ABSTRACT
Background
Maternal stress and psychopathology have a negative effect on mothers and neonates. Maternal stress may affect neonatal growth and development both physically and psychologically.
Purpose
To study the impact of pandemic-related pregnancy stress and maternal psychopathological symptoms during the COVID-19 lockdown in 2020 on neonatal development.
Methods
A two-phase prospective study was carried out on a sample of 181 pregnant women ranged from 18 to 40 years old in Spain (Europe). Phase 1: Pandemic-related pregnancy stress (PREPS), Prenatal Distress Questionnaire (PDQ), Perceived Stress Scale (PSS) and the revised version of the Symptom Checklist-90 (SCL-90-R) were used to assess psychological symptoms during the lockdown. In the follow-up (Phase 2), obstetric, birth-related and anthropometric variables were collected from 81 pregnant women-neonates dyads.
Results
Primiparous women showed higher psychopathological symptoms and higher levels of pandemic-related pregnancy stress than multiparous women. A multiple linear regression model showed that pandemic-related pregnancy stress could predict the length of neonate by adjusting for maternal age and gestational age, especially for primiparous women.
Implications for research
Studies assessing neonates development should evaluate the long-term effect of the COVID-19 pandemic on neonates´ length.
Implications for practice
States the relation between pandemic-related pregnancy stress and neonatal development by being able to track the effects on neonates whose mothers had high levels of stress during the COVID-19 pandemic.
Background and significance
Maternal stress is considered by the World Health Organization (WHO) as one of the main problems that affect the health and well-being of women during the perinatal period (Astbury, Citation2006; Baker, Citation2021; Flores-Ramos, Citation2013; Spanish Health Ministry, Citation2017, National Institute of Mental Health et al., Citation2021; World Health Organization [WHO], Citation2021). The magnitude of this stress worldwide demonstrates the presence of the pathology in different contexts. Prevalence of maternal stress has been reported to be above 27% in the first and second trimesters, and above 24% during the third trimester of pregnancy (Gokoel et al., Citation2021). During the COVID-19 pandemic, maternal stress has been informed to affect women's´ mental health dramatically, thus worsening existing pathologies or increasing cases of anxiety, depression or stress between others (Aryal & Pant, Citation2020; Jungari, Citation2020; Koenen, Citation2020; Saccone et al., Citation2020; Thapa et al., Citation2020; Wu et al., Citation2020). The risk increases in postpartum period (Fuente-Moreno et al., Citation2023). Globally, magnitude varies, but at least a third of pregnant women report experiencing stress of moderate intensity (Hou et al., Citation2018; Loomans et al., Citation2013; Pantha, Citation2014; Phelan et al., Citation2015; Tang et al., Citation2019; Yuksel et al., Citation2014). In this regard, high levels of maternal stress during the COVID-19 pandemic were reported to be associated with social isolation, lack of economic resources and intimate partner violence (Bueso-Izquierdo et al., Citation2021; Farrell et al., Citation2020; Mappa et al., Citation2020).
Women suffer from an increased risk of cognitive and behavioural problems due to stress. Besides, maternal stress can also have detrimental consequences on the fetus (Bleker et al., Citation2019; Glover, Citation2014; Thongsomboon et al., Citation2020). In this respect, high levels of stress during pregnancy were associated with prematurity, low birth weight, asthma and cognitive delay (Glover, Citation2014; Lautarescu et al., Citation2020; Staneva et al., Citation2015). The adverse effects maternal stress can have on the developing fetus has been explained through the Fetal Programming Hypothesis (Barker, Citation2003; Gallego, Citation2021). The Fetal Programming Hypothesis states that certain events that occurred during critical points of pregnancy may have permanent effects on the fetus and that probably continue long after the birth of the child (Barker, Citation2003; Gallego, Citation2021). The effect of fetal programming and the implications of modulating the health and development of the unborn baby has been described in different studies conducted previously (Barker, Citation2003; Caparros-Gonzalez et al., Citation2019; Cardwell, Citation2013; Garcia-Silva et al., Citation2021; Glover, Citation2014). If pregnant women have been exposed to stressful events, this may involve some adaptation to the characteristics of the intrauterine environment that may subsequently link to other functional changes that lastly affect the development of the neonate (Caparros-Gonzalez, Citation2020; Caparros-Gonzalez & Alderdice, Citation2020; Reynolds et al., Citation2019; Talge et al., Citation2007). Research has found that the pandemic had a negative impact on women’s mental health, increasing the incidence of mental health disorders, including stress that they suffered during pregnancy (Aryal & Pant, Citation2020; Jungari, Citation2020; Koenen, Citation2020; Orkaby et al., Citation2022; Saccone et al., Citation2020; Thapa et al., Citation2020; Wu et al., Citation2020). The implications on neonatal development must be studied in depth (Kotlar et al., Citation2021; van den Bergh et al., Citation2020; Vardi et al., Citation2022). The adverse effects of the pandemic-related stress are similar to those women who suffered for other reasons: including physical and psychological effects on both mother and neonate (Ceulemans et al., Citation2021; Garcia-Silva et al., Citation2021; Koenen, Citation2020; Kotlar et al., Citation2021; Preis, Mahaffey, Heiselman, et al., Citation2020; Vardi et al., Citation2022).
In addition, there are previously validated tools such as the Pandemic-Related Pregnancy Stress Scale (PREPS) in different contexts and cultures (Penengo et al., Citation2021; Preis, Mahaffey, & Lobel, Citation2020; Preis, Mahaffey, Heiselman, et al., Citation2020; Schaal et al., Citation2021). In the European Spanish-speaking context, PREPS showed adequate psychometric properties to measure the pandemic-related stress among pregnant women in Spain (Caparros-Gonzalez et al., Citation2019; Garcia-Silva et al., Citation2021). However, there is little research that studies the true impact of maternal stress, and neonatal development focusing on anthropological measurements, while there are many investigations related to neurodevelopment (Anifantaki et al., Citation2021; Chan et al., Citation2018; Lewis et al., Citation2015; Talge et al., Citation2007; Walsh et al., Citation2019). Thus, the associations between maternal stress and fetal growth need to be studied in depth (Mélançon et al., Citation2020; Wing et al., Citation2017).
Higher perceived stress during pregnancy has been shown to be associated with alterations in neonatal anthropometric measures at birth, as suggested by other researchers (Wing et al., Citation2017). In addition, some results showed that exposition to high psychological stress during the second trimester is associated with an increased risk of delivering a newborn with macrosomia (Mélançon et al., Citation2020). Pandemic-related stress in pregnant women is a current issue that may affect on how neonate develops physically and psychologically. Thus, our aim was to study the impact of pandemic-related pregnancy stress and maternal psychopathological symptoms during COVID-19 lockdown in 2020 on neonatal growth. Concretely in neonates´ length, assessing if those babies affected are smaller in size when comparing to babies from mothers without psychopathological symptoms during pandemic.
Material and methods
Design and participants
A prospective study was carried out on European Spanish-speaking pregnant women in Spain. Eligible participants were selected according to the definition established on the Pregnancy Health Document for a low-risk pregnancy (this document is a pregnancy-route-guide for every woman when being followed-up during pregnancy. Pregnant women are assessed and classified as low risk or high risk so that professionals involved in their care can set their follow-up) (de Andalucía, Citation2019). Inclusion criteria also included proficiency in the Spanish language and ≥18 years old.
This study was approved by the Biomedical Ethics Research Committee of Andalusia (Spain) named PEIBA, Number (XXXXX). The study also conformed to guidelines of the Helsinki Declaration (AMM) and the Good Clinical Practice Directive (Directive 2005/28/EC) of the European Union for human research. Participation was voluntary. An informed written consent document was signed by every participant.
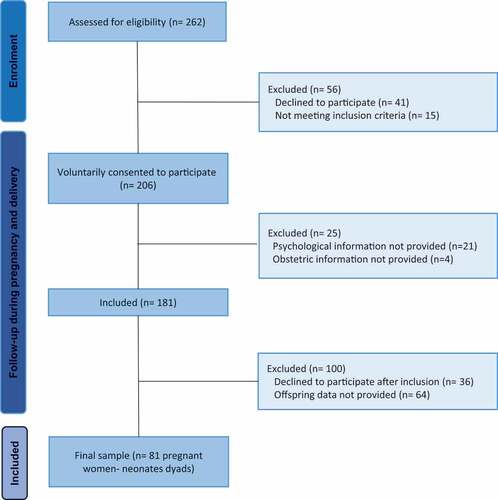
This study consisted of two phases: in the first phase (T1), the recruitment of pregnant women was carried out. A total of 262 women were invited to participate while attending an antenatal appointment with a midwife in the South of Spain during the COVID-19 pandemic from April to June 2020. Participants were recruited from the Department of Obstetrics and Gynecology of the Antequera Hospital through consecutive admissions. Forty-one women declined to participate due to lack of time, and15 women, although evaluated and accepted to participate, were excluded from analyses due to not meeting inclusion criteria. After consent to participate in the study, 25 women were excluded since they did not provide fully and comprehensive psychological or obstetric information. The sample consisted of 181 pregnant women (). The second phase of the study (T2) consisted of the follow-up of the participants (women and neonates) at the time of birth. Eighty-one participants participated in the follow-up. It was able to analyse the data of 81 pregnant women and their 81 neonates. Many of the women participating decided to discontinue their participation after hospital discharge due to lack of time. Thus, simply decided not to continue in the investigation (n = 36) and the rest of the sample declined to participate in the follow-up phase (n = 64).
Instruments and outcome variables
Pandemic-related pregnancy stress (PREPS)
The PREPS is a novel instrument consisting of 15 items to specifically assess COVID-19 related stress; the original version of this measure was developed in the U.S.A. during the COVID-19 pandemic (Preis, Mahaffey, Heiselman, et al., Citation2020). It was used the previously validated Spanish version (Garcia-Silva et al., Citation2021). The Cronbach´s alpha reliability coefficient of this version was α = 0.74. This 15-item version comprises three factors related to 1) stress about lack of preparation for birth, delivery and the postpartum (seven items, e.g. ‘I am concerned that the pandemic may ruin my birth plans’); 2) stress associated with worries and concerns about the infection (five items, e.g. ‘I am concerned that my baby may contract COVID-19 in the hospital after birth’); and 3) perceiving benefits of being pregnant during the pandemic (three items, e.g. ‘I feel that being pregnant is giving me strength during the pandemic’), with responses on a 5-point Likert scale ranging from 1 (Very little) to 5 (Very much). The three factors were, respectively, labelled as follows: PREPS-Preparedness, PREPS-Infection, and PREPS-Positive Appraisal. PREPS is a psychometrically validated and reliable instrument to measure pandemic-related stress for pregnant Spanish to assess COVID-19 related concerns out preparedness for birth and postpartum, infection, and positive appraisal, demonstrating its value for assessing stress and positive aspects that may reflect pregnant women’s resilience and successful coping. The three factors exhibited good inter-item correlations, (1) – Preparedness: 0.21; 2) – Infection: 0.23, and 3) – Positive Appraisal: 0.29. Convergent validity was examined through the Pearson’s correlation coefficients of the PREPS with the Perceived Stress Scale (PSS) and the Prenatal Distress Questionnaire (PDQ) (Garcia-Silva et al., Citation2021).
Prenatal Distress Questionnaire (PDQ)
The 12-item PDQ was used to assess pregnancy-specific stress, including worries and concerns pregnant women have about medical problems, labour and delivery, relationships, parenting and the health of the baby (Caparros-Gonzalez et al., Citation2019; Ibrahim & Lobel, Citation2020; Yali & Lobel, Citation1999). Responses are on a 5-point Likert scale ranging from 0 (Not at all) to 4 (Extremely). Responses are summed and provide a prenatal stress score ranging from 0 to 48. A higher score is indicative of a higher level of pregnancy-specific stress. The Cronbach’s alpha reliability coefficient for the Spanish version was α = 0.74 (Caparros-Gonzalez et al., Citation2019).
Perceived Stress Scale (PSS)
The PSS assesses perceptions of general stress during the last month. The Spanish version of the 14-item PSS was used (Cohen et al., Citation1983). Responses are on a 5-point Likert scale from 0 (never) to 4 (very often). The score ranges from 0 to 56. The European Spanish version PSS (14-item) demonstrated adequate reliability (internal consistency, α = 0.81, and test–retest, r = 0.73), validity (concurrent), and sensitivity (Remor, Citation2006).
Symptom Checklist-90-Revised (SCL-90-R)
The SCL-90-R is a self-administered questionnaire that has been widely used and translated into 18 languages, developed and reformed by Derogatis (Martínez-Azumendi et al., Citation2001). The Spanish version was developed by collaboration with the original author. The SCL-90-R questionnaire is used to assess the individual existence and intensity of 90 psychiatric and psychosomatic symptoms, evaluating the intensity of each symptom on a scale ranging from the total absence (0) to the maximum intensity of the symptom (4). The statistical test used was the level of significance of X2. All of the factorial models contrasted widely with no unsatisfactory fit according to this criterion. In addition, X2 complemented by GFi, AGFI, RN or IR shows an acceptable fit to all the models (Martínez-Azumendi et al., Citation2001).
Sociodemographic, medical and obstetric information
Socio-demographic and obstetric data were collected through the Pregnancy Health Document (de Andalucía, Citation2019). Obstetric variables included gravidity (primigravid vs. multigravid), weeks of gestation, maternal weight gain, numbers of neonates in the present pregnancy, the type of pregnancy, and previous miscarriages. Besides, pregnant women declared whether the pregnancy was wanted or unwanted. Birth-related variables were collected. This information included gestational age at birth, type of delivery (eutocic, caesarean, instrumental), spontaneous, induced or augmented labour, aminorexes (artificial or spontaneous), sex of the neonate and anthropometric measures (length, weight and head circumference at birth).
Statistical analysis
Analyses were performed using SPSS version 22.0 software (SPSS, Inc., Chicago, IL). Continuous variables were expressed as mean ± SD, and categorical variables were expressed as percentages. Kolmogorov–Smirnov test was used to test the normality of the variables.
To compare whether there were any differences between those participants in the final sample and those pregnant women who decided not to participate in the study before completing all the assessment points descriptive analyses of PREPS-S scores were completed using a two-sample-test for continuous variables (age, gestational age in T1, number of babies in the present pregnancy, number of previous miscarriages, the SCL-90-R score, total PREPS and PREPS factors, PSS and PDQ) and χ2 test to compare categorical variables (civil status, level of studies, country of origin, work situation and type of pregnancy) between groups. Significant differences in weeks of pregnancy were found between women who were finally included in the analysis and those that decided to not participate in the follow-up (Included in T2: M = 36.23 weeks, SD = 2.19 vs Rejected to participate in T2: M = 18.80 weeks, SD = 7.68).
Within women included in the follow-up, sociodemographic variables, level of studies, obstetric variables and the anthropometric data of the newborn were compared between primiparous and multiparous women. The χ2 test was used to compare categorical variables previously reported between groups.
Besides, in order to analyse the relationship between PREPS score and the baby length, a univariate analysis of variance using a general linear factorial model was performed. Considering that maternal age ranged from 18 to 40 years old, it was included as a confounding variable in the analyses. Likewise, gestational age was included as a confounding since it was significantly higher in final sample than in women who left the study before the T2 phase. In addition, fetal growth and anthropometric variables of the neonate are intimately related to weeks of pregnancy (Manuck et al., Citation2016).
Results
Descriptive sample characteristics
A total of 81 pregnant women-neonates dyads participated in this study. The participant’s age ranged from 18 to 40 years (Mean (M) = 32.13 years; SD = 5.64). Primiparous women were significantly younger than multiparous (M = 31.18, SD = 6.06 vs M = 33.16, SD = 4.98, p = 0.018). One hundred forty-five women (80.11%) were Spanish, 127 women (70.17%) were working, and 81 women (44.75%) had university studies. All women were similar gestational age when psychological scales were administered. However, it was shown that there were more primiparous women who became pregnant by an artificial technique (11.7 vs 3.4%, p = 0.038).
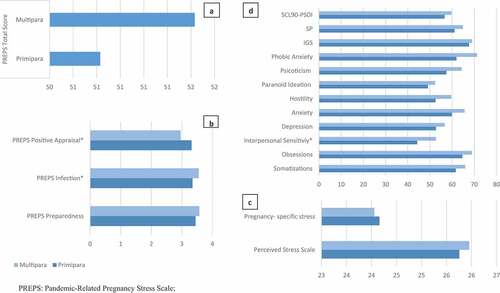
In respect to the 81 neonates (40 girls and 41 boys), all the infants were full term at the time of birth (mean gestational age = 39.05 weeks, SD = 1.35; mean weight = 3446.67 g, SD = 408.35) and there were no differences in head circumference between the neonates of primiparous and multiparous women. Descriptive analyses revealed significant differences for the length of the neonate, showing higher length the neonates of primiparous women (M = 50.46 cm, SD = 1.62) than multiparous women (M = 49.74, SD = 1.38, p=0.033) ().
Table 1. Means and standard errors and distributions for main study factors among pregnant women.
The results of the multiple linear regression model for the PREPS score as predictor, crude and adjusted for potential covariates (age and gestational age), and newborn length as the predicted variable are shown in . Total PREPS score could predict 26% of variance of length of newborn baby (R2 = 0.263, β = 0.513, p < 0.05) in total sample. For primiparous women group, PREPS accounted for 35% of variance of newborn length (R2 = 0.592, β = 0.513, p < 0.05). In multiparous women group, PREPS accounted for 16% of variance of baby length (R2 = 0.160, β = 0.400, p < 0.05).
Table 2. Association of total PREPS score and average length of neonate baby estimated through multiple linear regression.
shows differences between primiparous (n = 94) and multiparous women (n = 87) in psychological symptomatology measured though the psychometric scales PREPS, SCL90-R, Perceived Stress Scale and Pregnancy-specific stress. For PREPS scale, there were no significant differences between groups for total score, notwithstanding multiparous showed higher scores for the ‘Positive Appraisal’ items compared to primiparous women (M = 3.32, SD = 0.83 vs M = 2.96, SD = 0.81, p=0.004). Multiparous women showed higher mean scores for the ‘Infection’ dimension, compared to primiparous women (M = 3.55, SD = 0.65 vs M = 3.35, SD = 0.70, p=0.042). There were no differences between groups in terms of levels of stress measured though the Perceived Stress and Pregnancy-specific stress scales. However, first-time pregnant women showed slight but not significantly higher levels of stress than multiparous measured through the Pregnancy-specific stress scale (M = 24.16, SD = 3.79 vs M = 24.06, SD = 3.06) and lower mean scores than multiparous for stress measured through the Perceived Stress Scale (M = 25.95, SD = 3.41 vs M = 25.76, SD = 3.79). For the SCL90-R checklist items, multiparous women showed an exacerbated psychological symptomatology. However, those differences approached significance for the dimension of ‘Interpersonal Sensitivity’ (M = 44.26, SD = 31.95 vs M = 52.72, SD = 32.35, p = 0.078). The effect of those pandemic-related stress symptoms and its implications in neonatal growing and length can be seen in .
Discussion
The aim of the study was to study the impact of pandemic-related pregnancy stress and maternal psychopathological symptoms during COVID-19 lockdown in 2020 on neonatal development. Two of the instruments measured stress related to pregnancy with one of these addressing stress related to pandemic-induced stress and the third measured general stress. The main finding of this study was that PREPS score could predict 26% of variance of length of neonate baby. Multiparous showed higher scoring for the ‘Positive Appraisal’ items than primiparous. Multiparous women also showed higher mean scores for the ‘Infection’ dimension, than primiparous. Primiparous showed slightly higher levels of stress than multiparous measured through the Pregnancy-specific stress scale and lower mean scores than multiparous for stress measured through the Perceived Stress Scale although those symptoms were exacerbated in multiparous women.
Multiparous women showed more positivity compared to primiparous when approaching the conversation about the pandemic and pregnancy. This may show the lack of experience and facing maternity in a pandemic context (Caparros-Gonzalez, Citation2020; Jungari, Citation2020; Saccone et al., Citation2020). As has been found in other studies, primiparous women have more emergency care visits when exposed to psychosocial general stress (Phelan et al., Citation2015). It is possible that experiences with the previous pregnancy may facilitate the positive attitude towards pandemic, may become a risk factor to develop fear and insecurity towards infection on the other hand (Jungari, Citation2020; Kotlar et al., Citation2021; Saccone et al., Citation2020).
Primiparous women showed slightly higher levels of stress than multiparous measured through the Pregnancy-Specific Stress Scale as well as lower mean scores when compared to multiparous for pregnancy-specific stress, although, those symptoms were aggravated in multiparous women. Some authors found that the severity of symptoms is related to the trimester of pregnancy that women were in when the pandemic outbreak occurred (Saccone et al., Citation2020).
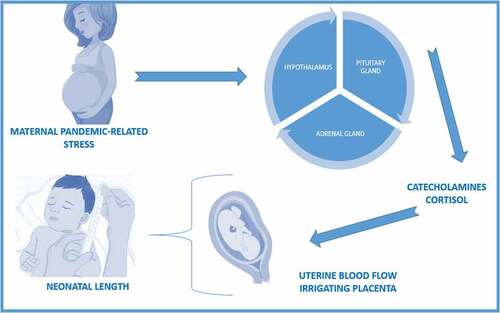
Our results showed that the PREPS score predicted 26% of the variance of the length of the neonate, which means using the questionnaire may help to predict the length of the neonate. Fetal growth is influenced by catecholamines and their relation with uterine blood flow, as well as cortisol levels (Rakers et al., Citation2020). The catecholamines can potentially reduce the blood flow to the uterus, thus reducing the oxygen and nutrients supply to the fetus will ultimately affects fetal growing. In addition, the higher cortisol levels can impact on how placental corticotropin releasing hormone acts, thus to a higher concentration, lower fetal growth predisposition (Rakers et al., Citation2020). The interactions with maternal pandemic-related stress and how they play a role in neonatal length can be seen in . The way in which the environment can cause changes during embryonic and fetal development that determine the future health of the newborn is known as fetal programming (Barker, Citation2003; Lautarescu et al., Citation2020; Lewis et al., Citation2015; O’Connor et al., Citation2003; Reynolds et al., Citation2019). Thus, our results showed the effect of the pandemic-related stress on neonatal growth and length; other researchers have only found implications on neurodevelopment (Bleker et al., Citation2019; Caparros-Gonzalez et al., Citation2019; Chan et al., Citation2018; Lautarescu et al., Citation2020; Lewis et al., Citation2015; Talge et al., Citation2007; Walsh et al., Citation2019). Moreover, not many investigations have been conducted that explore the implications of general stress and neonatal physical development. A previous study found an increased risk of fetal macrosomia when the woman is exposed to high psychological general stress during the second trimester (Mélançon et al., Citation2020). Although our findings focus on neonatal length, they are in line with other studies, which have found an association between neonatal weight and general stress suffered by the mother (Mélançon et al., Citation2020). Conversely, other authors found no impact of perceived stress and neonatal measurements (Wing et al., Citation2017). In a prospective, multicentre and longitudinal study of fetal growth, undertaken with a reference population of pregnant women ranging from 18 to 40 years old, as in our study, the authors did not find results in line with ours. However, all the women that took part in the study were low-risk pregnant women (Wing et al., Citation2017).
The questionnaires and tools used to collect data have been used previously in similar populations and contexts. The fact that all of them were previously used with good psychometric results made them suitable to collect data in a sample similar to the population of the study. In addition, to align the obstetric, medical, and sociodemographic information with the medical records, the Pregnancy Health Document was the information source chosen for those variables. Being a document that recalls the information of the whole pregnancy, characteristics of birth, and critical information about the neonate helps to keep track of everything that occurs during pregnancy, thus converting this record to the ideal source to obtain obstetric data (Kerkin et al., Citation2018). However, some researchers argue that paper record-keeping is not contemporaneous enough when compared to digital records such as digital voice recorders considering this factor a limitation (Pezaro & Lilley, Citation2015). As currently in the context of the study, a clear digital record-keeping policy does not exist, we consider this a strength rather than a limitation (Spanish Health Ministry, Citation2022). The study was conducted in two phases, being the first one exclusively to recruit the participants and the next one to follow-up the neonates of those women. Due to the design, selection bias although was considered as a possible limitation of the study, we do not believe is present due to recruitment method. The women were invited to participate during the antenatal appointment with the midwife and only 41 women declined to participate at this point. Moreover, the women should consent to participate and after selection provide psychological information, obstetric data, and offspring data as well. Thus, all the women had several steps to reaffirm their consent to take part in the study. Information bias cannot be completely ruled out and have to be considered as another possible limitation, despite the tools for data collection being adapted to any educational level (Althubaiti, Citation2016).
To our knowledge, research linking neonatal duration and development and pandemic-related stress has a major impact. It provides an opportunity for health professionals to include different tools to assess women’s stress levels at the antenatal visit and to follow up and to prevent negative consequences on neonatal growth.
Authorship statement
All authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors. All authors are in agreement with the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Althubaiti, A. (2016). Information bias in health research: Definition, pitfalls, and adjustment methods. Journal of Multidisciplinary Healthcare, 9, 211–217. https://doi.org/10.2147/JMDH.S104807
- Anifantaki, F., Pervanidou, P., Lambrinoudaki, I., Panoulis, K., Vlahos, N., & Eleftheriades, M. (2021). Maternal prenatal stress, thyroid function and neurodevelopment of the offspring: A mini review of the literature. Frontiers in Neuroscience, 15, 692446. https://doi.org/10.3389/fnins.2021.692446
- Aryal, S., & Pant, S. B. (2020). Maternal mental health in Nepal and its prioritization during COVID-19 pandemic: Missing the obvious. Asian Journal of Psychiatry, 54, 102281. https://doi.org/10.1016/j.ajp.2020.102281
- Astbury, J. (2006). Mental health aspects of women’s reproductive health. World Health Organization, 80–168. https://apps.who.int/iris/bitstream/handle/10665/43846/9789241563567_eng.pdf
- Baker, C. (2021). Mental Health Statistics: Prevalence, Services and Funding in England. https://commonslibrary.parliament.uk/research-briefings/sn06988/
- Barker, D. J. P. (2003). Coronary heart disease: A disorder of growth. Hormone Research in Paediatrics, 59(1), 35–41. https://doi.org/10.1159/000067843
- Bleker, L. S., de Rooij, S. R., & Roseboom, T. J. (2019). Prenatal psychological stress exposure and neurodevelopment and health of children. International Journal of Environmental Research and Public Health, 16(19). https://doi.org/10.3390/ijerph16193657
- Bueso-Izquierdo, N., Daugherty, J. C., Puente, A. E., & Caparros-Gonzalez, R. A. (2021). Intimate partner violence and pregnancy during the COVID-19 pandemic. Journal of Gender Studies, 31(5), 573–583. https://doi.org/10.1080/09589236.2021.1999794
- Caparros-Gonzalez, R. A. (2020). Consecuencias maternas y neonatales de la infección por coronavirus COVID-19 durante el embarazo: Una scoping review. Revista Española de Salud Pública, 94, 202004033. https://doi.org/10.4321/S1135-57272020000100025
- Caparros-Gonzalez, R. A., & Alderdice, F. (2020). The COVID-19 pandemic and perinatal mental health. Journal of Reproductive and Infant Psychology, 38(3), 223–225. https://doi.org/10.1080/02646838.2020.1786910
- Caparros-Gonzalez, R. A., Perra, O., Alderdice, F., Lynn, F., Lobel, M., García-García, I., & Peralta-Ramírez, M. I. (2019). Psychometric validation of the Prenatal Distress Questionnaire (PDQ) in pregnant women in Spain. Women & Health, 59(8), 937–952. https://doi.org/10.1080/03630242.2019.1584143
- Cardwell, M. S. (2013). Stress. Obstetrical & Gynecological Survey, 68(2), 119–129. https://doi.org/10.1097/OGX.0b013e31827f2481
- Ceulemans, M., Foulon, V., Ngo, E., Panchaud, A., Winterfeld, U., Pomar, L., Lambelet, V., Cleary, B., O’Shaughnessy, F., Passier, A., Richardson, J. L., Hompes, T., & Nordeng, H. (2021). Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic-A multinational cross-sectional study. Acta Obstetricia Et Gynecologica Scandinavica, 100(7), 1219–1229. https://doi.org/10.1111/aogs.14092
- Chan, J. C., Nugent, B. M., & Bale, T. L. (2018). Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biological Psychiatry, 83(10), 886–894. https://doi.org/10.1016/j.biopsych.2017.10.005
- Cohen, S., Kamarck, T., & Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health & Social Behavior, 24(4), 385–396. https://doi.org/10.2307/2136404
- de Andalucía, J. (2019). Documento de Salud de la Embarazada. Consejería de Salud y Familias. https://www.juntadeandalucia.es/export/drupaljda/csafa_documentoSaludEmbarazada_SE1738-2019.pdf
- Farrell, T., Reagu, S., Mohan, S., Elmidany, R., Qaddoura, F., Ahmed, E. E., Corbett, G., Lindow, S., Abuyaqoub, S. M., & Alabdulla, M. A. (2020). The impact of the COVID-19 pandemic on the perinatal mental health of women. Journal of Perinatal Medicine, 48(9), 971–976. https://doi.org/10.1515/jpm-2020-0415
- Flores-Ramos, M. (2013). La salud mental en la mujer embarazada. Perinatología y Reproducción Humana, 27(3), 143–144.
- Fuente-Moreno, M., Garcia-Terol, C., Domínguez-Salas, S., Rubio-Valera, M., & Motrico, E. (2023). Maternity care changes and postpartum mental health during the COVID-19 pandemic: A Spanish cross-sectional study. Journal of Reproductive and Infant Psychology. https://doi.org/10.1080/02646838.2023.2171375
- Gallego, B. R. (2021). Programación fetal: ambiente de crecimiento y desarrollo prenatal, de Rafael A. Caparrós González. Ediciones Pirámide, 231 pp., año 2019. Clínica Y Salud, 32(3), 151–152. https://doi.org/10.5093/clysa2021a19
- Garcia-Silva, J., Caracuel, A., Lozano-Ruiz, A., Alderdice, F., Lobel, M., Perra, O., & Caparros-Gonzalez, R. A. (2021). Pandemic-related pregnancy stress among pregnant women during the COVID-19 pandemic in Spain. Midwifery, 103, 103163. https://doi.org/10.1016/j.midw.2021.103163
- Glover, V. (2014). Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice & Research Clinical Obstetrics & Gynaecology, 28(1), 25–35. https://doi.org/10.1016/j.bpobgyn.2013.08.017
- Gokoel, A. R., Abdoel Wahid, F., Zijlmans, W. C. W. R., Shankar, A., Hindori-Mohangoo, A. D., Covert, H. H., MacDonald-Ottevanger, M.-S., Lichtveld, M. Y., & Harville, E. W. (2021). Influence of perceived stress on prenatal depression in Surinamese women enrolled in the CCREOH study. Reproductive Health, 18(1), 136. https://doi.org/10.1186/s12978-021-01184-x
- Hou, Q., Li, S., Jiang, C., Huang, Y., Huang, L., Ye, J., Pan, Z., Teng, T., Wang, Q., Jiang, Y., Zhang, H., Liu, C., Li, M., Mo, Z., & Yang, X. (2018). The associations between maternal lifestyles and antenatal stress and anxiety in Chinese pregnant women: A cross-sectional study. Scientific Reports, 8(1), 10771. https://doi.org/10.1038/s41598-018-28974-x
- Ibrahim, S. M., & Lobel, M. (2020). Conceptualization, measurement, and effects of pregnancy-specific stress: Review of research using the original and revised Prenatal Distress Questionnaire. Journal of Behavioral Medicine, 43(1), 16–33. https://doi.org/10.1007/s10865-019-00068-7
- Jungari, S. (2020). Maternal mental health in India during COVID-19. Public Health, 185, 97–98. https://doi.org/10.1016/j.puhe.2020.05.062
- Kerkin, B., Lennox, S., & Patterson, J. (2018). Making midwifery work visible: The multiple purposes of documentation. Women and Birth: Journal of the Australian College of Midwives, 31(3), 232–239. https://doi.org/10.1016/j.wombi.2017.09.012
- Koenen, K. C. (2020). Pregnant during a pandemic?. Psychology Today.
- Kotlar, B., Gerson, E., Petrillo, S., Langer, A., & Tiemeier, H. (2021). The impact of the COVID-19 pandemic on maternal and perinatal health: A scoping review. Reproductive Health, 18(1), 10. https://doi.org/10.1186/s12978-021-01070-6
- Lautarescu, A., Craig, M. C., & Glover, V. (2020). Prenatal stress: Effects on fetal and child brain development. International Review of Neurobiology, 150, 17–40. https://doi.org/10.1016/bs.irn.2019.11.002
- Lewis, A. J., Austin, E., Knapp, R., Vaiano, T., & Galbally, M. (2015). Perinatal maternal mental health, fetal programming and child development. Healthcare (Basel, Switzerland), 3(4), 1212–1227. https://doi.org/10.3390/healthcare3041212
- Loomans, E. M., van Dijk, A. E., Vrijkotte, T. G. M., van Eijsden, M., Stronks, K., Gemke, R. J. B. J., & van den Bergh, B. R. H. (2013). Psychosocial stress during pregnancy is related to adverse birth outcomes: Results from a large multi-ethnic community-based birth cohort. European Journal of Public Health, 23(3), 485–491. https://doi.org/10.1093/eurpub/cks097
- Manuck, T. A., Rice, M. M., Bailit, J. L., Grobman, W. A., Reddy, U. M., Wapner, R. J., Thorp, J. M., Caritis, S. N., Prasad, M., Tita, A. T. N., Saade, G. R., Sorokin, Y., Rouse, D. J., Blackwell, S. C., Tolosa, J. E., Varner, M., Hill, K., Sowles, A., & Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. (2016). Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. American Journal of Obstetrics & Gynecology, 215(1), e103.1–.e103.14. https://doi.org/10.1016/j.ajog.2016.01.004
- Mappa, I., Distefano, F. A., & Rizzo, G. (2020). Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: A prospective observational study. Journal of Perinatal Medicine, 48(6), 545–550. https://doi.org/10.1515/jpm-2020-0182
- Martínez-Azumendi, O., Fernández-Gómez, C., & Beitia-Fernández, M. (2001). Factorial variance of the SCL-90-R in a Spanish out-patient psychiatric sample. Actas espanolas de psiquiatria, 29(2), 95–102.
- Mélançon, J., Bernard, N., Forest, J.-C., Tessier, R., Tarabulsy, G. M., Bouvier, D., & Giguère, Y. (2020). Impact of maternal prenatal psychological stress on birth weight. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 39(12), 1100–1108. https://doi.org/10.1037/hea0001017
- National Institute of Mental Health, Mental Health America, National Alliance on Mental Illness, John Hopkins Medicine, Center for Disease Control, & Our World in Data. (2021). Mental Health Statistics.
- O’Connor, T. G., Heron, J., Golding, J., & Glover, V. (2003). Maternal antenatal anxiety and behavioural/emotional problems in children: A test of a programming hypothesis. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44(7), 1025–1036. https://doi.org/10.1111/1469-7610.00187
- Orkaby, N., Kalfon- Hakhmigari, M., Levy, S., Krissi, H., Peled, Y., & Handelzalts, J. E. (2022). COVID-19-Related worries mediate the association between attachment orientation and elevated depression levels at 21-month postpartum. Journal of Reproductive and Infant Psychology. https://doi.org/10.1080/02646838.2022.2132382
- Pantha, S. (201405). Prevalence of stress among pregnant women attending antenatal care in a tertiary maternity hospital in kathmandu. Journal of Women’s Health Care, 3 (5). https://doi.org/10.4172/2167-0420.1000183.
- Penengo, C., Colli, C., Garzitto, M., Driul, L., Sala, A., Degano, M., Preis, H., Lobel, M., & Balestrieri, M. (2021). Psychometric properties of the Italian version of the Pandemic-Related Pregnancy Stress Scale (PREPS) and its correlation with anxiety and depression. Journal of Affective Disorders, 294, 48–53. https://doi.org/10.1016/j.jad.2021.06.076
- Pezaro, S., & Lilley, L. (2015). Digital voice recorders - a conceptual intervention to facilitate contemporaneous record keeping in midwifery practice. Women and Birth: Journal of the Australian College of Midwives, 28(4), e171–6. https://doi.org/10.1016/j.wombi.2015.04.008
- Phelan, A. L., DiBenedetto, M. R., Paul, I. M., Zhu, J., & Kjerulff, K. H. (2015). Psychosocial stress during first pregnancy predicts infant health outcomes in the first postnatal year. Maternal and Child Health Journal, 19(12), 2587–2597. https://doi.org/10.1007/s10995-015-1777-z
- Preis, H., Mahaffey, B., Heiselman, C., & Lobel, M. (2020). Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Social Science & Medicine, 266(1982), 113348. https://doi.org/10.1016/j.socscimed.2020.113348
- Preis, H., Mahaffey, B., & Lobel, M. (2020). Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). Journal of Psychosomatic Obstetrics and Gynaecology, 41(3), 191–197. https://doi.org/10.1080/0167482X.2020.1801625
- Rakers, F., Rupprecht, S., Dreiling, M., Bergmeier, C., Witte, O. W., & Schwab, M. (2020). Transfer of maternal psychosocial stress to the fetus. Neuroscience & Biobehavioral Reviews, 117, 185–197. https://doi.org/10.1016/j.neubiorev.2017.02.019
- Remor, E. (2006). Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). The Spanish Journal of Psychology, 9(1), 86–93. https://doi.org/10.1017/s1138741600006004
- Reynolds, L. P., Borowicz, P. P., Caton, J. S., Crouse, M. S., Dahlen, C. R., & Ward, A. K. (2019). Developmental programming of fetal growth and development. The Veterinary Clinics of North America: Food Animal Practice, 35(2), 229–247. https://doi.org/10.1016/j.cvfa.2019.02.006
- Saccone, G., Florio, A., Aiello, F., Venturella, R., de Angelis, M. C., Locci, M., Bifulco, G., Zullo, F., & diSpiezio Sardo, A. (2020). Psychological impact of coronavirus disease 2019 in pregnant women. American Journal of Obstetrics & Gynecology, 223(2), 293–295. https://doi.org/10.1016/j.ajog.2020.05.003
- Schaal, N. K., La Marca-Ghaemmaghami, P., Preis, H., Mahaffey, B., Lobel, M., & Amiel Castro, R. (2021). The German version of the pandemic-related pregnancy stress scale: A validation study. European Journal of Obstetrics & Gynecology and Reproductive Biology, 256, 40–45. https://doi.org/10.1016/j.ejogrb.2020.10.062
- Spanish Health Ministry. (2017). Encuesta Nacional de Salud de España 2017. https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuesta2017.htm
- Spanish Health Ministry. (2022). Historia Clínica Digital del Sistema Nacional de Salud. https://www.sanidad.gob.es/profesionales/hcdsns/home.htm
- Staneva, A., Bogossian, F., Pritchard, M., & Wittkowski, A. (2015). The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: A systematic review. Women and Birth: Journal of the Australian College of Midwives, 28(3), 179–193. https://doi.org/10.1016/j.wombi.2015.02.003
- Talge, N. M., Neal, C., & Glover, V. (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(3–4), 245–261. https://doi.org/10.1111/j.1469-7610.2006.01714.x
- Tang, X., Lu, Z., Hu, D., & Zhong, X. (2019). Influencing factors for prenatal stress, anxiety and depression in early pregnancy among women in Chongqing, China. Journal of Affective Disorders, 253, 292–302. https://doi.org/10.1016/j.jad.2019.05.003
- Thapa, S. B., Mainali, A., Schwank, S. E., & Acharya, G. (2020). Maternal mental health in the time of the COVID-19 pandemic. Acta Obstetricia Et Gynecologica Scandinavica, 99(7), 817–818. https://doi.org/10.1111/aogs.13894
- Thongsomboon, W., Kaewkiattikun, K., & Kerdcharoen, N. (2020). Perceived stress and associated factors among pregnant women attending antenatal care in Urban Thailand. Psychology Research and Behavior Management, 13, 1115–1122. https://doi.org/10.2147/PRBM.S290196
- van den Bergh, B. R. H., van den Heuvel, M. I., Lahti, M., Braeken, M., de Rooij, S. R., Entringer, S., Hoyer, D., Roseboom, T., Räikkönen, K., King, S., & Schwab, M. (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews, 117, 26–64. https://doi.org/10.1016/j.neubiorev.2017.07.003
- Vardi, N., Zalsman, G., Madjar, N., Weizman, A., & Shoval, G. (2022). COVID-19 pandemic: Impacts on mothers’ and infants’ mental health during pregnancy and shortly thereafter. Clinical Child Psychology and Psychiatry, 27(1), 82–88. https://doi.org/10.1177/13591045211009297
- Walsh, K., McCormack, C. A., Webster, R., Pinto, A., Lee, S., Feng, T., Krakovsky, H. S., O’Grady, S. M., Tycko, B., Champagne, F. A., Werner, E. A., Liu, G., & Monk, C. (2019). Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proceedings of the National Academy of Sciences of the United States of America, 116(48), 23996–24005. https://doi.org/10.1073/pnas.1905890116
- Wing, D. A., Ortega-Villa, A. M., Grobman, W. A., Hediger, M. L., Grewal, J., Pugh, S. J., Kim, S., Newman, R., Chien, E., Owen, J., D’Alton, M. E., Wapner, R., Sciscione, A., Albert, P. S., & Grantz, K. L. (2017). Maternal stress and neonatal anthropometry: The NICHD fetal growth studies. American Journal of Obstetrics & Gynecology, 217(1), .e82.1–.e82.7. https://doi.org/10.1016/j.ajog.2017.02.039
- World Health Organization. (2021). Mental Health Definition . https://www.who.int/data/gho/data/major-themes/health-and-well-being
- Wu, Y., Zhang, C., Liu, H., Duan, C., Li, C., Fan, J., Li, H., Chen, L., Xu, H., Li, X., Guo, Y., Wang, Y., Li, X., Li, J., Zhang, T., You, Y., Li, H., Yang, S., Tao, X., & Huang, H.-F. (2020). Perinatal depressive and anxiety symptoms of pregnant women during the coronavirus disease 2019 outbreak in China. American Journal of Obstetrics & Gynecology, 223(2), .e240.1–.e240.9. https://doi.org/10.1016/j.ajog.2020.05.009
- Yali, A. M., & Lobel, M. (1999). Coping and distress in pregnancy: An investigation of medically high risk women. Journal of Psychosomatic Obstetrics & Gynecology, 20(1), 39–52. https://doi.org/10.3109/01674829909075575
- Yuksel, F., Akin, S., & Durna, Z. (2014). Prenatal distress in Turkish pregnant women and factors associated with maternal prenatal distress. Journal of Clinical Nursing, 23(1–2), 54–64. https://doi.org/10.1111/j.1365-2702.2012.04283.x