Abstract
Meal replacements and viscous soluble fibre represent safe and sustainable aids for weight loss. Our purpose was to determine if PGX® meal replacements and PGX® fibre complex in combination with a calorie-restricted diet would aid in weight loss in a clinical setting. Fifty-two overweight and obese participants (49 women, 3 men; average age 47.1 years) with a mean body mass index (BMI) of 33.8 ± 6.4 kg/m2 consumed 57 g of proprietary PGX® meal replacement product at breakfast and another 57 g at lunch for 12 weeks. In addition to the meal replacements, they were also asked to consume 5 g/day of PGX® fibre in the form of granules, powder or capsules together with 250 mlwater. A registered dietician recommended low-fat, low-glycaemic-index foods for snacks and the dinner menus such that each volunteer was consuming a total of 1200 kcal/day. All participants (n = 52) lost a significant amount of weight from baseline (−4.69 ± 3.73 kg), which was further reflected in the reductions in their waist (−7.11 ± 6.35 cm) and hip circumference (−5.59 ± 3.58 cm) over the 12-week study (p < 0.0001). BMI scores (n = 51) were reduced by 1.6 ± 1.4 kg/m2. The use of PGX® meal replacements and PGX® fibre along with a controlled dietary caloric intake is of benefit for short-term weight loss.
Introduction
Within the last 25 years, obesity and its associated co-morbidities have become a major public healthcare concern throughout the industrialized world. In 2008, the World Health Organization (WHO, 2008) estimated that 1.5 billion adults were overweight, of which 500 million were obese. Individual countries like Canada have also succumbed to this latter malady. In a recent survey of 5600 Canadians from March 2007 to February 2009, it was determined that, although 38% of adult Canadians are at a healthy weight, 37% were overweight and 24% were obese (Statistics Canada, Citation2010).
Given that obesity is a chronic disease, there are a number of treatment options available including bariatric surgery, pharmacotherapy, cognitive behavioural therapy, exercise and a structured dietary plan. In the execution of a multidisciplinary weight management programme, one type of approach that aids in the reduction of calories while providing basic macro- and micronutrient needs is the use of meal replacements. Numerous research initiatives have supported this latter approach as a safe and sustainable method of weight management (Poole et al., Citation2011; Smith et al., Citation2010; Lee et al., Citation2009). For example, during a 4-month weight-loss phase in 90 obese adults, weight reduction in the meal replacement group averaged 12.3% (13.5 ± 5.9 kg) compared with 6.7% (6.5 ± 6.8 kg) in the food-based diet group (Davis et al., Citation2010).
Meal replacements can be used for weight loss and weight maintenance as well. In a study by Vásquez et al. (Citation2009), 62 obese adults who had lost at least 5% of their initial body weight were randomized to a calorie-restricted diet (−400 to −500 kcal) or calorie restriction plus meal replacement at dinner for an additional 6 months. Participants in the meal replacement and diet group lost an additional 3.2% of body weight whereas the diet-only group lost an additional 1.3% (Vásquez et al., Citation2009).
Higher-fibre diets may support long-term weight management by helping to reduce digestible energy intake (Howarth, Salzman, & Roberts, Citation2001). Moreover, owing to its unique physical characteristics in the stomach and small intestine (i.e., high degree of viscosity), soluble fibre not only lowers post-prandial glycaemia but also helps promote satiety through the altered release of specific gut neuropeptides (i.e., glucagon-like peptide-1 [GLP-1], peptide YY [PYY], ghrelin; Papathanasopoulos & Camilleri, Citation2010). From a research perspective, several types of individual soluble fibre supplements favour weight loss including glucomannan, psyllium and guar gum (Walsh, Yaghoubian,k & Behforooz, Citation1984; Pal, Khossousi, Binns, Dhaliwal, & Ellis, Citation2011; Tuomilehto, Voutilainen, Huttunen, Vinni, & Homan, Citation1980).
Combinations of soluble fibre to promote a change in body composition have had clinically mixed results, with both positive and negative outcomes (Salas-Salvadó et al., Citation2008; Birketvedt, Shimshi, Erling, & Florholmen, Citation2005). However, a proprietary complex of three types of soluble fibre known as PGX® has shown favourable results for weight management, and the focus of this review is on the properties of this substance. Using 5 g of PGX® granules two to three times a day for 14 weeks, one open clinical investigation has determined that this fibre complex was of adjunctive value in treating overweight and obesity (Lyon & Reichert, Citation2010). Compared with controls, significant reductions have been observed in group weight loss (−5.79 ± 3.55 kg), waist measurements (−12.07 ± 5.56 cm) and percentage body fat (−2.43 ± 2.39%) in those participants using PGX® and other lifestyle modifications for 14 weeks (Lyon & Reichert, Citation2010).
Pgx®
PGX® (PolyGlycopleX®; Inovobiologic Inc., Calgary, Canada) is a unique polysaccharide that is manufactured by reacting glucomannan (an extract from Amorphophallus konjac) with other soluble polysaccharides (sodium alginate and xanthan gum) using a proprietary process (EnviroSimplex®). The resulting polysaccharide ((α-D-glucurono-α-D-manno-β-D-manno-β-D-gluco), (α-L-gulurono-β-D mannurono), β-D-gluco-β-D-mannan) is a novel entity with high water- and gel-holding properties (Figure ).
Figure 1 Viscosity of PGX compared with other water-soluble polysaccharides after hydrating for 3 h.
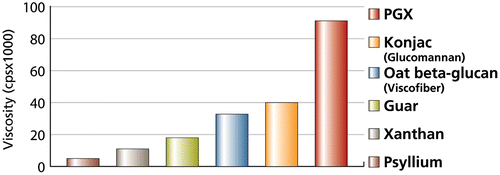
In vivo, PGX® has been shown to exert several remarkable physiological effects. For example, when PGX® was given to diabetic Zucker rats, 5% wt/wt in rodent chow, over a 12-week period, a marked reduction in haemoglobin A1c and an increase in GLP-1 level were reported (Grover et al., Citation2011). PGX® has also been shown to reduce hepatic steatosis and decrease serum hypertriglyeridemia in rats fed a high-sucrose diet (Reimer et al., Citation2011).
PGX® meal replacement (Slim Styles®) has been employed in a double-blind, placebo-controlled study in healthy non-obese teenagers in which the amount of food intake in the form of pizza was compared using different products with low, medium and high viscosities. The highly viscous PGX® meal replacement significantly reduced pizza consumption (278 ± 111 g) compared with medium (glucomannan) (313 ± 123 g) and low (cellulose; 316 ± 138 g) viscosity containing product pre-loads (Vuksan et al., Citation2009). In contrast to other types of commercially available meal replacements, the PGX® meal replacement employs a whey protein concentrate rather than casein, contains a lower overall carbohydrate content per serving and includes a unique viscous dietary fibre complex (PGX®; Table ).
Table 1. Characteristics of some commercially available meal replacements in Canada.
Several studies have confirmed that PGX® consumption in humans lowers the glycaemic index (GI) of foods including starches (Jenkins, Kacinik, Lyon, & Wolever, Citation2010). In one recent study, PGX® use in 10 healthy humans significantly lowered the GI of six different starchy foodstuffs, with a mean reduction of 19% for the 2.5-g dose (−7 GI units) and 30% for the 5-g dose (−15 GI units; Brand-Miller et al., Citation2012).
There is limited research on the effects of PGX® meal replacements in combination with additional PGX® fibre in overweight and obese adults. We now consider the results from a clinical analysis programme conducted to evaluate whether the use of a PGX® meal replacement and additional PGX® viscous fibre as part of a calorie-restricted diet was efficacious for weight loss over a 12-week period.
Materials and methods
Participants
Fifty-two overweight and obese adults (49 women; 3 men; average age 47.1 years), with a mean BMI of 33.8 ± 6.4 kg/m2 and mean weight of 94.09 ± 23.36 kg were invited to participate in a clinical weight-management programme through a series of advertisements placed in local newspapers. Participants provided their informed consent before participation in this observational clinical analysis programme, and the overall investigation was conducted in accordance with the ethical standards as set forth in the Helsinki Declaration of 1975.
Anthropometric and other measurements
Programme participants were evaluated at baseline and then on a bi-weekly basis for weight (kg), height (cm), and waist and hip (cm) measurements. Waist and hip measurements were recorded using a standard medical-type tape measure at consistent anatomical locations: approximately mid-way between the lowest rib and the iliac crest for the waist and at the level of the greater trochanter around the hip. BMI scores were calculated using an online BMI calculator available through the US Department of Health and Human Services (http://www.nhlbisupport.com/bmi/bmicalc.htm).
Diet and supplementation
Participants were given a proprietary meal replacement powder (Slim Styles® with PGX®) at a dose of 57 g at breakfast and another 57 g at lunch daily mixed with 250 ml of water, instead of their regular meals. Each serving of meal replacement contained 226 calories (945 kJ); 21 g of whey protein concentrate; 6.6 g of fat and 21 g of carbohydrate containing 5 g of soluble PGX® fibre. In addition to the meal replacements, participants were advised by a registered dietician to incorporate low-fat, moderate- to low- GI foods for their 500-kcal dinner meal and 250-kcal between-meal snacks (50–100-kcal per snack) throughout the day. Total caloric intake per day was set at 1200 kcal for all participants. Participants were encouraged to exercise but not given specific routines through the 12-week programme. Along with the use of meal replacements, participants were also given PGX® fibre in three optional forms. The additional 5 g of fibre could be consumed as PGX® granules, PGX® powder or PGX® capsules (10 capsules equivalent to 5 g PGX®) throughout the day, along with 250 ml of water per serving of product, needed for additional appetite control.
Statistical analysis
The null hypothesis of ‘no change from baseline’ was tested for each of the clinical parameters using the one-sample t-test on the change from baseline scores; 95% confidence intervals for the average 12-week change in each parameter were calculated. Correlations between changes from baseline in the different clinical variables and the baseline value, as well as age, were examined using Spearman’s correlation coefficient. All statistical tests were two-sided and used a 0.05 significance level.
Results
All the participants (n = 52) in the programme lost a significant amount of weight from baseline (−4.68 ± 3.73 kg Figure ) which was further reflected in their waist (−7.11 ± 6.35 cm) and hip circumference (−5.59 ± 3.58 cm) reductions over a 12-week period (p < 0.0001; Table ). The BMI scores in both the overall group and women only showed a significant reduction from baseline of 1.6 ± 1.4 and 1.6 ± 1.3 kg/m2, respectively (Table ). As a group, women showed a significant amount of weight loss and reduction in waist and hip circumferences (p < 0.0001) from the beginning of the 12-week programme (Table ).
Figure 2 Overall weight change in the group compared with weight change in women.Note: The overall weight reduction and weight reduction in women observed over the 12-week period are both significant from baseline (p < 0.0001).
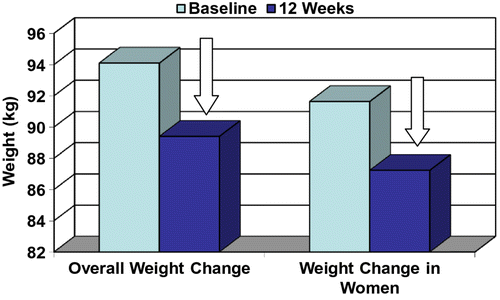
Table 2. Body weight, and waist and hip circumference measurements at baseline and after 12 weeks of the calorie-restricted diet with PGX® meal replacement and PGX® fibre supplement.
Table 3. Overall and women BMI scores at baseline and after 12 weeks of the calorie-restricted diet with PGX® meal replacement and PGX® fibre supplement.
Table 4. Body weight, waist and hip circumference measurements in women at baseline and after 12 weeks of the calorie-restricted diet with PGX® meal replacement and PGX® fibre supplement.
Discussion
In the clinical programme considered above, it has been demonstrated that PGX® meal replacement and additional PGX® fibre are of benefit in promoting weight loss over a 12-week period. Participants lost a significant amount of weight with a concomitant reduction in waist and hip measurements and BMI. On average, those who consumed the PGX® meal replacement and additional PGX® fibre lost 4.97% of their initial body weight. The majority of the participants (n = 49) in the clinical trial were women and had comparable but significant outcomes on all tested variables. The data from the small number of men (n = 3) that were available for analysis precluded meaningful statistical evaluation for males alone, although on observation the three male participants lost a substantial amount of weight (−2.59, −4.77 and −9.87 kg) during the 12-week programme.
While the observed reduction in weight (−4.69 kg) is lower than in other published results, the reduction (−7.11 cm) seen in waist circumference is consistent with the existing literature on the use of meal-replacement strategies for obesity management. In one clinical trial, 286 overweight and obese men and women (BMI 27.40–32.90 kg/m2) consuming two meal replacements along with snacks and a reduced-calorie dinner meal (approximately 1160 kcal/day) for 3 months had significant weight loss of −6.88 to −7.34 kg (Winick, Rothacker, & Norman, Citation2002). However compared with men (−8.45 to −8.47 kg), the women in the latter study lost less overall weight (−5.26 to −6.38 kg) at the 12-week mark (Winick et al., Citation2002). Another randomized controlled trial using a soy-based meal replacement in 100 obese subjects concluded that, compared with control, the treatment group lost more weight (−7 vs. −2.9 kg) and had a greater reduction in waist circumference (−6 vs. −2.9 cm) after 12 weeks of intervention (Allison et al., Citation2003). While there is some research indicating that soy-protein-based diets may augment weight loss and preserve muscle mass (Diebert et al., Citation2004), this conclusion regarding soy protein and weight loss is controversial (St-Onge, Claps, Wolper, & Heymsfield, Citation2007). A comparison of casein- and soy-based meal replacements determined that both groups of obese women lost weight in a similar fashion of 12.5% and 11.3% from baseline, respectively, after 4 months (Anderson, Fuller, Patterson, Blair, & Tabor Citation2007).
The primary protein source in the PGX® meal replacement was whey protein. High-protein supplements using either casein or whey (2 × 25 g/day) along with a low-fat diet in healthy obese subjects after losing weight on a very low-calorie diet resulted in better weight maintenance compared with the high-carbohydrate group (−2.3 kg difference) after 3 months of treatment (Claessens, Van Baak, Monsheimer, & Saris, Citation2009). This improvement in weight control may be due to whey protein and its peptides in increasing satiety through its physiological effect on promoting cholecystokinin (CCK) and GLP-1 release while reducing ghrelin (Luhovvy, Akhavan, & Anderson, Citation2007). Whey as a functional food has positive effects on satiety and may be superior to other types of proteins (i.e., tuna, turkey and egg) in reducing appetite (Pal & Ellis, Citation2010). Moreover, whey protein seems to decrease hunger more than soy or casein, but this action may be dependent on a lower (10%) or higher (25%) quantity of whey protein consumed (Veldhorst et al., Citation2009).
Like whey protein, the use of viscous polysaccharides has been shown to influence satiety and reduce food intake (Lyly et al., Citation2009). PGX® is a novel functional fibre complex (Abdelhameed et al., Citation2010; Harding, Smith, Lawson, Gahler, & Wood, Citation2011). PGX® has been shown to be safe and well tolerated in rodents (Matulka et al., Citation2009) and humans (Carabin et al., Citation2009). No mutagenic effects of PGX® were demonstrated in genotoxicity studies using bacterial reverse mutation and mouse micronucleus assays (Marone et al., Citation2009). A double-blind, randomized controlled trial has demonstrated the potential for PGX® as a candidate for weight modification (Lyon et al., Citation2011). In this 15-week trial, non-dieting obese and overweight women were supplemented with 5–15 g/day of PGX® or inulin. Results showed that there was a statistically significant reduction in both average body weight (−1.6 kg) and waist circumference (−2.8 cm) in the PGX® group compared with the inulin group (Lyon et al., Citation2011).
Several factors could account for this improvement in body weight. Specific PGX®-related research has determined that PGX® not only reduces post-prandial blood glucose levels and the GI of foodstuffs, but it may improve the physiological actions of appetite hormones, notably increasing PYY levels in humans (Reimer et al., Citation2010). The limitations of our preliminary clinical programme is that we did not collect a food diary from our participants and as such it is not clear how much their restriction in calories may have been due to the introduction of the meal replacement.
Consumption of whey-protein-based meal replacement in conjunction with highly viscous soluble fibre in the form of PGX® can have a significant and beneficial effect on weight loss on overweight and obese participants. Given the worldwide obesity epidemic and the initial positive outcomes obtained in this open clinical programme, the use of PGX® meal replacements and additional PGX® fibre along with a controlled dietary intake should be further investigated as a natural solution for short-term and long-term weight management.
Conflict of interest
RGR is a consultant to the parent company of the sponsor. RAR receives consulting fees from InovoBiologic Inc. VK is an employee of the CCFM. SP has no conflict of interest. SW is a consultant to InovoBiologic Inc. RJG is the owner of the Factors Group of Nutritional Companies and retains an interest in PGX®.
PGX® and PolyGlycopleX® are trademarks of InovoBiologic Inc. Slim Styles® is a trademark of Natural Factors. All other marks are the property of their respective owners.
Acknowledgements
We acknowledge InovoBiologic Inc., Calgary, AB, Canada for the support of this programme and the supply of PGX®.
References
- Abdelhameed , A. S. , Ang , S. , Morris , G. A. , Smith , I. , Lawson , C. , Gahler , R. , … and Harding , S. E. 2010 . An analytical ultracentrifuge study on ternary mixtures of konjac glucomannan supplemented with sodium alginate and xanthan gum . Carbohydrate Polymers , 81 : 141 – 148 .
- Allison , D. B. , Gadbury , G. , Schwartz , L. G. , Murugesan , R. , Kraker , J. L. , Heshka , S. , … and Heymsfield , S. B. 2003 . A novel soy-based meal replacement formula for weight loss among obese individuals: A randomized controlled trial . European Journal of Clinical Nutrition , 57 : 514 – 522 .
- Anderson , J. W. , Fuller , J. , Patterson , K. , Blair , R. and Tabor , A. 2007 . Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: Randomized controlled trial . Metabolism Clinical and Experimental , 56 : 280 – 288 .
- Birketvedt, G. S., Shimshi, M., Erling, T., & Florholmen, J. (2005). Experiences with three different fiber supplements in weight reduction. Medical Science Monitor, 11, PI5–8.
- Brand-Miller , J. C. , Atkinson , F. S. , Gahler , R. J. , Kacinik , V. , Lyon , M. R. and Wood , S. 2012 . Effects of added PGX®, a novel functional fibre, on the glycaemic index of starchy foods . British Journal of Nutrition , 108 : 245 – 248 .
- Carabin , I. G. , Lyon , M. R. , Wood , S. , Pelletier , X. , Donazzolo , Y. and Burdock , G. A. 2009 . Supplementation of the diet with the functional fiber PolyGlycoplex is well tolerated by healthy subjects in a clinical trial . Nutrition Journal , 8 : 9
- Claessens , M. , Van Baak , M. A. , Monsheimer , S. and Saris , W. H. 2009 . The effect of a low-fat, high protein or high carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors . International Journal of Obesity , 33 : 296 – 304 .
- Davis , L. M. , Coleman , C. , Kiel , J. , Rampolla , J. , Hutchisen , T. , Ford , L. , … and Hanlon-Mitola , A. 2010 . Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: A randomized controlled trial . Nutrition Journal , 9 : 11
- Deibert , P. , König , D. , Schmidt-Trucksaess , A. , Zaenker , K. S. , Frey , I. , … and Berg , A. 2004 . Weight loss without losing muscle mass in pre-obese and obese subjects induced by a high-soy-protein diet . International Journal of Obesity and Related Metabolic Disorders , 28 : 1349 – 1352 .
- Grover , G. J. , Koetzner , L. , Wicks , J. , Gahler , R. J. , Lyon , M. R. , … and Wood , S. 2011 . Effects of the soluble fiber complex PolyGlycopleX® (PGX®) on glycemic control, insulin secretion, and GLP-1 levels in Zucker diabetic rats . Life Sciences , 88 : 392 – 399 .
- Harding , S. E. , Smith , I. H. , Lawson , C. J. , Gahler , R. J. and Wood , S. 2011 . Studies on macromolecular interactions in ternary mixtures of konjac glucomannan, xanthan gum and sodium alginate . Carbohydrate Polymers , 83 : 329 – 338 .
- Howarth , N. C. , Saltzman , E. and Roberts , S. B. 2001 . Dietary fiber and weight regulation . Nutrition Reviews , 59 : 129 – 139 .
- Jenkins , A. L. , Kacinik , V. , Lyon , M. and Wolever , T. M. S. 2010 . Effect of adding the novel fiber, PGX®, to commonly consumed foods on glycemic response, glycemic index and GRIP: A simple and effective strategy for reducing post prandial blood glucose levels – a randomized, controlled trial . Nutrition Journal , 9 : 58
- Lee , K. , Lee , J. , Bae , W. K. , Choi , J. K. , Kim , H. J. and Cho , B. 2009 . Efficacy of low-calorie, partial meal replacement diet plans on weight and abdominal fat in obese subjects with metabolic syndrome: A double-blind, randomised controlled trial of two diet plans – one high in protein and one nutritionally balanced . International Journal of Clinical Practice , 63 : 195 – 201 .
- Luhovvy , B. L. , Akhavan , T. and Anderson , G. H. 2007 . Whey proteins in the regulation of food intake and satiety . Journal of the American College of Nutrition , 26 : 704S – 712S .
- Lyly , M. , Liukkonen , K. H. , Salmenkallio-Marttila , M. , Karhunen , L. , Poutanen , K. and Lähteenmäki , L. 2009 . Fibre in beverages can enhance perceived satiety . European Journal of Nutrition , 48 : 251 – 258 .
- Lyon , M. R. and Reichert , R. G. 2010 . The effect of a novel viscous polysaccharide along with lifestyle changes on short-term weight loss and associated risk factors in overweight and obese adults: an observational retrospective clinical program analysis . Alternative Medicine Review , 15 : 68 – 75 .
- Lyon , M. , Wood , S. , Pelletier , X. , Donazzolo , Y. , Gahler , R. and Bellisle , F. 2011 . Effects of a 3-month supplementation with a novel soluble highly viscous polysaccharide on anthropometry and blood lipids in nondieting overweight or obese adults . Journal of Human Nutrition and Dietetics , 24 : 351 – 359 .
- Marone , P. A. , Lyon , M. , Gahler , R. , Donath , C. , Hofman-Hüther , H. and Wood , S. 2009 . Genotoxicity studies of PolyGlycopleX (PGX): A novel dietary fiber . International Journal of Toxicology , 28 : 318 – 331 .
- Matulka , R. A. , Lyon , M. R. , Wood , S. , Marone , P. A. , Merkel , D. J. and Burdock , G. A. 2009 . The safety of PolyGlycopleX® (PGX®) as shown in a 90-day rodent feeding study . Nutrition Journal , 8 : 1
- Pal , S. and Ellis , V. 2010 . The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men . British Journal of Nutrition , 104 : 1241 – 1248 .
- Pal , S. , Khossousi , A. , Binns , C. , Dhaliwal , S. and Ellis , V. 2011 . The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin, and other metabolic syndrome risk factors in overweight and obese individuals . British Journal of Nutrition , 105 : 90 – 100 .
- Papathanasopoulos , A. and Camilleri , M. 2010 . Dietary fiber supplements: Effects in obesity and metabolic syndrome and relationship to gastrointestinal functions . Gastroenterology , 138 : 65 – 72 .
- Poole , C. N. , Roberts , M. D. , Dalbo , V. J. , Tucker , P. S. , Sunderland , K. L. , DeBolt , N. D. , … and Kerksick , C. M. 2011 . The combined effects of exercise and ingestion of a meal replacement in conjunction with a weight loss supplement on body composition and fitness parameters in college-aged men and women . Journal of Strength and Conditioning Research/National Strength & Conditioning Association , 25 : 51 – 60 .
- Reimer , R. A. , Grover , G. J. , Koetzner , L. , Gahler , R. J. , Lyon , M. R. and Wood , S. 2011 . The soluble fiber complex PolyGlycopleX lowers serum triglycerides and reduces hepatic steatosis in high-sucrose-fed rats . Nutrition Research , 31 : 296 – 301 .
- Reimer , R. A. , Pelletier , X. , Carabin , I. G. , Lyon , M. , Gahler , R. , … and Wood , S. 2010 . Increased plasma PYY levels following supplementation with the functional fiber PolyGlycopleX in healthy adults . European Journal of Clinical Nutrition , 64 : 1186 – 1191 .
- Salas-Salvadó , J. , Farrés , X. , Luque , X. , Narejos , S. , Borrell , M. , Basora , J. , … and Balanza , R. 2008 . Effect of two doses of a mixture of soluble fibres on body weight and metabolic variables in overweight or obese patients: A randomised trial . British Journal of Nutrition , 99 : 1380 – 1387 .
- Smith , T. J. , Sigrist , L. D. , Bathalon , G. P. , McGraw , S. , Karl , J. P. and Young , A. J. 2010 . Efficacy of a meal-replacement program for promoting blood lipid changes and weight and body fat loss in US Army soldiers . Journal of the American Dietetic Association , 110 : 268 – 273 .
- Statistics Canada (2010). Canadian Health Measures Survey: Cycle 1 data tables 2007 to 2009. Statistics Canada. Retrieved May 19, 2011, from http://www.statcan.gc.ca/daily-quotidien/100113/dq100113a-eng.htm
- St-Onge , M. P. , Claps , N. , Wolper , C. and Heymsfield , S. B. 2007 . Supplementation with soy-protein-rich foods does not enhance weight loss . Journal of the American Dietetic Association , 107 : 500 – 505 .
- Tuomilehto , J. , Voutilainen , E. , Huttunen , J. , Vinni , S. and Homan , K. 1980 . Effect of guar gum on body weight and serum lipids in hypercholesterolemic females . Acta Medica Scandinavica , 208 : 45 – 48 .
- Vázquez , C. V. , Montagna , C. , Alcaraz , F. , Balsa , J. A. , Zamarrón , I. , Arrieta , F. and Botella-Carretero , J. I. 2009 . Meal replacement with a low-calorie diet formula in weight loss maintenance after weight loss induction with diet alone . European Journal of Clinical Nutrition , 63 : 1226 – 1232 .
- Veldhorst , M. A. , Nieuwenhuizen , A. G. , Hochstenbach-Waelen , A. , van Vught , A. J. , Westerterp , K. R. , … and Westerterp-Plantenga , M. S. 2009 . Dose-dependent satiating effect of whey relative to casein or soy . Physiology & Behavior , 96 : 675 – 682 .
- Vuksan , V. , Panahi , S. , Lyon , M. , Rogovik , A. L. , Jenkins , A. L. and Leiter , L. A. 2009 . Viscosity of fiber preloads affects food intake in adolescents . Nutrition, Metabolism & Cardiovascular Diseases , 19 : 498 – 503 .
- Walsh , D. E. , Yaghoubian , V. and Behforooz , A. 1984 . Effect of glucomannan on obese patients: a clinical study . International Journal of Obesity , 8 : 289 – 293 .
- Winick , C. , Rothacker , D. Q. and Norman , R. L. 2002 . Four worksite weight loss programs with high-stress occupations using a meal replacement product . Occupational Medicine , 52 : 25 – 30 .
- World Health Organization (WHO) (2011). Obesity and overweight. World Health Organization. Retrieved May 19, 2011, from http://www.who.int/mediacentre/factsheets/fs311/en/index.html