Abstract
Zebrafish offer a unique vertebrate model for research areas such as drug development, disease modeling and other biological exploration. There is significant conservation of genetics and other cellular networks among zebrafish and other vertebrate models, including humans. Here we discuss the recent work and efforts made in different fields of biology to explore the potential of zebrafish. Along with this, we also reviewed the concept of systems biology. A biological system is made up of a large number of components that interact in a huge variety of combinations. To understand completely the behavior of a system, it is important to know its components and interactions, and this can be achieved through a systems biology approach. At the end of the paper we present a concept of integrating zebrafish into the systems biology approach.
Introduction
The dispute between two philosophies, reductionism and holism, has persisted for years. On one hand, the reductionist approach is useful to understand accurately the functions of the smallest unit at an individual level but loses the information of the synergistic effects of multiple units involved in an entity; the opposite is true for a holistic approach (Figure ). The time has come to combine these two approaches in a more flexible and open approach. This is what is known as systems biology. The definition of systems biology is still a subject of discussion. It can be defined in many ways, for example as .an approach to measure a multiple component system quantitatively’ (Way & Silver, Citation2007) or as ‘an exploration of different biological parts functionality and intractability which give rise to the system and its behavior’ (Kohl, Crampin, Quinn, & Noble, 2010) or as ‘the understanding of this biological system and systematic understanding and examining of various variables and components making this system’ (Ideker, Galitski, & Hood, Citation2001). A biological system is made up of a large number of components, which can interact in a huge variety of combinations. Thus, complex interactions and synergism are a vital part of the biological system. Systems biology is not really a new concept: it can be traced back to the first knowledge of a human being about its system or its interaction with the system. Today, we knowingly or unknowingly apply this knowledge in our daily life, when we choose what is good for ourselves or how we are going to interact with the system in which we live. If we translate this concept into science, the only difference will be in its application, as the use of a more systematic approach assisted by advanced techniques and/or computational models will lead us to a better understanding. According to Kitano (Citation2007), a system is not tangible, but its parts – genes or metabolites, for example – can be identified and even quantified. These, however, represent just a snapshot of a part, but the center or strength of the systems biology lies in the dynamics of the system that one can try to understand eventually, by joining different snapshots. Thus, defining a system is difficult, and while one can analyze the smallest unit, say, a nucleus of the cell as a part of the system, the nucleus could, in fact, be a whole new system. Systems biology is, therefore, an approach rather than a new discipline. As an approach, systems biology can be applied to a small or large biological singleton (Kohl et al., Citation2010). Thus, unlike the reductionist approach, Systems Biology relies upon the understanding or investigation of system dynamics as a whole instead of studying the individual components of the system. So we can say that systems biology is a sort of data integration approach, in which information from all relevant different fields including biology, pharmacology, omics, engineering, chemistry, computation, mathematics and physics will be considered.
The reductionist approach is based on a hypothesis that selects a model that subsequently is validated or falsified by means of a series of experiments. Conversely, in a typical systems approach, one makes as many different observations of the system as possible under different conditions and analyzes all data to look for possible correlations between the observations.
In the past years, the development of novel drugs seems to have been stalling. The single compound–single-target approach has been more or less exhausted and the search for new drugs for existing targets is no longer economically feasible. A paradigm shift in drug development has been long overdue. The basis of a new approach could be the hypothesis that most discoveries are multifunctional, thus novel drugs should have an effect on different targets, either because the compound itself has activity on several targets, like one of the most successful drugs ever, salicylic acid, or by using mixtures of compounds. In the latter case, synergy in any of its many forms could play an important role. Unfortunately, synergism is not easy to develop other than in simple forms as in augmentin, in which the effect of an antibiotic is improved by decreasing its availability (reducing metabolism). Particularly in traditional medicine, one might find examples of synergism, for example the antifungal effect of Solanum alkaloids (Fewell & Roddick, Citation1993). One of the challenges for finding novel drugs could thus be to develop tools to detect synergistic effects in medicinal plants as a lead for drug development.
Because of the complexity of the chemical composition of traditional medicines, it is difficult to detect synergism when applying classical bioassay-guided fractionation schemes. It is reasonable to attribute the loss of activity in any step of the fractionation to synergism, but it could also due to the decomposition of compounds. In methods like Hippocratic screening, the activity of a whole organism is measured, including all sorts of synergism at a pharmacokinetic and pharmacodynamic level (Malone & Robichaud, Citation1962; Taesotikul, Panthong, Kanjanapothi, Verpoorte, & Scheffer, Citation1989). Hippocratic screening was based on a rodent model in which regular observations were made after the administration of a drug or an extract. The activity of unknown samples could be classified by validation with all major drug classes, after which additional, more specific statistics on the activity could be performed. Certain inherent characteristics of Hippocratic screening, i.e., its high labor intensity and dependence on animal experiments that are increasingly restricted, led to its replacement by other, mainly target-based approaches, including molecular modeling. So, is it possible to recover the Hippocratic screening method by keeping to its principle but dropping its drawbacks? The idea would be to apply it to develop novel drugs or drug mixtures from traditional medicines. The requirements would be that it should be modified to (i) use living cells, (ii) allow high throughput, (iii) avoid animal experiments and (iv) have a broad range of activation. Obviously, screening for antibiotics and antitumor compounds can be done with microbe or cancer cell lines respectively. For other bioactivities, however, the whole organism would be the ideal model. This is where zebrafish come into the picture. In the past years, there have been reports of a number of specific test systems that use zebrafish. The question is whether the zebrafish could be used for a ‘Hippocratic screening’, that is, if they can be used to observe a whole system with the purpose of classifying and quantifying a certain biological activity. Most of the present models of zebrafish are, like the classic Hippocratic screening, based on phenotyping observations (e.g., morphology). Nowadays, however, the ‘omics’ offer new possibilities to observe the total system and get a better insight into the possible modes of action related to the phenotypic changes after certain treatments of an organism.
In this review, therefore, we consider the use of omics for zebrafish and discuss the possibilities of applying ‘Hippocratic screening’ to medicinal plants using zebrafish.
Different ways of implementing a systems biology approach
There are different ways to implement a systems biology approach, a few of which are briefly discussed below.
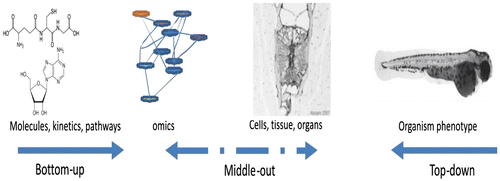
Top-down approach
In simple words, a top-down approach is like a bird’s eye view of a whole system (Bruggeman & Westerhoff, Citation2007): start from the top and move towards the bottom, zooming in for more detail. With the development of more sophisticated techniques, tools and knowledge, this approach seems to be quite attractive and useful. Thus, one can start by applying genomics, proteomics, metabolomics or fluxomics and then use analysis and integration techniques to examine the correlation between different micro parts of systems. This would allow the prediction of some new concepts and the formulation of hypotheses that could be tested by using the same approach and different sets of experiments further or using a more targeted (Katagiri, Citation2003), reductionist approach to validate the models and hypothesis.
Bottom-up approach
Conversely, the bottom-up approach can be likened to a worm’s eye view. In simple words, this approach starts from the lowest or smallest unit of the system. For reasons of convenience we can consider a gene as the beginning of the organization of the system in an organism. Genes are static elements that are, in principle, present in all cells of the system. This stored information is regulated from the dynamics of the system in order to adapt to its need to function under different conditions. The goal of this approach is to study these micro-components of the system, understand their dynamics and kinetics (Forst, Citation2006) and through the understanding of their interactions and correlations move up in the system from the genes to mRNA, proteins and, finally, to metabolites and the metabolic network. Bottom-up approaches use these data on the hypothesis that regulation of the following entity is responsible for specific changes and can be tested by using an omics approach. This approach, however, becomes more difficult as one moves toward more complex interactions of the system.
Middle-out approach
A more moderate approach, or as the name indicates, an approach that is midway between top-down and bottom-up, was proposed by a Canadian group (Munkittrick & McCarty, Citation1995) as a way of overcoming difficulties in understanding the link in a large ecosystem and the pharmacokinetics in an individual organism in that ecosystem. The core data obtained by the middle-out approach can be used to move upwards (population level) or downwards (molecular level; McCarty & Mackay, Citation1993). The middle-out system is of particular importance in understanding chemical-impact-derived ecological problems, while the bottom- up and top-down approaches are more suited to measuring a single organism under well-controlled experimental conditions, thus focusing on a single organism. These three approaches are illustrated in Figure .
Zebrafish as a model for systems biology
Zebrafish first appeared as a vertebrate model after the pioneering work of Dr. George Streisinger (Streisinger, Walker, Dower, Knauber, & Singer, Citation1981). From there it made its way into developmental biology and genetics. As a vertebrate model, it is quite small compared with other vertebrate models used for research purposes, such as mice or rats. The adult fish can grow to 3 cm or more in length and because of its small size is very easy to maintain and manage within a small space. During the early stages of the embryonic larval life, zebrafish are only a few millimeters long. They can survive 6–7 days post-fertilization (dpf) solely on the nutrients stored in the yolk sac. Owing to their small larval size, they can be managed in standard 96- or 348-well plates with a minimum media requirement (100–250 ul). There are many advantages of zebrafish over other vertebrate models. One is their high speed of development, since within 72 h post-fertilization (hpf), embryogenesis is almost complete and most organs are fully developed and functional (Parng, Seng, Semino, & McGrath, 2002). Another advantage is that because of external fertilization, their minimal parental care reduces any epigenetic parental influence. An interesting feature is their transparency as it allows real-time life imaging. Yet another advantage is that, given their low mass, the amount of drugs required for tests is very small compared with other vertebrate models, a requirement that is especially important in the case of expensive or novel drugs. Transparency and ease of genetic manipulation of zebrafish embryo resulted in the production of transgenic lines for different type of bioassays (Kishi et al., Citation2008; Trede et al., Citation2008; Chen et al., Citation2011; Parsons et al., Citation2002; Tong et al., Citation2009). This kind of transgenic lines or mutants makes real-time observation of organs and other systems possible, especially after combining with fluorescence proteins as markers (Detrich & Kevin, Citation2008; Gong et al., Citation2002; Higashijima., Okamoto, Ueno, Hotta, & Eguchi, Citation1997). All these advantages make zebrafish a unique model for research in all fields. A comparison of different features of zebrafish and other models is shown in Table .
Table 1. A general comparison of different features among vertebrate models.
Various methods for screening of bioactivities have been reported. Here we discuss a few of them and consider the applications of zebrafish in research, particularly when following a systems biology approach.
Zebrafish in genetics and disease modeling
Zebrafish have proven to be quite interesting for developing disease models. Before developing models for human diseases, one important question has been the conservation of the candidate genes for disease (Cox et al., Citation2009). The zebrafish model is relatively new compared with pre-existing models such as mice, guinea pigs or primates. Surprisingly, conservation of genes among humans and fish is quite high, for example Barbazuyk et al. (2000) have found that ∼ 80% of 523 orthologous genes fall in conserved synteny. Zebrafish have almost the same number of chromosomes (25) as humans (23). Evolutionary data indicate that, after the separation of tetrapod lineage from fish lineage, a whole genome duplication process occurred in early stages of the teleost lineage (Spitsbergen & Kent, Citation2003). As a result, many duplicate genes of mammalian species occur in different chromosomes of teleost fishes. Not all genes, though, were preserved during the course of time and it is estimated that 20% of the genes are conserved (Spitsbergen & Kent, Citation2003). It has been suggested that, although there are large blocks of synteny conservation between human and zebrafish, gene orders are frequently inverted and transposed (Postlethwait et al., Citation2000). With the advancement in genetics and especially in the field of reverse genetics, scientists are able to mimic many traits of interest in zebrafish embryo. The early work done by Streisinger’s group provides a good basis for of genetic studies (Dodd, Curtis, Williams, & Love, Citation2000). As mentioned above, among the analyzed genes, 80% fall into the conserved synteny group. In disease modeling, it is important to isolate genes that are analogous to genes identified in humans previously. In the case of zebrafish it has been suggested that during evolution events of gene duplication may have resulted in paralogous genes (Sun et al., Citation2008). This complicates the isolation of orthologues genes for disease modeling. But through the zebrafish genome-sequencing project it is possible to identify and correlate genes that are orthologs, so isolation and identification of genes seem to be a good tool. Different approaches have been developed to produce and analyze different mutants. One of them uses ethylnitrosourea (ENU), which offers random mutation as a source of mutagenesis in zebrafish; while another approach is the use of retroviral techniques that cause point mutation in embryos (D. Wang et al., Citation2007; Knapik, Citation2000). Owing to the transparency of the embryo, phenotypic assessment is much easier than with other mammalian models (Lieschke and Currie, Citation2007). These approaches produced many useful mutants with similar phenotypic and genetic attributes of human diseases (Muto et al., Citation2005). Other approaches used a more targeted mutation that included the production of the transgenic lines, by injecting RNA or DNA construct into embryos in their early developmental stages. Studies on the production of transgenic lines that are similar to human diseases have been extensively reviewed (Sager, Bai, & Burton, Citation2010; Paquet, Schmid, & Haass, Citation2010; Liu & Leach, Citation2011). One major breakthrough in this field has been the production of lines with a fluorescent protein as a marker to record and quantify the promoter activity or to determine the function of specific cells or organs in the embryo. For example, Jung et al. (Citation2009) developed the Tg(mbp:egfp) line to understand the myelination process, and similar lines were developed to understand diseases such as hemochromatosis, leukemia, inflammation and steatohepatitis (Bian et al., Citation2011; Langenau et al., Citation2003; Mathias et al., Citation2006; Amali et al., Citation2006). The TILLING (targeting induced local lesions in genomes) and antisense morpholino oligoneucleotides approaches also help to produce mutations in a localized manner, and these techniques have been applied successfully to produce zebrafish mutants with orthologs to human diseases (Moens, Donn, Wolf-Saxon, & Ma, Citation2008; Amsterdam & Hopkins, Citation2006; Goldsmith & Jobin, Citation2012). Below are some examples of the use of zebrafish as a disease model (Table ).
Table 2. Some examples of mutation based disease models in zebrafish.
Zebrafish and omics
The omics approach involves the systemic characterization of an organism at all four levels of life: genes, mRNA, proteins and metabolites – that is, the profiling of genome, transcriptome, proteome and metabolome. Obviously the omics are the basis of systems biology. Genomics were discussed previously, so we now deal with the other three omics: transcriptomics, proteomics and metabolomics. Omics are widely used to reveal the complex nature of living systems and the interrelation of different components (Wishart, Citation2008; Kültz et al., Citation2007; Nibbe, Koyutürk, & Chance, Citation2010). In the case of zebrafish, the use of omics is relatively new compared with other models. Here we discuss mainly three omics approaches, namely zebrafish in transcriptomics, proteomics and metabolomics.
Zebrafish in transcriptomics
This approach deals with the total set of transcripts under a specific condition of an organism or cell. The main goal of transcriptomics is to measure all kind of transcripts, including mRNA, non-coding RNA or any other small RNA, to determine the expression of genes in specific conditions (Z. Wang, Gerstein, & Snyder, 2009). Different methods are available to measure transcripts such as micro-arrays, cDNA sequence methods, Tag base methods and RNA sequence-based methods (Shiraki et al., Citation2003; X.S. Liu, Citation2007; Gerhard et al., Citation2004; Harbers and Carninci, Citation2005; Z. Wang et al., Citation2009; Ingolia, Brar, Rouskin, McGeachy, & Weissman, Citation2012). A discussion of the advantages or limitations of the technique is outwith the scope of this review, so we shall focus mainly on its usage on zebrafish.
As mentioned above, there is already evidence of genetic homology between zebrafish and humans, which makes it a very convenient model for developmental studies. Vesterlund, Jiao, Unneberg, Hovatta, and Kere, (Citation2011) used an RNA sequence technique to measure the transcript profile of four early developmental stages of embryos (1-cell stage, 16-cell stage, 512-cell stage, 50% epiboly) and found that at the onset of 50% epiboly, molecular functions of genes change significantly from previous stages and exhibit a switch in the developmental transcriptome. Similarly, in another study, Aanes et al. (Citation2011) have demonstrated the dynamics of the mid-blastula stage and the role of the maternal transcripts in the dynamics of some new transcribed regions.
Along with developmental studies, transcriptomics has also been applied in the field of disease modeling in zebrafish. The regeneration capacity of zebrafish organs led researchers to investigate its mechanism, with the hope of finding clues for human heart regeneration, for example. Applying microarray analysis, several researchers found an upregulation of the expression of the genes cyclin A2 (ccna2) and cell division cycle 2 (cdc2; Sleep et al., Citation2010); the constitutive expression of the former gene also seems to be involved in prolonged postnatal hyperplasia in mice (Chaudhry et al., Citation2004). Zheng et al. (Citation2011) have investigated the homology between the zebrafish swim bladder and lungs of mammals, including humans. They found that the genes responsible for the human lungs proteins, namely SP-A, SP-B and SP-C, have their orthologs expressed during the development and functioning of the swim bladder of zebrafish.
Thus, transcriptomics can help to unravel the role of genes in various physiological functions using zebrafish as a model.
Zebrafish in proteomics
Proteins are synthesized from genes through transcription and translation. To understand the role of genes and measure their function, it is very important to identify the end product of gene expression. Proteins play different roles at different levels. They may form complexes with other macromolecules and membranes to achieve different functions at a cellular level. After translation, a protein will suffer more modifications such as phosphorylation and glucosylation. So, for every single gene, several proteins are usually formed through post-translational processing. Therefore, to identify, quantify and characterize the proteins in an organism, it is very important to understand the system. This is where proteomics comes into play; by definition, proteomics is the global profiling of proteins or the large-scale measurement of all the proteins, their isoforms and all post-translational changes.
Proteomics surpasses genomics and transcriptomics in terms of understanding functions more elaborately, since mRNA or DNA analyses are unable to explain post-translational changes that often can modify the function of proteins. Proteomics is a kind of middle approach as it complements transcriptomics data by linking them to metabolic pathways (Sukardi, Ung, Gong, & Lam, Citation2010).
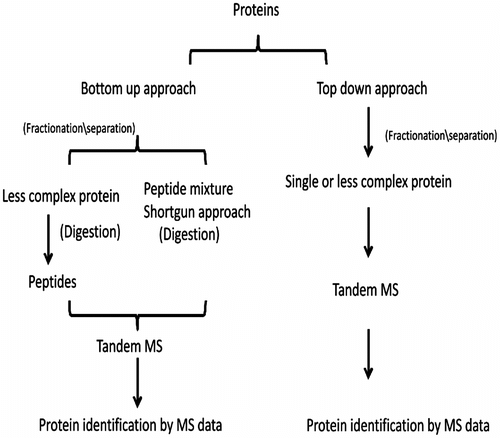
There are several techniques that can be applied to identify and quantify proteins, but the most important is mass spectrometry (Gygi, Corthals, Zhang, Rochon, & Aebersold, Citation2000; Gstaiger & Aebersold, Citation2009). Generally, two types of approach (Figure ) are used for proteomics, i.e., the top-down or bottom-up approach, both of which have been discussed. Thus we can start from complex proteins and move downwards to the amino acids configuration level or start from simple peptides and predict more complex proteins. The pros and cons of these approaches are well reviewed by (Han, Aslanian, & Yates, Citation2008; Guerrera & Kleiner, Citation2005). Because of the complexity of protein structures, it is important to fractionate and separate them from mixtures by means of 2D gel electrophoresis before subjecting them to mass spectrometry (Wu, Wang, Baek, & Shen, Citation2006). Monteoliva & Albar (Citation2004) and Herbert et al. (Citation2001) have reviewed different methods of using gel electrophoresis for this purpose. Modern closed chromatography systems (such as high performance liquid chromatography, HPLC) and capillary electrophoresis are gaining interest for the separation of proteins, offering the advantage of online mass spectrometry (Mitulović & Mechtler, Citation2006; Reinders, Zahedi, Pfanner, Meisinger, & Sickmann, Citation2006; Issaq, Chan, Hanini, Conrads, & Veenstra, Citation2005). The possibility of quantifying proteins in proteomics makes it a very valuable tool in systems biology and in the same way as different methodologies allow their identification and profiling, several techniques can be used for their quantitative analysis. One of these involves the addition of isobaric tags that split the proteins in specific sequences and work as an internal standard, as described in detail by Ross et al., Citation2004). Another method incorporates stable isotopes (2H, 13C, 15N and 18O) in peptides through labeled amino acids (stable isotope labeling by amino acids in cell culture, SILAC; S.E. Ong et al., Citation2002). The presence of these isotopes in peptides produces peaks with a mass shift and can thus be used to quantify the proteins (Monetti, Nagaraj, Sharma, & Mann, Citation2011). Another option is a label-free method, in which case quantification can be done either by spectral counting or peptide signal abundance and comparison of the extracted ion chromatogram (XIC), both of which provide an idea of the relative abundance of the protein in question (M. Wang, You, Bemis, Tegeler, & Brown, Citation2008; Higgs, Knierman, Gelfanova, Butler, & Hale, Citation2005; Gilchrist et al., Citation2006).
To understand and decipher complex processes and reactions to development, disease and drug interaction, the precise quantitation and identification of involved proteins is very important and, thanks to its many advantages, zebrafish have proved to be an acquiescent model of choice. The development of zebrafish at two advanced stages (72 hpf, 120 hpf) has been successfully studied by Lucitt et al. (Citation2008) using 2D polyacrylamide gel electrophoresis (PAGE)–matrix assisted laser desorption ionization-tandem time of flight (MALDI-TOF/TOF) and 2D liquid chromatography electron spray ionization tandem mass spectrometer (LC ESI-MS/MS) techniques. Having detected 1384 proteins, they then applied 2D differential gel electrophoresis (DIGE) and observed that those proteins involved in energy production and translation were more abundant at 72 hpf than at 120 hpf owing to the requirement of increased synthesis of cellular protein. Global changes in protein profiles of the developing embryos from 10 different stages covering the gastrulation period (6, 8 and 10 hpf), segmentation period (12, 14 and 18 hpf), start of the pharyngula stage (24 hpf), beginning (48 hpf) and end (72 hpf) of the hatching phase, and 1-week-old larvae have been studied by Tay et al. (Citation2006) using 2D gels combined with MALDI TOF/TOF MS. Their results indicate clear changes in protein abundance after 18 hpf with 49% of the proteins from 6 hpf still present in up to 1-week-old larvae. Among the identified proteins, 108 proteins are derived by 55% of genes and are cytosolic, cytoskeletal and nuclear proteins. Zebrafish can also be used in the investigation of age-related diseases such as Alzheimer’s or Parkinson’s disease. In an attempt to understand the changes and abundance of different proteins during aging, Abramsson et al. (Citation2010) analyzed the proteome profile of different organs of adult zebrafish and compared them with 120-hpf embryos. They found that the proteins matrilin, opsin and tenascin that are more involved in translation, signal transduction and receptor activity were more abundant in embryos, while proteins with transporter activity were more abundant in adult zebrafish.
The proteomics approach was used for toxicity testing of different drugs and toxins using zebrafish. In a study by Ponnudurai et al. (Citation2012), cyclosporine A, an immune suppressor, caused an alteration in the levels of proteins involved in the cytoskeleton, lipid binding, stress response and metabolism. The effect of microcystin-RR (known as cyanotoxin from cyanobacteria) was studied using 2D gel electrophoresis and MALDI-TOF MS (Zhao et al., Citation2012). In a brain-related proteomics study using zebrafish, researchers (Biales, Bencic, Billeneuve, Ankley, & Lattier, Citation2011; Huang, Huang, & Huang, Citation2010) showed that, after the administration of prochloraz and methylparathion, the regulation of binding proteins, cytoskeletal proteins and anti-apoptopic proteins of brain can increase, resulting in cell death and less neuro-protection. In another study Zhuo, Huang, Huang, and Cai (Citation2012), evaluated the effect of tramadol exposure and found that the proteins that were affected were similar to those affected by the use of other abusive agents such as morphine, cocaine and alcohol. They also found out that the identified proteins were mainly involved in four processes: insulin/insulin-like pathway, growth factor signaling pathway, synaptic vesicle cycling process, protein catabolism and carbohydrate catabolism.
Zebrafish in metabolomics
Metabolomics is the quantitative and qualitative measurement of all the metabolites that take part in the metabolic reactions needed for the primary functions in an organism and all secondary functions specific for the survival of an organism (Dunn, Bailey, & Johnson, 2005; Verpoorte, Choi, Mustafa, & Kim, 2008). As mentioned above, gene identification and quantification are not sufficient to understand the complexity of living organisms as there may be post-translational changes or modifications that cannot be measured only by transcriptomics. Hence, the impact of proteins can only be understood by studying phenotypic responses, and to understand these responses we need to identify the metabolic pathways and metabolites. This is where metabolomics comes into the picture (Verpoorte et al., Citation2008).
Different technologies have been applied to measure metabolites in different organisms, for example high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS), gas chromatography mass spectrometry (GC-MS), capillary electrophoresis mass spectrometry (CE-MS), nuclear magnetic resonance spectroscopy (NMR) and MS-MS spectrometry. Pros and cons of the above-listed technologies and their use have been extensively reviewed (Dunn et al., Citation2005; Verpoorte et al., Citation2008; Shulaev, Citation2006; Moco, Vervoort, Bino, De Vos, & Bino, Citation2007).
Metabolomics also seems to be a very promising technology to apply on zebrafish. Though not much work has been done on this so far, recent studies have revealed the huge potential of zebrafish as a model system for addressing various questions about functional genomics and to study bioactivities in the future. Papan and Chen (Citation2009) and Hayashi et al. (Citation2009) have performed metabolomics studies of zebrafish embryogenesis using LC-MS and GC-MS and reported the correlation of metabolites with different developmental stages. They found that the developmental stages are metabolically distinct and reproducible and that there is also a very dynamic pattern of metabolic regulation between different stages. Soanes et al. (Citation2011) have used the multi-curve resolution-weighted alternating least square (MCR-wALS) approach to fuse the data from different platforms such as transcriptomics and metabolomics from NMR and LC-MS. This study showed that changes at a transcript level of different developmental stages follow similar trends to the regulation of metabolites, whereas the housekeeping metabolites (like the housekeeping genes) show a steady-state presence throughout development. Metabolic profiling of the livers of male and female zebrafish was also done (E.S. Ong, Chor, Zou, & Ong, Citation2009)) using different platforms such as NMR, GC-MS and LC-MS. This study showed that, while there is a significant difference between the amino acid and unsaturated lipid profile of male and female zebrafish, there is no difference in their carbohydrate profile. Jang et al. (Citation2012) studied the alcohol-induced fatty liver disease in adult zebrafish and found metabolite regulations to be similar to long-term alcohol consumption, making zebrafish a good model for future studies.
Zebrafish in toxicology and investigations of phenotype
The effect of chemicals or toxins on humans is usually done by screening on rodent models, but these assays are both expensive and time-consuming. Zebrafish could potentially provide an alternative to overcome this problem. A major question in zebrafish toxicological studies is whether the observations are valid for mammalians. As mentioned above, there is a high level of conservation between the zebrafish and human genome and during the early developmental stages of all vertebrates; there is a significant conservation not only in the genetic makeup but also in morphology (Hill, Teraoka, Heideman, & Peterson, Citation2005). This places zebrafish on the high end of the debate. Parng et al. (Citation2002) and Ali, Champagne, Alia, and Richardson (2011) showed comparable LD50 values of different compounds in zebrafish and rodents. Similarly, the screening of 100 compounds that are known to produce bradycardia in humans also showed similar symptoms in zebrafish (Milan, Peterson, Ruskin, Peterson, & MacRae, Citation2003). Among the many advantages that make zebrafish a valuable tool, two prominent ones are their small size and high facsimile rates that allow high-throughput (HT) analysis.
Advancement in robotics engineering in biology makes it possible to screen chemicals and drugs at high rates and in a short time (W. Wang, Liu, Gelinas, Ciruna, & Sun, Citation2007). Development of micro fluid systems (Akagi et al., Citation2012; Wielhouwer et al., Citation2011) have also assisted in screening chemicals under controlled environments (e.g., temperature, flow rate). This can not only reduce the time and cost of the testing system, but also enables the screening of novel and expensive compounds using very small amounts.
Transparency of the zebrafish embryo is another advantage of this model over other organisms, especially in the field of toxicology. It is possible to see almost every organ and follow the development in real time by using optic visualization techniques. The optical clarity of zebrafish, which is impossible in higher-order vertebrates, helps to locate embryos in different developmental stages by visual analysis under a simple dissecting microscope. For example, the effect of different chemicals, including ethanol, during embryogenesis revealed the sensitivity of the stages to ethanol (Ali et al., Citation2011). In situ hybridization techniques can also be applied after chemical perturbation to assess the expression of genes without the need to dissect the tissues or organisms (Thisse and Thisse, Citation2008; Chandrasekar, Arner, Kitambi, Dahlman-Wright, & Lendahl, Citation2011; Parng, Roy, Ton, Lin, & McGrath, Citation2007). Another advantage of optical transparency is the use of staining dyes or fluorescent probes for specific organs or whole embryos; for example, acridine orange and alcian blue have been used in zebrafish embryos to visualize apoptosis for the bone formation process after chemical exposure (Schwend & Ahlgren, Citation2009).
As the embryonic development of zebrafish occurs outside the maternal body, it is much easier to get toxicological readouts. Phenotypic readouts such as animal size or malformation in any organ represent very valuable information in terms of toxicity. By using the appropriate magnification, malformations in cartilage, jaw, brain, ear, notochord and animal size can be quantitatively assessed. Also, by using special movement-tracking devices, neuro-toxicological effects of exposure to chemicals can be analyzed both in zebrafish embryos and adults (De Castro et al., Citation2009; Creton, Citation2009). Other examples of the use of zebrafish in toxicological analysis are shown in Table .
Table 3. Examples of using zebrafish in different toxicological analyses.
Modeling of zebrafish in systems biology
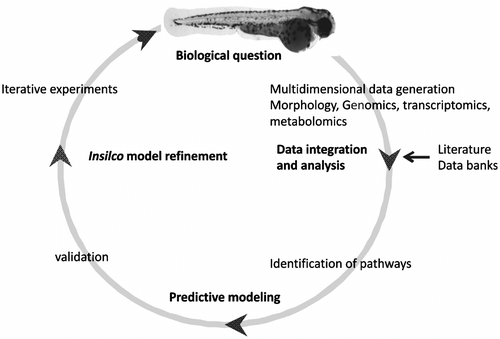
From the discussion above, it is clear that using modern technologies it is possible to generate a huge number of data. Powerful data-processing technology can digitalize the data, for example DNA and RNA sequencing, genomics, proteomics, metabolomics and phenotypic perturbation, and link them through networks. With the aid of different genetic and chemical perturbations, it is possible to develop different study models using zebrafish. As mentioned above, systems biology is mainly a data integration technique, so if we are able to produce and store data related to specific changes phenotypically (top-down approach) or at a molecular level (bottom-up approach), it is quite possible to integrate these data computationally and extract some useful information. But this requires storage data banks. For instance, so far, the only data available for zebrafish are related to gene sequencing and this provides some insight into the genes that are present, but more information is needed to understand how genes are regulated in complex cellular functioning. If we were able to develop a repository for phenotypic, transcriptomics, proteomics and metabolomics data, zebrafish could be a very useful tool in assessing the effect of different perturbations in the whole system by using a systems biology approach (Figure ). Iterative modeling of zebrafish by using the previously mentioned techniques can lead us to more refined in silico information and subsequent modeling (Deo & MacRae, Citation2010). The unique advantages of zebrafish over other models is their ease of manipulation and tractability of genetic material, which can help researchers use a system approach and thus understand correlations between different underlying mechanisms in different conditions, developmental stages and time.
Acknowledgements
MYM is grateful to the Higher Education Commission of Pakistan for its financial support. The authors are also grateful to the National Institute of Horticultural and Herbal Science, Rural Development Administration of Korea (Project No: PJ008700) for their financial support.
Notes
References
- Aanes , H. , Winata , C. L. , Ling , C. H. , Chen , J. P. , Srinivasan , K. G. , Lee , S. G. , … and Aanes , S. 2011 . Zebrafish mRNA sequencing deciphers novelties in transcriptome dynamics during maternal to zygotic transition . Genome Research , 21 : 1328 – 1338 .
- Abramsson , A. , Westman-Brinkmalm , A. , Pannee , J. , Gustavsson , M. , Otter , M. V. , Blennow , K. , … and Zetterberg , H. 2010 . Proteomics profiling of single organs from individual adult zebrafish . Zebrafish , 7 : 161 – 168 .
- Akagi , J. , Khoshmanesh , K. , Evans , B. , Hall , C.J. , Crosier , K. E. , Cooper , J. M. , … and Wlodkowic , D. 2012 . Miniaturized embryo array for automated trapping, immobilization and microperfusion of zebrafish embryos . PLoS One , 7 : e36630
- Ali , S. , Champagne , D. L. , Alia , A. and Richardson , M. K. 2011 . Large-scale analysis of acute ethanol exposure in zebrafish development: A critical time window and resilience . PLoS One , 6 : e20037
- Amali , A. , Rekha , R. , Lin , C. , Wang , W. L. , Gong , H. Y. , Her , G. M. and Wu , J. L. 2006 . Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis . Journal of Biomedical Science , 13 : 225 – 232 .
- Amsterdam , A. and Hopkins , N. 2006 . Mutagenesis strategies in zebrafish for identifying genes involved in development and disease . Trends in Genetics , 22 : 473 – 478 .
- Barbazuk , W. B. , Korf , I. , Kadavi , C. , Heyen , J. , Tate , S. , Wun , E. , … and Johnson , S. L. 2000 . The syntenic relationship of the zebrafish and human genomes . Genome Research , 10 : 1351 – 1358 .
- Biales , A. D. , Bencic , D. C. , Villeneuve , D. L. , Ankley , G. T. and Lattier , D. L. 2011 . Proteomic analysis of zebrafish brain tissue following exposure to the pesticide prochloraz . Aquatic Toxicology , 105 : 618 – 628 .
- Bian , Y. H. , Xu , C. , Li , J. , Xu , J. , Zhang , H. and Du , S. 2011 . Development of a transgenic zebrafish model expressing GFP in the notochord, somite and liver directed by the hfe2 gene promoter . Transgenic Research , 20 : 787 – 798 .
- Brownlie , A. , Donovan , A. , Pratt , S. J. , Paw , B. H. , Oates , A. C. , Brugnara , C. , … and Zon , L. I. 1998 . Positional cloning of the zebrafish sauternes gene: A model for congenital sideroblastic anaemia . Nature Genetics , 20 : 244 – 250 .
- Bruggeman , F. J. and Westerhoff , H. V. 2007 . The nature of systems biology . Trends in Microbiology , 15 : 45 – 50 .
- Chandrasekar , G. , Arner , A. , Kitambi , S. S. , Dahlman-Wright , K. and Lendahl , M. A. 2011 . Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish . Neurotoxicology and Teratology , 33 : 752 – 764 .
- Chaudhry , H. W. , Dashoush , N. H. , Tang , H. , Zhang , L. , Wang , X. , Wu , E. X. and Wolgemuth , D. J. 2004 . Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium . Journal of Biological Chemistry , 279 : 35858 – 35866 .
- Chen , C.-F. , Chu , C.-Y. , Chen , T. H. , Lee , S. J. , Shen , C. N. and Hsiao , C.-D. 2011 . Establishment of a transgenic zebrafish line for superficial skin ablation and functional validation of apoptosis modulators in vivo . PLoS One , 6 : e20654
- Cox , B. , Kotlyar , M. , Evangelou , A. I. , Ignatchenko , V. , Ignatchenko , A. , Whiteley , K. , … and Kislinger , T. 2009 . Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology . Molecular System Biology , 5 : 279
- Creton , R. 2009 . Automated analysis of behavior in zebrafish larvae . Behavioural Brain Research , 203 : 127 – 136 .
- De Castro , M. R. , Lima , J. V. , Salomao De Freitas , D. P. , De Souza Valente , R. , Dummer , N. S. , De Aguiar , R. B. , … and Barros , D. M. 2009 . Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae) . Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology , 150 : 337 – 342 .
- Deo , R. C. and MacRae , C. A. 2010 . The zebrafish: Scalable in vivo modeling for systems biology . Wiley Interdisciplinary Reviews: Systems Biology and Medicine , 3 : 335 – 346 .
- Detrich, H. W., III, & Kevin, F. S. (2008). Fluorescent proteins in zebrafish cell and developmental biology. In F. S. Kevin (Ed.), Methods in Cell Biology (pp. 219–241). Oxford: Academic Press.
- Ding , Y. J. and Chen , Y. H. 2012 . Developmental nephrotoxicity of aristolochic acid in a zebrafish model . Toxicology and Applied Pharmacology , 261 : 59 – 65 .
- Dodd , A. , Curtis , P. M. , Williams , L. C. and Love , D. R. 2000 . Zebrafish: Bridging the gap between development and disease . Human Molecular Genetics , 9 : 2443 – 2449 .
- Drummond , I. A. , Majumdar , A. , Hentschel , H. , Elger , M. , Solnica-Krezel , L. , Schier , A.F. , … and Fishman , M. C. 1998 . Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function . Development , 125 : 4655 – 4667 .
- Dunn , W. B. , Bailey , N. J. C. and Johnson , H. E. 2005 . Measuring the metabolome: Current analytical technologies . Analyst , 130 : 606 – 625 .
- Fewell , A. M. and Roddick , J. G. 1993 . Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine . Phytochemistry , 33 : 323 – 328 .
- Fisher , S. , Jagadeeswaran , P. and Halpern , M. E. 2003 . Radiographic analysis of zebrafish skeletal defects . Developmental Biology , 264 : 64 – 76 .
- Forst , C. V. 2006 . Host-pathogen systems biology . Drug Discovery Today , 11 : 220 – 227 .
- Garrity , D. M. , Childs , S. and Fishman , M. C. 2002 . The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome . Development , 129 : 4635 – 4645 .
- Gerhard, D. S., Wagner, L., Feingold, E. A., Shenmen, C. M., Grouse, L. H., Schuler, G., … Malek, J.; MGC Project Team. (2004). The status, quality, and expansion of the NIH full-length cDNA project: The Mammalian Gene Collection (MGC). Genome Research, 14, 2121–2127.
- Gilchrist , A. , Au , C. E. , Hiding , J. , Bell , A. W. , Fernandez-Rodriguez , J. , Lesimple , S. , … and Bergeron , J. J. M. 2006 . Quantitative proteomics analysis of the secretory pathway . Cell , 127 : 1265 – 1281 .
- Goldsmith , J. R. and Jobin , C. 2012 . Think small: Zebrafish as a model system of human pathology . Journal of Biomedicine and Biotechnology , 2012 : 12
- Gong , Z. , Ju , B. , Wang , X. , He , J. , Wan , H. , Sudha , P. M. and Yan , T. 2002 . Green fluorescent protein expression in germ-line transmitted transgenic zebrafish under a stratified epithelial promoter from Keratin8 . Developmental Dynamics , 223 : 204 – 215 .
- Gstaiger , M. and Aebersold , R. 2009 . Applying mass spectrometry-based proteomics to genetics, genomics and network biology . Natue Reviews. Genetics , 10 : 617 – 627 .
- Guerrera , I. and Kleiner , O. 2005 . Application of mass spectrometry in proteomics . Bioscience Reports , 25 : 71 – 93 .
- Gygi , S. P. , Corthals , G. L. , Zhang , Y. , Rochon , Y. and Aebersold , R. 2000 . Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology . Proceedings of the National Academy of Sciences of the United States of America , 97 : 9390 – 9395 .
- Han , X. , Aslanian , A. and Yates , J. R. III . 2008 . Mass spectrometry for proteomics . Current Opinion in Chemical Biology , 12 : 483 – 490 .
- Hanai , J. I. , Cao , P. , Tanksale , P. , Imamura , S. , Koshimizu , E. , Zhao , J. , … and Lecker , S. H. 2007 . The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity . Journal of Clinical Investigation , 117 : 3940 – 3951 .
- Harbers , M. and Carninci , P. 2005 . Tag-based approaches for transcriptome research and genome annotation . Nature Methods , 2 : 495 – 502 .
- Hayashi , S. , Akiyama , S. , Tamaru , Y. , Takeda , Y. , Fujiwara , T. , Inoue , K. , … and Fukusaki , E. 2009 . A novel application of metabolomics in vertebrate development . Biochemical and Biophysical Research Communications , 386 : 268 – 272 .
- Herbert , B. R. , Harry , J. L. , Packer , N. H. , Gooley , A. A. , Pedersen , S. K. and Williams , K. L. 2001 . What place for polyacrylamide in proteomics? . Trends in Biotechnology , 19 : S3 – S9 .
- Higashijima , S. I. , Okamoto , H. , Ueno , N. , Hotta , Y. and Eguchi , G. 1997 . High-frequency generation of transgenic zebrafish which reliably express gfp in whole muscles or the whole body by using promoters of zebrafish origin . Developmental Biology , 192 : 289 – 299 .
- Higgs , R. E. , Knierman , M. D. , Gelfanova , V. , Butler , J. P. and Hale , J. E. 2005 . Comprehensive label-free method for the relative quantification of proteins from biological samples . Journal of Proteome Research , 4 : 1442 – 1450 .
- Hill , A. J. , Teraoka , H. , Heideman , W. and Peterson , R. E. 2005 . Zebrafish as a model vertebrate for investigating chemical toxicity . Toxicological Sciences , 86 : 6 – 19 .
- Huang , Q. Y. , Huang , L. and Huang , H. Q. 2010 . Proteomic analysis of methyl parathion-responsive proteins in zebrafish (Danio rerio) brain . Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology , 153 : 67 – 74 .
- Ideker , T. , Galitski , T. and Hood , L. 2001 . A new approach to decoding life: Systems biology . Annual Review of Genomics and Human Genetics , 2 : 343 – 372 .
- Ingolia , N. T. , Brar , G. A. , Rouskin , S. , McGeachy , A. M. and Weissman , J. S. 2012 . The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments . Nature Protocol , 7 : 1534 – 1550 .
- Issaq , H. J. , Chan , K. C. , Janini , G. M. , Conrads , T. P. and Veenstra , T. D. 2005 . Multidimensional separation of peptides for effective proteomic analysis . Journal of Chromatography B , 817 : 35 – 47 .
- Jang , Z. H. , Chung , H. C. , Ahn , Y. G. , Kwon , Y. K. , Kim , J. S. , Ryu , J. H. , … and Hwang , G. S. 2012 . Metabolic profiling of an alcoholic fatty liver in zebrafish (Danio rerio) . Molecular BioSystems , 8 : 2001 – 2009 .
- Jung , S. H. , Kim , S. , Chung , A. Y. , Kim , H. T. , So , J. H. , Ryu , J. , … and Kim , C. H. 2009 . Visualization of myelination in GFP-transgenic zebrafish . Developmental Dynamics , 239 : 592 – 597 .
- Katagiri , F. 2003 . Attacking complex problems with the power of systems biology . Plant Physiology , 132 : 417 – 419 .
- Kishi , S. , Bayliss , P. E. , Uchiyama , J. J. , Koshimizu , E. , Qi , J. , Nanjappa , P. , … and Roberts , T. M. 2008 . The identification of zebrafish mutants showing alterations in senescence-associated biomarkers . PLoS Genetics , 4 : e1000152
- Kitano , H. 2007 . “ Scientific challanges in system biology ” . In Introduction to system biology , Edited by: Choi , S. 3 – 11 . Totowa , NJ : Humana Press .
- Knapik , E. W. 2000 . ENU mutagenesis in zebrafish from genes to complex diseases . Mammalian Genome , 11 : 511 – 519 .
- Kohl , P. , Crampin , E. J. , Quinn , T. A. and Noble , D. 2010 . Systems biology: An approach . Clinical Pharmacology and Therapeutics , 88 : 25 – 33 .
- Kültz , D. , Fiol , D. , Valkova , N. , Gomez-Jimenez , S. , Chan , S. Y. and Lee , J. 2007 . Functional genomics and proteomics of the cellular osmotic stress response in ‘non-model’ organisms . Journal of Experimental Biology , 210 : 1593 – 1601 .
- Langenau , D. M. , Traver , D. , Ferrando , A. A. , Kutok , J. L. , Aster , J. C. , Kanki , J. P. , … and Look , A. T. 2003 . Myc-induced T cell leukemia in transgenic zebrafish . Science , 299 : 887 – 890 .
- Langheinrich , U. , Vacun , G. and Wagner , T. 2003 . Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia . Toxicology and Applied Pharmacology , 193 : 370 – 382 .
- Lieschke , G. J. and Currie , P. D. 2007 . Animal models of human disease: Zebrafish swim into view . Nature Review Genetics , 8 : 353 – 367 .
- Liu , S. and Leach , S. D. 2011 . Zebrafish models for cancer . Annual Review of Pathology: Mechanisms of Disease , 6 : 71 – 93 .
- Liu , X. S. 2007 . Getting started in tiling microarray analysis . PLoS Computational Biology , 3 : e183
- Lucitt , M. B. , Price , T. S. , Pizarro , A. , Wu , W. , Yocum , A. K. , Seiler , C. , … and Grosser , T. 2008 . Analysis of the zebrafish proteome during embryonic development . Molecular & Cellular Proteomics , 7 : 981 – 994 .
- Madsen , E. C. , Morcos , P. A. , Mendelsohn , B. A. and Gitlin , J. D. 2008 . In vivo correction of a Menkes disease model using antisense oligonucleotides . Proceedings of the National Academy of Sciences of the United States of America , 105 : 3909 – 3914 .
- Malone , M. H. and Robichaud , R. C. 1962 . Hippocratic screening for pure or crude drug materials . Lloydia , 25 : 320 – 332 .
- Mathias , J. R. , Perrin , B. J. , Liu , T. X. , Kanki , J. , Look , A. T. and Huttenlocher , A. 2006 . Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish . Journal of Leukocyte Biology , 80 : 1281 – 1288 .
- McCarty , L. S. and Mackay , D. 1993 . Enhancing ecotoxicological modeling and assessment. Body residues and modes of toxic action . Environmental Science & Technology , 27 : 1718 – 1728 .
- Milan , D. J. , Peterson , T. A. , Ruskin , J. N. , Peterson , R. T. and MacRae , C. A. 2003 . Drugs that induce repolarization abnormalities cause bradycardia in zebrafish . Circulation , 107 : 1355 – 1358 .
- Mitulović , G. and Mechtler , K. 2006 . HPLC techniques for proteomics analysis-a short overview of latest developments . Briefings in Functional Genomics & Proteomics , 5 : 249 – 260 .
- Moco , S. , Vervoort , J. , Bino , R. J. , De Vos , R. C. H. and Bino , R. 2007 . Metabolomics technologies and metabolite identification . TrAC Trends in Analytical Chemistry , 26 : 855 – 866 .
- Moens , C. B. , Donn , T. M. , Wolf-Saxon , E. R. and Ma , T. P. 2008 . Reverse genetics in zebrafish by TILLING . Briefings in Functional Genomics & Proteomics , 7 : 454 – 459 .
- Monetti , M. , Nagaraj , N. , Sharma , K. and Mann , M. 2011 . Large-scale phosphosite quantification in tissues by a spike-in SILAC method . Nature Methods , 8 : 655 – 658 .
- Monteoliva , L. and Albar , J. P. 2004 . Differential proteomics: An overview of gel and non-gel based approaches . Briefings in Functional Genomics & Proteomics , 3 : 220 – 239 .
- Munkittrick , K. R. and McCarty , L. S. 1995 . An integrated approach to aquatic ecosystem health: Top-down, bottom-up or middle-out? . Journal of Aquatic Ecosystem Stress and Recovery , 4 : 77 – 90 .
- Muto , A. , Orger , M. B. , Wehman , A. M. , Smear , M. C. , Kay , J. N. , Page-Mccaw , P. S. , … and Baier , H. 2005 . Forward genetic analysis of visual behavior in zebrafish . PLoS Genetics , 1 : e66
- Nibbe , R. K. , Koyutürk , M. and Chance , M. R. 2010 . An integrative omics approach to identify functional sub-networks in human colorectal cancer . PLoS Computational Biology , 6 : e1000639
- Ong , E. S. , Chor , C. F. , Zou , L. and Ong , C. N. 2009 . A multi-analytical approach for metabolomic profiling of zebrafish (Danio rerio) livers . Molecular BioSystems , 5 : 288 – 298 .
- Ong , S. E. , Blagoev , B. , Kratchmarova , I. , Kristensen , D. B. , Steen , H. , Pandey , A. and Mann , M. 2002 . Stable isotope labeling by amino acids in cell culture, silac, as a simple and accurate approach to expression proteomics . Molecular & Cellular Proteomics , 1 : 376 – 386 .
- Papan , C. and Chen , L. 2009 . Metabolic fingerprinting reveals developmental regulation of metabolites during early zebrafish embryogenesis . OMICS: A Journal of Integrative Biology , 13 : 397 – 405 .
- Paquet , D. , Bhat , R. , Sydow , A. , Mandelkow , E.-M. , Berg , S. , Hellberg , S. , … and Haass , C. 2009 . A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation . Journal of Clinical Investigation , 119 : 1382 – 1395 .
- Paquet , D. , Schmid , B. and Haass , C. 2010 . Transgenic zebrafish as a novel animal model to study tauopathies and other neurodegenerative disorders in vivo . Neurodegenerative Diseases , 7 : 99 – 102 .
- Parng , C. , Roy , N. M. , Ton , C. , Lin , Y. and McGrath , P. 2007 . Neurotoxicity assessment using zebrafish . Journal of Pharmacological and Toxicological Methods , 55 : 103 – 112 .
- Parng , C. , Seng , W. L. , Semino , C. and McGrath , P. 2002 . Zebrafish: A preclinical model for drug screening . Assay and Drug Development Technologies , 1 : 41 – 48 .
- Parsons , M. J. , Pollard , S. M. , Saude , L. , Feldman , B. , Coutinho , P. , Hirst , E. M. A. and Stemple , D. L. 2002 . Zebrafish mutants identify an essential role for laminins in notochord formation . Development , 129 : 3137 – 3146 .
- Petrie , H. L. , Hohn , C. and Hanson , L. 2009 . Characterization of rag1 mutant zebrafish leukocytes . BMC Immunology , 10 : 1 – 8 .
- Peng , H. C. , Wang , Y. H. , Wen , C. C. , Wang , W. H. , Cheng , C. C. and Chen , Y. H. 2010 . Nephrotoxicity assessments of acetaminophen during zebrafish embryogenesis . Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology , 151 : 480 – 486 .
- Ponnudurai , R. P. , Basak , T. , Ahmad , S. , Bhardwaj , G. , Chauhan , R. K. , Singh , R. A. , … and Sengupta , S. 2012 . Proteomic analysis of zebrafish (Danio rerio) embryos exposed to cyclosporine A . Journal of Proteomics , 75 : 1004 – 1017 .
- Postlethwait , J. H. , Woods , I. G. , Ngo-Hazelett , P. , Yan , Y. L. , Kelly , P. D. , Chu , F. , … and Talbot , W. S. 2000 . Zebrafish comparative genomics and the origins of vertebrate chromosomes . Genome Research , 10 : 1890 – 1902 .
- Reinders , J. , Zahedi , R. P. , Pfanner , N. , Meisinger , C. and Sickmann , A. 2006 . Toward the complete yeast mitochondrial proteome: Multidimensional separation techniques for mitochondrial proteomics . Journal of Proteome Research , 5 : 1543 – 1554 .
- Ross , P. L. , Huang , Y. N. , Marchese , J. N. , Williamson , B. , Parker , K. , Hattan , S. , … and Pappin , D. J. 2004 . Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents . Molecular and Cellular Proteomics , 3 : 1154 – 1169 .
- Sager , J. , Bai , Q. and Burton , E. 2010 . Transgenic zebrafish models of neurodegenerative diseases . Brain Structure and Function , 214 : 285 – 302 .
- Schwend , T. and Ahlgren , S. 2009 . Zebrafish con/disp1 reveals multiple spatiotemporal requirements for Hedgehog-signaling in craniofacial development . BMC Developmental Biology , 9 : 59
- Shepard , J. L. , Amatruda , J. F. , Finkelstein , D. , Ziai , J. , Finley , K. R. , Stern , H. M. , … and Zon , L. I. 2007 . A mutation in separase causes genome instability and increased susceptibility to epithelial cancer . Genes & Development , 21 : 55 – 59 .
- Shiraki , T. , Kondo , S. , Katayama , S. , Waki , K. , Kasukawa , T. , Kawaji , H. , … and Hayashizaki , Y. 2003 . Cap analysis gene expression for high-throughput analysis of transcriptional starting point and identification of promoter usage . Proceedings of the National Academy of Sciences of the United States of America , 100 : 15776 – 15781 .
- Shulaev , V. 2006 . Metabolomics technology and bioinformatics . Briefings in Bioinformatics , 7 : 128 – 139 .
- Sleep , E. , Boué , S. , Jopling , C. , Raya , M. , Raya , Á. and Belmonte , J. C. I. 2010 . Transcriptomics approach to investigate zebrafish heart regeneration . Journal of Cardiovascular Medicine , 11 : 369 – 380 .
- Soanes , K. H. , Achenbach , J. C. , Burton , I. W. , Hui , J. P. M. , Penny , S. L. and Karakach , T. K. 2011 . Molecular characterization of zebrafish embryogenesis via dna microarrays and multiplatform time course metabolomics studies . Jounal of Proteome Research , 10 : 5102 – 5117 .
- Spitsbergen , J. M. and Kent , M. L. 2003 . The state of the art of the zebrafish model for toxicology and toxicologic pathology research advantages and current limitations . Toxicologic pathology , 31 : 62 – 87 .
- Stehr , C. M. , Linbo , T. L. , Incardona , J. P. and Scholz , N. L. 2006 . The developmental neurotoxicity of fipronil: Notochord degeneration and locomotor defects in zebrafish embryos and larvae . Toxicological Sciences , 92 : 270 – 278 .
- Streisinger , G. , Walker , C. , Dower , N. , Knauber , D. and Singer , F. 1981 . Production of clones of homozygous diploid zebra fish (Brachydanio rerio) . Nature , 291 : 293 – 296 .
- Sukardi , H. , Ung , C. Y. , Gong , Z. Y. and Lam , S. H. 2010 . Incorporating zebrafish omics into chemical biology and toxicology . Zebrafish , 7 : 41 – 52 .
- Sun , X. J. , Xu , P. F. , Zhou , T. , Hu , M. , Fu , C. T. , Zhang , Y. , … and Chen , Z. 2008 . Genome-wide survey and developmental expression mapping of zebrafish set domain-containing genes . PLoS One , 3 : e1499
- Taesotikul , T. , Panthong , A. , Kanjanapothi , D. , Verpoorte , R. and Scheffer , J. J. C. 1989 . Hippocratic screening of ethanolic extracts from two Tabernaemontana species . Journal of Ethnopharmacology , 27 : 99 – 106 .
- Tay , T. L. , Lin , Q. , Seow , T. K. , Tan , K. H. , Hew , C. L. and Gong , Z. 2006 . Proteomic analysis of protein profiles during early development of the zebrafish, Danio rerio . Proteomics , 6 : 3176 – 3188 .
- Thisse , C. and Thisse , B. 2008 . High-resolution in situ hybridization to whole-mount zebrafish embryos . Nature Protocols , 3 : 59 – 69 .
- Ton , C. and Parng , C. 2005 . The use of zebrafish for assessing ototoxic and otoprotective agents . Hearing Research , 208 : 79 – 88 .
- Tong , S. K. , Mouriec , K. , Kuo , M. W. , Pellegrini , E. , Gueguen , M.-M. , Brion , F. , … and Chung , B. C. 2009 . A cyp19a1b-gfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells . Genesis , 47 : 67 – 73 .
- Trede , N. S. , Ota , T. , Kawasaki , H. , Paw , B. H. , Katz , T. , Demarest , B. , … and Zon , L. I. 2008 . Zebrafish mutants with disrupted early T-cell and thymus development identified in early pressure screen . Developmental Dynamics , 237 : 2575 – 2584 .
- Ung , C. , Lam , S. , Hlaing , M. , Winata , C. , Korzh , S. , Mathavan , S. and Gong , Z. 2010 . Mercury-induced hepatotoxicity in zebrafish: In vivo mechanistic insights from transcriptome analysis, phenotype anchoring and targeted gene expression validation . BMC Genomics , 11 : 212
- Verpoorte , R. , Choi , Y. H. , Mustafa , N. and Kim , H. K. 2008 . Metabolomics: Back to basics . Phytochemistry Reviews , 7 : 525 – 537 .
- Vesterlund , L. , Jiao , H. , Unneberg , P. , Hovatta , O. and Kere , J. 2011 . The zebrafish transcriptome during early development . BMC Developmental Biology , 11 : 30
- Wang , W. , Liu , X. , Gelinas , D. , Ciruna , B. and Sun , Y. 2007 . A fully automated robotic system for microinjection of zebrafish embryos . PLoS One , 2 : e862
- Wang , Z. , Gerstein , M. and Snyder , M. 2009 . RNA-Seq: A revolutionary tool for transcriptomics . Nature Review Genetics , 10 : 57 – 63 .
- Wang , D. , Jao , L.-E. , Zheng , N. , Dolan , K. , Ivey , J. , Zonies , S. , … and Burgess , S. M. 2007 . Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions . Proceedings of the National Academy of Sciences of the United States of America , 104 : 12428 – 12433 .
- Wang , M. , You , J. , Bemis , K. G. , Tegeler , T. J. and Brown , D. P. G. 2008 . Label-free mass spectrometry-based protein quantification technologies in proteomic analysis . Briefings in Functional Genomics & Proteomics , 7 : 329 – 339 .
- Way , J. C. and Silver , P. A. 2007 . Systems engineering without an engineer: Why we need systems biology . Complexity , 13 : 22 – 29 .
- Wielhouwer , E. M. , Ali , S. , Al-Afandi , A. , Blom , M. T. , Olde Riekerink , M. B. , Poelma , C. , … and Richardson , M. K. 2011 . Zebrafish embryo development in a microfluidic flow-through system . Lab on a Chip , 11 : 1815 – 1824 .
- Wishart , D. S. 2008 . Applications of metabolomics in drug discovery and development . Drugs in R & D , 9 : 307 – 322 .
- Wu , W. W. , Wang , G. , Baek , S. J. and Shen , R.-F. 2006 . Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D Gel- or LC-MALDI TOF/TOF . Journal of Proteome Research , 5 : 651 – 658 .
- Xi , Y. , Ryan , J. , Noble , S. , Yu , M. , Yilbas , A. E. and Ekker , M. 2010 . Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function . European Journal of Neuroscience , 31 : 623 – 633 .
- Zhang , Y. , Wang , C. , Huang , L. , Chen , R. , Chen , Y. and Zuo , Z. 2012 . Low-level pyrene exposure causes cardiac toxicity in zebrafish (Danio rerio) embryos . Aquatic Toxicology , 114–115 : 119 – 124 .
- Zhao , S. , Xie , P. , Li , G. , Jun , C. , Cai , Y. , Xiong , Q. and Zhao , Y. 2012 . The proteomic study on cellular responses of the testes of zebrafish (Danio rerio) exposed to microcystin-RR . Proteomics , 12 : 300 – 312 .
- Zheng , W. , Wang , Z. , Collins , J. E. , Andrews , R. M. , Stemple , D. and Gong , Z. 2011 . Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung . PLoS One , 6 : e24019
- Zhuo , H.-Q. , Huang , L. , Huang , H.-Q. and Cai , Z. 2012 . Effects of chronic tramadol exposure on the zebrafish brain: A proteomic study . Journal of Proteomics , 75 : 3351 – 3364 .