Abstract
The interleukin 12 (IL-12) family comprises a group of heterodimeric cytokines that can cope with a great variety of immune conditions as the microenvironment demands. By sharing cytokine and receptor subunits, IL-12 (comprised of p40/p35 subunits), IL-23 (p40/p19), IL-27 (p28/EBI3), and IL-35 (p35/EBI3) represent, as a whole, a highly versatile system participating in controlling the continuum from inflammation to tolerance. Promiscuity, a peculiar feature of those cytokines, is a powerful and economic means of producing individual factors with distinct activities via different combinations of a single set of subunits. Whereas IL-12 and IL-23 have a clearly dominant immunostimulatory functional profile and IL-35 is a potent immunosuppressive agent, IL-27 can exert both adjuvant and regulatory effects, depending on the cytokine milieu. Promiscuity itself, however, may significantly hamper the therapeutic use of heterodimeric cytokines. The subunits of a recombinant cytokine, when administered in its native form, will rapidly dissociate in vivo and reassociate with alternative partners, thus generating different heterodimeric or even homodimeric molecules (i.e., p40/p40) with unwanted effects. As in other areas, bioengineering has provided a formidable tool to overcome the constraints associated with the potential use of IL-12 family cytokines. The generation of several gene constructs expressing IL-12, IL-23, IL-27, IL-35, or even the homodimer p40/p40, in their monomerized, single-chain form has allowed us to unveil the efficacy of those molecules in several experimental settings, including neoplasia, viral infection, chronic inflammation, allergy and autoimmunity. Although work is still needed to obtain an overall picture of therapeutic vs. adverse effects of individual molecules before any use in humans, the new frontiers of bioengineering are now driving the production of completely new combinations of cytokine subunits that may further extend the potential clinical use of such eclectic proteins.
Keywords:
Introduction
Cytokines are soluble factors released by a large variety of different cell types, capable of mediating several immunologic processes, including differentiation, proliferation, migration and apoptosis of their target cells. The actions of cytokines are pleiotropic, synergistic and mutually regulated by positive or negative feedbacks, and range over activating to suppressive outcomes. The IL-12 cytokine family is a prototypic example of this wide spectrum of immunological functions, encompassing both proinflammatory and immunoregulatory members. This family includes four heterodimeric proteins, namely IL-12, IL-23, IL-27 and IL-35 (Figure ), which are mainly produced by activated antigen presenting cells (APCs) with the exception of IL-35, which is instead expressed by various populations of thymus-derived natural regulatory T (nTreg) cells (Hunter, Citation2005; Trinchieri, Citation2003; Vignali, Collison, & Workman, Citation2008).
Figure 1 Heterodimeric cytokines belonging to IL-12 family: structural and functional characteristics.Naturally occurring IL-12, IL-23, IL-27, and IL-35 are heterodimeric cytokines with a high sharing rate. The p40 β-chain is shared by IL-12 and IL-23, EBI3 β-chain is shared by IL-27 and IL-35, p35 α-chain is shared by IL-12 and IL-35, whereas IL-23 and IL-27 have unique α-chains, p19 and p28, respectively. Only IL-12 and IL-23 are disulfide-linked heterodimers. The receptor complexes of heterodimeric cytokines also include shared subunits; IL-12Rβ1 is present in receptors for IL-12 and IL-23, IL-12Rβ2 in those for IL-12 and IL35, and gp130 is common to IL-27 and IL35 receptor complexes. Jak-STAT signaling partners of these receptors are indicated, as well as the functional effects of related cytokines.
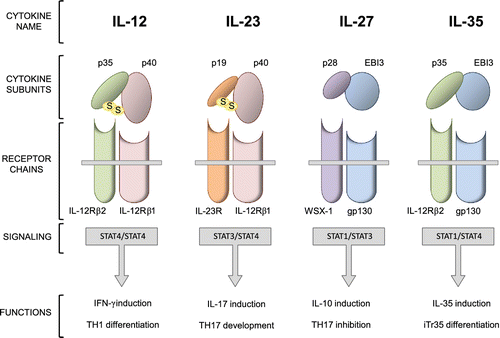
IL-12, the first discovered heterodimeric cytokine, is a pivotal proinflammatory cytokine produced in response to microbial pathogens by APCs such as dendritic cells (DCs), macrophages and B cells. Acting as a potent promoter of interferon-γ (IFN-γ) production by T lymphocytes and natural killer (NK) cells, IL-12 triggers a positive feedback loop for its induction through T-cell-derived IFN-γ and thus drives the differentiation of T helper type 1 (TH1) cells (O’Shea & Paul, Citation2002; Trinchieri, Citation2003). IL-12 is composed of two subunits, p35 (α-chain) and p40 (β-chain). p35, owing to a four-α-helix bundle structure as the other IL-12 family α-chains, is homologous to IL-6 and granulocyte colony-stimulating factor (G-CSF), whereas p40 is homologous to the nonsignaling extracellular portion of the IL-6 receptor (IL-6R) (Trinchieri, Citation2003). Clustering of the two receptor chains, interleukin-12 receptor (IL-12R) β1 and IL-12Rβ2, allows the formation of the complete cytokine membrane receptor, which signals via the Jak-STAT pathway by the dominant activation of signal transducers and activators of transcription 4 (STAT4) homodimers (Thierfelder et al., Citation1996).
Similarly to IL-12, IL-23 is also produced by APCs in response to multiple infectious and/or inflammatory stimuli and, therefore, likewise belongs in the array of immunostimulatory cytokines capable of initiating and mediating inflammation. Unlike IL-12, the most important role of IL-23 is the induction of IL-17 by T cells that promotes the development of TH17 lymphocytes, a T-cell subset widely involved in autoimmune and chronic inflammatory pathologies (Parham et al., Citation2002). The IL-23 cytokine and its receptor share one chain with IL-12 and IL-12R, respectively, because the IL-12 p40 subunit combines with p19 – the IL-23 α-chain – to form the IL-23 heterodimer (Oppmann et al., Citation2000), whereas IL-12Rβ1 associates with IL-23R, a gp130-like receptor chain unique to IL-23, to form the complete IL-23 receptor complex (Lyakh, Trinchieri, Provezza, Carra, & Gerosa, Citation2008). IL-23 signal transduction involves the activation of Tyk2, Jak2 and STAT1, STAT3, STAT4, with a prevalent nuclear translocation of STAT3/4 complexes (Lyakh et al., Citation2008).
The p28 β-chain and Epstein-Barr virus induced gene 3 subunit (EBI3; both related to IL-6R) are components of IL-27, which is primarily produced by APCs after stimulation by microbial products or inflammatory mediators. Although originally characterized as a proinflammatory cytokine (Pflanz et al., Citation2002), IL-27 has been later revealed to have an anti-inflammatory nature in studies of infectious and autoimmune disease models. Moreover, recent work showed that IL-27 inhibits the development of TH17 cells (Fitzgerald, Zhang et al., 2007) and will also induce the production of the anti-inflammatory cytokine IL-10 by T cells (Awasthi et al., Citation2007). Signaling of IL-27 occurs via clustering of WSX-1, a type I cytokine receptor, and gp130 – a receptor subunit used by several other IL-6 family members – followed by subsequent activation of STAT3 and STAT4 (Wojno & Hunter, Citation2012).
Among the heterodimeric cytokines belonging in the IL-12 family, IL-35 has the stronger immunosuppressive connotation, since it is produced by mouse and human regulatory T (Treg) cell populations and drives tolerogenic mechanisms, such as suppression of T-cell proliferation and induction of a specific Treg cell subset (iTr35, induced T regulatory cells producing IL-35) which produces IL-35 but does not express forkhead box P3 (Foxp3), IL-10, or the immunoregulatory cytokine transforming growth factor-β (TGF-β; Collison et al., Citation2010; Vignali et al., Citation2008). Again, whilst sharing both cytokine and receptor subunits with other members of the family, IL-35 is composed of IL-12 p35 and IL-27 EBI3 (Collison et al., Citation2012), and binds the gp130/IL-12Rβ2 receptor complex, although two additional receptor-chain homodimers (gp130–gp130 and IL-12Rβ2–IL-12Rβ2) are also activated (Collison et al., Citation2012). Functional effects are then mediated by the activation of the Jak-STAT pathway and phosphorylation of STAT1 and STAT3 (Delgoffe, Murray, & Vignali, Citation2011).
Promiscuity in chain combination gives different cytokines and receptors
An intriguing feature of cytokines belonging to the IL-12 family is chain-sharing among members of the group, which could be viewed phylogenetically as an economic expedient of synthesizing different biologically active factors by using a minimum of precursor molecules. In general, one α-chain (p19, p28 or p35) and one β-chain (p40 or EBI3) couple to generate these heterodimeric cytokines. α-chains have a four-helix bundle structure characteristic of the IL-6 superfamily, whereas β-chains share homology with nonsignaling receptors of the IL-6 family (IL-6R, IL-11R, ciliary neurotrophic factor receptor; Jones et al., Citation2012). Although IL-12 (p40/p35), IL-23 (p40/p19), IL-27 (EBI3/p28) and IL-35 (EBI3/p35) might potentially be produced by all cells (activated DCs, macrophages, or B cells) expressing the subunits they are made of, their production is qualitatively and quantitatively controlled at multiple levels. In fact, different expression rate of individual subunits, competition in combining each other and secretion to different extents – either alone or paired with other members – are all factors contributing to the final, diversified availability of biologically active cytokines. In particular, secretion ability and formation of disulfide linkage are critical for released heterodimers to be formed to sufficient extents. Thus, despite the presence of p19 and p35 that are not easily secreted alone (Vignali & Kuchroo, Citation2012), IL-12 and IL-23 are abundantly released by virtue of p40 secretion output and further sustained by inter-chain disulfide linkages. On the contrary, IL-27 and more so IL-35, are greatly affected by poorly secreted subunits (EBI3 for IL-27 and EBI3 plus p35 for IL-35), and the absence of inter-chain disulfide linkages strongly contributes to the poorness of their secretion (Vignali & Kuchroo, Citation2012). In addition, p28 and p40 can be secreted also as monomeric or homodimeric soluble factors, respectively, and can both act as antagonist molecules, the former preventing IL-6 binding to the gp130 receptor chain (Stumhofer et al., Citation2010) and the latter competing with IL-12 for IL-12Rα (Stumhofer et al., Citation2010).
Promiscuity in chain combination characterizes not only the IL-12 family cytokine architecture, but also their receptor assembly. Awareness of receptor chain-sharing among this group of ligands has gradually grown when, one after the other, heterodimeric cytokines were discovered. At the beginning, when IL-12 was the only known cytokine, IL-12Rβ1 and IL-12Rβ2 chains were characterized as proper subunits of IL-12 receptor and their clustering was described as being necessary to start signaling through phosphorylation of Jak2 and Tyk2 kinases and the prevalent recruitment of STAT4 transcription factors, although activation of STAT1, STAT3 and STAT5 can also be observed (Presky et al., Citation1996; Thierfelder et al., Citation1996). In 2002, two years after its identification (Oppmann et al., Citation2000), IL-23, similarly to IL-12, was demonstrated to bind the β1 but not the β2 subunit of IL-12R (Parham et al., Citation2002). In fact, IL-23R, a newly discovered member of the hemopoietin receptor family, was found to pair with IL-12Rβ1 to confer IL-23 responsiveness on cells expressing both subunits. This was the first evidence for receptor chain-sharing in the group of heterodimeric cytokines, which was accompanied by the observation that such different combinations result in the activation of the same receptor-associated kinases (Jak2 and Tyk2), yet in different STAT phosphorylation patterns, as STAT3 and STAT4 dimers translocate into the nucleus for IL-23 signal transduction (Parham et al., Citation2002).
In the same year, IL-27 and its receptor were identified (Pflanz et al., Citation2002). IL-27 can be included in the IL-12 cytokine family for the p28 and EBI3 homology to IL-12p35 and IL-12p40, respectively, and for its binding to the receptor complex WSW-1/gp130, whose subunits are type I transmembrane proteins belonging in the structural family of IL-6/IL-12 receptors (Pflanz et al., Citation2004). IL-27 signaling only in part resembles that of IL-12 and IL-23 receptors, since STAT1 and STAT3 are activated as a consequence of Jak1 and Jak2 phosphorylation (Collison & Vignali, Citation2008). Nevertheless, the IL-27 receptor was later discovered to share the gp130 subunit with the IL-35 receptor, which is, in fact, characterized by high-level flexibility in receptor-chain composition, including three possible homo- and hetero-combinations of gp130 and of IL-12Rβ2 as well. Among these, the gp130/IL-12Rβ2 heterodimeric form is the one required for a ‘canonical’ triggering of the IL-35 receptor, fully activating the transduction pathway mediated by Jak1/Jak2 kinases and STAT1/STAT4 heterodimers, thus providing maximal T-cell suppression, IL-35 induction and conversion of T cells into an iTr35 functional profile. However, it has been shown that gp130/gp130 and IL-12Rβ2/ IL-12Rβ2 pairs, generating phosphorylated STAT1 or STAT4, respectively, also contribute effectively to IL-35 signaling, because the absence of either one of the IL-35R chains in vivo ablates the suppressive effects of an intact IL-35 form (Collison et al., Citation2012).
The wide sharing of receptor and ligand chains in this group of cytokines raises the question of whether competition could occur in dimer formation, and how the higher/lower expression of paired subunits in different cell types can be controlled and manipulated. For receptor complexes, in addition to the expression degree regulated by environmental conditions, membrane fluidity and intrinsic mobility of single subunits are supposed to represent important factors capable of skewing the clustering to one or the other receptor combination. For heterodimeric cytokines, a strong transcriptional control of distinct chains may be hypothesized such that the subsequent combination of specific subunits might be a matter of timing in their synthesis and assembly. Despite such multiple pairing systems, promiscuity represents a skillful nature strategy in guaranteeing a saving program and a fine modulation of IL-12 family cytokine inputs. However, from a therapeutic perspective, this feature imposes the need of identifying an appropriate method to make ectopic cytokine subunits be expressed in the desired combination.
Recombinant heterodimeric cytokines produced as single-chain fusion proteins
In initial studies, the production of biologically active IL-12 in transfected mammalian cells was obtained by simultaneous transfection of the two different cytokine genes, p35 and p40, cloned in distinct retroviral vectors (Tahara & Lotze, Citation1995), or linked through an internal ribosome entry site (IRES) in a unique vector (Zitvogel et al., Citation1994). However, both strategies did not guarantee that p40 and p35 – produced as distinct protein subunits – would eventually assemble to form IL-12, since this process was affected by an imbalanced transfection efficiency of the two plasmids and by the typical p40 and p35 capacity in combining with other IL-12 family members. The risk of subunit mismatches was found to be extendable to IL-23, IL-27 and IL-35 as well and, in general, did not guarantee a secure and rigorous ectopic expression of the planned heterodimeric cytokine. Therefore, a series of bioengineering studies aimed at solving these problems has subsequently been performed. The new strategies were all based on the generation of fusion proteins consisting of the two subunits encoded in tandem by a single gene, in the same vector. The construction of recombinant single-chain cytokines of the IL-12 family requires the identification of (i) appropriate space linkers, in order to maintain the physical and physiological distance between the two subunits, and (ii) appropriate tags, in order to favor purification procedures and overcome solubility constraints of the fusion protein, as described below.
Importance of the linker
Since the possibility of evoking cytokine-specific effects is inextricably linked to the reliability of transfected DNA in producing the desired combination of subunits, an improvement in recombinant heterodimeric cytokine expression can be gained using single-chain gene constructs assuring equal subunit transcription and translation, in addition to the correct assembly of resulting fusion protein (Belladonna et al., Citation2002; Collison et al., Citation2007; Lieschke, Rao, Gately, & Mulligan, Citation1997; Oniki et al., Citation2006; Peng, Penichet, & Morrison, Citation1999). Importantly, direct fusion of functional domains should be avoided in these constructs, since it may lead to unwanted protein misfolding. Rather, cytokine subunits can be spaced by linker peptide sequences to build successfully a recombinant fusion protein endowed with improved folding and stability, facilitated expression, increased intrinsic biological activities, enhanced ability in targeting specific sites in vivo and suitably modified pharmacokinetic properties (Chen, Zaro, & Shen, Citation2012).
The current knowledge of linker design in the synthesis of recombinant fusion proteins is quite extensive and has been inspired by the study of naturally occurring multidomain proteins composed of two or more functional domains joined by linker peptides. In initial studies, properties of natural linkers (length, composition, hydrophobicity and secondary structure) served as a general reference for creating rational empirical linkers in recombinant fusion proteins. This approach has been widely applied to drug targeting (toxins or cytokines linked to single-chain antibodies or to cell surface receptor ligands) and for transporting protein drugs across biological barriers (i.e., upon conjugating protein drugs with carrier moieties such as cell penetrating peptides, antibodies or transferrin; Leader, Baca, & Golan, Citation2008; Manoukian & Hagemeister, Citation2009; Pardridge, Citation2010). In single-chain gene constructs of IL-12 family cytokines, empirical, flexible linkers allowing movements or interactions between fusion protein domains are preferentially used. The most common flexible linker has a sequence primarily consisting of Gly stretches and Ser residues – also known as the G4S linker or (Gly4Ser)n – whose length can be optimized by adjusting the copy number n to achieve appropriate separation of the functional domains and their proper independent folding (Belladonna et al., Citation2002; Collison et al., Citation2007; Ha, Kim, Baek, Yun, & Sung, Citation2004; P. Hu et al., Citation2009; Oniki et al., Citation2006; Yoshimoto et al., Citation2004; Yuan, Hu, Belladonna, Black, & Yu, Citation2006). The small size of these amino acids provides flexibility and allows for appropriate mobility of the connecting functional domains. Moreover, the specific incorporation of Ser stabilizes the linker in aqueous solutions by forming hydrogen bonds with water molecules, thus reducing the unfavorable interaction between the linker and protein moieties. Therefore, the G4S linker seems to offer both proper flexibility – resembling the fusion protein closely to the endogenous heterodimeric cytokine – and sufficient subunits’ spatial separation needed for the correct folding of protein domains, without reciprocal structural perturbation (Chen et al., Citation2012). Further optimization of the linker design for single-chain heterodimeric cytokines is, however, still possible and may lead to an improvement of their expression yield (especially for the low level-secreted cytokines such as IL-27 and IL-35) and their bioactivity, two crucial aspects strictly wanted when fusion proteins should be used as drugs.
Importance of the tag
The success of fusion protein production depends on their effective expression, solubility, and purification.
While techniques for protein expression and purification have improved dramatically in the past decade, the expression of soluble mammalian proteins still remains difficult, often requiring alternative expression conditions. The majority of work in the field has been focused on the discovery, development and refinement of fusion tags that guarantee solubility to the fusion protein. These improving-solubility fusion tags are proteins or peptides attached to the protein of interest that provide help for a proper folding while enhancing solubility. However, the addition of fusion tags raises the problem of the ultimate removal of these sequences, since proteins made in this way might not retain their native structure and activity; on the other hand, some tags themselves can improve the pharmacokinetic properties of the protein they are joined to (Esposito & Chatterjee, Citation2006). When tag removal is required, a protease cleavage site is often engineered between the solubility tag and the partner protein, permitting an in vitro directed proteolysis of the purified fusion protein to remove the fusion tag. Proteases commonly used to cleave fusion tags include thrombin, factor Xa, enterokinase, tobacco etch virus (TEV) protease and the small ubiquitin-like modifier (SUMO) protease. By placing an affinity tag at the N terminus of the fusion partner, one can purify the protein, cleave the tag and then re-purify on the same affinity matrix to remove the cleaved tag (Waugh, Citation2005). Alternatively, self-cleaving tags, a special group of fusion tags that are based on protein modules possessing inducible proteolytic activities, can be used. Fusion proteins containing self-cleaving tags can undergo site-specific self-cleavage upon triggering by a low-molecular weight compound or a change in conformation. Combined with appropriate affinity handles, self-cleaving tags enable fusion purification, cleavage and target protein separation achievable in a single step. However, problems are sometimes encountered; in fact, even if the protease cleavage is successful, the ‘passenger’ protein (i.e., transported by its tag-linked, hydrophilic portion) may not remain soluble once the fusion partner is removed and can revert to its naturally insoluble and precipitating form.
Today, a number of common solubility-enhancing fusion tags are available, facilitating protein expression in a soluble form and, at least for some of them, increasing the efficiency of protein purification (Box 1), but only few of them have been selected as tags for the bioengineering of single-chain fusion heterodimeric cytokines. The application of the Fc (crystallizable fragment) portion of immunoglobulin G (IgG) has become quite usual in the past few years as a tag to obtain a therapeutic cytokine aimed at modulating inflammatory and immune responses. The rationale of such protein engineering relies on the structural and pharmacokinetic features of these molecules, which, however, in their monomeric form, are relatively small and typically have short serum half-lifes, thus requiring frequent administration that limits their clinical utility. Nevertheless, Fc-containing proteins tend to form homodimers, much the same way as the constant heavy chains of natural IgGs, which instead display quite long serum half-lifes (> 20 days), owing to their binding of the neonatal Fc receptor (FcRn: see below). In fact, chimeric proteins derived from the ligation of Fc domains and recombinant cytokines do show highly improved pharmacokinetics when compared with either monomeric Fc or cytokine alone. However, to guarantee a proper rate of diffusion through the mucus, gene constructs should be engineered to produce molecules as small as possible (i.e., avoiding fusion of multiple cytokine genes to a single Fc portion; Jazayeri & Carroll, Citation2008). In some instances, attempts have been made to improve the stability of bioengineered cytokines in vivo by means of modification using polyethylene glycol (PEG; a procedure known as PEGylation), a highly hydrophilic reagent that can confer higher water solubility and serum half-life on recombinant proteins and reduce renal clearance as well as immunogenicity (Zhang, Yang, Yuan, Pu, & Liao, Citation2012).
Some tags are endowed with both affinity purification-favoring and solubility-enhancing properties.
 The simple hexahistidine (His6) tag allows the fusion partner to maintain its solubilizing function and also to behave as an affinity purification tag. The His-tag is, indeed, the most commonly used for affinity purification of proteins. By means of binding metal ions and of allowing the tagged protein to be purified by immobilized metal affinity chromatography, the His-tag presents several advantages: the small size makes it less likely to interfere with the structure or activity of the target protein; it works under both native and denaturing conditions; and elution can be accomplished under mild conditions by adding imidazole as a competitor (Gaberc-Porekar & Menart, Citation2001).
The simple hexahistidine (His6) tag allows the fusion partner to maintain its solubilizing function and also to behave as an affinity purification tag. The His-tag is, indeed, the most commonly used for affinity purification of proteins. By means of binding metal ions and of allowing the tagged protein to be purified by immobilized metal affinity chromatography, the His-tag presents several advantages: the small size makes it less likely to interfere with the structure or activity of the target protein; it works under both native and denaturing conditions; and elution can be accomplished under mild conditions by adding imidazole as a competitor (Gaberc-Porekar & Menart, Citation2001).
 Glutathione S-transferase (GST) from Schistosoma japonicum is a 26-kDa protein that has been used for single-step purification of fusion proteins by means of its affinity for immobilized glutathione and the possibility of elution under nondenaturing conditions with reduced glutathione at 10 mM (Smith & Johnson, Citation1988). In many cases, the GST-tag protects against intracellular proteolysis and stabilizes the recombinant protein in the soluble fraction as monomers or homodimers (Kaplan et al., Citation1997). However, some GST fusion proteins are wholly or partly insoluble. GST expression vectors commonly include specific protease cleavage sites between the tag and the partner protein. Thus, being the GST-tag readily removed from GST fusion proteins after or during purification, harvest of the protein of interest may result greatly simplified.
Glutathione S-transferase (GST) from Schistosoma japonicum is a 26-kDa protein that has been used for single-step purification of fusion proteins by means of its affinity for immobilized glutathione and the possibility of elution under nondenaturing conditions with reduced glutathione at 10 mM (Smith & Johnson, Citation1988). In many cases, the GST-tag protects against intracellular proteolysis and stabilizes the recombinant protein in the soluble fraction as monomers or homodimers (Kaplan et al., Citation1997). However, some GST fusion proteins are wholly or partly insoluble. GST expression vectors commonly include specific protease cleavage sites between the tag and the partner protein. Thus, being the GST-tag readily removed from GST fusion proteins after or during purification, harvest of the protein of interest may result greatly simplified.
 The maltose-binding protein (MBP), a 42-kDa protein encoded by the malE gene of Escherichia coli K12, is used for single-step purification by affinity chromatography using cross-linked amylose (a linear polymer made up of d-glucose units; di Guan, Li, Riggs, & Inouye, Citation1988). MBP fusion proteins are eluted under nondenaturing conditions with maltose at 10 mM and generally show improved expression, folding and solubility of eukaryotic fusion proteins in bacteria (Riggs, Citation2000). Vectors for generating MBP-fused proteins contain a specific protease cleavage site in the region between the MBP tag and the multiple cloning site, allowing an easy release of the untagged protein.
The maltose-binding protein (MBP), a 42-kDa protein encoded by the malE gene of Escherichia coli K12, is used for single-step purification by affinity chromatography using cross-linked amylose (a linear polymer made up of d-glucose units; di Guan, Li, Riggs, & Inouye, Citation1988). MBP fusion proteins are eluted under nondenaturing conditions with maltose at 10 mM and generally show improved expression, folding and solubility of eukaryotic fusion proteins in bacteria (Riggs, Citation2000). Vectors for generating MBP-fused proteins contain a specific protease cleavage site in the region between the MBP tag and the multiple cloning site, allowing an easy release of the untagged protein.
 The small ubiquitin-like modifier (SUMO) protein, when used as an N-terminal carrier protein during prokaryotic expression, promotes folding and structural stability, leading to enhanced functional production to the fusion protein compared with its untagged counterpart (Marblestone et al., Citation2006). Unlike GST and MBP, SUMO itself does not serve as a means of purifying fusion proteins, which can, however, be obtained placing the His6 in series with the SUMO tag. Moreover, a specific SUMO protease (Saccharomyces cerevisiae UlpI) recognizes SUMO’s tertiary structure at a specific Gly-Gly motif, with a low probability of cleaving within the protein of interest. The wild-type SUMO tag is an excellent carrier protein in prokaryotic expression systems but fails to work in eukaryotes, since it can be efficiently removed in vivo by naturally occurring SUMO proteases.
The small ubiquitin-like modifier (SUMO) protein, when used as an N-terminal carrier protein during prokaryotic expression, promotes folding and structural stability, leading to enhanced functional production to the fusion protein compared with its untagged counterpart (Marblestone et al., Citation2006). Unlike GST and MBP, SUMO itself does not serve as a means of purifying fusion proteins, which can, however, be obtained placing the His6 in series with the SUMO tag. Moreover, a specific SUMO protease (Saccharomyces cerevisiae UlpI) recognizes SUMO’s tertiary structure at a specific Gly-Gly motif, with a low probability of cleaving within the protein of interest. The wild-type SUMO tag is an excellent carrier protein in prokaryotic expression systems but fails to work in eukaryotes, since it can be efficiently removed in vivo by naturally occurring SUMO proteases.
 The FLAG epitope is a short, hydrophilic octapeptide (DYKDDDDK) that can be used for antibody-based purification. A unique aspect of FLAG use is the inherent enterokinase cleavage site located within the five C-terminal residues of the peptide sequence. FLAG-tagged fusion proteins can be purified using an immobilized monoclonal antibody matrix under nondenaturing conditions and eluted by lowering the pH or adding chelating agents such as ethylenediaminetetraacetic acid (EDTA) (Einhauer, Schuster, Wasserbauer, & Jungbauer, Citation2002).
The FLAG epitope is a short, hydrophilic octapeptide (DYKDDDDK) that can be used for antibody-based purification. A unique aspect of FLAG use is the inherent enterokinase cleavage site located within the five C-terminal residues of the peptide sequence. FLAG-tagged fusion proteins can be purified using an immobilized monoclonal antibody matrix under nondenaturing conditions and eluted by lowering the pH or adding chelating agents such as ethylenediaminetetraacetic acid (EDTA) (Einhauer, Schuster, Wasserbauer, & Jungbauer, Citation2002).
 The Fc portion of IgGs specifically binds protein A, a bacterial protein containing an IgG-binding domain that allowed the development of the first gene-fusion approach for affinity purification of recombinant proteins (Uhlen et al., Citation1983). The Z domain of protein A is a 7-kDa synthetic IgG-binding fragment that has been synthesized on the B domain sequence of the protein A, which, in single or double form, has replaced the full-length protein as an affinity handle in most cases (Nilsson et al., Citation1987). A disadvantage of the protein A-based system and bioengineered derivatives thereof is that elution of the fusion protein requires denaturing conditions such as low pH.
The Fc portion of IgGs specifically binds protein A, a bacterial protein containing an IgG-binding domain that allowed the development of the first gene-fusion approach for affinity purification of recombinant proteins (Uhlen et al., Citation1983). The Z domain of protein A is a 7-kDa synthetic IgG-binding fragment that has been synthesized on the B domain sequence of the protein A, which, in single or double form, has replaced the full-length protein as an affinity handle in most cases (Nilsson et al., Citation1987). A disadvantage of the protein A-based system and bioengineered derivatives thereof is that elution of the fusion protein requires denaturing conditions such as low pH.
Many single-chain constructs for heterodimeric cytokines include an Fc tag at the C-terminal (Belladonna et al., Citation2002; J. Hu et al., Citation2006; Yuan et al., Citation2006), allowing an optimal means of purifying the fusion protein by protein A affinity columns (Box 1) which adds to a significant increase in half-life when administered in vivo. In fact, it has been demonstrated that therapeutic proteins conjugated to the Fc domain of IgG can be bound by the FcRn expressed on cell membranes of several tissues and, through its trafficking pathway for IgG bidirectional transcytosis, can be protected from catabolism (Kuo & Aveson, Citation2011). However, the Fc of IgGs can also bind and activate proinflammatory Fcγ receptors (CD16 and CD32), complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity, all of which can promote inflammatory mechanisms exerting adjuvant effects in antitumor immunotherapy. When instead an immunosuppressive effect is designed, Fc-dependent proinflammatory effects should be avoided. As a matter of fact, the Fc portion of Abatacept®, a well-known immunosuppressor used in rheumatoid arthritis, has been genetically modified in order to reduce the proinflammatory effects linked to the Fc portion (Fiocco et al., Citation2008). On the other hand, an entire anti-Her2/neu IgG3 has been attached to single-chain IL-12 to obtain an IL-12–IgG fusion protein capable of combining the therapeutic potential of IL-12 with the specific tumor-targeting ability of the antibody, thus increasing drug selectivity and reducing secondary effects (Peng et al., Citation1999).
Tags particularly suitable for protein purification have also been experimented in heterodimeric cytokine constructs. As a representative example, a His-tag has been used for the production of IL-35 as a single polypeptide, i.e., containing mouse IL-12p35 subunit and mouse EBI3 fused via a bovine elastin linker (VPGVGVPGVG; Kochetkova, Golden, Holderness, Callis, & Pascual, Citation2010); moreover, a 3 × FLAG has been attached at the NH2 terminus for the preparation of single-chain IL-12, IL-23 and IL-27, all made by joining with the (G4S)3 linker (Oniki et al., Citation2006).
Production of single-chain, heterodimeric cytokines
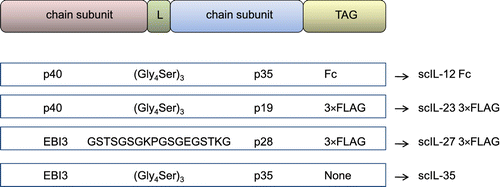
Natural occurring subunits of heterodimeric cytokines are encoded by distinct genes, but, when they must be overexpressed in a cell system, the stoichiometric production of both subunits can be preferentially obtained by means of a single gene construct generally comprising, from the start to the stop codon, the first cytokine subunit sequence (comprehensive of its signal peptide), the linker (joining and spacing the two portions of the fusion protein), the mature protein sequence of the second cytokine subunit (i.e., without its signal peptide), and, in most cases, a proper tag for improved stability and/or easier purification of the translated fusion protein (Figure ). Expression of the fusion construct can be achieved by transfection of mammalian or yeast cells in vitro followed by a purification step, through ectopic expression of the desired protein by cell transfectants in vivo, or by direct gene transfer in vivo.
Purification of the fusion protein from supernatants of transfected cells
Many different cell lines and transfection protocols are available for the expression of recombinant proteins and their subsequent purification from in vitro cell culture supernatants. Among these, several combinations of DNA construct/transfection technique/recipient cell can be found in the literature related to single-chain cytokines. Eukaryotic cell lines such as human embryonic kidney 293 (HEK293) and Chinese hamster ovary (CHO) are commonly used for the expression of recombinant proteins because of the great compatibility with many transfection reagents and techniques, in addition to the high process yield. For these characteristics, the transfection of single-chain constructs has soon found the proper approach using these cells.
Figure 2 General architecture of constructs encoding for engineered single-chain cytokines of the IL-12 family.Schematic diagrams of the DNA constructs for single-chain IL-12, IL-23, IL-27, and IL-35 fusion proteins are reported, showing that the β chain (p40 and EBI3 subunits) and the α chain (p35, p19 and p28 subunits) can be sequentially and genetically fused with a DNA linker (L). Resulting constructs can be further fused with an appropriate tag sequence (mainly consisting of Fc of IgG and 3×FLAG).
Yoshimoto et al. (Citation2004) produced a recombinant single-chain IL-27 by fusion of EBI3 and the p28 mature protein, spaced by the (G4S)3 linker, containing the preprotrypsin signal peptide in addition to a 3×FLAG-epitope-tag sequence at N-terminal. The 3×FLAG-tagged single-chain IL-27 was obtained from culture supernatants of HEK293 cells transiently transfected using 293fectin (a cationic lipid-based formulation) and was purified by affinity chromatography using an anti-FLAG affinity gel (M2). Both mouse and human IL-35 (EBI3 and IL-12p35 connected by the (G4S)3 linker and expressed as a single-chain protein) were also obtained from the culture supernatant of HEK293 cells, transfected by TransIT transfection reagent, which can be used without any purification step to avoid easy degradation of this unstable cytokine (Collison et al., Citation2007, Citation2010, Citation2012). Human single-chain IL-35-Fc was also built by linking EBI3 and p35 along with human IgG1 Fc; the EBI3-p35-Fc expression construct was then transfected into CHO cells by lipofectamine and the recombinant protein was purified via a protein A-Agarose column (Niedbala et al., Citation2007). A variant of CHO cells (CHO-K1) was also used for optimizing the transfection of a single-chain IL-27. Specifically, CHO-K1 cells allow the use of the glutamine synthetase gene expression system, which facilitates the high-level gene expression of proteins. The glutamine synthetase gene is used as a selectable marker when transfected cells are cultured in glutamine-free medium added with methionine sulfoximine, a glutamine synthetase inhibitor, at a concentration sufficient to block the CHO-K1 endogenous enzyme to obtain the needed selection pressure. Here, the IL-27 construct (consisting of EBI3 fused with a (G4S)3 linker followed by the coding sequence of the p28 mature chain) was cloned in the glutamine synthetase expression vector pEE12.4 carrying the glutamine synthetase gene for the catalysis of glutamine into glutamate and ammonia. The IL-27-containing vector was then transfected into CHO-K1 cells, whose culture supernatants were purified by consecutive column purification steps (UNOSphere S column, ceramic hydroxyapatite column type I, and heparin sepharose FF column), thus allowing fast and reliable separations of biomolecule (Sasaoka et al., Citation2011).
A different combination of cells and transfection techniques was instead adopted for IL-12-IgG3 and IL-23-IgG3 fusion proteins, both containing the (G4S)3 linker and the Fc tag from IgG3. P1.HTR mastocytoma cells were stably transfected by the calcium phosphate method and fusion proteins were affinity-purified from collected supernatants by means of a protein A–sepharose column (Belladonna et al., Citation2002). As mentioned above, IL-12 has been also expressed as an antibody fusion protein in which the mouse single-chain cytokine (p40-(G4S)3 linker-p35) was fused to an anti-Her2/neu antibody at the amino terminus of its H chain (mscIL-12.her2.IgG3). This fusion protein, retaining antibody specificity against Her2/neu cell-surface oncogene and exhibiting IL-12 bioactivity, was obtained after transfection of P3X63Ag8.653 myeloma cells by electroporation and purification of mscIL-12.her2.IgG3 from culture supernatants through a protein A column (Peng et al., Citation1999).
Also yeast cells are suitable for recombinant protein expression. For example, IL-35 was produced as a single polypeptide fusing mouse IL-12p35 subunit and mouse EBI3 via a bovine elastin linker (VPGVGVPGVG). The construct was cloned in the Pichia pastoris expression vector pPicZB containing a His-tag sequence for protein purification. Pichia pastoris cells were transformed via electroporation, and the fusion protein encoded by the gene construct was purified from yeast cell lysates by a Talon affinity resin column which binds the His-tag (Kochetkova et al., Citation2010).
In vivo transfer of in vitro transfected cells
IL-27, IL-23, and IL-12, sometimes in studies of mutual comparison, have been successfully tested in many different tumor experimental models, in which cytokine immunotherapy was performed by transferring properly transfected cells into the animal for the subsequent in vivo production of single-chain fusion proteins. Thus, B16F10 melanoma cells have been transfected by lipofectamine with the DNA constructs for single-chain (sc)IL-12, scIL-23, or scIL-27 (all linked using the (G4S)3 tag), cloned into a vector containing a preprotrypsin signal peptide and the 3×FLAG-epitope tag sequence at the NH2 terminus. The transfectants were injected subcutaneously (s.c.) in the flank of syngeneic mice to observe the timing of cytokine-transfected tumor growth and, as a vaccine, to assess the development of protective immunity against parental B16F10 tumors (Oniki et al., Citation2006). Later on, MM45T.Li hepatocellular carcinoma cells were engineered with the same constructs to investigate whether antitumor responses induced by IL-23 and IL-27 would occur at an early or a late stage of tumor cell growth and whether these cytokines could induce a long-term systemic immunologic memory response against parental MM45T.Li tumor cells (P. Hu et al., Citation2009). Efficacy of IL-23 was also investigated using MBT2 cells (a mouse bladder tumor) transduced with an scIL-23-encoding vector and injected s.c. into the mouse right-flank, in terms of tumor rejection and inhibition of parental tumor growth in vaccinated mice (Kuramoto et al., Citation2011).
As an alternative to lipofectamine, the Fugene 6 transfection reagent has been used to overexpress scIL-12 or scIL-27, both containing the (G4S)3 linker and the 3×FLAG tag, in colon carcinoma and melanoma cells. In the first tumor model, mice inoculated s.c. with C26/scIL-27 transfectant cells were used to study the antitumor effects of IL-27 in vivo and its tumor-specific protective immunity to a subsequent challenge with parental C26 tumor cells (Hisada et al., Citation2004). In the second model, s.c. or intravenous (i.v.) transfer of scIL-27-transfected B16F10 cells were used to enlighten the antiangiogenic activities of IL-27 and its antitumor potential against experimental pulmonary metastasis (Shimizu et al., Citation2006). The suppression of tumorigenesis by IL-27 has been also investigated using different single-chain gene constructs, built by inserting a synthetic linker between EBI3 and p28 sequences, either with or without a tag. A DNA construct containing the GSTSGSGKPGSGEGSTKG linker and no tag was used to establish a scIL-27-producing murine Lewis lung carcinoma cell line (LLC-1) to be inoculated in vivo for tumor progression studies (Ho et al., Citation2009). An scIL-27 gene sequence linked by GSGSGGSGGSGSGKL and tagged with 3×FLAG was used for the engineering of TBJ neuroblastoma tumor cells to be implanted either s.c. or orthotopically in the adrenal gland (Salcedo et al., Citation2004).
Besides transfection of tumor cell lines, other cell types have been engineered to overexpress single-chain cytokines to be transferred in vivo with the aim of generating local dispensers of antitumor effects. For instance, bone-marrow-derived DCs were transduced with adenoviral vectors bearing genes for scIL-23 or scIL-12, both containing a (G4S)3 linker and an Fc-IgG3 tag, and intratumorally injected in mice previously implanted intracranially with GL26 glioma cells (J. Hu et al., Citation2006). Moreover, to extend the immunotherapeutic potential application of the antiglioma function induced by IL-23, bone-marrow-derived neural stem-like cells (BM-NSC), transduced with the same viral vector bearing scIL-23, were also used. Remarkably, BM-NSC have been shown to be endowed with the peculiar ability of tracking migratory glioma cells and can thus be exploited as in situ carriers of IL-23 (Yuan et al., Citation2006).
In vivo administration of DNA constructs
Immunization involving priming with plasmid DNA and boosting with recombinant virus represents a successful protocol of DNA immunization for induction of cytotoxic T lymphocytes (CTLs), particularly useful in immunostimulating approaches. By this strategy, the DNA used for the priming and encoding the same antigen of recombinant virus can elicit low-level but persistent immunity that allows subsequent strong boost with virus to be able to provide an efficient CTL induction. Adjuvant effects of IL-23, IL-27 and IL-12 for epitope-specific CTL induction has been studied by means of (G4S)3-linked and 3×FLAG-tagged single-chain cytokine constructs in prime-boost immunization experiments. Here, mice transgenic for human histocompatibility leukocyte antigen HLA-A0201 were primed and boosted with a mixture of DNA including recombinant adenoviruses expressing both structural and nonstructural proteins of hepatitis C virus (HCV) and additional plasmids for expression of scIL-12, scIL-23, or scIL-27, before an additional boosting with HCV. This vaccination protocol has revealed that both IL-23 and IL-27, as well as IL-12, can exert an adjuvant effect in epitope-specific CTL induction and that they are potential prophylactic and therapeutic agents against HCV (Matsui et al., Citation2004). Another study on DNA vaccination against HCV showed the suitability of an scIL-23-encoding vector as efficient genetic adjuvant in evoking durable memory T-cell immunity, since co-immunization experiments with plasmids expressing both (G4S)3-linked IL-23 and HCV envelope protein 2 could enhance and maintain E2-specific TH1 and CTL immune responses (Ha et al., Citation2004).
Bioengineered heterodimeric cytokines as therapeutic proteins
IL-12 and IL-23 are cytokines with a main proinflammatory and prostimulatory functional profile and play key roles in the development and/or expansion of TH1 and TH17 subsets, respectively (Vignali & Kuchroo, Citation2012). IL-12 is the major signal required for the development of TH1 cells, which are particularly important in the control/eradication of tumors as well as of intracellular microorganisms. In response to IL-12, TH1 cells – but also NK cells – produce very high levels of IFN-γ, which, in turn, represents the major stimulus for the activation of CTLs and macrophages, both required for the elimination of tumor cells, viral infected cells, intracellular bacteria and some fungi (Trinchieri, Citation2003). The later identification of the second member of the family, IL-23, led to the discovery of TH17 cells that mainly produce IL-17A, a highly proinflammatory cytokine implicated in the pathogenesis of autoimmune diseases and chronic inflammation (Boniface, Blom, Liu, & de Waal Malefyt, Citation2008). It is now clear that IL-23 is not absolutely required for the TH17 development, but rather favors their expansion and terminal differentiation. Compared with TH1 cells, the TH17 subset is more plastic, meaning that in the presence of appropriate signals such as TGF-β alone or combined with IL-6, TH17 can convert into Treg cells and vice versa, respectively (Weaver & Hatton, Citation2009). Thus these observations indicate that therapeutic interventions acting on the TH17/Treg balance may also be effective on a late stage of the disease course – as in fact it has been shown to be the case for IL-23 in tumor immunotherapy (P. Hu et al., Citation2009) – whereas IL-12 efficacy should be mostly pronounced at early disease stages.
Previously thought to be a proinflammatory cytokine, IL-27 is now considered more immunoregulatory than immunostimulatory, thus functionally more similar to IL-35 than IL-12 or IL-23 (Vignali & Kuchroo, Citation2012; Yoshida & Miyazaki, Citation2008). However, there is evidence that IL-27 can be beneficial also in cancer, in which it acts via effects on immune cells but also on nonimmune tissues such as endothelium (Nagai et al., Citation2010). Nevertheless, in a more pronounced fashion than do other IL-12 family members, the immunostimulatory versus immunoregulatory functions of IL-27 critically depend on the presence of other cytokines, namely IL-12/IL-2 or IL-10, respectively (Vignali & Kuchroo, Citation2012).
IL-35 is the ‘newborn’ heterodimeric cytokine, which clearly shows potent immunoregulatory effects (Vignali & Kuchroo, Citation2012). In fact, IL-35 seems to be specifically produced by Treg cells and is required for their maximal suppressive activity (Collison et al., Citation2007). In addition, it induces the development of the iTr35 subset, producing IL-35, which does not express Foxp3 nor produces IL-10 or TGF-β (Chaturvedi, Collison, Guy, Workman, & Vignali, Citation2011; Collison et al., Citation2010). Its being produced by T cells represents an additional unique feature of this cytokine, since the main source of IL-12, IL-23 and IL-27 is instead represented by APCs, such as DCs and macrophages. Because EBI3 is a subunit shared by both IL-35 and IL-27, it might be hypothesized that the immunosuppressive functions of IL-27 may also depend on EBI3.
Additional layers of complexity in the IL-12 family can be ascribed to the nonstoichiometric production of the subunits composing a specific heterodimer, which can lead to the release of nonassociated, monomeric (such as p40, p28 and EBI3) or of associated but homodimeric compounds (such as p402). These molecules can either promote (agonist function) or inhibit (antagonist) the receptor activation, depending again on the circumstances (Vignali & Kuchroo, Citation2012). Moreover, the fact that the IL-35 signaling implies subunits not belonging to the ‘classical’ IL-12 receptor family but rather to the IL-6 receptor family – such as gp130 – would suggest that the signaling networks of IL-12 family cytokines goes far beyond the IL-12/IL-23/IL-27/IL-35 world.
For all of these reasons, the use of engineered, single-chain cytokines belonging to the IL-12 family seem to be particularly appropriate for therapeutic purposes.
The ‘pros and cons’ of IL-12 in tumor immunotherapy
Soon after its discovery, IL-12 was recognized to possess a great potential for use in tumor immunotherapy. IL-12, in fact, by activating both T (Stern et al., Citation1990) and NK (Kobayashi et al., Citation1989) cells and stimulating naive CD4+ T cells to differentiate toward the TH1 phenotype (Trinchieri, Citation1994), has potent antitumor and antimetastatic effects. The TH1 response involves the secretion of a cytokine profile dominated by IFN-γ, which activates CTLs and macrophages, both effects being highly desirable in tumor immunity. In addition, IL-12 induces the production of nitric oxide (NO) and inducible protein-10, which can lead to the cell-cycle arrest of tumor cells and antiangiogenic effects, respectively (Del Vecchio et al., Citation2007). To be effective, however, high concentrations of the cytokine should reach the tumor site (Colombo et al., Citation1996). Disappointingly, a short-term (7 days) i.v. administration of rIL-12 in early clinical trials resulted in severe toxicity, including hemorrhagic colitis, sepsis and death, without perceptible therapeutic effects (Car, Eng, Lipman, & Anderson, Citation1999).
In order to reduce the systemic toxic effects of rIL-12, Peng et al. (Citation1999) generated a fusion protein composed of a single-chain mouse IL-12 and an IgG3 humanized monoclonal antibody specific for Her/neu, a cell-surface oncogene product that is overexpressed in a significant percentage of human ovarian cancer with poor prognosis. The novel fusion protein, termed mscIL-12.her2.IgG3, exhibited IL-12 bioactivity in vitro comparable to mouse rIL-12. In vivo, a short-term i.v. administration of the molecule reduced the growth of CT26/Her2 mouse tumor cells with no apparent signs of toxicity. Although this approach has not reached the clinic yet, these data confirm the power of bioengineering in designing ‘artificial’ therapeutic proteins that may successfully overcome the poor pharmacokinetics and possible high toxicity of a potent cytokine such as IL-12.
Another approach has consisted of the intratumoral expression of IL-12, by means of transfection with a combination of IL-12p35- and IL-12p40-encoding plasmids or retroviral transduction ex vivo, which has proven to induce tumor regression in experimental in vivo models. However, results were often compromised by the formation of inhibitory p40 homodimers (see below). Although over the years the co-transfection of the two genes encoding IL-12 subunits has been substituted by the use of constructs containing the gene coding for monomerized IL-12 (Lieschke et al., Citation1997), no significant therapeutic benefit has been shown in clinical trials yet. Lack of efficacy may depend on an insufficient expression of the cytokine in tumors, and thus efficient vectors such as those of the adenoviral type may overcome this problem (Wulff et al., Citation2007). As an alternative to tumor transduction, tumor infiltrating CD8+ T cells genetically modified to overproduce supraphysiologic amounts of single-chain IL-12 have been recently shown to be highly effective in tumor-bearing, lymphodepleted mice (Kerkar et al., Citation2010).
Vaccines composed of either tumor peptide and IL-12 or tumor peptide-pulsed DCs (the most efficient cells in antigen presentation to naive T cells) engineered to overproduce IL-12 have also been tested in clinical trials (Del Vecchio et al., Citation2007). Infiltration of tumor-specific CD8+ lymphocytes was often detected, but a partial response was observed in only one patient (Mazzolini et al., Citation2005). Interesting in this regard are our previous observations, in that pretreatment of DCs with either rIL-12 or p35-p40-Ig (instead of cell transfection with IL-12 gene constructs) and prior pulsing with a tumor-specific peptide and i.v. administration to recipient mice, induce a strong tumor antigen-specific immune response, mediated by both CD4+ and CD8+ T lymphocytes (Belladonna et al., Citation2002; Grohmann et al., Citation1999).
Overall, the immunostimulatory potential of IL-12, the first member of the family of heterodimeric cytokines identified so far, still appears difficult for use in clinical settings (Del Vecchio et al., Citation2007). Nevertheless, the huge number of studies on the possible therapeutic use of this cytokine and on its biotechnological modifications has certainly paved the way for more fruitful and straightforward investigations on other members of the same family.
Therapeutic perspectives of IL-23: Not just an IL-12 ‘duplicate’
The discovery of IL-23 as a novel pro-inflammatory and immunostimulatory member of the IL-12 family in 2000 (Oppmann et al., Citation2000) prompted several groups to replace IL-12 in therapeutic studies with IL-23 or to try combinations with the two cytokines. In fact, it has been proposed that IL-12 and IL-23 promote distinct immunological pathways that have separate but complementary proinflammatory functions, thus providing a synergism that might be effective in the control of a multifaceted disease such as cancer (Langrish et al., Citation2004; Weiss, Subleski, Wigginton, & Wiltrout, Citation2007).
The main effect of IL-23 relies on its ability to promote expansion and maintenance of TH17 cells, which secrete the proinflammatory cytokine IL-17A and are implicated in the pathogenesis of many chronic inflammatory and autoimmune disorders (Boniface et al., Citation2008). IL-23, in the form of mouse p40-(G4S)3-p19-Fc (i.e., scIL-23–Fc), can also act in vitro on mouse DCs to promote immunogenic presentation by these cells in vivo of an otherwise tolerogenic tumor peptide (Belladonna et al., Citation2002) via stimulation of autocrine production of the potent proinflammatory cytokine IL-6 (Puccetti, Belladonna, & Grohmann, Citation2002).
Possibly because of the previous experience with IL-12, only few studies have been done so far using mouse IL-23 administered systemically in vivo in the form of recombinant cytokine (rIL-23), i.e., in the two subunits/native form. In particular, using experimental models of fungal infections and IL-12p40–/– or IL-12p35–/– mice (to exclude possible interference by endogenous IL-12), it was shown that rIL-23 is less effective than rIL-12 in protecting against acute fungal infection and can rather complement the more dominant therapeutic role of IL-12 in chronic fungal infections (Ehigiator, McNair, & Mead, Citation2007; Kleinschek et al., Citation2006). On the other hand, repeated subcutaneous injections of rIL-23 were found to cause skin inflammation in mice (Rizzo et al.). In addition to immunostimulatory effects, a direct antiproliferative activity in human leukemic cells in vitro has been observed for human rIL-23 (Cocco et al., Citation2010).
In contrast to the small number of studies based on the use of rIL-23, several successful results have been generated upon transfection of constructs encoding mouse single-chain IL-23 (scIL-23), particularly in tumor models. For example, the immunostimulatory power of scIL-23 could be clearly established when the cytokine was overproduced by engineered tumor cells, including poorly immunogenic B16F10 melanoma (Oniki et al., Citation2006), mouse hepatocellular carcinoma (P. Hu et al., Citation2009), and mouse bladder carcinoma (Kuramoto et al., Citation2011). In particular, the study on the hepatocellular carcinoma suggested that scIL-23-transduced tumor cells are highly effective as antitumor vaccines, since mice develop a long-term systemic immunologic memory response against parental tumor cells. Moreover, the therapeutic effects of scIL-23-transduced cells are more evident at late stages of neoplasia as compared to tumor cells transduced with either scIL-12- or scIL27-encoding (see below) constructs.
scIL-23 constructs were also transduced into bone marrow-derived DCs, which showed potent immunostimulatory effects towards experimental intracranial tumors (J. Hu et al., Citation2006). In this model, DC transduction with an scIL-12 encoding gene was less therapeutically effective than scIL-23. Interestingly, BM-NCS, which have the ability to track intracranial tumors, can induce immune resistance to cranial tumors when transduced with scIL-12 (Yuan et al., Citation2006).
Direct administration of plasmids containing the scIL-23 gene in combination with cDNA expressing viral antigens was also attempted in a mouse infection model with HCV and compared with plasmids containing either the scIL-12 or scIL-27 gene (Matsui et al., Citation2004). The results showed that the gene transfer with scIL-12, scIL-23 or scIL-27 expressing plasmids was equally effective in inducing virus-specific CTLs in vivo. However, in another study, the scIL-23-containing plasmid was superior to the scIL-12 counterpart as an adjuvant of anti-HCV vaccines. Moreover, modification of the scIL-23 encoding gene into an scIL-23 mutant lacking the N-glycosylation site in the p40 domain further increased the adjuvant potential of the plasmid because of a stronger TH1-mediated response, indicating that the glycan portion of p40 may restrain the immunostimulatory activity of IL-23 (Ha et al., Citation2004).
Thus, therapies with cells transduced with scIL-23 or with scIL-23-containing plasmids seem to induce significant protective immune responses much like scIL-12 in several experimental models of tumor and infection. Particularly interesting is the observation of the long-lasting effects that would be highly desirable for a complete eradication of tumors or chronic infection such as those sustained by HCV. Since IL-23 induces lower levels of IFN-γ compared with IL-12, IL-23 may represent an alternative and safer therapeutic agent in cancer and chronic infections. In fact, it seems that the severe side effects caused by IL-12 administration are mainly caused by the extremely high levels of IFN-γ it induces (Cohen, Citation1995). On the other hand, the role played by IL-23 in the induction/progression of chronic inflammatory diseases raises obvious concerns for possible side effects not shared by IL-12.
Thus, although the available data would indicate the IL-12 family member IL-23 as a very promising drug, additional studies should be done to optimize the therapeutic profile and pharmacologic use of bioengineered IL-23.
IL-27: The most functionally flexible member
Among the IL-12 family members, IL-27 may represent the most functionally flexible cytokine, since it is endowed with either immunostimulatory or immunoregulatory properties, depending on the circumstances. This feature can be ascribed to its capacity to exert differential effects on distinct TH subsets, namely inhibition of the development of proinflammatory TH17 cells (Diveu et al., Citation2009) and promotion of early proinflammatory TH1-mediated responses (Owaki et al., Citation2005). Most of all, however, the IL-27 contribution to the induction of Treg cells is particularly intriguing. The ability of IL-27 to induce anti-inflammatory IL-10 seems to be implicated in its immunoregulatory effects (Awasthi et al., Citation2007). Interestingly, IL-27 can be protective in cancer, and thus immunostimulatory, but also in autoimmune diseases, in which the cytokine clearly mediates immunoregulatory effects. IL-27 immunostimulatory properties appear to require the co-presence of IL-12 and/or IL-2 (Trinchieri, Pflanz, & Kastelein, Citation2003), whereas immune regulation relies on the presence of IL-10 (Awasthi et al., Citation2007). IL-27 does not share any subunit with either IL-12 or IL-23, but it does share the EBI3 subunit with the potent immunoregulatory cytokine IL-35.
Previous studies showed that IL-27-transduced colon carcinoma (Hisada et al., Citation2004), as well as neuroblastoma (Salcedo et al., Citation2004), can increase CD8+ T cell-dependent IFN-γ production, cytotoxicity and tumor clearance. In addition, IL-27 has antiangiogenic and antiproliferative activities that inhibit tumor growth and metastasis in murine melanoma (Shimizu et al., Citation2006; Yoshimoto et al., Citation2008). In murine Lewis lung carcinoma, IL-27 suppresses expression of the enzyme cyclooxygenase 2 and thus the production of prostaglandin E2, known to be associated with a poor prognosis in cancer, when transduced in the form of single-chain gene into tumor cells administered in vivo or used in vitro in the form of purified single-chain cytokine (Ho et al., Citation2009). Overall, IL-27 seems to induce immune and nonimmune effects, both of which are involved in the antitumor effects of the cytokine. Particularly interesting is the observation that, at least in the model of B16F10 mouse melanoma, a poorly immunogenic tumor, the therapeutic antitumor and antimetastatic effects of IL-27 are detectable also in mice lacking the gene encoding IFN-γ or in immunodeficient nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice (Shimizu et al., Citation2006). These data therefore indicate that nonimmune effects, such as those on endothelial cells, may be particularly relevant in the antitumor efficacy of IL-27 compared with IL-12. Moreover, because IL-27 can activate STAT1, it has been speculated that IL-27 itself may mimic the function of IFN-γ (Shimizu et al., Citation2006). On the other hand, the dynamics of appearance of IL-27 therapeutic effects appear to be very rapid, and thus more similar to IL-12 than IL-23, at least in experimental models of hepatocellular carcinoma and B16F10 melanoma cells, both transduced with the gene expressing the monomerized form of the cytokine (P. Hu et al., Citation2009; Oniki et al., Citation2006).
The other side of the coin, i.e., the immunoregulatory potential of IL-27 (Hunter, Citation2005), has instead been evaluated in experimental models of autoimmune diseases. The rationale of these studies was based on the capacity of IL-27 to induce Treg cells and inhibit the development of the proinflammatory TH17 subset. A continuous delivery of rIL-27 (in the native form) by an osmotic pump implanted s.c. significantly suppressed experimental autoimmune encephalomyelitis (EAE), a model for human multiple sclerosis, via inhibition of encephalitogenic TH17 responses in vivo (Fitzgerald, Ciric et al., 2007). In two experimental mouse models of colitis, treatment with a subcutaneous osmotic pump containing human single-chain IL-27 (hscIL-27; produced from CHO cells using the glutamine synthetase (GS) system) significantly improved the colon length, extent of necrosis, ulceration and several pathological scores in a dose-dependent manner (Sasaoka et al., Citation2011). The therapeutic effect was evident even after active colitis was established and was associated with a suppression of TH17 responses, thus suggesting that hscIL-27 may be effective in the management of Crohn’s disease and ulcerative colitis.
The possibility of an ‘IL-27 therapy’ opens undoubtedly a manifold perspective in the treatment of several diseases, from cancer to autoimmunity and chronic inflammation. Interestingly, no significant toxicity has been reported so far with either rIL-27 or scIL-27. Besides positive results in preclinical models, the current challenge would be to define clearly the co-signals and stimuli that tip the balance of IL-27 effects in favor of either immune stimulation or suppression. In addition, it would be important to evaluate the specific role of each IL-27 subunit (p28 and EBI3), either singly or homodimerized by bioengineering, in order to understand whether the opposite functions of this cytokine may be attributed to specific subunits.
IL-35: The authentic immune regulator of the IL-12 family
In 2007, the IL-12p35/EBI3 heterodimer or IL-35 was identified as a novel anti-inflammatory and immunosuppressive cytokine (Collison et al., Citation2007). IL-35 is unusual in that it can also signal via two peculiar receptor compositions: IL-12Rβ2-IL-12Rβ2 homodimers and gp130-gp130 (Collison et al., Citation2012; Vignali & Kuchroo, Citation2012). The capacity of activating gp130-containing receptors may uncover an important cross-talk between IL-12 family members, such as IL-35, with IL-6 family cytokines; and in fact IL-35 has been indicated as the first inter-familiar IL-6/IL-12 family member (Garbers et al., Citation2012). These findings may catalyze new exploratory platforms at the interface between apparently distinct cytokine families.
EBI3 and p35 subunits from human and mouse pair effectively with each other, indicating there is no species barrier to IL-35 dimerization and suggesting a conserved dimerization interface (Jones et al., Citation2012). However, whilst the role of IL-35 in human immune regulation still remains unclear, the functional effect of this cytokine in mouse models has clearly been observed. In the human system, a very recent report indicates that IL-35 is not constitutively expressed in tissues, but it is inducible in response to inflammatory stimuli (Li et al., Citation2012). In the mouse, IL-35 is likewise not constitutively expressed in tissues, is produced mainly by Treg cells (Banchereau, Pascual, & O’Garra, Citation2012) and can induce the transformation of CD4+ effector T cells into Treg cells (iTr35 cells) that in turn express IL-35 but lack expression of Foxp3, TGF-β and IL-10, classical markers of Treg cells (Collison et al., Citation2007, Citation2010).
Interestingly, a new Treg cell subset has been described based on its expression of CD39, an extracellular ectonucleoside triphosphate diphosphohydrolase-1 enzyme, capable of controlling activated lymphocytes through conversion of extracellular ATP (Borsellino et al., Citation2007). When administered as a single-chain fusion protein to mice with collagen-induced arthritis (CIA), IL-35 was recently found to increase the frequency of a CD4+CD39+ T-cell population that exerts immunosuppressive, therapeutic effects via production of IL-10 (Kochetkova et al., Citation2010). In addition, the in vivo effects of single-chain IL-35 (scIL-35) were also associated with an inhibition of both TH1 and TH17 subsets. However, in a previous study, the in vivo administration of an Fc fusion protein of scIL-35 (IL-35-Fc) in a CIA model did promote the expansion of suppressive CD4+CD25+ T cells and inhibited the differentiation of TH17 cells, but the production of the TH1 cytokine IFN-γ seemed to be enhanced (Niedbala et al., Citation2007). Although IL-35−Fc suppressed the IFN-γ secretion by activated CD4+CD25− T cells in vitro, the in vivo differences on TH1 effects by scIL-35 and IL-35-Fc may be ascribed by the presence of Fc in one construct or also by a different glycosylation of the therapeutic proteins, since scIL-35 was expressed in yeasts (Kochetkova et al., Citation2010), whereas the IL-35-Fc construct was produced using mammalian CHO cells (Niedbala et al., Citation2007).
In a very recent study, expression of IL-35 was targeted to pancreatic β-cells in nonobese diabetic (NOD; the prototypic model of autoimmune diabetes) mice via the rat insulin promoter II (Bettini, Castellaw, Lennon, Burton, & Vignali, Citation2012). Particularly innovative in this study was the use of a construct containing the genes coding for two chains of IL-35 protein (EBI3 and p35) connected by a self-cleaving P2A peptide that allowed for the stoichiometric expression of the two separate subunits. Ectopic expression of IL-35 by pancreatic β-cells led to substantial, long-term protection against autoimmune diabetes, despite limited intra-islet IL-35 secretion. Local islet expression of IL-35 was sufficient in controlling both CD4+ and CD8+ T-cell responses and did not seem to have any systemic effects on T cells or developmental abnormalities of the islets. Nevertheless, constant exposure to IL-35 was necessary for continued suppression of the islet infiltrating, antigen-specific T cells and protection from diabetes. The authors hypothesized that IL-35 could also act on APCs, such as DCs, suppressing their activation, maturation and/or trafficking from the islets to the pancreatic lymph nodes, where in turn they altered T-cell activation and expansion.
Given the immunoregulatory potential in both chronic inflammatory diseases such as CIA and autoimmune pathologies such as type 1 diabetes and also the apparent lack of toxic effects in vivo, it might be easily envisioned that a burst of information on IL-35 mode of action and therapeutic use will arrive soon (Banchereau et al., Citation2012).
IL-12p402: Not just an undesired product
Several pieces of evidence indicate that the two subunits composing IL-12p70, namely p35 and p40, are differentially expressed. As an example, the secretion of p40 by monocytes can be 5- to 500-fold greater than IL-12p70 (D’Andrea et al., Citation1992). Moreover, approximately 20–40% of p40 in the serum of normal and endotoxin-treated mice is in the form of p40 homodimer (IL-12p402), indicating the existence of a possible biological role of this molecule (Heinzel, Hujer, Ahmed, & Rerko, Citation1997). The p40 homodimer, which binds to IL-12Rβ1, has been shown to exert an antagonistic activity on IL-12Rβ1 in in vitro systems by inhibiting the biological activity of IL-12p70 (Germann, Rude, Mattner, & Gately, Citation1995; Gillessen et al., Citation1995; Jana, Dasgupta, Saha, Liu, & Pahan, Citation2003; X. Wang et al., Citation1999). However, it is now known that IL-12p402 also displays IL-12Rβ1-mediated agonistic activity that, unexpectedly, appears to be more proinflammatory than immunosuppressive. Specifically, in vitro, IL-12p402 has been shown to induce the expression of inducible NO synthase and thus the production of NO but also the secretion of IL-6 and IL-16, i.e., a leukocyte chemoattractant factor, in mouse microglia and macrophages via activation of the proinflammatory NF-κB pathway (Jana & Pahan, Citation2009; Pahan et al., Citation2001). Moreover, IL-12p402 downregulates the expression of Foxp3 in Treg cells either directly or indirectly, i.e. via NO induction (Brahmachari & Pahan, Citation2009). In vivo, administration of recombinant mouse p402 (i.e., in the native form) at the disease onset exacerbated the clinical symptoms of acute EAE in susceptible mice (Mondal, Roy, & Pahan, Citation2009), an effect associated with increased infiltration of mononuclear cells into the central nervous system and increased permeability of the brain–blood barrier.
Thus, despite the paucity of information regarding the p40 homodimer effects in vivo, the available in vitro data would indicate the existence of IL-12p402 dichotomic effects, either anti-inflammatory or proinflammatory, that may render its therapeutic use rather difficult and unpredictable. Compared with IL-27, which can also mediate opposite effects yet in distinct and defined microenvironments, we do not know whether the dichotomic behavior of the cytokine IL-12p402 may depend on specific conditions. In any case, the current knowledge on the p40 homodimer further underlines the importance of the use of single-chain heterodimeric IL-12 cytokines, particularly when the p40 chain is involved. In this regard, it should also be noted that, at variance with p35 that is not apparently secreted in the absence of p40, the p40 monomer is efficiently secreted in the extracellular milieu and can display functions on its own, in a similar yet less potent fashion than the homodimer (Vignali & Kuchroo, Citation2012).
IL-12 family subunits and bioengineering: Novel combinations, novel therapeutic perspectives?
The IL-12 family of heterodimeric cytokines is built on the existence of a discrete number of subunits, most of which can be shared among distinct molecules. Although the complete range of combinations would lead to 10 heterodimeric members plus five homodimeric molecules, only four heterodimeric and one homodimeric cytokine are known at present (Figure ). However, the biochemistry and biology of the known IL-12 family members would indicate that not all combinations could be really feasible (Vignali & Kuchroo, Citation2012). Nevertheless, we could predict that additional IL-12 family members may appear on the scene in the near future.
Figure 3 Schematic diagram of all possible combinations for IL-12 family subunits.The diagram shows that the combination of five types of subunits belonging to the IL-12 family may lead to ten heterodimeric and five homodimeric distinct proteins, in addition to five monomers. Of these, the natural (nat.) existence of only four heterodimers (IL-12, IL-23, IL-27, and IL-35), one homodimer (p402), and three monomers (p40, EBI3 and p28) is known, whereas only one engineered (engin.) combination (p28/p40) has been generated so far. Where identified, immunological functions – either immunostimulatory (imm.) or tolerogenic (toler.), or both – are indicated.
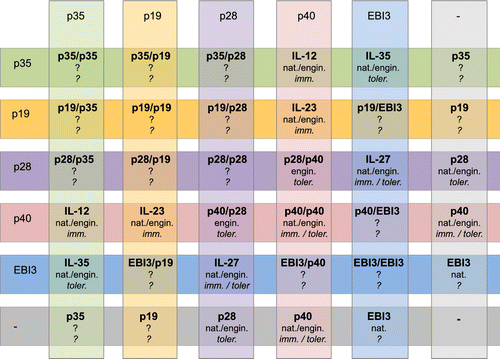
Combining previous information that IL-27p28 (p28) antagonizes gp130-mediated signaling (Stumhofer et al., Citation2010) and that the IL-12p40 homodimer is a potent IL-12 antagonist (Gillessen et al., Citation1995), a novel IL-27p28/IL-12p40 heterodimeric cytokine (p28/p40) has been very recently engineered in a bicistronic vector (R.X.Wang, Yu, Mahdi, & Egwuagu, Citation2012). When administered in vivo, p28/p40 heterodimer suppressed experimental autoimmune uveitis (EAU) by inhibiting the differentiation and inflammatory responses of both TH1 and TH17 cells while promoting expansion of IL-10+ and Foxp3+ Treg cells. In addition, lymph node cells from mice treated with p28/p40 blocked the adoptive transfer of EAU to naive syngeneic mice by immunopathogenic T cells. In vitro experiments demonstrated that p28/p40 inhibits the signaling downstream both IL-12Rβ1 and gp130 receptor subunits, an effect that may explain the concomitant suppression of TH1 (mediated by IL-12Rβ1) and TH17 (mediated by IL-6 and thus gp130) responses. The inhibition of IL-6 signaling may in turn push the balance from TH17 to Treg cells favoring the increase in immunosuppressive Treg-mediated responses in vivo.
Although the antagonistic effects of p28/p40 has not been entirely dissected at the molecular level, the creation of a novel heterodimeric cytokine (Figure ) represents an important proof-of-concept that may promote the discovery of additional IL-12 family members but also of new therapeutic agents.
Conclusions
Bioengineering and preclinical testing of cytokines belonging in the IL-12 family at the moment represent a very fertile ground of investigations, still full of likely surprises. On the one hand, the multitude of studies in several preclinical experimental models of disease has been providing clear clues of therapeutic effects of the subunit members of the IL-12 family in the heterodimeric but, unexpectedly, also in the homodimeric and monomeric form. On the other hand, the availability of predetermined subunit combinations has been allowing us to perform additional studies in order to better define the complex world of these cytokines. Although the new wave of basic biology may perhaps delay the translation into the clinic, heterodimeric cytokines already fulfill the drug property of ‘pharmacological promiscuity’ (i.e., the capacity of a compound to be active at multiple targets) that seems to represent one of the most appealing aims in modern pharmacology (Hopkins, Citation2008).
References
- Awasthi , A. , Carrier , Y. , Peron , J. P. , Bettelli , E. , Kamanaka , M. , Flavell , R. A. , … and Weiner , H. L. 2007 . A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells . Nature Immunology , 8 : 1380 – 1389 .
- Banchereau , J. , Pascual , V. and O’Garra , A. 2012 . From IL-2 to IL-37: The expanding spectrum of anti-inflammatory cytokines . Nature Immunology , 13 : 925 – 931 .
- Belladonna , M. L. , Renauld , J. C. , Bianchi , R. , Vacca , C. , Fallarino , F. , Orabona , C. , … and Puccetti , P. 2002 . IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells . Journal of Immunology , 168 : 5448 – 5454 .
- Bettini , M. , Castellaw , A. H. , Lennon , G. P. , Burton , A. R. and Vignali , D. A. 2012 . Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35 . Diabetes , 61 : 1519 – 1526 .
- Boniface , K. , Blom , B. , Liu , Y. J. and de Waal Malefyt , R. 2008 . From interleukin-23 to T-helper 17 cells: Human T-helper cell differentiation revisited . Immunological Reviews , 226 : 132 – 146 .
- Borsellino , G. , Kleinewietfeld , M. , Di Mitri , D. , Sternjak , A. , Diamantini , A. , Giometto , R. , … and Falk , K. 2007 . Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression . Blood , 110 : 1225 – 1232 .
- Brahmachari , S. and Pahan , K. 2009 . Suppression of regulatory T cells by IL-12p40 homodimer via nitric oxide . Journal of Immunology , 183 : 2045 – 2058 .
- Car , B. D. , Eng , V. M. , Lipman , J. M. and Anderson , T. D. 1999 . The toxicology of interleukin-12: A review . Toxicologic Pathology , 27 : 58 – 63 .
- Chaturvedi , V. , Collison , L. W. , Guy , C. S. , Workman , C. J. and Vignali , D. A. 2011 . Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance . Journal of Immunology , 186 : 6661 – 6666 .
- Chen, X., Zaro, J.L., & Shen, W.C. (2012). Fusion protein linkers: Property, design and functionality. Advanced Drug Delivery Reviews. Retrieved from http://dx.doi.org/10.1016/j.addr.2012.09.039.
- Cocco , C. , Canale , S. , Frasson , C. , Di Carlo , E. , Ognio , E. , Ribatti , D. , … and Airoldi , I. 2010 . Interleukin-23 acts as antitumor agent on childhood B-acute lymphoblastic leukemia cells . Blood , 116 : 3887 – 3898 .
- Cohen , J. 1995 . IL-12 deaths: Explanation and a puzzle . Science , 270 : 908
- Collison , L. W. , Chaturvedi , V. , Henderson , A. L. , Giacomin , P. R. , Guy , C. , Bankoti , J. , … and Vignali , D. A. 2010 . IL-35-mediated induction of a potent regulatory T cell population . Nature Immunology , 11 : 1093 – 1101 .
- Collison , L. W. , Delgoffe , G. M. , Guy , C. S. , Vignali , K. M. , Chaturvedi , V. , Fairweather , D. , … and Vignali , D. A. 2012 . The composition and signaling of the IL-35 receptor are unconventional . Nature Immunology , 13 : 290 – 299 .
- Collison , L. W. and Vignali , D. A. 2008 . Interleukin-35: Odd one out or part of the family? . Immunological Reviews , 226 : 248 – 262 .
- Collison , L. W. , Workman , C. J. , Kuo , T. T. , Boyd , K. , Wang , Y. , Vignali , K. M. , … and Vignali , D. A. 2007 . The inhibitory cytokine IL-35 contributes to regulatory T-cell function . Nature , 450 : 566 – 569 .
- Colombo , M. P. , Vagliani , M. , Spreafico , F. , Parenza , M. , Chiodoni , C. , Melani , C. and Stoppacciaro , A. 1996 . Amount of interleukin 12 available at the tumor site is critical for tumor regression . Cancer Research , 56 : 2531 – 2534 .
- D’Andrea , A. , Rengaraju , M. , Valiante , N. M. , Chehimi , J. , Kubin , M. , Aste , M. , … and Trinchieri , G. 1992 . Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells . Journal of Experimental Medicine , 176 : 1387 – 1398 .
- Del Vecchio , M. , Bajetta , E. , Canova , S. , Lotze , M. T. , Wesa , A. , Parmiani , G. and Anichini , A. 2007 . Interleukin-12: Biological properties and clinical application . Clinical Cancer Research , 13 : 4677 – 4685 .
- Delgoffe , G. M. , Murray , P. J. and Vignali , D. A. 2011 . Interpreting mixed signals: The cell’s cytokine conundrum . Current Opinion in Immunology , 23 : 632 – 638 .
- di Guan , C. , Li , P. , Riggs , P. D. and Inouye , H. 1988 . Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein . Gene , 67 : 21 – 30 .
- Diveu , C. , McGeachy , M. J. , Boniface , K. , Stumhofer , J. S. , Sathe , M. , Joyce-Shaikh , B. , … and Kastelein , R. A. 2009 . IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells . Journal of Immunology , 182 : 5748 – 5756 .
- Ehigiator , H. N. , McNair , N. and Mead , J. R. 2007 . Cryptosporidium parvum: The contribution of Th1-inducing pathways to the resolution of infection in mice . Experimental Parasitology , 115 : 107 – 113 .
- Einhauer , A. , Schuster , M. , Wasserbauer , E. and Jungbauer , A. 2002 . Expression and purification of homogenous proteins in Saccharomyces cerevisiae based on ubiquitin-FLAG fusion . Protein Expression and Purification , 24 : 497 – 504 .
- Esposito , D. and Chatterjee , D. K. 2006 . Enhancement of soluble protein expression through the use of fusion tags . Current Opinion in Biotechnology , 17 : 353 – 358 .
- Fiocco , U. , Sfriso , P. , Oliviero , F. , Pagnin , E. , Scagliori , E. , Campana , C. , … and Punzi , L. 2008 . Co-stimulatory modulation in rheumatoid arthritis: The role of (CTLA4-Ig) abatacept . Autoimmunity Reviews , 8 : 76 – 82 .
- Fitzgerald , D. C. , Ciric , B. , Touil , T. , Harle , H. , Grammatikopolou , J. , Das Sarma , J. , … and Rostami , A. 2007a . Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis . Journal of Immunology , 179 : 3268 – 3275 .
- Fitzgerald , D. C. , Zhang , G. X. , El-Behi , M. , Fonseca-Kelly , Z. , Li , H. , Yu , S. , … and Rostami , A. 2007b . Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells . Nature Immunology , 8 : 1372 – 1379 .
- Gaberc-Porekar , V. and Menart , V. 2001 . Perspectives of immobilized-metal affinity chromatography . Journal of Biochemical and Biophysical Methods , 49 : 335 – 360 .
- Garbers , C. , Hermanns , H. M. , Schaper , F. , Muller-Newen , G. , Grotzinger , J. , Rose-John , S. and Scheller , J. 2012 . Plasticity and cross-talk of interleukin 6-type cytokines . Cytokine and Growth Factor Reviews , 23 : 85 – 97 .
- Germann , T. , Rude , E. , Mattner , F. and Gately , M. K. 1995 . The IL-12 p40 homodimer as a specific antagonist of the IL-12 heterodimer . Immunology Today , 16 : 500 – 501 .
- Gillessen , S. , Carvajal , D. , Ling , P. , Podlaski , F. J. , Stremlo , D. L. , Familletti , P. C. , … and Gately , M. K. 1995 . Mouse interleukin-12 (IL-12) p40 homodimer: A potent IL-12 antagonist . European Journal of Immunology , 25 : 200 – 206 .
- Grohmann , U. , Bianchi , R. , Belladonna , M. L. , Vacca , C. , Silla , S. , Ayroldi , E. , … and Puccetti , P. 1999 . IL-12 acts selectively on CD8 alpha- dendritic cells to enhance presentation of a tumor peptide in vivo . Journal of Immunology , 163 : 3100 – 3105 .
- Ha , S. J. , Kim , D. J. , Baek , K. H. , Yun , Y. D. and Sung , Y. C. 2004 . IL-23 induces stronger sustained CTL and Th1 immune responses than IL-12 in hepatitis C virus envelope protein 2 DNA immunization . Journal of Immunology , 172 : 525 – 531 .
- Heinzel , F. P. , Hujer , A. M. , Ahmed , F. N. and Rerko , R. M. 1997 . In vivo production and function of IL-12 p40 homodimers . Journal of Immunology , 158 : 4381 – 4388 .
- Hisada , M. , Kamiya , S. , Fujita , K. , Belladonna , M. L. , Aoki , T. , Koyanagi , Y. , … and Yoshimoto , T. 2004 . Potent antitumor activity of interleukin-27 . Cancer Research , 64 : 1152 – 1156 .
- Ho , M. Y. , Leu , S. J. , Sun , G. H. , Tao , M. H. , Tang , S. J. and Sun , K. H. 2009 . IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities . Journal of Immunology , 183 : 6217 – 6226 .
- Hopkins , A. L. 2008 . Network pharmacology: the next paradigm in drug discovery . Nature Chemical Biology , 4 : 682 – 690 .
- Hu , J. , Yuan , X. , Belladonna , M. L. , Ong , J. M. , Wachsmann-Hogiu , S. , Farkas , D. L. , … and Yu , J. S. 2006 . Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells . Cancer Research , 66 : 8887 – 8896 .
- Hu , P. , Hu , H. D. , Chen , M. , Peng , M. L. , Tang , L. , Tang , K. F. , … and Ren , H. 2009 . Expression of interleukins-23 and 27 leads to successful gene therapy of hepatocellular carcinoma . Molecular Immunology , 46 : 1654 – 1662 .
- Hunter , C. A. 2005 . New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions . Nature Reviews. Immunology , 5 : 521 – 531 .
- Jana , M. , Dasgupta , S. , Saha , R. N. , Liu , X. and Pahan , K. 2003 . Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages . Journal of Neurochemistry , 86 : 519 – 528 .
- Jana , M. and Pahan , K. 2009 . IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages . Molecular Immunology , 46 : 773 – 783 .
- Jazayeri , J. A. and Carroll , G. J. 2008 . Fc-based cytokines: prospects for engineering superior therapeutics . BioDrugs , 22 : 11 – 26 .
- Jones , L. L. , Chaturvedi , V. , Uyttenhove , C. , Van Snick , J. and Vignali , D. A. 2012 . Distinct subunit pairing criteria within the heterodimeric IL-12 cytokine family . Molecular Immunology , 51 : 234 – 244 .
- Kaplan , W. , Husler , P. , Klump , H. , Erhardt , J. , Sluis-Cremer , N. and Dirr , H. 1997 . Conformational stability of pGEX-expressed Schistosoma japonicum glutathione S-transferase: A detoxification enzyme and fusion-protein affinity tag . Protein Science , 6 : 399 – 406 .
- Kerkar , S. P. , Muranski , P. , Kaiser , A. , Boni , A. , Sanchez-Perez , L. , Yu , Z. , … and Restifo , N. P. 2010 . Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts . Cancer Research , 70 : 6725 – 6734 .
- Kleinschek , M. A. , Muller , U. , Brodie , S. J. , Stenzel , W. , Kohler , G. , Blumenschein , W. M. and Alber , G. 2006 . IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12 . Journal of Immunology , 176 : 1098 – 1106 .
- Kobayashi , M. , Fitz , L. , Ryan , M. , Hewick , R. M. , Clark , S. C. , Chan , S. , … and Trinchieri , G. 1989 . Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes . Journal of Experimental Medicine , 170 : 827 – 845 .
- Kochetkova , I. , Golden , S. , Holderness , K. , Callis , G. and Pascual , D. W. 2010 . IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10 . Journal of Immunology , 184 : 7144 – 7153 .
- Kuo , T. T. and Aveson , V. G. 2011 . Neonatal Fc receptor and IgG-based therapeutics . MAbs , 3 : 422 – 430 .
- Kuramoto , T. , Fujii , R. , Nagai , H. , Belladonna , M. L. , Yoshimoto , T. , Kohjimoto , Y. , … and Hara , I. 2011 . IL-23 gene therapy for mouse bladder tumour cell lines . BJU International , 108 : 914 – 921 .
- Langrish , C. L. , McKenzie , B. S. , Wilson , N. J. , de Waal Malefyt , R. , Kastelein , R. A. and Cua , D. J. 2004 . IL-12 and IL-23: Master regulators of innate and adaptive immunity . Immunological Reviews , 202 : 96 – 105 .
- Leader , B. , Baca , Q. and Golan , D. E. 2008 . Protein therapeutics: A summary and pharmacological classification . Nature Reviews. Drug Discovery , 7 : 21 – 39 .
- Li , X. , Mai , J. , Virtue , A. , Yin , Y. , Gong , R. , Sha , X. , … and Yang , X. F. 2012 . IL-35 is a novel responsive anti-inflammatory cytokine – A new system of categorizing anti-inflammatory cytokines . PLoS One , 7 : e33628
- Lieschke , G. J. , Rao , P. K. , Gately , M. K. and Mulligan , R. C. 1997 . Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo . Nature Biotechnology , 15 : 35 – 40 .
- Lyakh , L. , Trinchieri , G. , Provezza , L. , Carra , G. and Gerosa , F. 2008 . Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans . Immunological Reviews , 226 : 112 – 131 .
- Manoukian , G. and Hagemeister , F. 2009 . Denileukin diftitox: A novel immunotoxin . Expert Opinion on Biological Therapy , 9 : 1445 – 1451 .
- Marblestone , J. G. , Edavettal , S. C. , Lim , Y. , Lim , P. , Zuo , X. and Butt , T. R. 2006 . Comparison of SUMO fusion technology with traditional gene fusion systems: Enhanced expression and solubility with SUMO . Protein Science , 15 : 182 – 189 .
- Matsui , M. , Moriya , O. , Belladonna , M. L. , Kamiya , S. , Lemonnier , F. A. , Yoshimoto , T. and Akatsuka , T. 2004 . Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A∗0201 transgenic mice . Journal of Virology , 78 : 9093 – 9104 .
- Mazzolini , G. , Alfaro , C. , Sangro , B. , Feijoo , E. , Ruiz , J. , Benito , A. , … and Melero , I. 2005 . Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas . Journal of Clinical Oncology , 23 : 999 – 1010 .
- Mondal , S. , Roy , A. and Pahan , K. 2009 . Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis . Journal of Immunology , 182 : 5013 – 5023 .
- Nagai , H. , Oniki , S. , Fujiwara , S. , Xu , M. , Mizoguchi , I. , Yoshimoto , T. and Nishigori , C. 2010 . Antitumor activities of interleukin-27 on melanoma. Endocrine . Metabolic and Immune Disorders. Drug Targets , 10 : 41 – 46 .
- Niedbala , W. , Wei , X. Q. , Cai , B. , Hueber , A. J. , Leung , B. P. , McInnes , I. B. and Liew , F. Y. 2007 . IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells . European Journal of Immunology , 37 : 3021 – 3029 .
- Nilsson , B. , Moks , T. , Jansson , B. , Abrahmsen , L. , Elmblad , A. , Holmgren , E. , … and Uhlen , M. 1987 . A synthetic IgG-binding domain based on staphylococcal protein A . Protein Engineering , 1 : 107 – 113 .
- Oniki , S. , Nagai , H. , Horikawa , T. , Furukawa , J. , Belladonna , M. L. , Yoshimoto , T. , … and Nishigori , C. 2006 . Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma . Cancer Research , 66 : 6395 – 6404 .
- Oppmann , B. , Lesley , R. , Blom , B. , Timans , J. C. , Xu , Y. , Hunte , B. , … and Kastelein , R. A. 2000 . Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12 . Immunity , 13 : 715 – 725 .
- O’Shea , J. J. and Paul , W. E. 2002 . Regulation of T(H)1 differentiation – Controlling the controllers . Nature Immunology , 3 : 506 – 508 .
- Owaki , T. , Asakawa , M. , Morishima , N. , Hata , K. , Fukai , F. , Matsui , M. , … and Yoshimoto , T. 2005 . A role for IL-27 in early regulation of Th1 differentiation . Journal of Immunology , 175 : 2191 – 2200 .
- Pahan , K. , Sheikh , F. G. , Liu , X. , Hilger , S. , McKinney , M. and Petro , T. M. 2001 . Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells . Journal of Biological Chemistry , 276 : 7899 – 7905 .
- Pardridge , W. M. 2010 . Biopharmaceutical drug targeting to the brain . Journal of Drug Targeting , 18 : 157 – 167 .
- Parham , C. , Chirica , M. , Timans , J. , Vaisberg , E. , Travis , M. , Cheung , J. , … and Moore , K. W. 2002 . A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R . Journal of Immunology , 168 : 5699 – 5708 .
- Peng , L. S. , Penichet , M. L. and Morrison , S. L. 1999 . A single-chain IL-12 IgG3 antibody fusion protein retains antibody specificity and IL-12 bioactivity and demonstrates antitumor activity . Journal of Immunology , 163 : 250 – 258 .
- Pflanz , S. , Hibbert , L. , Mattson , J. , Rosales , R. , Vaisberg , E. , Bazan , J.F. , … and Kastelein , R. A. 2004 . WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27 . Journal of Immunology , 172 : 2225 – 2231 .
- Pflanz , S. , Timans , J. C. , Cheung , J. , Rosales , R. , Kanzler , H. , Gilbert , J. , … and Kastelein , R. A. 2002 . IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells . Immunity , 16 : 779 – 790 .
- Presky , D. H. , Yang , H. , Minetti , L. J. , Chua , A. O. , Nabavi , N. , Wu , C. Y. , … and Gubler , U. 1996 . A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits . Proceedings of the National Academy of Sciences of the United States of America , 93 : 14002 – 14007 .
- Puccetti , P. , Belladonna , M. L. and Grohmann , U. 2002 . Effects of IL-12 and IL-23 on antigen-presenting cells at the interface between innate and adaptive immunity . Critical Reviews in Immunology , 22 : 373 – 390 .
- Riggs , P. 2000 . Expression and purification of recombinant proteins by fusion to maltose-binding protein . Molecular Biotechnology , 15 : 51 – 63 .
- Rizzo , H. L. , Kagami , S. , Phillips , K. G. , Kurtz , S. E. , Jacques , S. L. and Blauvelt , A. 2011 . IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A . Journal of Immunology , 186 : 1495 – 1502 .
- Salcedo , R. , Stauffer , J. K. , Lincoln , E. , Back , T. C. , Hixon , J. A. , Hahn , C. , … and Wigginton , J. M. 2004 . IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: Role for CD8+ T cells . Journal of Immunology , 173 : 7170 – 7182 .
- Sasaoka , T. , Ito , M. , Yamashita , J. , Nakajima , K. , Tanaka , I. , Narita , M. , … and Kaneko , K. 2011 . Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. American Journal of Physiology . Gastrointestinal and Liver Physiology , 300 : G568 – 576 .
- Shimizu , M. , Shimamura , M. , Owaki , T. , Asakawa , M. , Fujita , K. , Kudo , M. , … and Yoshimoto , T. 2006 . Antiangiogenic and antitumor activities of IL-27 . Journal of Immunology , 176 : 7317 – 7324 .
- Smith , D. B. and Johnson , K. S. 1988 . Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase . Gene , 67 : 31 – 40 .
- Stern , A. S. , Podlaski , F. J. , Hulmes , J. D. , Pan , Y. C. , Quinn , P. M. , Wolitzky , A. G. , … and Gately , M. K. 1990 . Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells . Proceedings of the National Academy of Sciences of the United States of America , 87 : 6808 – 6812 .
- Stumhofer , J. S. , Tait , E. D. , Quinn , W. J. 3rd , Hosken , N. , Spudy , B. , Goenka , R. , … and Hunter , C. A. 2010 . A role for IL-27p28 as an antagonist of gp130-mediated signaling . Nature Immunology , 11 : 1119 – 1126 .
- Tahara , H. and Lotze , M. T. 1995 . Antitumor effects of interleukin-12 (IL-12): Applications for the immunotherapy and gene therapy of cancer . Gene therapy , 2 : 96 – 106 .
- Thierfelder , W. E. , van Deursen , J. M. , Yamamoto , K. , Tripp , R. A. , Sarawar , S. R. , Carson , R. T. , … and Ihle , J. N. 1996 . Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells . Nature , 382 : 171 – 174 .
- Trinchieri , G. 1994 . Interleukin-12: A cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes . Blood , 84 : 4008 – 4027 .
- Trinchieri , G. 2003 . Interleukin-12 and the regulation of innate resistance and adaptive immunity . Nature Reviews. Immunology , 3 : 133 – 146 .
- Trinchieri , G. , Pflanz , S. and Kastelein , R. A. 2003 . The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses . Immunity , 19 : 641 – 644 .
- Uhlen , M. , Nilsson , B. , Guss , B. , Lindberg , M. , Gatenbeck , S. and Philipson , L. 1983 . Gene fusion vectors based on the gene for staphylococcal protein A . Gene , 23 : 369 – 378 .
- Vignali , D. A. , Collison , L. W. and Workman , C. J. 2008 . How regulatory T cells work . Nature Reviews. Immunology , 8 : 523 – 532 .
- Vignali , D. A. and Kuchroo , V. K. 2012 . IL-12 family cytokines: Immunological playmakers . Nature Immunology , 13 : 722 – 728 .
- Wang , R. X. , Yu , C. R. , Mahdi , R. M. and Egwuagu , C. E. 2012 . Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells . Journal of Biological Chemistry , 287 : 36012 – 36021 .
- Wang , X. , Wilkinson , V. L. , Podlaski , F. J. , Wu , C. , Stern , A. S. , Presky , D. H. and Magram , J. 1999 . Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits . European Journal of Immunology , 29 : 2007 – 2013 .
- Waugh , D. S. 2005 . Making the most of affinity tags . Trends in Biotechnology , 23 : 316 – 320 .
- Weaver , C. T. and Hatton , R. D. 2009 . Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective . Nature Reviews. Immunology , 9 : 883 – 889 .
- Weiss , J. M. , Subleski , J. J. , Wigginton , J. M. and Wiltrout , R. H. 2007 . Immunotherapy of cancer by IL-12-based cytokine combinations . Expert Opinion on Biological Therapy , 7 : 1705 – 1721 .
- Wojno , E. D. and Hunter , C. A. 2012 . New directions in the basic and translational biology of interleukin-27 . Trends in Immunology , 33 : 91 – 97 .
- Wulff , H. , Krieger , T. , Kruger , K. , Stahmer , I. , Thaiss , F. , Schafer , H. and Block , A. 2007 . Cloning and characterization of an adenoviral vector for highly efficient and doxycycline-suppressible expression of bioactive human single-chain interleukin 12 in colon cancer . BMC Biotechnology , 7 : 35 – 43 .
- Yoshida , H. and Miyazaki , Y. 2008 . Regulation of immune responses by interleukin-27 . Immunological Reviews , 226 : 234 – 247 .
- Yoshimoto , T. , Morishima , N. , Mizoguchi , I. , Shimizu , M. , Nagai , H. , Oniki , S. , … and Mizuguchi , J. 2008 . Antiproliferative activity of IL-27 on melanoma . Journal of Immunology , 180 : 6527 – 6535 .
- Yoshimoto , T. , Okada , K. , Morishima , N. , Kamiya , S. , Owaki , T. , Asakawa , M. , … and Mizuguchi , J. 2004 . Induction of IgG2a class switching in B cells by IL-27 . Journal of Immunology , 173 : 2479 – 2485 .
- Yuan , X. , Hu , J. , Belladonna , M. L. , Black , K. L. and Yu , J. S. 2006 . Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma . Cancer Research , 66 : 2630 – 2638 .
- Zhang , C. , Yang , X. L. , Yuan , Y. H. , Pu , J. and Liao , F. 2012 . Site-specific PEGylation of therapeutic proteins via optimization of both accessible reactive amino acid residues and PEG derivatives . BioDrugs , 26 : 209 – 215 .
- Zitvogel , L. , Tahara , H. , Cai , Q. , Storkus , W. J. , Muller , G. , Wolf , S. F. , … and Lotze , M. T. 1994 . Construction and characterization of retroviral vectors expressing biologically active human interleukin-12 . Human Gene Therapy , 5 : 1493 – 1506 .