 159(3), 330–338. https://doi.org/10.1016/j.psychres.2005.08.028
- Navon, D. (1977). Forest before trees: The precedence of global features in visual perception. Cognitive Psychology, 9(3), 353–383. https://doi.org/10.1016/0010-0285(77)90012-3
- Nijhof, A. D., Catmur, C., Brewer, R., Coll, M. P., Wiersema, J. R., & Bird, G. (2024). Differences in own-face but not own-name discrimination between autistic and neurotypical adults: A fast periodic visual stimulation-EEG study. cortex, 171, 308–318. https://doi.org/10.1016/j.cortex.2023.10.023
- Nunnally, J. C., & Bernstein, I. H. (2007). Psychometric theory: Nunnally and Bernstein. Academic Internet Publishers.
- Palermo, R., Willis, M. L., Rivolta, D., McKone, E., Wilson, C. E., & Calder, A. J. (2011). Impaired holistic coding of facial expression and facial identity in congenital prosopagnosia. Neuropsychologia, 49(5), 1226–1235. https://doi.org/10.1016/j.neuropsychologia.2011.02.021
- Pertzov, Y., Krill, D., Weiss, N., Lesinger, K., & Avidan, G. (2020). Rapid forgetting of faces in congenital prosopagnosia. Cortex, 129, 119–132. https://doi.org/10.1016/j.cortex.2020.04.007
- Piepers, D., & Robbins, R. (2012). A review and clarification of the terms “holistic,” “configural,” and “relational” in the face perception literature. Frontiers in Psychology, 3, 559. https://doi.org/10.3389/fpsyg.2012.00559
- Retter, T. L., & Rossion, B. (2015). Global shape information increases but color information decreases the composite face effect. Perception, 44(5), 511–528. https://doi.org/10.1068/p7826
- Revelle, W. (2023). psych: Procedures for psychological, psychometric, and personality research (Version 2.3.3) [Software]. https://cran.r-project.org/package=psych
- Rezlescu, C., Danaila, I., Miron, A., & Amariei, C. (2020). More time for science: Using testable to create and share behavioral experiments faster, recruit better participants, and engage students in hands-on research. Progress in Brain Research, 253, 243–262. https://doi.org/10.1016/bs.pbr.2020.06.005
- Rezlescu, C., Pitcher, D., & Duchaine, B. (2012). Acquired prosopagnosia with spared within-class object recognition but impaired recognition of degraded basic-level objects. Cognitive Neuropsychology, 29(4), 325–347. https://doi.org/10.1080/02643294.2012.749223
- Rezlescu, C., Susilo, T., Wilmer, J. B., & Caramazza, A. (2017). The inversion, part-whole, and composite effects reflect distinct perceptual mechanisms with varied relationships to face recognition. Journal of Experimental Psychology: Human Perception and Performance, 43(12), 1961–1973. https://doi.org/10.1037/xhp0000400
- Richler, J. J., Cheung, O. S., & Gauthier, I. (2011). Holistic processing predicts face recognition. Psychological Science, 22(4), 464–471. https://doi.org/10.1177/0956797611401753
- Richler, J. J., & Gauthier, I. (2014). A meta-analysis and review of holistic face processing. Psychological Bulletin, 140(5), 1281–1302. https://doi.org/10.1037/a0037004
- Rossion, B. (2008). Picture-plane inversion leads to qualitative changes of face perception. Acta Psychologica, 128(2), 274–289. https://doi.org/10.1016/j.actpsy.2008.02.003
- Rossion, B. (2013). The composite face illusion: A whole window into our understanding of holistic face perception. Visual Cognition, 21(2), 139–253. https://doi.org/10.1080/13506285.2013.772929
- Rossion, B., & Gauthier, I. (2002). How does the brain process upright and inverted faces? Behavioral and Cognitive Neuroscience Reviews, 1(1), 63–75. https://doi.org/10.1177/1534582302001001004
- Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V., Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology, 10(11), 634–642. https://doi.org/10.1038/nrneurol.2014.181
- Shah, P., Gaule, A., Gaigg, S. B., Bird, G., & Cook, R. (2015). Probing short-term face memory in developmental prosopagnosia. Cortex, 64, 115–122. https://doi.org/10.1016/j.cortex.2014.10.006
- Stumps, A., Saad, E., Rothlein, D., Verfaellie, M., & DeGutis, J. (2020). Characterizing developmental prosopagnosia beyond face perception: Impaired recollection but intact familiarity recognition. Cortex, 130, 64–77. https://doi.org/10.1016/j.cortex.2020.04.016
- Susilo, T., & Duchaine, B. (2013). Advances in developmental prosopagnosia research. Current Opinion in Neurobiology, 23(3), 423–429. https://doi.org/10.1016/j.conb.2012.12.011
- Susilo, T., McKone, E., Dennett, H., Darke, H., Palermo, R., Hall, A., Pidcock, M., Dawel, A., Jeffery, L., Wilson, C. E., & Rhodes, G. (2010). Face recognition impairments despite normal holistic processing and face space coding: Evidence from a case of developmental prosopagnosia. Cognitive Neuropsychology, 27(8), 636–664. https://doi.org/10.1080/02643294.2011.613372
- Tanaka, J. W., & Farah, M. J. (1993). Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology Section A, 46(2), 225–245. https://doi.org/10.1080/14640749308401045
- Tanaka, J. W., & Simonyi, D. (2016). The “parts and wholes” of face recognition: A review of the literature. Quarterly Journal of Experimental Psychology, 69(10), 1876–1889. https://doi.org/10.1080/17470218.2016.1146780
- Tardif, J., Morin Duchesne, X., Cohan, S., Royer, J., Blais, C., Fiset, D., Duchaine, B., & Gosselin, F. (2019). Use of face information varies systematically from developmental prosopagnosics to super-recognisers. Psychological Science, 30(2), 300–308. https://doi.org/10.1177/0956797618811338
- Towler, J., Fisher, K., & Eimer, M. (2018). Holistic face perception is impaired in developmental prosopagnosia. Cortex, 108, 112–126. https://doi.org/10.1016/j.cortex.2018.07.019
- Tsantani, M., Gray, K. L., & Cook, R. (2020). Holistic processing of facial identity in developmental prosopagnosia. Cortex, 130, 318–326. https://doi.org/10.1016/j.cortex.2020.06.003
- Ulrich, P. I., Wilkinson, D. T., Ferguson, H. J., Smith, L. J., Bindemann, M., Johnston, R. A., & Schmalzl, L. (2017). Perceptual and memorial contributions to developmental prosopagnosia. Quarterly Journal of Experimental Psychology, 70(2), 298–315. https://doi.org/10.1080/17470218.2016.1177101
- Verfaillie, K., Huysegems, S., De Graef, P., & Van Belle, G. (2014). Impaired holistic and analytic face processing in congenital prosopagnosia: Evidence from the eye-contingent mask/window paradigm. Visual Cognition, 22(3-4), 503–521. https://doi.org/10.1080/13506285.2014.881446
- Verhallen, R. J., Bosten, J. M., Goodbourn, P. T., Lawrance-Owen, A. J., Bargary, G., & Mollon, J. D. (2017). General and specific factors in the processing of faces. Vision Research, 141, 217–227. https://doi.org/10.1016/j.visres.2016.12.014
- Wang, R., Li, J., Fang, H., Tian, M., & Liu, J. (2012). Individual differences in holistic processing predict face recognition ability. Psychological Science, 23(2), 169–177. https://doi.org/10.1177/0956797611420575
- Wong, A. C. N., Bukach, C. M., Yuen, C., Yang, L., Leung, S., & Greenspon, E. (2011). Holistic processing of words modulated by Reading experience. PLoS One, 6(6), e20753. https://doi.org/10.1371/journal.pone.0020753
- Wong, H. K., Estudillo, A. J., Stephen, I. D., & Keeble, D. R. (2021). Plasma Hsp90 levels in patients with systemic sclerosis and relation to lung and skin involvement: A cross-sectional and longitudinal study. Scientific Reports, 11(1), 1–15. https://doi.org/10.1038/s41598-020-79139-8
- Yardley, L., McDermott, L., Pisarski, S., Duchaine, B., & Nakayama, K. (2008). Psychosocial consequences of developmental prosopagnosia: A problem of recognition. Journal of Psychosomatic Research, 65(5), 445–451. https://doi.org/10.1016/j.jpsychores.2008.03.013
- Yin, R. K. (1969). Looking at upside-down faces. Journal of Experimental Psychology, 81(1), 141–145. https://doi.org/10.1037/h0027474
- Young, A. W., Hellawell, D., & Hay, D. C. (1987). Configurational information in face perception. Perception, 16(6), 747–759. https://doi.org/10.1068/p160747
- Yovel, G., & Duchaine, B. (2006). Specialised face perception mechanisms extract both part and spacing information: Evidence from developmental prosopagnosia. Journal of Cognitive Neuroscience, 18(4), 580–593. https://doi.org/10.1162/jocn.2006.18.4.580
- Yovel, G., Wilmer, J. B., & Duchaine, B. (2014). What can individual differences reveal about face processing? Frontiers in Human Neuroscience, 8, 56. https://doi.org/10.3389/fnhum.2014.00562
