Abstract
L-asparaginase is a vital enzyme of medical importance, and renowned as a chemotherapeutic agent. The relevance of this enzyme is not only limited as an anti-cancer agent, it also possesses a wide range of medical application. The application includes the antimicrobial property, treatment of infectious diseases, autoimmune diseases, canine and feline cancer. Apart from the health care industry, its significance is also established in the food sector as a food processing agent to reduce the acrylamide concentration. L-asparaginase is known to be produced from various bacterial, fungal and plant sources. However, there is a huge market demand due to its wide range of application. Therefore, the industry is still in the search of better-producing source in terms of high yield and low immunogenicity. It can be produced by both submerged and solid state fermentation, and each fermentation process has its own merits and demerits. This review paper focuses on its improved production strategy by adopting statistical experimental optimization techniques, development of recombinant strains, through mutagenesis and nanoparticle immobilization, adopting advanced and cost-effective purification techniques. Available research literature proves the competence and therapeutic potential of this enzyme. Therefore, research orientation toward the exploration of this clinical significant enzyme has to be accelerated. The objectives of this review are to discuss the high yielding sources, current production strategies, improvement of production, effective downstream processing and therapeutic application of L-asparaginase.
1. Introduction
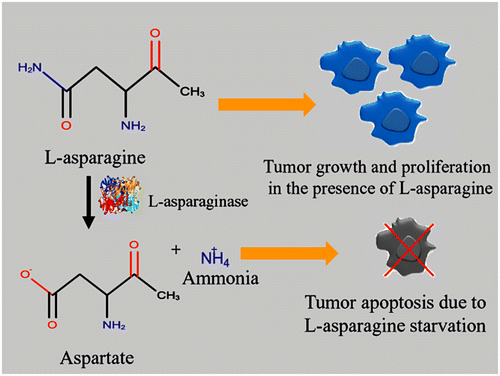
L-asparaginase (L-asparagine amidohydrolase, EC 3.5.1.1, ASNase) is an enzyme of prime therapeutic importance that contributes to 40% of the total worldwide enzyme demands. It contributes one-third of global requirements as anti-leukemic and anti-lymphoma agents (Warangkar & Khobragade, Citation2009). It is mainly used to treat acute lymphoblastic leukemia (ALL) in combination with vincristine and a glucocorticoid (e.g. dexamethasone) (Batool, Makky, Jalal, & Yusoff, Citation2016). It inhibits the growth of tumor cells by depriving them from nutrition, necessary for their growth and proliferation. For normal cells, L-asparagine is a non-essential amino acid, as it is synthesized by the action of L-asparagine synthetase. On the other hand, tumor cells lack L-asparagine synthetase activity that restricts their ability to produce L-asparagine. Tumor cells necessitate L-asparagine for protein synthesis essential for pyrimidine synthesis. It also acts as a nitrogen donor for purine synthesis by means of adenylosuccinate synthetase1 (Martinez-outschoorn, Pestell, Sotgia, & Lisanti, Citation2016). They use L-asparagine available in plasma pool to meet their growth and development requirement. When L-asparaginase is administrated, its concentration gets lower down in the blood. The introduction of this therapeutic enzyme causes inhibition of protein synthesis in tumor cells leading to G1 cell-cycle arrest. It grounds for the fatal starvation of tumor cells, whereas normal cells remain unaffected because of their ability to synthesize L-asparagine naturally (Shrivastava et al., Citation2015). The mechanism of action of tumor cell starvation in the presence of this enzyme is depicted in Figure . The enzyme is active in the alkaline pH range and at temperature 37 °C that makes it extremely valuable in the chemotherapeutic use. It has been proved a potent antineoplastic agent in animals and has given complete remission in human leukemias (Dange & Peshwe, Citation2013). The reason of its preferred use is its high biodegradability, non-toxicity and can be administered at the local site quite easily. Other agents are found quite painful when administered to the patient and are relatively costly (Prema, Devi, & Alagumanikumaran, Citation2013). However, there are some side effects associated with existing therapy like anaphylaxis, coagulation abnormality, thrombosis, liver dysfunction, pancreatitis, hyperglycemia, and cerebral dysfunction. The production of anti-asparaginase antibody or the presence of L-glutaminase and urease activity causes such immunogenic response in the treated patient (Doriya & Kumar, Citation2016). The review describes the key issues related to L-asparaginase source, improved production strategy, and its multifarious application.
2. Production of L-asparaginase from various sources
It is broadly distributed among plants, animals, and micro-organisms. A wide range of micro-organisms that includes bacteria, yeasts, and filamentous fungi have been considered as an ideal source of this potent enzyme. However, microbial enzymes are preferred over plant and animal sources because it facilitates economic production, consistency, ease of process modification, optimization, and purification. The enzymes isolated from these sources are relatively more stable than corresponding enzymes derived from plants or animals (Balakrishnan, Nair, & Kumar, Citation2013). Moreover, it is easy to genetically modify them in order to increase the yield (Lopes et al., Citation2015).
2.1. Bacterial sources
It is produced by several bacterial species, but the enzymes (type-II) from E. coli and Erwinia chrysanthemi are produced on the industrial scale for the clinical application. The drugs from both sources have identical mechanisms of action and toxicities, however, their pharmacokinetic properties are different, and patients that are allergic to one source of the enzyme are frequently resistant to the other (Sanches, Krauchenco, & Polikarpov, Citation2007). Moreover, the enzyme from E. coli has a higher affinity (Km = 18 ± 3 μM) than enzyme from E. chrysanthemi (Km = 33 ± 6 μM) toward the substrate L-asparagine (Lanvers-Kaminsky, Citation2017). Most of the microbial L-asparaginase are intracellular in nature except a few that are secreted outside the cell. From industrial prospects, extracellularly secreted enzyme is more beneficial as it could be produced abundantly in culture under normal condition. Additionally, the purification process will be easier and economical (Hosamani & Kaliwal, Citation2011). Gram-positive bacteria do not have a periplasmic space and therefore they secrete several enzymes into surrounding medium (exoenzymes) that ordinarily would be periplasmic in gram-negative bacteria (Singh & Srivastava, Citation2012). This implies that the screening of gram positive bacteria would be more advantageous in order to get extracellular enzyme. There are several reported bacterial sources of this enzyme and few of them are listed in Table .
Table 1. L-asparaginase producing micro-organisms.
2.2. Fungal sources
It is discussed earlier that commercial production of this enzyme is primarily done from bacterial sources for the treatment of ALL and Non-Hodgkin Lymphoma (NHL). However, the enzyme produced from the bacterial sources causes some immunological reaction like hypersensitivity, coagulation abnormality, pancreatitis, anaphylaxis, thrombosis, liver dysfunction, allergic reactions, hyperglycemia, and cerebral dysfunction (Doriya & Kumar, Citation2016). For minimizing such immunological problems, an alternative source of the enzyme is obligatory. The present situation demands a noble source with minimum or no cross-reactivity and that too with higher yield. As fungi are evolutionarily closer to human beings (as compared to bacteria), it is estimated that the enzyme isolated from them will cause less immunogenicity (Shrivastava, Khan, Shrivastav, Jain, & Singhal, Citation2012). The various reported cases of L-asparaginase producing fungi are discussed in Table .
2.3. Plant and other source
Plant sources are less explored in terms L-asparaginase production. In plants, asparagine is one of the major nitrogen storage and transport compound. It accumulates during physiological processes like seed germination as well as during stress condition like drought, mineral deficiencies, and pathogen attack. L-asparaginase releases ammonia from the stored asparagine necessary for plant growth and development. Literature reports many plant sources like Tamarindus indica, Capsicum annum, Withania somnifera, Vicia faba, Lupinus angustifolius, Phaseoulus vulgaris as L-asparaginase producers that are summarized in Table (Lea, Sodek, Parry, Shewry, & Halford, Citation2007; Oza, Parmar, Kumar, & Subramanian, Citation2010). Apart from bacteria, fungi, and plants, the enzyme is also produced by yeast, algae, and animals that are incorporated in Table .
3. Production strategies of L-asparaginase
Fermentation is the pre-eminent technique for the production of various enzymes. Both bacteria and fungi are exploited to yield a series of enzymes when fermented on appropriate substrates. L-asparaginase production is carried out by submerged fermentation (SmF), solid state fermentation (SSF), and recombinant DNA technology.
3.1. Submerged fermentation (SmF)
This fermentation technique is generally employed for bacterial enzyme production. The L-asparaginase production is mostly carried out by submerged fermentation throughout the world. The reason is that SmF is well established, manipulation of medium components is comparatively easier that leads to high yield along with quality. The utility of SmF over SSF are (i) no pre-treatment of substrate required, (ii) easy control of process parameters (heat, pH, etc.), (iii) supports the utilization of genetically modified organisms to a greater extent than SSF, and (iv) purification of products is easier (Subramaniyam & Vimala, Citation2012). However, there are numerous shortcomings associated with SmF that encouraged researchers to move toward SSF. The process inadequacies are higher energy demand (in agitation and aeration), higher risk of contamination, low yield, higher cost of production, and a huge amount of waste generation that is difficult to dispose of. At present, the market demand of L-asparaginase is met by SmF using genetically modified strains but the cost of production is very high that promotes the requirement for an alternative method like SSF. Table summarizes the fermentation condition for the production of L-asparaginase employing SmF technique.
Table 2. L-asparaginase production by submerged fermentation at various operating condition.
3.2. Solid state fermentation
In past recent years, SSF is encouraged for the production of enzymes especially where the crude fermented product can be directly used as enzyme source. In most of the studies, it was found that L-asparaginase production is higher in SSF as compared to SmF. The culture condition in SSF mimic natural growth condition of microbes promotes high enzyme growth. The process utilizes cheap agriculture waste such as rice bran, wheat bran, sesame oil cake, soya bean meal, corn cob, gram husk, orange peel, coconut oil cake, groundnut cake, and tea waste. The use of agricultural wastes makes the process cost effective as well as reduces environmental pollution. In this process, the moisture level is low that means the low volume of medium per unit weight of the substrate. Hence, enzyme specific activity is usually very high (Soniyamby, Sundaram, & Vasantha, Citation2011). Another advantage is that the process involves less polluting effluents and requires a small quantity of dissolvent for product extraction since product concentration is high. The process also offers benefits like ease of downstream processing, low energy requirement (less or no mechanical agitation), and simple media formulation (Ghosh, Murthy, Govindasamy, & Chandrasekaran, Citation2013). The various cases where L-asparaginase was produced using SSF was summarized in Table .
Table 3. L-asparaginase production by solid state fermentation at various operating condition.
3.3. Recombinant DNA technology (RDT)
RDT can be used to achieve targeted production of this enzyme with higher recovery. There are two isoforms of this enzyme reported that are named as type-I and type-II. Type-I is produced in cytoplasm whereas type-II in the periplasm. In E. coli, AnsA gene code for type-I that has low affinity for asparagine whereas AnsB gene code for type-II that have a higher affinity for asparagine. It is the type-II enzyme that is used in the treatment of acute lymphoblastic leukemia (Costa et al., Citation2016). In a similar study, protoplasts of two fungal isolates i.e. Trichoderma sp. and Cladosporium sp. were fused in order to increase the yield. The recombinant strain developed has 2.58-fold more enzyme activity than parental isolates (El-Gendy, Al-Zahrani, & El-Bondkly, Citation2017). The knowledge of L-asparaginase coding gene and recombinant engineering techniques will provide insight into higher production along with reduced immunogenicity and cost. One of such attempt is where higher producer strain was constructed using protoplast fusion technique employing two strain (Bacillus subtilis and B. cereus). The recombinant strain obtained showed 2.5 times higher activity (Hegazy & Moharam, Citation2010). Literature suggests that in Saccharomyces cerevisiae ASP1 gene encodes for L-asparaginase-I (ScASNase1). In a recent study, recombinant ScASNase1 was reported to have properties of both type-I and type-II L-asparaginase as of E. coli. It has allosteric nature like type-I and anti-tumor activity like type-II. Another notable thing is that it has activity similar to commercial E. coli strain but with low immunogenicity (due to low glutaminase activity) (Costa et al., Citation2016). Other recombinant strains with low glutaminase activity and high L-asparaginase activity was developed by researchers by cloning and expressing the type-II gene from the Cyanobacterium synechococcus (Kebeish, El-Sayed, Fahmy, & Ghany, Citation2016).
4. Improvement in production of L-asparaginase
The optimization of process parameters may lead to higher yield resulting in commercial success. Traditional/classical methods use one factor at a time that makes the process expensive, time-consuming as well as interaction among various factors remains hidden. On the other hand, strain improvement and development of a process for high-titer enzyme production are the fast methods for the improvement of the enzyme production. Therefore, researchers have to shift from conventional method to modern optimization techniques for achieving higher production of L-asparaginase. The methods like factorial design help in screening and selection of major influencing fermentation parameters from a large number of process variables. The statistical methods like Plackett–Burman experimental design are usually used to screen out most influential parameter from a large set of process parameters. The advantage of this approach is that it predicts the most significant parameters in minimum possible runs. The limitation of this approach is the lack of determining interaction among different process parameters. The interactions among selected factors can be determined by applying Response Surface Methodology (RSM) approach. In the context of L-asparaginase production, a comparable study was done in Serratia marcescens to improve its activity. It was found that enzyme activity was improved 33% as compared to the classical method (Ghosh et al., Citation2013).
For the economical production of the enzyme on the industrial scale, techniques like whole cell immobilization can be applied. The method offers several advantages like (i) ease to separate cell mass from the bulk liquid for its reusability, (ii) facilitate the continuous operation for longer time period, and (iii) higher catalysis efficiency. One such example is where Streptomyces gulbargensis and its mutant Streptomyces gulbargensis mu24 were immobilized. This helps in enhancing 30% enzyme production as compared to conventional floating cell fermentation (Kattimani, Amena, Nandareddy, & Mujugond, Citation2009). Immobilization on nanoparticles is also a potential alternative. This can be exampled as immobilization of this enzyme on hydrogel-magnetic nanoparticles. It was found that the residual activity of the immobilized enzyme remains the same even after six months when stored at 4 °C (Teodor, Litescu, Lazar, & Somoghi, Citation2009). Another strategy that can be employed for improving the efficiency of mutagenesis of potent enzyme producing strains. There was a successful attempt reported in the literature where production was enhanced by applying strain improvement technique, UV and EMS treatment on Pseudomonas fluorescens. The UV- and EMS-mutated strain showed 1.6-fold and 1.54-fold more activity as compared to the wild strain, respectively (Prema et al., Citation2013).
5. Downstream processing
As this enzyme is of pharmaceutical value, the high purity is the foremost thing that is desirable for its medical application. The enzyme with higher purity generates less toxic and allergic response in the patient. Its purification is a multi-step process that individually accounts for about 80% of total production cost (Tundisi et al., Citation2017). The major steps of purification are (i) removal of insoluble material, (ii) concentration, (iii) fractionation, and (iv) purification. Some of the common purification techniques include filtration, centrifugation, salt precipitation followed by dialysis, liquid two-phase extraction, and chromatography (ion exchange, affinity chromatography, size exclusion, gel filtration) (Cachumba et al., Citation2016). One of such illustration is where scientists partially purified L-asparaginase from Talaromyces pinophilus by ammonium sulfate precipitation (at 60–70% saturation) followed by dialysis and get 7-fold higher specific activity. Further, by applying gel-filtration chromatography 19.6-fold higher specific activity was obtained that is more that commercially available enzyme Oncaspar (Krishnapura & Belur, Citation2016). Another literature reported work is where Bacillus licheniformis was purified by a series of steps starting from ultra-filtration then followed by acetone precipitation, anion exchange, and gel filtration. A remarkable increase in specific activity from 23.1 to 697.09 IU/mg (30.18-fold) was achieved. However, there are few drawbacks associated with the classical approach of purification like long processing hours, high cost, low yield and productivity, difficulty in scale up. The industry demands for economical, fast, scalable process/method that should also be environmental friendly. The membrane processes, Aqueous Two-Phase Systems (ATPS) integrated with precipitation and/or fractionation steps, Countercurrent Chromatography (CCC) are examples of such advanced process (Tundisi et al., Citation2017).
6. Therapeutic application of L-asparaginase
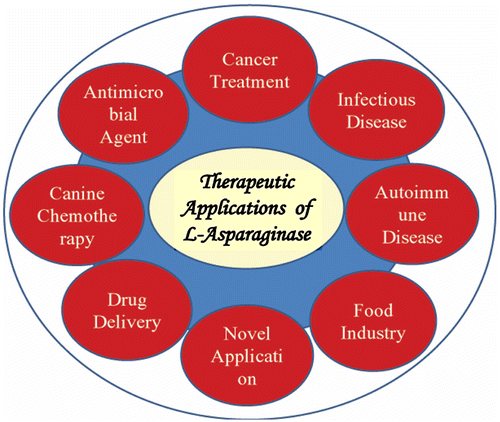
L-asparaginase is non-toxic and one of the most widely applicative medical enzyme; therefore it could be a promising therapeutic agent (Figure ).
6.1. L-asparaginase: polymers and nanoparticles as drug delivery agents
In past few decades, nanoparticles (size range 1–1000 nm) are employed as drug delivery agents for the treatment of cancer, HIV, infectious diseases, and several other diseases. Along with reducing the immunogenicity of drugs, nanoparticles also improves their solubility, improves half-life, and enhances their therapeutic index. There are many nanoparticle delivery systems used so far for various drug deliveries. They are polymeric, liposomal, magnetic, gold, silver, quantum dots, carbon nanotubes, and dendrimers. In 1994, PEG–L-asparaginase (Oncospar; Enzon) became the first polymeric therapeutic to receive FDA, USA approval, for the treatment of acute lymphocytic leukemia (Petros & DeSimone, Citation2010). Other polymers used for the formulation of L-asparaginase loaded nanoparticles are poly (lactic-co-glycolide) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHPV) nano-capsules. Scientists formulated silk fibroin nanoparticle conjugated with this enzyme that offers better trypsin resistance in addition to better stability in serum (Wang & Zhang, Citation2015). Other delivery systems employed are magnetic nanoparticles, as they are used as MRI contrast agent. The enzyme is also immobilized by entrapping it in hydrogel magnetic nanoparticles with a successive layer of chitosan followed by hyaluronic acid (Teodor et al., Citation2009). Recently immobilization of this enzyme was done in magnetic nanoparticles. Further, it is functionalized by a biocompatible reactive polymer, poly (2-vinyl-4, 4-dimethylazlactone). It retains 95.7% of the activity even after 10 repeated uses. It also maintains more than 72.6% of the activity after 10 weeks storage (Mu, Qiao, Qi, Dong, & Ma, Citation2014). The researcher also developed a nanobiocomposite that contains asparaginase, carbon nanoparticles, methotrexate (Mtx) coupled with fluorescein isothiocyanate (FITC). FITC imaging property helps in monitoring drug pathways whereas Mtx (a folic acid analog) helps in targeted drug delivery (Muthukumar et al., Citation2014). One of the major issues associated with this medically significant enzyme is its non-human origin, leading to the formation of antibody against it. In order to overcome this problem, it was encapsulated in a dual pore hollow nanoparticle which allows only L-asparagine to move inside. Inside the pore, the reaction takes place whereas antibody and protease remain outside the hollow nanoparticle. This prevents the degradation of the immobilized enzyme by body immune system (Ortac et al., Citation2014).
6.2. Antimicrobial activities
Industries are in continuous search of the new source of L-asparaginase with higher yield and novel property. The reason is its wide application increases high market demand that is challenging to meet with current production rate. It is reported that sources of this enzyme also possesses antimicrobial property. This property can be exploited for L-asparaginase bioprocessing to prevent contamination. The enzyme produced from such sources also has some of this property against the pathogens. Marine sources are more diverse and least explored for the production this enzyme. The micro-organisms from this source are expected to have unique property due to tolerance of diverse environmental condition like temperature, pH, and salinity. A report suggests marine isolate of Streptomyces species from the Bay of Bengal has 25.90 IU enzyme activity along with the antimicrobial property (Sivasankar et al., Citation2013). The other study discovers that the leaves of Amaranthus polygonoides showing L-asparaginase activity also has antibacterial and antifungal activity against pathogens such as Staphylococcus aureus, S. epidermidis, Micrococcus luteus, B. cereus, B. subtilis, E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Aspergillus niger, and A. fumigates (Naveena, Narayani, Sakthiselvan, & Partha, Citation2012).
6.3. Cancer treatment
The presence of L-asparaginase was first detected by Clementi in 1922 in guinea pig serum (Clementi, Citation1922). Further, its anti-lymphoma activity was explored by Broome in 1961 on lymphoma 6C3HED cells (Broome, Citation1961). Afterward, Mashburn and Wriston in 1963 experimentally determine that asparaginase extracted from E. coli inhibits the tumor growth (Mashburn & Wriston, Citation1964). The discovery of the anti-tumorigenic property of this enzyme has contributed to the rapid development of the production of the enzyme. For more than 40 years it is utilized in the chemotherapeutic treatment of certain kinds of lymphoblastic malignancy, mainly in ALL and lymphosarcoma (Singh & Srivastava, Citation2012). The FDA and WHO organizations have approved L-asparaginase that can be effectively used in ALL (Acute Lymphoblastic Leukemia) and lymph sarcoma treatment (Patil, Patil, & Maheshwari, Citation2012). It is also used for the treatment of acute myeloblastic leukemia, chronic lymphocytic leukemia, Hodgkin’s disease, melonosarcoma, non-Hodgkin’s lymphoma, pancreatic carcinoma, and bovine lymphosarcoma (Abd El Baky & El Baroty, Citation2016). At present, the largest pharmaceutical firms in the USA, England, Federal Republic of Germany and Japan are manufacturing a highly purified form of his enzyme (Warangkar & Khobragade, Citation2009). There are three formulations for L-asparaginase that have been used as a drug for the treatment of ALL. This includes asparaginase (from E. coli), Erwinia asparaginase (from Erwinia caratovora) and Pegaspargase (L-asparaginase from E. coli and attached to polyethylene glycol (Priya, Radhakrishnan, Balagurunathan, Arts, & Nadu, Citation2011). In the year 2016, a recombinant L-asparaginase from E. coli has been introduced. It can only be administered intravenously while the previous three forms are licensed for both intravenous and intramuscular administration (Lanvers-Kaminsky, Citation2017). Cristanaspase (USA), Elspar (USA), L-Asnase (USA), Crasntin (Germany), Leumase (Japan), Oncaspar, Erwinase, Kidrolase, Crisantas, Pasum, PEG-asparaginase, Pegasparagasum are commercially available (Doriya, Jose, Gowda, & Kumar, Citation2016). Currently, these drugs are also used in the formulation, along with some other chemotherapeutic agents such as methotrexate, vincristine, and prednisone.
6.4. Infectious disease
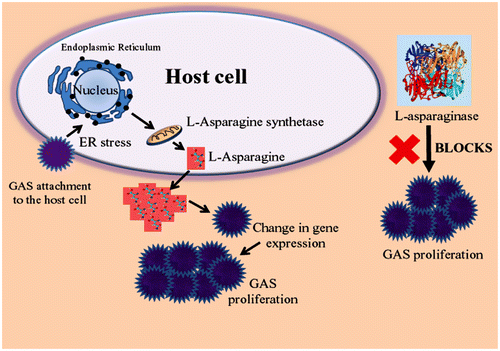
A recent study suggests that L-asparaginase inhibits Group A streptococcus (GAS) growth in human blood as well as in a mouse model of bacterial infection. Streptococcus pyogenes, or GAS is known for causing a wide range of infections. It ranges from mild to deadly like flesh eating disease (necrotizing fasciitis), pharyngitis, glomerulonephritis, scarlet fever, toxic shock syndrome, meningitis, and rheumatic fever. When GAS adheres to host cell, it releases streptolysin toxins (streptolysin O and streptolysin S) that cause endoplasmic reticulum stressed condition. This results in enhanced expression and production of asparagine synthetase, in turn, the high level of L-asparagine in blood. At the elevated level of L-asparagine, the gene expression of the GAS is upregulated, triggering proliferation of GAS that causes a fatal effect in the host (Baruch et al., Citation2014). The mechanism of GAS infection and release of L-asparagine is illustrated in Figure . The L-asparagine released (due to GAS infection) can mitigate by the L-asparaginase to check the proliferation of pathogen inside the host. This finding also grabs attention toward the metabolic changes that occur during the pathogenic invasion and can be targeted for an efficient treatment of infectious disease. Researchers are also making presumptions that other Gram-positive bacteria such as Staphylococcus aureus, Listeria monocytogenes, and Clostridium botulinum that secrete similar streptolysin toxins may also follow the same mechanism of proliferation in the host. Hence, an infection caused by them may also be treated using L-asparaginase (Baruch et al., Citation2014).
6.5. Autoimmune diseases
It is observed that L-asparaginase also has immunosuppressive and anti-inflammatory effects in the patients. It is highly capable of suppressing T-cell-mediated B-cell responses. Its effect on the lymphatic system increases its chances to be used in the treatment of autoimmune diseases associated with abnormal T cell responses. An attempt was made by treating male DBA/1 mice having collagen-induced arthritis (CIA) with pegylated L-asparaginase from E. coli. It was found that the enzyme effectively reduces the severity of arthritis in CIA mice. A comparative study showed that it is also more efficacious and less toxic than cyclophosphamide (standard drug) in treating CIA. The study explores the hidden quality of this enzyme to cure autoimmune diseases such as rheumatoid arthritis in future (Reiff et al., Citation2001).
7. Various applications of L-asparaginase other than therapeutics
7.1. Food and health industry
Along with its therapeutic and medicinal value, L-asparaginase is also used as a processing aid in the food industry. It is used for reducing the acrylamide content in the fried and baked foods. On the basis of several studies, International Agency for Research on Cancer (IARC report, 1994) has classified acrylamide as ‘probably carcinogenic to human’. Scientific Committee on Toxicity, Ecotoxicity, and the Environment has also established its intrinsic toxic effects (neurotoxicity, genotoxicity to both somatic and germ cells, carcinogenicity, and reproductive toxicity). Acrylamide is a chemical that is found in some starchy foods during high-temperature cooking, such as frying, roasting, and baking above 120 °C. It is formed by the chemical reaction between carbonyl groups of reducing sugars and the free amino group of the amino acid asparagine that is naturally present in the starchy food. The process is known as ‘Millard reaction’. To reduce the formation of acrylamide, asparaginase is added before baking or frying the food that converts asparagine into aspartic acid and ammonium ions. As a food processing aid, it effectively reduces the level of acrylamide up to 90% in a variety of starchy foods. It does not interfere with the taste and appearance of the end product (Batool et al., Citation2016). However, complete acrylamide removal is probably not possible due to other minor asparagine independent formation pathways (Kushwaha, Ahmed, Jayanand, & Singh, Citation2012). Acrylaway and PreventASe are the two brand names of asparaginase available in the market for the reduction of acrylamide in starchy food. The food industry also demands new sources of this enzyme to mitigate acrylamide with some desirable properties. The enzyme should be stable over a wide range of pH and temperature and possess high substrate conversion rate and specificity so that the processing time is shortened. Apart from this, other components of the foodstuff remain unaffected during the process (Krishnapura, Belur, & Subramanya, Citation2015). Table shows a decrease in acrylamide percentage in different food stuff after treating with Acrylaway. Another application of the enzyme in the food industry is that the aspartic acid produced in asparagine hydrolysis gives sour and savory taste to the food. Bacillus subtilis is commonly used in food industry for this purpose. B. subtilis natto, a Japanese fermented food is one of the common examples of such type of fermented food. Along with enhancing the taste, aspartic acid is also involved in improving the liver function and helps to get control over fatigue (Yano, Minato, Thongsanit, Tachiki, & Wakayama, Citation2008).
Table 4. Reduction in acrylamide content in different food items after using Acrylaway.
7.2. Canine and feline chemotherapy
Lymphoma is the most common type of cancer found in dogs and cats. Along with human, L-asparaginase is also used as a chemotherapeutic agent to treat canine and feline lymphomas. It is used in multidrug resistance protocol in canine cancer along with cyclophosphamide and prednisone (Saba, Hafeman, Vail, & Thamm, Citation2009). In the most treated cases of canine cancer, it relapses and requires rescue therapy. The reason for relapses may because cytotoxic drug concentrations are not achieved at specific anatomic sites, such as the central nervous system. Veterinary oncologist administers L-asparaginase to decrease tumor for some time allowing doctors to consider available alternative curing therapies. (Kitchell & Ho, Citation2011). However, its high cost restricts its frequent usage in the veterinary application.
7.3. Novel application
In some homo-oligomeric protein, it is observed that the monomers exist in different conformations. These monomers (of the same type) when joined together give rise to the different quaternary structure. Each different quaternary structure of the protein exhibits a different function. Such proteins are called morpheein. A reversible rearrangement in quaternary structure creates new allosteric sites in these morpheeins which can be exploited as a potential drug target (Selwood & Jaffe, Citation2012). L-asparaginase is observed as a dimer, tetramer, and inactive octamer. Type-I L-asparaginase shows an allosteric reorganization of the tetramer upon binding L-asparagine. This protein quaternary structure dynamics may serve the basis for new drug discovery (Vimal & Kumar, Citation2016)
L-asparaginase is reported as a virulence factor in many diseases. It is reported that Salmonella typhimurium uses L-asparaginase-II mediated T cell immunosuppression mechanism to cause infections in the mammal and avoids clearance by host immune system (Kullas et al., Citation2012). Helicobacter pylori, the causative agent of chronic gastritis, peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma of the stomach, exploits L-asparaginase as a relevant virulence factor. It causes infection through cell-cycle inhibition in the host (Scotti et al., Citation2010). Mycobacterium tuberculosis (the causative agent of Tuberculosis), uses asparagine transporter AnsP2 and secreted asparaginase AnsA to resist acidic stress as well as for pH buffering within macrophages for its survival (Gouzy et al., Citation2014). In all such cases, morpheein model of allosteric regulation of L-asparaginase can be explored to improve current therapies.
8. Conclusion
L-asparaginase has very imperative role as a chemotherapeutic agent in the medical field. The various lab studies support its other medicinal utility in the treatment of infectious and autoimmune diseases. The role of this enzyme as food processing aid is also well established. The multiple application and massive market requirement of this enzyme are demanding its high production with cost effectiveness. Submerged fermentation is well established for its industrial production. However, the mode of operation greatly affects its productivity. A comparative study of batch and fed-batch mode of production of this enzyme using Pectobacterium carotovorum MTCC 1428 was done. The production was almost double in the case of fed-batch fermentor (38. 8 U/ml) as compared to the batch reactor (17.97 U/ml) (S. Kumar, Prabhu, Dasu, & Pakshirajan, Citation2017). SSF could also be a promising alternative because of high productivity, usage of cheap agricultural wastes, and generation of less agricultural waste. SSF using fishery wastes or any other unique substrate instead of conventional substrates could also be an alternative for higher productivity. The production can also be enhanced by adopting statistical method of process optimization and whole cell immobilization techniques. There is also a necessity of superior producer strains for improved enzyme production and reduced immunogenicity. It can be possible by exploring novel enzyme source or developing higher producer strains. Marine sources have a diverse range of micro-organisms with unique properties. One such explored example is of Spirulina maxima, nutrient-rich blue-green microalgae. The advantages associated with blue-green microalgae-based enzyme production is high efficiency, economical, no seasonal variation, easy scale up, extraction, and higher yields and purification through the simple protocol (Abd El Baky & El Baroty, Citation2016). So, we can explore the marine sources for the production of this medically important enzyme in future. The potential strains can be generated in the laboratory through various biotechnological methods like mutagenesis, recombinant DNA technology, protoplast fusion, or combined strategy. The key issue of immunogenicity can be addressed by isolating the new strain that is free from glutaminase and urease activity. Another way to deal with this problem is immobilizing or conjugating the enzymes with suitable polymers. It was found that half-life of native and PEG-asparaginase are 7–8 and 384-600 h, respectively (Fortier, Citation1994). It reduces the immunogenicity by decreasing need of frequent doses as well as causes less antibody generation. There is an equal need to develop highly efficient, cost effective, easy scale up separation, and purification techniques to decrease the cost and immunogenicity. The methods like membrane processes, Aqueous Two-Phase Systems (ATPS) integrated with precipitation and/or fractionation steps, and CCC holds tremendous potential for industrial scale applicability. It is expected that such a review would be a comprehensive guide on this particular topic and currently available information about the L-asparaginase and collectively it would broaden the scope of its cost effective production and application in future.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgment
We are greatly thankful for the support provided by NIT Raipur (CG), India for providing space and an opportunity to frame out this piece of review.
References
- Abd El Baky, H. H., & El Baroty, G. S. (2016). Optimization of growth conditions for purification and production of L-asparaginase by Spirulina maxima. Evidence-Based Complementary and Alternative Medicine, 2016(1), 1–7. doi:10.1155/2016/1785938
- Balakrishnan, K., Nair, A., & Kumar, R. (2013). Screening of microbial isolates for the fermentative production of L-asparaginase in submerged fermentation. Research and Reviews: Journal of Pharmacy and Pharmaceutical Sciences, 2, 95–100.
- Bano, M., & Sivaramakrishnan, V. M. (1980). Preparation and properties of L-asparaginase from green chilies (Capsicum annum L.). Journal of Biosciences, 2, 291–297.10.1007/BF02716861
- Baruch, M., Belotserkovsky, I., Hertzog, B. B., Ravins, M., Dov, E., McIver, K. S., … Hanski, E. (2014). An extracellular bacterial pathogen modulates host metabolism to regulate its own sensing and proliferation. Cell, 156, 97–108. doi:10.1016/j.cell.2013.12.007
- Baruch, M., Hertzog, B. B., Ravins, M., Anand, A., Cheng, C. Y., Biswas, D., & Hanski, E. (2014, August). Induction of endoplasmic reticulum stress and unfolded protein response constitutes a pathogenic strategy of group A streptococcus. Frontiers in Cellular and Infection Microbiology, 4, 1–5. doi:10.3389/fcimb.2014.00105
- Baskar, G., & Sahadevan, R. (2011). Optimization of media components and operating conditions for exogenous production of fungal L-asparaginase. Chiang Mai Journal of Science, 38, 270–279.
- Batool, T., Makky, E. A., Jalal, M., & Yusoff, M. M. (2016). A comprehensive review on L-asparaginase and its applications. Applied Biochemistry and Biotechnology, 178, 900–923. doi:10.1007/s12010-015-1917-3
- Broome, J. D. (1961). Evidence that the L-asparaginase activity of guinea pig serum is responsible for its anti lymphoma effects. Nature, 191, 1114–1115.10.1038/1911114a0
- Cachumba, J. J. M., Antunes, F. A. F., Peres, G. F. D., Brumano, L. P., Dos Santos, J. C., & Da Silva, S. S. (2016). Current applications and different approaches for microbial L-asparaginase production. Brazilian Journal of Microbiology, 47, 77–85. doi:10.1016/j.bjm.2016.10.004
- Carta de-Angeli, L., Pocchiari, F., Russi, S., Tonolo, A., Zurita, V. E., Ciaranfi, E., & Perin, A. (1970). Effect of L-asparaginase from Aspergillus terreus on ascites sarcoma in the rat. Nature, 225, 549–550.10.1038/225549a0
- Clementi, A. (1922). La d_esamidation enzymatique de l’asparagine chez les diff_erentes esp_eces animales et la signification physio logique de sa presence dans l’organisme. Archives Internationales de Physiologie, 19, 369–398.10.3109/13813452209145156
- Costa, I. M., Schultz, L., de Araujo Bianchi Pedra, B., Leite, M. S. M., Farsky, S. H. P., de Oliveira, M. A., … Monteiro, G. (2016, April). Recombinant L-asparaginase 1 from Saccharomyces cerevisiae: An allosteric enzyme with antineoplastic activity. Scientific Reports, 6, 893. doi:10.1038/srep36239
- Dange, V. U., & Peshwe, S. A. (2013). Screening and characterization of actinomycetes for production of anti-leukemic L-asparaginase. Indian Journal of Applied Research, 3, 543–545.
- Doriya, K., & Kumar, D. S. (2016). Isolation and screening of L-asparaginase free of glutaminase and urease from fungal sp. 3. Biotech, 6(2), 1–10. doi:10.1007/s13205-016-0544-1
- Doriya, K., Jose, N., Gowda, M., & Kumar, D. S. (2016). Solid-state fermentation vs submerged fermentation for the production of L-asparaginase. Advances in food and nutrition research (1st ed., Vol. 78). Elsevier. Retrieved from https://doi.org/10.1016/bs.afnr.2016.05.003
- El-Bessoumy, A. A., Sarhan, M., & Mansour, J. (2004). Production, isolation, and purification of L-asparaginase from Pseudomonas aeruginosa 50071 using solid-state fermentation. Journal of Biochemistry and Molecular Biology, 37, 387–393. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15469724
- El-Gendy, M. M. A. A., Al-Zahrani, S. H. M., & El-Bondkly, A. M. A. (2017). Construction of potent recombinant strain through intergeneric protoplast fusion in endophytic fungi for anticancerous enzymes production using rice straw. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-017-2429-0
- Erva, R. R., Goswami, A. N., & Suman, P. (2016). Optimization of L-asparaginase production from novel Enterobacter Sp. by submerged fermentation using response surface methodology. Preparative Biochemistry & Biotechnology, 47, 219–228. doi:10.1080/10826068.2016.1201683
- Fortier, G. (1994). Biomedical applications of enzymes and their polyethylene glycol adducts. Biotechnology and Genetic Engineering Reviews, 12, 329–356. doi:10.1080/02648725.1994.10647915
- Ghosh, S., Murthy, S., Govindasamy, S., & Chandrasekaran, M. (2013). Optimization of L-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustainable Chemical Processes, 1, 9. doi:10.1186/2043-7129-1-9
- Gouzy, A., Larrouy-Maumus, G., Bottai, D., Levillain, F., Dumas, A., Wallach, J. B., … Neyrolles, O. (2014). Mycobacterium tuberculosis exploits asparagine to assimilate nitrogen and resist acid stress during infection. PLoS Pathogens, 10(2), e1003928. doi:10.1371/journal.ppat.1003928
- Hegazy, W., & Moharam, M. (2010). L-asparaginase hyperproducing recombinant Bacillus strains obtained by interspecific protoplast fusion. Journal of Genetic Engineering and Biotechnology, 8, 67–74.
- Hosamani, R., & Kaliwal, B. B. (2011). L-asparaginase-an anti tumor agent production by Fusarium equiseti using solid state fermentation. International Journal of Drug Discovery, 3, 88–99.
- Jayaramu, M., Hemalatha, N. B., Rajeshwari, C. P., Siddalingeshwara, K. G., & Sunil, P. L. N. S. N. (2010). A novel approach for detection, confirmation, and optimization of L-asparaginase from Emericella Nidulans. Current Pharma Research, 1, 20–24.
- Kattimani, L., Amena, S., Nandareddy, V., & Mujugond, P. (2009). Immobilization of Streptomyces gulbargensis in polyurethane foam: A promising technique for L-asparaginase production. Iranian Journal of Biotechnology, 7, 199–204.
- Kavitha, A., & Vijayalakshmi, M. (2012). A study on L-asparaginase of Nocardia levis MK-VL_113. The Scientific World Journal, 2012, 1–5. doi:10.1100/2012/160434
- Kebeish, R., El-Sayed, A., Fahmy, H., & Ghany, A. A. (2016). Molecular cloning, biochemical characterization, and antitumor properties of a novel L-asparaginase from Synechococcus elongatus PCC6803. Biochemistry (Moscow), 81, 1173–1181.10.1134/S000629791610014X
- Kil, J. O., Kim, G. N., & Park, I. (1995). Extraction of extracellular L-Asparaginase from Candida utilis. Bioscience, Biotechnology, and Biochemistry, 59, 749–750. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/777284510.1271/bbb.59.749
- Kitchell, B. E., & Ho, H.-Y. (2011, September). Rescue therapy for canine lymphoma. Cutting Edge Oncology, 61–64.
- Krishnapura, P. R., & Belur, P. D. (2016). Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. Journal of Molecular Catalysis B: Enzymatic, 124, 83–91. doi:10.1016/j.molcatb.2015.12.007
- Krishnapura, P. R., Belur, P. D., & Subramanya, S. (2015, September). A critical review on properties and applications of microbial L-asparaginases. Critical Reviews in Microbiology, 96, 1–18. doi:10.3109/1040841X.2015.1022505
- Kullas, A. L., McClelland, M., Yang, H., Tam, J. W., Torres, A., Porwollik, S., … Andrews-polymenis, H. (2012). L-asparaginase II produced by Salmonella Typhimurium inhibits T cell responses and mediates virulence. Cell Host and Microbe, 12, 791–798. doi:10.1016/j.chom.2012.10.018
- Kumar, D. P., Thangabalan, B., Venkata, R. M., Vadivel, K., Manohar, B. S., & Rao. D. S. (2011). Optimization of parameters for the production of L-asparaginase by Serratia marcescens. Journal of Pharmaceutical and Biomedical Sciences, 7, 5–8.
- Kumar, K., Punia, S. S., & Verma, N. (2012). Media optimization for the production of anti-leukemic enzyme L-asparaginase from E coli K-12. Annals of Biological Research, 3, 4828–4837.
- Kumar, S., Prabhu, A. A., Dasu, V. V., & Pakshirajan, K. (2017). Batch and fed-batch bioreactor studies for the enhanced production of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Preparative Biochemistry and Biotechnology, 47, 74–80. doi:10.1080/10826068.2016.1168841
- Kushwaha, A., Ahmed, F., Jayanand, & Singh, P. (2012). Production and purification of L-asparaginase from bacterial source. International Journal of Universal Pharmacy and Life Sciences, 2, 39–62.
- Lanvers-Kaminsky, C. (2017). Asparaginase pharmacology: Challenges still to be faced. Cancer Chemotherapy and Pharmacology, 79, 439–450. doi:10.1007/s00280-016-3236-y
- Lea, P. J., Sodek, L., Parry, M. A. J., Shewry, P. R., & Halford, N. G. (2007). Asparagine in plants. Annals of Applied Biology, 150(1), 1–26. doi:10.1111/j.1744-7348.2006.00104.x
- Lopes, A. M., de Oliveira-Nascimento, L., Ribeiro, A., Tairum, C. A., Breyer, C. A., de Oliveira, M. A., … Pessoa, A. (2015, December). Therapeutic L-asparaginase: Upstream, downstream and beyond. Critical Reviews in Biotechnology, 8551, 1–18. doi:10.3109/07388551.2015.1120705
- Martinez-outschoorn, U. E., Pestell, R. G., Sotgia, F., & Lisanti, M. P. (2016, May). Cancer metabolism: A therapeutic perspective. Nature Reviews Clinical Oncology, 1–21. https://doi.org/10.1038/nrclinonc.2016.60.
- Mashburn, L. T., & Wriston, J. C. (1964). Tumor inhibitory effect of L-asparaginase from Escherichia coli. Archives of Biochemistry and Biophysics, 105, 450–453.10.1016/0003-9861(64)90032-3
- Mishra, A. (2006). Production of L-Asparaginase, an anticancer agent, from Aspergillus niger using agricultural waste. Applied Biochemistry and Biotechnology, 135, 33–42.10.1385/ABAB:135:1
- Moorthy, V., Ramalingam, A., Sumantha, A., & Shankaranaya, R. T. (2010). Production, purification, and characterization of extracellular L-asparaginase from a soil isolate of Bacillus sp. African Journal of Microbiology Research, 4, 1862–1867.
- Mu, X., Qiao, J., Qi, L., Dong, P., & Ma, H. (2014). Poly(2-Vinyl-4,4-dimethylazlactone)-functionalized magnetic nanoparticles as carriers for enzyme immobilization and its application. ACS Applied Materials & Interfaces, 6, 21346–21354. doi:10.1021/am5063025
- Muthukumar, T., Chamundeeswari, M., Prabhavathi, S., Gurunathan, B., Chandhuru, J., & Sastry, T. P. (2014). Carbon nanoparticle from a natural source fabricated for folate receptor targeting, imaging and drug delivery application in A549 lung cancer cells. European Journal of Pharmaceutics and Biopharmaceutics, 88, 730–736. doi:10.1016/j.ejpb.2014.09.011
- Nair, A., Kumar, R., Devi, R. A., & Balakrishnan, K. (2013). Screening of commonly available solid process residues as substrate for L-asparaginase production by Aspergillus terreus MTCC 1782. Research Journal of Pharmaceutical, Biological and Chemical Sciences journal of Pharmaceutical, Biological, and Chemical Sciences, 4, 1731–1737.
- Narayana, K. J. P., Kumar, K. G., & Vijayalakshmi, M. (2008). L-asparaginase production by Streptomyces albidoflavus. Indian Journal of Microbiology, 48, 331–336. doi:10.1007/s12088-008-0018-1
- Naveena, B., Narayani, T. G., Sakthiselvan, P., & Partha, N. (2012). Antioxidant and antimicrobial efficacies of Amaranthus polygonoides and its impact on L-asparaginase production. African Journal of Biotechnology, 11, 12483–12490. doi:10.5897/AJB12.098
- Ortac, I., Simberg, D., Yeh, Y., Yang, J., Messmer, B., Trogler, W. C., … Esener, S. (2014). Dual-porosity hollow nanoparticles for the immunoprotection and delivery of nonhuman enzymes. Nano Letters, 14, 3023–3032. doi:10.1021/nl404360 k
- Oza, V. P., Parmar, P. P., Kumar, S., & Subramanian, R. B. (2010). Anticancer properties of highly purified L-asparaginase from Withania somnifera L. against acute lymphoblastic leukemia. Applied Biochemistry and Biotechnology, 160, 1833–1840. doi:10.1007/s12010-009-8667-z
- Pallem, C., Nagarjun, V., & Srikanth, M. (2011). Production of a tumor inhibitory enzyme, L-asparaginase through solid state fermentation using Fusarium oxysporum. International Journal of Pharmaceutical Sciences Review and Research, 7, 189–192.
- Patil, M. P., Patil, R. H., & Maheshwari, V. L. (2012). A novel and sensitive agar plug assay for screening of asparaginase-producing endophytic fungi from Aegle marmelos. Acta Biologica Szegediensis, 56, 175–177.
- Paul, J. H., & Cooksey, K. E. (1981). Regulation of L-asparaginase in a Chlamydomonas species in response to ambient concentrations of combined nitrogen. Journal of Bacteriology, 147, 9–12. Retrieved from https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=216001&tool=pmcentrez&rendertype=abstract
- Petros, R. A., & DeSimone, J. M. (2010). Strategies in the design of nanoparticles for therapeutic applications. Nature Reviews Drug Discovery, 9, 615–627. doi:10.1038/nrd2591
- Prema, P., Devi, M. N., & Alagumanikumaran, N. (2013). Production of tumor inhibitory L-asparaginase by wild and mutant strains of Pseudomonas fluorescens. International Journal of Advanced Research, 1, 163–171.
- Priya, M., Radhakrishnan, M., Balagurunathan, R., Arts, S. S., & Nadu, T. (2011). Production and optimization of L-asparaginase from Streptomyces sp (TA22) isolated from Western Ghats, India. Journal of Chemical and Pharmaceutical Research, 3, 618–624.
- Reiff, A., Zastrow, M., Sun, B. C., Takei, S., Mitsuhada, H., Bernstein, B., & Durden, D. L. (2001). Treatment of collagen induced arthritis in DBA/1 mice with L-asparaginase. Clinical and Experimental Rheumatology, 19, 639–646. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11791634
- Saba, C. F., Hafeman, S. D., Vail, D. M., & Thamm, D. H. (2009). Combination chemotherapy with continuous L-asparaginase, lomustine, and prednisone for relapsed canine lymphoma. J Vet Intern Med, 23, 1058–1063.10.1111/jvim.2009.23.issue-5
- Sanches, M., Krauchenco, S., & Polikarpov, I. (2007). Structure, substrate complexation and reaction mechanism of bacterial asparaginases. Current Chemical Biology, 1, 75–86. doi:10.2174/187231307779814057
- Scotti, C., Sommi, P., Pasquetto, M. V., Cappelletti, D., Stivala, S., Mignosi, P., & Vannini, V. (2010). Cell-cycle inhibition by Helicobacter pylori L-asparaginase. PLoS ONE, 5(11), e13892. doi:10.1371/journal.pone.0013892
- Selwood, T., & Jaffe, E. K. (2012). Dynamic dissociating homo-oligomers and the control of protein function. Archives of Biochemistry and Biophysics, 519, 131–143. doi:10.1016/j.abb.2011.11.020
- Shrivastava, A., Khan, A. A., Shrivastav, A., Jain, S. K., & Singhal, P. K. (2012). Kinetic studies of L-asparaginase from Penicillium digitatum. Preparative Biochemistry & Biotechnology, 42, 574–581. doi:10.1080/10826068.2012.672943
- Shrivastava, A., Khan, A. A., Khurshid, M., Kalam, M. A., Jain, S. K., & Singhal, P. K. (2015). Recent developments in L-asparaginase discovery and its potential as anticancer agent. Critical Reviews in Oncology Hematology, pii, S1040, 1–12. doi:10.1016/j.critrevonc.2015.01.002
- Singh, Y., & Srivastava, S. K. (2012). Screening and characterization of microorganisms capable of producing antineoplastic drug, L-asparaginase. International Journal of Biological & Medical Research, 3, 2548–2554.
- Sivasankar, P., Sugesh, S., Vijayanand, P., Sivakumar, K., Vijayalakshmi, S., Balasubramanian, T., & Mayavu, P. (2013). Efficient production of L-asparaginase by marine Streptomyces sp. isolated from Bay of Bengal, India. African Journal of Microbiology Research, 7, 4015–4021. doi:10.5897/AJMR12.2184
- Soniyamby, A. R., Sundaram, L., & Vasantha, P. B. (2011). In vitro antioxidant and anticancer activity of L-asparaginase from Aspergillus flavus (KUFS20). Asian Journal of Pharmaceutical and Clinical Research, 4, 2–5.
- Subramaniyam, R., & Vimala, R. (2012). Solid state and submerged fermentation for the production of bioactive substances: A comparative study. International Journal of Science and Nature, 3, 480–486.
- Suresh, J. V., & Raju, K. J. (2013). Studies on the production of L-asparaginase by Aspergillus terreus MTCC 1782 using agro-residues under mixed substrate solid state fermentation. Journal of Chemical, Biological and Physical Sciences, 3, 314–325.
- Teodor, E., Litescu, S.-C., Lazar, V., & Somoghi, R. (2009). Hydrogel-magnetic nanoparticles with immobilized L-asparaginase for biomedical applications. Journal of Materials Science. Materials in Medicine, 20, 1307–1314. doi:10.1007/s10856-008-3684-y
- Tundisi, L. L., Coêlho, D. F., Zanchetta, B., Moriel, P., Pessoa, A., Tambourgi, E. B., … Mazzola, P. G. (2017). L-asparaginase purification. Separation & Purification Reviews, 46, 35–43. doi:10.1080/15422119.2016.1184167
- Varalakshmi, V., & Raju, K. J. (2013). Optimization of L-asparaginase production by Aspergillus terrus MTCC 1782 using bajra seed flour under solid state fermentation. International Journal of Research in Engineering and Technology, 2, 121–129.
- Venil, C., & Lakshmanaperumalsamy, P. (2013). Solid state fermentation for production of L-asparaginase in rice bran by Serratia marcescens SB08. The Internet Journal of Microbiology, 7(1). Retrieved from https://ispub.com/IJMB/7/1/10456#
- Vimal, A., & Kumar, A. (2016). The morpheein model of allosterism: A remedial step for targeting virulent L-asparaginase. Drug Discovery Today, pii, S1359. doi:10.1016/j.drudis.2016.10.004
- Vimal, A., & Kumar, A. (2017). In vitro screening and in silico validation revealed key microbes for higher production of significant therapeutic enzyme L-asparaginase. Enzyme and Microbial Technology, 98, 9–17. doi:10.1016/j.enzmictec.2016.12.001
- Vuddaraju, S. P., Nikku, M. Y., Chaduvula, A. I. R., Dasari, V. R. R. K., & Donthireddy, S. R. R. (2010). Application of statistical experimental designs for the optimization of medium constituents for the production of L-asparaginase by Serratia Marcescens. Journal of Microbial & Biochemical Technology, 2, 089–094. doi:10.4172/1948-5948.1000030
- Wang, F., & Zhang, Y. (2015). Bioconjugation of silk fibroin nanoparticles with enzyme and peptide and their characterization. Advanced protein chemistry and structural biology. (1st ed., Vol. 98). Elsevier. https://doi.org/10.1016/bs.apcsb.2014.11.005
- Warangkar, S. C., & Khobragade, C. N. (2009). Screening, enrichment and media optimization for L-asparaginase production. Journal of Cell and Tissue Research, 9, 1963–1968.
- Wriston, J. C., & Yellin, T. O. (1973). L-asparaginase: A review. Advances in Enzymology and Related Areas of Molecular Biology, 39, 185–248. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4583638
- Yano, S., Minato, R., Thongsanit, J., Tachiki, T., & Wakayama, M. (2008). Overexpression of type I L-asparaginase of Bacillus subtilis in Escherichia coli, rapid purification and characterization of recombinant type I L-asparaginase. Annals of Microbiology, 58, 711–716.10.1007/BF03175579