Abstract
Viruses hijack the host cell machinery and recruit host proteins to aid their replication. Several host proteins also play vital roles in inhibiting viral replication. Emerging class of host proteins central to both of these processes are the DEAD-box helicases: a highly conserved family of ATP-dependent RNA helicases, bearing a common D-E-A-D (Asp–Glu–Ala–Asp) motif. They play key roles in numerous cellular processes, including transcription, splicing, miRNA biogenesis and translation. Though their sequences are highly conserved, these helicases have quite diverse roles in the cell. Interestingly, often these helicases display contradictory actions in terms of the support and/or clearance of invading viruses. Increasing evidence highlights the importance of these enzymes, however, little is known about the structural basis of viral RNA recognition by the members of the DEAD-box family. This review summarizes the current knowledge in the field for selected DEAD-box helicases and highlights their diverse actions upon viral invasion of the host cell. We anticipate that through a better understanding of how these helicases are being utilized by viral RNAs and proteins to aid viral replication, it will be possible to address the urgent need to develop novel therapeutic approaches to combat viral infections.
Introduction
Viruses have stood the test of time, evolving and adapting to the various environmental and host changes, affecting every domain of life. Their ability to take over the host cells, using host replicative machinery for their own benefit, has enabled these small hijackers to persist and spread to other hosts. An emerging class of host enzymes that are central to the viral replication process are the DEAD-box helicases, whose precise role in the viral replication process remains unknown.
The DEAD-box helicases are a family of RNA helicases that hydrolyse ATP to carry out their function. So, named because of their common D-E-A-D (Asp–Glu–Ala–Asp) motif, DEAD-box helicases are found in essentially every domain of life from bacteria and fungi to humans and have motifs that are highly conserved among them. In humans, there have been 37 such DEAD-box helicases described. They function in essential cellular processes including transcription, splicing, miRNA biosynthesis, translation and RNA degradation. In fact, in experimental knockout models of DEAD-box helicases, many are shown to be essential for life (Chen et al., Citation2016; Fukuda et al., Citation2007; Jalal, Uhlmann-Schiffler, & Stahl, Citation2007; Janknecht, Citation2010). With such a critical role in cellular functioning and replication, it follows that these helicases may also be involved in the cell’s interaction with a viral invader. Like the cells’ requirement for helicases to unwind, splice and edit nucleotides, viral genomes which are comprised of analogous single- and double-stranded DNA and RNA components, also require mechanisms to process and reproduce their genetic material. Given that viruses do not carry all the replicative proteins required for their reproduction, any structural and non-structural proteins encoded by viruses are there either to directly aid viral replication or to aid in the hijacking of the necessary aspects of the host’s machinery. One of the groups of involved proteins are the DEAD-box helicases, but the question of whether these proteins are in fact aiding and abetting the virus or are acting to inhibit it in a form of innate immune response, remains to be determined. As well, there is growing evidence that they may function in roles that extend beyond their capacity as helicases.
This review will examine the structure and function of the DEAD-box helicases, with focus on DDX1, DDX3X, DDX5 and DDX17 helicases. In particular, we will focus on the helicases’ interactions with invading viruses and provide details on the current understanding about DEAD-box helicases in either aiding or preventing replication.
Motifs and structural similarities
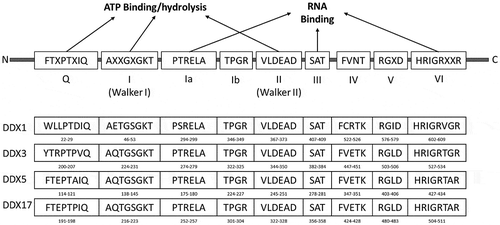
The DEAD-box helicases belong to the Superfamily 2 of helicases. As a class, they share nine conserved motifs – Q, Motif I, IA, IB, II (D-E-A-D motif), Motif III, IV, V and VI within their highly conserved core domain (Figure ). The sequences of the four DEAD-boxes described here are also included in Figure . Flanking the helicase core are the N‑terminal and C‑terminal domains, ranging from a few to several hundred amino acids in length. These domains are thought to facilitate interactions with other proteins and/or RNA (Fairman-Williams, Guenther, & Jankowsky, Citation2010). As much of the focus has been on functions of the segments of the core domain, there is limited structural data available on these terminal domain interactions (Linder & Jankowsky, Citation2011).
The N- and C-terminal domains of helicases are difficult to express and are known to have disordered secondary structures (Dutta et al., Citation2012). As a result, these domains have not been characterized widely using biophysical or structural biology tools. The core domains, however, are structurally very conserved across the species and have been better characterized experimentally. In general, the DEAD-box proteins are composed of two analogous RecA-like globular αβ-domains that come together to create the ATP hydrolysis site (Figure (a)–(c)), where the adenosine of ATP is anchored via π–π stacking with an aromatic amino acid (Tyr200 in DDX3X) and coordination with a glutamine (Gln207) and arginine (Arg202) of the Q motif. At the same time, the most proximal phosphate group forms hydrogen bonds with the core of the I (Walker I) motif (Gly227 and 229, Lys230 and Thr231 in DDX3X); while the most distal interacts with the DEAD motif (Asp347–Asp350 in DDX3X), enabling the critical ATP hydrolysis step.
Figure 2. Comparison of high-resolution structural information of DEAD-box helicases. (a) Surface representation of DDX3 with AMP (PDB file: 5E7J); (b) Ribbon representation of helicase core domains of DDX3(grey) and DDX5(green) overlaid with their associated nucleotide phosphate, AMP and ADP, respectively (PDB files: 5E7J and 3FE2); (c) Coordinating residues with AMP within the Q and Walker I motifs (for DDX3). Surface (d) and ribbon (e) representation of helicase core domain structure of DDX3 presenting RNA-binding site (produced with comparative modelling of the helicase eI4A, PDB file: 2HYI). (f) DDX1 SPRY domain tertiary structure indicating two stacked concave β-sheets (pink and blue) with a third lower β-sheet (yellow) (PDB ID 4XW3). Images were produced using PyMOL (PyMOL https://pymol.org/2/).
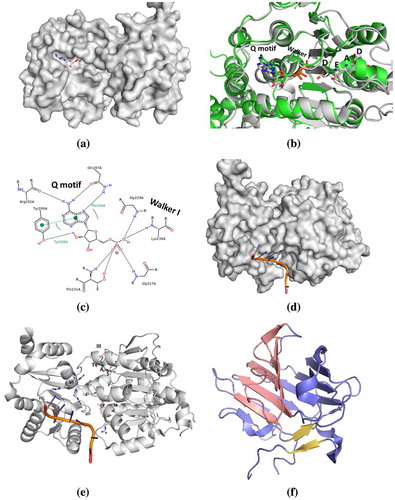
While no high-resolution structural information is yet available for the DDX17, its sequence analysis and comparison with DDX5 reveal approximately 90% sequence homology with the core of DDX5, and overall sequence homology of approximately 70%, suggesting that the secondary and tertiary structures of DDX17 are likely also well-conserved (Figure (d),(e)) (Lamm, Nicol, Fuller-Pace, & Lamond, Citation1996). For DDX1, structural information is similarly lacking, however, its unique 220-amino acid insertion – the SPRY domain – has been crystallized (Figure (f)) (Kellner & Meinhart, Citation2015). This domain is arranged as two layers of stacked, concave, antiparallel β-sheets, with a third β-sheet residing beneath (Figure (f)) (Kellner & Meinhart, Citation2015). This arrangement appears to be conserved among other eukaryotic SPRY-domain structures (Kellner & Meinhart, Citation2015).
Classical function of DEAD-box RNA helicases
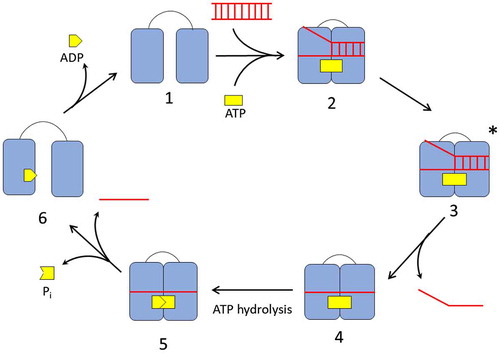
The catalytic cycle of DEAD-box helicase’s RNA unwinding is an ATP-driven process. The two RecA-like globular domains of the helicase come together to form the helicase core and create the binding sites for both the RNA and ATP (Hilbert, Karow, & Klostermeier, Citation2009; Linder & Jankowsky, Citation2011). Typically, the helicase exists in an ‘open’ conformation in the absence of RNA and ATP (Liu, Putnam, & Jankowsky, Citation2008; Mallam, Campo, Gilman, Sidote, & Lambowitz, Citation2012; Mallam et al., Citation2011; Schutz et al., Citation2010; Theissen, Karow, Köhler, Gubaev, & Klostermeier, Citation2008). Binding of both the dsRNA and ATP molecule causes the enzyme to change its conformation to a ‘closed’ conformation, aligning the catalytic site for ATP hydrolysis, and creating a kink in the RNA strand. This results in local destabilization of the RNA, causing the single strand to dissociate from the helicase (Mallam et al., Citation2012; Steimer & Klostermeier, Citation2012). Upon ATP hydrolysis, the helicase shifts back into ‘open’ conformation, distorting the RNA-binding site and causing the remaining RNA strand to leave and resetting the helicase for further catalysis (Figure ).
Figure 3. The catalytic cycle of DEAD-box helicase. In the absence of ligands, the DEAD-box helicase exists in an open conformation (1). Binding of RNA and ATP cause the helicase to switch to a closed conformation (2) aligning the ATP active site for hydrolysis and locally destabilizing the RNA in its binding site. In the activated complex (3), the first RNA strand dissociates. ATP hydrolysis (4) and release of the phosphate and second RNA strand (5) enables the helicase to re-open (6) and reset for the next cycle.
Despite the numerous structural similarities and common helicase functioning, their roles and actions upon viral invasion/replication can vary greatly – many appearing to maintain their actions as a helicase; others taking on distinctly unique roles, some to the benefit or detriment of the viruses themselves.
DEAD-box helicases and their relationships with viruses
Viruses require the host cells’ components (e.g. enzymes, lipids), including helicases, to carry out their RNA processing/ viral replication process. It follows that if the virus does not come with its own helicase (as does Hepatitis C Virus (HCV) with the non-structural protein NS3 segment coding for its own helicase), it must scavenge/hijack the host cell’s own machinery to fulfil this function. However, beyond their actions as a helicase, the DEAD-box proteins appear to be taken advantage of by viruses for many of its non-classical functions to complete its replication cycle. Perhaps the most well-described incorporation of DEAD-box helicases into the viral replication process is with that of HIV-1, where numerous helicases including DDX1 (Fang et al., Citation2004), DDX3 (Naji et al., Citation2012), DDX5, DDX17 (Lorgeoux, Pan, Le Duff, & Liang, Citation2013) among others are involved. As research into the host factors involved in viral replication continues, more DEAD-box helicases are being implicated in viral survival and maintenance.
In the following section, we summarize how a selected group of helicases play vital roles in viral life cycle, either via directly interacting with viral RNA/proteins or via modulating immune activity.
DEAD-box helicases 1 (DDX1)
DDX1 is unique amongst other DEAD-box family members in that it contains an insertion of a 220 amino acid sequence, known as the SPRY domain, located between two motifs – I (Walker A) and Ia, within the core domain (Godbout, Hale, & Bisgrove, Citation1994; Jankowsky & Fairman, Citation2007). It has been shown to be involved in various cellular processes including mRNA/miRNA processing (Bleoo et al., Citation2001; Han et al., Citation2014), regulation of transcription and translation through complexes with hnRNPK (Chen, Lin, Tsay, Lee, & Chang, Citation2002), and involvement in NFκB-mediated transcription (Ishaq et al., Citation2009). DDX1 is upregulated in breast cancer (Germain et al., Citation2011), neuroblastoma and retinoblastoma (Godbout, Packer, & Bie, Citation1998), as well as in testicular cancers where it is also required for tumorigenesis (Tanaka, Okamoto, Ishikawa, Tamura, & Hara, Citation2009). There have also been associations with DDX1 in colorectal cancers, where it is found to bind with the upregulated protein, PRMT1, however, its true role is unclear and may, in fact, be linked with an immune response (Akter, Mansour, Hyodo, & Senga, Citation2016; He et al., Citation2017). On the other hand, DDX1 has been found to inhibit ovarian tumour progression and may serve as a negative prognostic factor in ovarian cancer (Han et al., Citation2014).
DDX1-viral interactions
Aiding the virus in essentially all cases for which it has been studied, DDX1 supports the replication of Human Immunodeficiency Virus-1 (HIV-1), HCV, Severe acute respiratory syndrome (SARS) coronavirus, Venezuelan Equine Encephalitis (VEE) virus and John Cunningham virus (see Table ).
Table 1. DEAD-box helicase interactions with viruses.
In HIV-1, replication is facilitated through the Rev (regulator of expression of virion proteins) protein, which overcomes cellular restrictions on the transport of unspliced and incompletely spliced mRNA out of the nucleus (Fang et al., Citation2004; Hope & Pomerantz, Citation1995). These pre-mRNAs carry a recognition sequence called Rev responsive element (RRE) located in the env gene, that is not present in fully spliced viral mRNAs (Cullen, Citation2003; Harris & Hope, Citation2000). DDX1 is a co-factor for Rev, enabling the oligomerization of Rev with the RRE on the viral pre-mRNAs (Edgcomb et al., Citation2012; Fang et al., Citation2004). More specifically, a 15-residue N-terminal segment of HIV-1 Rev (10E-24Q) recruits DDX1 at the segment spanning 189H to 333L (Fang et al., Citation2004; Kubota & Pomerantz, Citation2000). DDX1 can also bind the mRNA independent of the complex. Experimental models suggest DDX1 does not appear to favour bound over unbound, and that its ATPase activity is stimulated to a similar degree by free RNA or Rev-bound RNA, implying only that the helicase requires RNA to be bound before ATPase stimulation (Edgcomb et al., Citation2012). Taken together, this helicase acts as an essential linker that likely prepares the Rev–RRE–RNA complexes for more efficient export through the Chromosomal Maintenance 1 (CRM1, also known as Exportin 1) pathway, the major mammalian nucleocytoplasmic transport pathway. Indeed, a down-regulation of DDX1 has been shown to lead to an alternative splicing pattern of RRE-containing mRNA, while overexpression, via co-transfection of a DDX1 expression vector with HIV-1, significantly increased viral production (Edgcomb et al., Citation2012; Lin et al., Citation2014). In studies of the HIV-1 Tat protein, a protein also linked to the Rev-dependent pathway in HIV-1 synthesis, DDX1 was found to directly interact with Tat (Lin et al., Citation2014). These studies were also able to link DDX1’s role in Rev subcellular localization in a Rev-independent manner, and when disrupted with use of a mutant Tat (Nullbasic), overexpression of DDX1 in cells was able to restore Rev-dependent mRNA export and gene expression, as well as the altered Rev localization (Lin et al., Citation2014).
These findings indicate that DDX1 is a critical cellular co-factor for Rev function, which maintains the proper subcellular distribution of this HIV-1 regulatory protein. Alterations in DDX1-Rev interactions, similar to that of other DEAD-boxes, could induce HIV-1 persistence and targeting DDX1 may lead to novel anti-HIV-1 strategies and therapeutics (Krishnan & Zeichner, Citation2004).
In the case of HCV, while the virus itself codes for a helicase (the NS3 helicase), DEAD-box helicases are still required for replication implying roles for the host proteins that go beyond their helicase functioning. DDX1 binds with the 3′-TR (terminal region) and reverse complementary 5′(-)-TR of HCV RNA (Tingting, Caiyun, Zhigang, Pengyuan, & Zhenghong, Citation2006). In siRNA knockdown of DDX1 expression, a significant reduction in subgenomic RNA replication was noted, implicating DDX1’s role in this process (Tingting et al., Citation2006). In another study, RNA affinity capture assays were employed to pull down host proteins. This experiment identified interaction of 3′-TR with DEAD-boxes DDX3, DDX5 and DDX17, but not DDX1 (Harris, Zhang, Chaubey, & Pandey, Citation2006). The reason for the differences in overall findings is not entirely clear, however, but likely relates to the differing proportions of the 3′-TR used in the capture assays as well as the concentrations of the respective proteins in their starting material (cell lysates).
In coronavirus, DDX1 is involved in the delicate balance of subgenomic mRNAs, encoding structural proteins and genomic RNAs, encoding non-structural proteins. Knockdown of DDX1 or loss of its helicase activity markedly reduced the levels of longer subgenomic mRNAs, impacting the virus’ ability to replicate (Wu, Chen, & Yeh, Citation2014).
DDX1 (as well as DDX3) has been found to interact with the non-structural protein 3 (NSP3) of VEE to aid its replication (Amaya et al., Citation2016). Knockdown studies with siRNA of DDX1 alone and of both DDX1 and DDX3 together are inhibitory to the virus, but their roles do not appear to be additive (Amaya et al., Citation2016). Additionally, given the underlying interaction of NSP3 with the host kinase IKKβ (inhibitor of nuclear factor kappa-B kinase subunit β), a protein essential for the production of infectious viral progeny, DDX1’s role in viral support is further validated (Amaya et al., Citation2014).
DDX1 has also been shown to form a complex with the cleavage stimulation factor and bind to the JC virus transcriptional control region (Sunden et al., Citation2007b). As the levels of DDX1 are much higher in JCV-susceptible cells (i.e. IMR-32) compared with non-susceptible cells (i.e. HeLa, HEK293, A549 and SW480), it is speculated that DDX1 plays a role in cell specificity for the virus (Sunden et al., Citation2007b). The same group also demonstrated an increase in transactivation of the JCV promoter when DDX1 is overexpressed in IMR-32 cells, however, it had little effect on replication efficiency (Sunden et al., Citation2007a). They further suggested that exogenous DDX1 enhances expression of JCV proteins in infected cells, while in the DDX1 siRNA knockdown model, protein expression is markedly suppressed (Sunden et al., Citation2007a).
DEAD-box helicase 3 (DDX3)
DDX3 (also known as DDX3X (X-linked) and DBX) is involved in several steps of gene expression, such as transcription, mRNA maturation, mRNA export and translation (Ariumi, Citation2014; Sharma & Jankowsky, Citation2014). Moreover, in malignant cellular states, DDX3 is involved in breast cancers, with upregulation and implication in inducing various tumour-promoting pathways (Botlagunta et al., Citation2008; Heerma van Voss et al., Citation2017). In colorectal cancers, there appear to be conflicting results – some reporting positive prognostication where low levels lead to more metastasis and progression (Su et al., Citation2015); others claiming an oncogenic role with overexpression and pathway stimulation (Heerma van Voss et al., Citation2015). Similarly, in hepatocellular carcinoma, opposing findings also exist, with downregulation and growth suppression reported in some (Chang et al., Citation2006; Chao et al., Citation2006); while overexpression in others (Huang et al., Citation2004). There is some suggestion that this difference in hepatocellular carcinoma may be related to the virus predisposing to cancer – Hepatitis B Virus (HBV) – versus HCV-related hepatocellular carcinoma, the former have the more prominent links with downregulation (Chang et al., Citation2006). It remains unclear, however, whether the malignant links with DDX3 are directly related to its helicase functioning or perhaps some alternate pathway and even whether the change in DDX3 levels are an active or secondary process.
A similarly perplexing association is with DDX3’s role in viruses where it again demonstrates a duality – on the one hand influencing innate immunity pathways against the virus; while on the other, aiding viral replication. In the former role, DDX3 stimulates interferon (IFN) type I production through binding with IκB kinase ε (IKKε) and TANK-binding kinase 1 (TBK1), leading to activation of IRF3 and ultimately viral clearance (Gu, Fullam, Brennan, & Schroder, Citation2013; Schroder, Citation2011; Soulat et al., Citation2008). DDX3 is also linked with stress granule formation which is thought to aid in viral RNA degradation (Shih et al., Citation2012). Taking on the contrary role, DDX3 also promotes the replication of many viruses, including HIV-1, Hepatitis B Virus (HBV), HCV, HSV-1, West Nile Virus (WNV), norovirus, Japanese encephalitis Virus (JEV) and influenza virus (see Table ).
DDX3-viral interactions
Analogous to the actions of DDX1, in HIV-1, DDX3 is also a critical member of the Rev–RRE pathway, escorting pre-mRNA out of the nucleus of infected cells (Yedavalli et al., Citation2004). During HIV-1 infection, DDX3 localizes to nuclear pores and employs the CRM1 export pathway through direct binding with CRM1. Various pull-down and immunoprecipitation assays determined the key region for this interaction was on the DDX3’s C-terminal 260–517 amino acids (Yedavalli et al., Citation2004). Additionally, when CRM1 export was inhibited by leptomycin B (an inhibitor of the nuclear export receptor CRM1), DDX3 accumulated in the nucleus; while in the absence of the inhibitor, the DDX3 was mainly present in the cytoplasm (Yedavalli et al., Citation2004). Additionally, DDX3 provides further support of viral propagation via aiding the release of infectious p24 particles (Naji et al., Citation2012). Knockdown studies of a series of DEAD-boxes in HIV-1-infected cells showed ~25% decrease in p24 release with DDX3 knockdown (Naji et al., Citation2012). Another unique function for DDX3 is related to the Tat protein. While other DEAD-box helicases are able to affect Rev in HIV-1 (DDX1, DDX3, DDX5, DDX17, DDX21 and DDX56), and incidentally can synergize to enhance Rev, only DDX3 enhances Tat-induced transcription of the HIV-1 genome (Yasuda-Inoue, Kuroki, & Ariumi, Citation2013). This link was established through both knockdown and overexpression of DDX3. The same group demonstrated that wildtype DDX3 colocalizes with Tat in cytoplasmic foci and is also found in stress granules (Yasuda-Inoue et al., Citation2013). Subsequently, it was demonstrated that DDX3 localization does not appear to be affected when Tat is mutated (Lin et al., Citation2014). The presence of DDX3 in these stress granules appears to be a function of the cell’s homeostatic, if not immune, mechanisms. In normal host cell functioning, DDX3’s role in these stress granules appears to be essential in maintaining cell viability following various stressors, the N-terminus performing the critical interactions for their formation (Shih et al., Citation2012). The importance of stress granules in viral infections are becoming increasingly realized, displaying the capacity to prevent viral mRNA translation (Beckham & Parker, Citation2008; McCormick & Khaperskyy, Citation2017). It appears that DDX3’s role in HIV-1 infection is quite extensive, whether purposefully acting in favour of enhancing the viral replication, actively preventing replication, or as a bystander, attempting to maintain cellular homeostasis in a stressful cellular environment.
In contrast to its actions in several other viral processes, DDX3 inhibits the replication of HBV. The knockdown of endogenous DDX3 resulted in increased viral DNA synthesis, while transfection with exogenous DDX3 reduced synthesis, both in a dose-dependent manner (Wang, Kim, & Ryu, Citation2009). Although it was demonstrated that DDX3 forms a complex with the HBV polymerase (Wang et al., Citation2009), subsequent studies indicated that DDX3 inhibits HBV replication independent of this HBV–Pol interaction (Ko, Lee, Windisch, & Ryu, Citation2014). Through similar DDX3 mutant studies as above, it was also determined that the ATPase activity, but not the helicase activity, was essential for the inhibition of HBV replication (Ko et al., Citation2014; Wang et al., Citation2009). Since DDX3 influences RNA decay in cells, the authors also examined whether the reduction in viral synthesis products was related to the degradation of the RNA itself, however, this was not found to be the case (Ko et al., Citation2014). Furthermore, DDX3 was also shown to be incorporated into the HBV virions (Wang et al., Citation2009). Interestingly, DDX3 was also found to be present in other viral particles, including Herpes simplex virus type-1(HSV-1) and human cytomegalovirus (Stegen et al., Citation2013; Varnum et al., Citation2004).
DDX3 has been shown to be essential for HCV viral replication. Knockdown studies of DDX3 resulted in a 95% reduction in HCV RNA accumulation compared to controls (Ariumi et al., Citation2007). One of the key interactions considered to impact this is that of the HCV core protein with the C-terminal domain of DDX3 (Ariumi et al., Citation2007; Owsianka & Patel, Citation1999). More specifically, the N-terminal domain of the core (aa 16–36) interacts with the C-terminal domain of DDX3 (aa 553–622) (Mamiya & Worman, Citation1999; Owsianka & Patel, Citation1999; Sun, Pager, Luo, Sarnow, & Cate, Citation2010; You et al., Citation1999). The DDX3 helicase localization shifts from nuclear to the cytoplasmic in HCV-infected cells, appearing in discrete foci in the endoplasmic reticulum – where the HCV core protein is also found to localize (Mamiya & Worman, Citation1999). Even an ectopic overexpression of HCV core results in a proportion of DDX3 from the nucleus to redistribute to the cytoplasm, in particular, at the sites of HCV core protein (Angus et al., Citation2010). It was speculated that the DDX3–core complex may act to unwind the double-stranded HCV RNA or possibly contribute to the function of HCV’s own NS3 helicase (Ariumi et al., Citation2007). However, the significance of this interaction has been questioned, as mutated core protein, with subsequent disrupted interaction, did not impact viral RNA replication or infectious viral particle production (Angus et al., Citation2010). There is some suggestion that DDX3’s colocalization may be related with HCV assembly and may incorporate into the HCV virion, functionally acting as a chaperone for the HCV RNA (Sato et al., Citation2006). Another explanation for this colocalization is that of a viral hijacking event, whereby the HCV core protein steals components of the processing bodies (P-bodies), trapping them in the lipid droplets to avoid the mRNA degradation associated with the p-body complexes (Angus et al., Citation2010; Ariumi et al., Citation2011). Additionally, DDX3 has been shown to bind to the 3′-TR, activating IKK-α and cellular lipogenesis pathways that enable viral particle assembly (Li, Pene, Krishnamurthy, Cha, & Liang, Citation2013; Pene, Li, Sodroski, Hsu, & Liang, Citation2015).
In HSV-1, DDX3 has been shown to be essential for replication, where siRNA knockdown studies reveal a 50% reduction in viral particles (Stegen et al., Citation2013). DDX3 has been shown to incorporate into virions (Loret, Guay, & Lippe, Citation2008), and depletion of the DDX3 in these viral pools or subsequent cell culture resulted in significant decreases in viral replication on in vitro serial infection studies (Stegen et al., Citation2013). DDX3 was shown to modulate transcription of numerous viral genes, including those coding for HSV-1’s transactivating proteins (ICP0, ICP4 and VP16), irrevocably implicating it in the virus’s propagation (Khadivjam et al., Citation2017). This role of DDX3 appeared to be independent of DDX3’s ability to stimulate IFN-g production (Khadivjam et al., Citation2017). On the other hand, WNV recruits DDX3 from the P-bodies to aid its replication, with a 45% reduction in virus-infected cells upon knockdown, and a ~30% reduction in viral mRNA as measured via qPCR methods (Chahar, Chen, & Manjunath, Citation2013). In the case of the Murine norovirus, the culturable surrogate to human norovirus, also appears to require DDX3 in its replication, showing an 80–90% reduction in viral NS7, viral yield and viral RNA levels in siRNA knockdown (Vashist, Urena, Chaudhry, & Goodfellow, Citation2012).
The absence of DDX3 during JEV infection resulted in a 15-fold reduction of viral replication and significant decreases in viral genomic RNA and protein expression levels, although no effect on viral assembly or release was observed (Li, Ge, et al., Citation2014). In knockdown-rescue studies using wild-type versus mutant-DDX3 lacking helicase (K230E) and ATPase (S382L) activity, respectively, DDX3’s helicase functioning was shown to be critical for the viral replication (Li, Ge, et al., Citation2014). DDX3 also interacts with JEV non-structural proteins (NS3 and NS5) and 3′-TR and colocalizes with these proteins/RNA upon infection of host cells with JEV (Li, Ge, et al., Citation2014). Additionally, these authors demonstrated that DDX3 also interacts with the 5′ and 3′-TR, both segments that are essential for viral RNA replication and translation, specifically in flaviviruses (Chien, Liao, & Lin, Citation2011; Li, Ge, et al., Citation2014).
Lastly, DDX3 also affects influenza viral replication (Bortz et al., Citation2011; Thulasi Raman et al., Citation2016). Through an initial RNAi screening of influenza polymerase activity in attenuated H5N1 influenza virus, several of the DEAD-box helicases (DDX3, DDX5 and DDX17) were identified, and confirmed to be vital for viral replication using siRNA-based knockdown studies where a significant decrease in viral titres was observed in the absence of these helicases (Bortz et al., Citation2011). Interestingly, another group employed a non-structural protein 1(NS1)-deficient virus to study the role of DDX3 in influenza viral replication and discovered that DDX3 actually inhibits viral replication, in contrast to what was observed earlier (Thulasi Raman et al., Citation2016). DDX3 was first demonstrated to interact with the NS1 and nucleoprotein of influenza virus. Subsequently, it was shown to be involved in the production of stress granules in the NS1-deficient virus, whereby the antiviral stress granules are able to form in the absence of NS1, but not in their presence (Thulasi Raman et al., Citation2016). Structurally for this colocalization in stress granules to occur, the core helicase and N-terminal domains of DDX3 are required (Thulasi Raman et al., Citation2016). In the DDX3 knockdown studies of this model, stress granule formation was inhibited and subsequent elevation in viral titres was seen (Thulasi Raman et al., Citation2016). It is clear that NS1 plays a key part in preventing DDX3 from performing its cellular homeostatic role, however, there are many questions that exist surrounding the fact that how DDX3 can perform such diverse roles during viral infection.
Given DDX3’s highly integrated and supporting role in many processes, there is a drive to produce DDX3 inhibitors for use as novel antivirals and/or anticancer agents (Bol, Xie, & Raman, Citation2015; Maga et al., Citation2011; Meyerhans et al., Citation2018; Radi et al., Citation2011, 2012, 2012).
DEAD-box helicase 5 (DDX5)
DDX5, or p68, was one of the first and perhaps prototypical DEAD-box helicases described (Hirling, Scheffner, Restle, & Stahl, Citation1989; Linder et al., Citation1989). It plays vital roles in mRNA splicing, transcription regulation, ribosomal biogenesis and turnover of mRNAs (Fuller-Pace, Citation2013; Naji et al., Citation2012). DDX5 is frequently overexpressed in colon (Causevic et al., Citation2001; Shin, Rossow, Grande, & Janknecht, Citation2007), breast (Iyer et al., Citation2014; Wang, Huang, & Hu, Citation2012), oesophageal (Ma et al., Citation2017), and prostate cancers (Clark et al., Citation2008) as well as various leukaemias (S. Lin et al., Citation2013; Mazurek et al., Citation2014). A single-nucleotide polymorphism has also been identified in the DDX5 gene that is associated with an increased risk of advanced fibrosis in patients with chronic HCV (Huang et al., Citation2006). This association has increased interest in DDX5 as a target for anti-cancer therapies (Dai et al., Citation2014; Taniguchi et al., Citation2016). At the same time, studies have demonstrated that DDX5 acts as a potent coactivator for the tumour suppressor p53, subsequently inducing transcription of genes involved in cell cycle arrest and apoptosis (Nicol & Fuller-Pace, Citation2010).
DDX5–viral interactions
In terms of DDX5’s role in viral replication, the literature overall suggests that this helicase is a supporter of viral reproduction (see Table ).
In HIV-1, as with several other helicases noted, DDX5 increases viral replication through the binding of HIV-1 mRNAs at the RRE-driving CRM1 export (Wang, Gao, Huang, Yang, & Liu, Citation2009; Zhou et al., Citation2013). Mutations in the DEAD-box motif of DDX5 result in loss of its ability to bind to Rev, indicating that the DEAD-box region in DDX5 is required for the interaction between RRE and Rev (Zhou et al., Citation2013). Interestingly, the knockdown studies of DDX5 with subsequent ‘rescue’ with exogenous DDX1 or DDX3 (other known RRE-binders) during HIV-1 infection demonstrated the only partial restoration of the activity (p24 production), indicating a unique need for the DDX5 in the process (Zhou et al., Citation2013).
The DDX5 helicase interacts with the RNA-dependent RNA polymerase (NS5B) of HCV via two independent NS5B-binding sites in DDX5, one located at the N-terminus and another at the C-terminus (Dutta et al., Citation2012; Goh et al., Citation2004). Co-immunoprecipitation studies confirmed that neither the ATP-binding site nor the DEAD-box was required for this DDX5–NS5B interaction (Dutta et al., Citation2012). In overexpression studies, the DDX5 was observed to be redistributed from nucleus to the cytoplasm (Goh et al., Citation2004). If the C-terminus of DDX5 is deleted, the helicase loses its ability to re-distribute, highlighting the importance of this region for transport (Goh et al., Citation2004). In siRNA knockdown of endogenous DDX5 in cells that were transiently transfected with a full-length HCV expression construct, reduced transcription of negative-strand HCV RNA was observed (Goh et al., Citation2004). The same researchers employed a different cell line infected with a chimeric HCV strain (J6/JFH-1), and found that siRNA knockdown in this system resulted in a slight increase in HCV RNA, implying an anti-viral role for DDX5 (Upadya, Aweya, & Tan, Citation2014).
DDX5 interacts with the JEV non-structural proteins 3 and 5 (both the MTase and RdRp), as well as with the 3′-TR – collectively, aiding viral replication (Li, Ge, et al., Citation2013). Knockdown studies of DDX5 in BHK-21 cells showed a 13-fold decrease in viral titres compared to control and decreased viral genome and protein expression levels (Li, Ge, et al., Citation2013). In the same model, the knockdown rescue using the wild-type helicase or helicase mutants (deficient in ATPase activity (K144E) or unwinding ability (S279L)), showed that both functional aspects of the helicase are required by the virus (Li, Ge, et al., Citation2013). DDX5’s necessity is also shown in the leptomycin B studies, where DDX5, normally residing in the nucleus, is recruited to the cytoplasm after viral infection, and where its transport can be blocked by leptomycin B, greatly reducing viral titres (Li, Ge, et al., Citation2013; Wang et al., Citation2009). Mimicking experiments performed in other viruses for protein incorporation into virions, DDX5 was assessed for the same in JEV infected BHK-21 cells, however, there did not appear to be any incorporation (Li, Ge, et al., Citation2013; Stegen et al., Citation2013; Varnum et al., Citation2004; Wang et al., Citation2009). Lastly, DDX5 was shown to bind the 3′-TR of JEV, positively regulating RNA synthesis, but not protein translation (Li, Ge, et al., Citation2013).
DDX5 also interacts with the SARS coronavirus helicase (non-structural protein 13), likely as a transcription cofactor, enhancing viral replication (Chen et al., Citation2009). Furthermore, in porcine reproductive and respiratory syndrome virus, DDX5 has been shown to interact with the virus’ RNA-dependent RNA polymerase (RdRp on non-structural protein 9) (Zhao et al., Citation2015). Despite the critical nature of the viral helicase and RdRp pair of proteins in the viruses noted above, the analogous pull-downs for the opposite protein have yet to be described. Such work would enable further structural and functional comparisons of the host DEAD-box RNA helicases.
DEAD-box helicase 17 (DDX17)
DDX17 (also known as RH70, p72 or p72/p82), has two isoforms – p72 and p82, that result from alternative mRNA translation (Uhlmann-Schiffler, Rossler, & Stahl, Citation2002). Both these isoforms are produced in relatively the same abundance and function as ATP-dependent RNA helicases, among other emerging roles (Jalal et al., Citation2007). In the host cell, DDX17 is involved with regulation of gene transcription, ribosomal biogenesis and RNA splicing through various cellular interactions, including HDAC1, estrogen receptor alpha and U1snRNP (Lee, Citation2002; Lorgeoux et al., Citation2013; Wilson et al., Citation2004; Wortham et al., Citation2009). Given the close structural similarity of DDX17 with DDX5, a comparison of the two DEAD-boxes is warranted.
DDX17 vs. DDX5
DDX17 (p72/p82) and DDX5 (p68) proteins, are closely related in terms of their sequences and many functions. As noted above, there is about a 90% sequence homology of the DDX5 and DDX17 core regions and an overall homology of about 70% (Lamm et al., Citation1996). The two helicases display similar functions in the cells in terms of transcriptional regulation, RNA splicing and ribosomal biogenesis (Fuller-Pace, Citation2013; Jalal et al., Citation2007; Janknecht, Citation2010; Rössler, Straka, & Stahl, Citation2001). Some studies have looked at silencing and/or knockdown models to determine whether there is complete functional overlap or that they act as unique entities. In U2OS (human osteosarcoma) cells, siRNA silencing of DDX5 and DDX17 revealed that DDX5 silencing caused increased levels of DDX17, but not vice versa (Moy et al., Citation2014). Similar interdependencies were seen in the HeLa cell (cervical cancer) and RKO cell (colon cancer) models (Lorgeoux et al., Citation2013; Shin et al., Citation2007). As well in the U2OS model, infection with Rift Valley Fever virus (RVFV) or Lacrosse virus (LACV) (both bunyaviruses) was unchanged by DDX5 depletion, but greatly increased with DDX17 depletion, implying the DDX17, but not DDX5, played a central and unique role to the control of these viruses (Moy et al., Citation2014). The same group did a comparable study with another virus – vesicular stomatitis virus (VSV) and found no effect on viral replication indicating a viral selectivity to the DDX17’s actions (Moy et al., Citation2014). As well, in HIV-1, DDX5 and DDX17 were shown to have opposing influences on viral protein production and infectivity with their siRNA knockdown (Naji et al., Citation2012). Some of these discrepancies may be able to be explained on a structural basis. Differing from other DEAD-box RNA helicases, p72 has a unique N-terminal domain containing repeats of the sequence RGG and a C-terminal domain rich in serine and glycine and terminating with a polyproline region (Lamm et al., Citation1996). Given the importance of the terminal regions in mediating various protein interactions, the sequence differences may be enough to account for specificity in its actions.
DDX17–viral interactions
DDX17 can positively and negatively affect the replication of several viruses, including HIV-1, Rift Valley Fever virus (RVFV) and influenza A virus through interactions with viral components (see Table ).
In HIV-1, DDX17 has mixed influences on the virus’ replication. As with DDX1 and DDX3 noted above, DDX17 also promotes the Rev-dependent export of HIV-1 RNA (Fang et al., Citation2004; Lorgeoux et al., Citation2013; Naji et al., Citation2012). Additionally, DDX17 is involved in the production of infectious HIV-1 particles, as knockdown studies show the dramatic reduction in their generation (Lorgeoux et al., Citation2013; Naji et al., Citation2012). This action was further elicited to require the helicase functioning, as a mutated DEAD-region resulted in up to 10-fold reduction in infectious particles, 5- to 10-fold reduction in viral RNA packaging, specifically with reversed ratios of full-length to multiply-spliced mRNA transcripts (wild type predominantly full-length RNA)(Lorgeoux et al., Citation2013). As well, DDX17 was determined to modulate HIV-1 Gag processing via the Gag-Pol frameshift mechanism critical to HIV-1 enzyme production, maintaining the proper ratios of expression required for optimal infectivity (Lorgeoux et al., Citation2013). Specific interactions for these activities are not known, but again, appears to be related to its helicase functioning given the converse observed effects in its mutated version.
Despite all the HIV-1 promotory effects, DDX17 has also been reported as a cofactor for the zinc finger antiviral protein (ZAP) that is involved in the breakdown of HIV-1 mRNA (Chen, Guo, Lv, Xu, & Gao, Citation2008; Zhu et al., Citation2011). The ZAP binds to a specific region of the mRNA, the ZAP-responsive element, and recruits DDX17 and the exosome for degradation (Guo, Ma, Sun, & Gao, Citation2007). Knockdown of DDX17 results in 50% less efficient ZAP-functioning (Chen et al., Citation2008). This mechanism of mRNA degradation has been shown in numerous viruses, including Sindbis and Moloney murine leukaemia virus (Chen et al., Citation2008; Guo et al., Citation2007); filoviruses, including Ebola and Marburg virus (Müller et al., Citation2007); and HIV-1 (Zhu et al., Citation2011), the prerequisite being the presence of the ZAP-responsive element (Chen et al., Citation2008).
DDX17 inhibits the replication of Rift Valley Fever virus (RVFV). Moy et al. (Citation2014) first showed that RNAi interference studies on the Drosophila homolog of DDX17, Rm62, led to enhanced RVFV infection in Drosophila cells, as well as in a related bunyavirus, LaCrosse virus (Moy et al., Citation2014). The same studies performed on other arboviruses, VSV (a rhabdovirus), Sindbis virus (an alphavirus) and drosophila C virus (a picornavirus), found no effect on the virus with the silencing suggesting a degree of viral selectivity to this action (Moy et al., Citation2014). As well, overexpression of DDX17 led to decreased RVFV protein accumulation and percentage of infected cells. CLIP-Seq studies were subsequently employed to show that DDX17 interacts with RVFV RNAs, preferentially binding to mature mRNA and showing a bias for CT- and CA-repeat elements (Moy et al., Citation2014). Furthermore, DDX17 was found to bind to a subset of pri-miRNA hairpins, including one well-described hairpin from the S segment of the RVFV genome (Emery & Bishop, Citation1987; Moy et al., Citation2014). An analogous hairpin in another bunyavirus (Punta Toro virus) was shown to control transcription termination, giving further mechanistic explanation for the resulting effects of this interaction (Emery & Bishop, Citation1987). Given the involvement of DDX17 in miRNA biogenesis and the displayed biased binding to miRNAs in the CLIP-Seq study, to determine whether miRNA involvement was directly linked to DDX17’s antiviral activity, researchers silenced Drosha, an enzyme involved in the processing of pri-miRNA to pre-miRNA, and found no effect on viral infection, thus highlighting DDX17’s actions to be independent of Drosha (Moy et al., Citation2014).
For influenza A virus, in contrast, the DDX17 helicase supports replication. An siRNA knock-down model of human lung adenocarcinoma cells (A549) showed a significant reduction in viral titres when DDX17, DDX5 or DDX3X were knocked down (Bortz et al., Citation2011). As well, viral polymerase activity, as measured via minigenome reporter assays, were reduced when these helicases were absent, indicating an influence on the viral polymerase through either direct interaction, or a related cofactor for transcription. Interestingly, DDX17 appeared to enhance polymerase activity in the H5N1 human isolate (PB2 627 K genotype) but antagonize that in the avian mutant (PB2 627E genotype) – a finding also confirmed in exogenous overexpression studies (Bortz et al., Citation2011). Comparing this to the avian cell model (chicken fibroblasts), targeted knockdown of the avian DDX17 homolog again showed the necessity of DDX17 for replication, indicating host selectivity, but also an additional role in influenza adaptation to human cells (Bortz et al., Citation2011). This could provide evidence for a potential interaction site, given the single mutation selectivity and corresponding to other studies highlighting specific residues for influenza adaptability (Long et al., Citation2013; Yamada et al., Citation2010).
DEAD-box helicases as potential immunomodulators
Recent studies have highlighted the role of various DEAD-box helicases in innate immunity. For example, DDX1 has been shown to play a role in the interferon response pathway, whereby DDX1 interacts with the non-structural protein 14 of porcine enteropathogenic coronavirus, a transmissible gastroenteritis virus to induce interferon-β upregulation in response to infection (Zhou et al., Citation2017). This was further confirmed with inhibition of this pathway with siRNA DDX1 knockdown (Zhou et al., Citation2017).
Furthermore, DDX3 has been thoroughly implicated in the innate immune response to viruses by interacting with IκB kinase ε (IKKε) and TANK-binding kinase (TBK-1) to regulate interferon type-1 activation downstream of virus recognition (Schroder, Citation2011; Soulat et al., Citation2008). Interestingly, in HBV, it appears that this DDX3-IKKε interaction is disrupted by the HBV polymerase – a unique role for the polymerase, and perhaps an explanation for HBV’s immune evasion (Kato et al., Citation2006; Wang & Ryu, Citation2010). Supporting this concept further is the fact that another unrelated virus, vaccinia virus, has also developed a similar mechanism to subvert the immune system, interfering with the DDX3–IKKε interaction of the TBK1/IKKε-interferon pathway suggesting a common viral evolutionary drive for interfering with this interaction (Schroder, Baran, & Bowie, Citation2008). In host cells, DDX3 has been shown to be upregulated during an immune response to lipopolysaccharide-induced inflammation and during interferon treatment (De Vries et al., Citation2009; de Veer et al., Citation2001; Saban et al., Citation2006). It has also been shown to play roles in the development of stress granules and p-bodies and in the degradation of mRNA (Beckham & Parker, Citation2008; Fuller-Pace, Citation2006; Parker & Sheth, Citation2007). The former of this list may be linked in that the stress granules are being produced in response to any number of perturbations in cellular homeostasis, including the responses to lipopolysaccharide or interferon.
In contrast to DDX3, DDX5 has a less clear role in immunity. A recent study has established a link between DDX5 and Th17 cell differentiation – a group of lymphocytes that protect mucosal surfaces against infections, whereby the nuclear receptor, RAR-related orphan receptor γ (RORγt), partners with DDX5 to transcribe certain Th17 genes (Huang et al., Citation2015). In the autoimmune sense, this pathway is thus associated with Th17-mediated inflammatory diseases (Huang et al., Citation2015). On the other hand, DDX17’s role in innate immunity appears to be circumstantial thus far. In the RVFV-U2OS and VSV-U2OS models, DDX17 was studied to determine whether it regulated interferon production, but there appeared to be no influence of the helicase on the gene expression of Ifit1 or IkBa, IRF7 and DDX58 (other genes involved in interferon response) (Moy et al., Citation2014). In the mouse model, the miRNA processing was determined to be initiated by the binding of DDX17 to the miR-132 loop of the RNA (Remenyi, Bajan, Fuller-Pace, Arthur, & Hutvagner, Citation2016). Simultaneously, other papers report the centrality of this loop region, miR-132, in the response to viral innate immunity (Chiang, Liu, & Rice, Citation2013; Jiang et al., Citation2014; Lagos et al., Citation2010; Sathish & Yuan, Citation2011).
Thus, while there is mounting evidence of the DEAD-box helicases acting in an immunomodulatory manner, there is much that remains to be fully-explored.
Conclusions
The DEAD-box helicases possess a Yin-and-Yang duality in viral infections, playing roles central to both viral replication and viral destruction. Study of their diverse and occasionally overlapping functional roles in these settings continues to reveal critical links and information about the viruses which may be used to ultimately develop effective management strategies.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the NSERC Discovery (Natural Sciences and Engineering Research Council of Canada) [grant number RGPIN-2017-04003].
References
- Akter, K. A., Mansour, M. A., Hyodo, T., & Senga, T. (2016). FAM98A associates with DDX1-C14orf166-FAM98B in a novel complex involved in colorectal cancer progression. Journal of Biochemistry & Cell Biology, 84, 1–13. doi:10.1016/j.biocel.2016.12.013
- Amaya, M., Brooks-Faulconer, T., Lark, T., Keck, F., Bailey, C., Raman, V., & Narayanan, A. (2016). Venezuelan equine encephalitis virus non-structural protein 3 (nsP3) interacts with RNA helicases DDX1 and DDX3 in infected cells. Antiviral Research, 131, 49–60. doi:10.1016/j.antiviral.2016.04.008.
- Amaya, M., Voss, K., Sampey, G., Senina, S., de la Fuente, C., Mueller, C., … Narayanan, A. (2014). The role of IKKβ in Venezuelan equine encephalitis virus infection. PLoS ONE, 9(2), e86745. doi:10.1371/journal.pone.0086745.
- Angus, A. G. N., Dalrymple, D., Boulant, S., McGivern, D. R., Clayton, R. F., Scott, M. J., … Patel, A. H. (2010). Requirement of cellular DDX3 for hepatitis C virus replication is unrelated to its interaction with the viral core protein. Journal of General Virology, 91(Pt 1), 122–132. doi:10.1099/vir.0.015909-0.
- Ariumi, Y. (2014). Multiple functions of DDX3 RNA helicase in gene regulation, tumorigenesis, and viral infection. Frontiers in Genetics, 5, 423. doi:10.3389/fgene.2014.00423.
- Ariumi, Y., Kuroki, M., Abe, K., Dansako, H., Ikeda, M., Wakita, T., & Kato, N. (2007). DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. Journal of Virology, 81(24), 13922–13926. doi:10.1128/JVI.01517-07.
- Ariumi, Y., Kuroki, M., Kushima, Y., Osugi, K., Hijikata, M., Maki, M., … Kato, N. (2011). Hepatitis C virus hijacks P-body and stress granule components around lipid droplets. Journal of Virology, 85(14), 6882–6892. doi:10.1128/jvi.02418-10.
- Beckham, C.J., & Parker, R. (2008). P-bodies, stress granules and viral life cycles. Cell Host & Microbe, 3(4), 206–212. doi:10.1016/j.chom.2008.03.004.
- Bleoo, S., Sun, X., Hendzel, M. J., Rowe, J. M., Packer, M., & Godbout, R. (2001). Association of human DEAD box protein DDX1 with a cleavage stimulation factor involved in 3′-end processing of pre-MRNA. Molecular Biology of the Cell, 12(10), 3046–3059.10.1091/mbc.12.10.3046
- Bol, G.M., Xie, M., & Raman, V. (2015). DDX3, a potential target for cancer treatment. Molecular Cancer, 14, 499. doi:10.1186/s12943-015-0461-7.
- Bortz, E., Westera, L., Maamary, J., Steel, J., Albrecht, R. A., Manicassamy, B., … Garcia-Sastre, A. (2011). Host- and strain-specific regulation of influenza virus polymerase activity by interacting cellular proteins. MBio, 2(4), doi:10.1128/mBio.00151-11.
- Botlagunta, M., Vesuna, F., Mironchik, Y., Raman, A., Lisok, A., Winnard, P., Jr, … Raman, V. (2008). Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene, 27(28), 3912–3922. doi:10.1038/onc.2008.33.
- Causevic, M., Hislop, R. G., Kernohan, N. M., Carey, F. A., Kay, R. A., Steele, R. J., & Fuller-Pace, F. V. (2001). Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene, 20(53), 7734–7743. doi:10.1038/sj.onc.1204976.
- Chahar, H. S., Chen, S., & Manjunath, N. (2013). P-body components LSM1, GW182, DDX3, DDX6 and XRN1 are recruited to WNV replication sites and positively regulate viral replication. Virology, 436(1), 1–7. doi:10.1016/j.virol.2012.09.041.
- Chang, P. C., Chi, C. W., Chau, G. Y., Li, F. Y., Tsai, Y. H., Wu, J. C., & Wu Lee, Y. H. (2006). DDX3, a DEAD box RNA helicase, is deregulated in hepatitis virus-associated hepatocellular carcinoma and is involved in cell growth control. Oncogene, 25(14), 1991–2003. doi:10.1038/sj.onc.1209239.
- Chao, C. H., Chen, C. M., Cheng, P. L., Shih, J. W., Tsou, A. P., & Lee, Y. H. (2006). DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Research, 66(13), 6579–6588. doi:10.1158/0008-5472.CAN-05-2415.
- Chen, H. C., Lin, W. C., Tsay, Y. G., Lee, S. C., & Chang, C. J. (2002). An RNA helicase, DDX1, interacting with poly(A) RNA and heterogeneous nuclear ribonucleoprotein K. Journal of Biological Chemistry, 277(43), 40403–40409. doi:10.1074/jbc.M206981200.
- Chen, C. Y., Chan, C. H., Chen, C. M., Tsai, Y. S., Tsai, T. Y., Wu Lee, Y. H., & You, L. R. (2016). Targeted inactivation of murine Ddx3x: Essential roles of Ddx3x in placentation and embryogenesis. Human Molecular Genetics, 25(14), 2905–2922. doi:10.1093/hmg/ddw143.
- Chen, G., Guo, X., Lv, F., Xu, Y., & Gao, G. (2008). p72 DEAD box RNA helicase is required for optimal function of the zinc-finger antiviral protein. Proceedings of the National Academy of Sciences, 105(11), 4352–4357. doi:10.1073/pnas.0712276105.
- Chen, J. Y., Chen, W. N., Poon, K. M., Zheng, B. J., Lin, X., Wang, Y. X., & Wen, Y. M. (2009). Interaction between SARS-CoV helicase and a multifunctional cellular protein (Ddx5) revealed by yeast and mammalian cell two-hybrid systems. Archives of Virology, 154(3), 507–512. doi:10.1007/s00705-009-0323-y.
- Chiang, K., Liu, H., & Rice, A. P. (2013). miR-132 enhances HIV-1 replication. Virology, 438(1), 1–4. doi:10.1016/j.virol.2012.12.016.
- Chien, H. L., Liao, C. L., & Lin, Y. L. (2011). FUSE binding protein 1 interacts with untranslated regions of Japanese encephalitis virus RNA and negatively regulates viral replication. Journal of Virology, 85(10), 4698–4706. doi:10.1128/jvi.01950-10.
- Clark, E. L., Coulson, A., Dalgliesh, C., Rajan, P., Nicol, S. M., Fleming, S., … Robson, C. N. (2008). The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Research, 68(19), 7938–7946. doi:10.1158/0008-5472.can-08-0932
- Cullen, B.R. (2003). Nuclear RNA export. Journal of Cell Science, 116(4), 587–597.10.1242/jcs.00268
- Dai, T.-Y., Cao, L., Yang, Z.-C., Li, Y.-S., Tan, L., Ran, X.-Z., & Shi, C.-M. (2014). P68 RNA helicase as a molecular target for cancer therapy. Journal of Experimental & Clinical Cancer Research, 33(1), 271. doi:10.1186/s13046-014-0064-y.
- de Veer, M. J., Holko, M., Frevel, M., Walker, E., Der, S., Paranjape, J. M., … Williams, B. R. (2001). Functional classification of interferon-stimulated genes identified using microarrays. Journal of Leukocyte Biology, 69(6), 912–920.
- De Vries, D. R., Ter Linde, J. J. M., Van Herwaarden, M. A., Schwartz, M. P., Shephard, P., Geng, M. M., … Samsom, M. (2009). In GERD patients, mucosal repair associated genes are upregulated in non-inflamed oesophageal epithelium. Journal of Cellular and Molecular Medicine, 13(5), 936–947. doi:10.1111/j.1582-4934.2008.00626.x.
- Dutta, S., Gupta, G., Choi, Y. W., Kotaka, M., Fielding, B. C., Song, J., & Tan, Y. J. (2012). The variable N-terminal region of DDX5 contains structural elements and auto-inhibits its interaction with NS5B of hepatitis C virus. Biochemical Journal, 446(1), 37–46. doi:10.1042/BJ20120001.
- Edgcomb, S. P., Carmel, A. B., Naji, S., Ambrus-Aikelin, G., Reyes, J. R., Saphire, A. C., … Williamson, J. R. (2012). DDX1 is an RNA-dependent ATPase involved in HIV-1 Rev function and virus replication. Journal of Molecular Biology, 415(1), 61–74. doi:10.1016/j.jmb.2011.10.032.
- Emery, V. C., & Bishop, D. H. (1987). Characterization of Punta Toro S mRNA species and identification of an inverted complementary sequence in the intergenic region of Punta Toro phlebovirus ambisense S RNA that is involved in mRNA transcription termination. Virology, 156(1), 1–11. doi:10.1016/0042-6822(87)90430-2.10.1016/0042-6822(87)90430-2
- Fairman-Williams, M. E., Guenther, U. P., & Jankowsky, E. (2010). SF1 and SF2 helicases: Family matters. Current Opinion in Structural Biology, 20(3), 313–324. doi:10.1016/j.sbi.2010.03.011.
- Fang, J., Kubota, S., Yang, B., Zhou, N., Zhang, H., Godbout, R., & Pomerantz, R. J. (2004). A DEAD box protein facilitates HIV-1 replication as a cellular co-factor of Rev. Virology, 330(2), 471–480. doi:10.1016/j.virol.2004.09.039.
- Fukuda, T., Yamagata, K., Fujiyama, S., Matsumoto, T., Koshida, I., Yoshimura, K., … Kato, S. (2007). DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nature Cell Biology, 9(5), 604–611. doi:10.1038/ncb1577.
- Fuller-Pace, F. V. (2006). DExD/H box RNA helicases: Multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Research, 34(15), 4206–4215. doi:10.1093/nar/gkl460.
- Fuller-Pace, F. V. (2013). The DEAD box proteins DDX5 (p68) and DDX17 (p72): multi-tasking transcriptional regulators. Biochimica et Biophysica Acta (BBA) – Gene Regulatory Mechanisms, 1829(8), 756–763. doi:10.1016/j.bbagrm.2013.03.004.
- Germain, D. R., Graham, K., Glubrecht, D. D., Hugh, J. C., Mackey, J. R., & Godbout, R. (2011). DEAD box 1: A novel and independent prognostic marker for early recurrence in breast cancer. Breast Cancer Research and Treatment, 127(1), 53–63. doi:10.1007/s10549-010-0943-7
- Godbout, R., Hale, M., & Bisgrove, D. (1994). A human DEAD box protein with partial homology to heterogeneous nuclear ribonucleoprotein U. Gene, 138(1–2), 243–245. doi:10.1016/0378-1119(94)90816-8.10.1016/0378-1119(94)90816-8
- Godbout, R., Packer, M., & Bie, W. (1998). Overexpression of a DEAD box protein (DDX1) in neuroblastoma and retinoblastoma cell lines. Journal of Biological Chemistry, 273(33), 21161–21168. doi:10.1074/jbc.273.33.21161.
- Goh, P. Y., Tan, Y. J., Lim, S. P., Tan, Y. H., Lim, S. G., Fuller-Pace, F., & Hong, W. (2004). Cellular RNA helicase p68 relocalization and interaction with the hepatitis C virus (HCV) NS5B protein and the potential role of p68 in HCV RNA replication. Journal of Virology, 78(10), 5288–5298. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15113910. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC400326/pdf/1485-03.pdf10.1128/JVI.78.10.5288-5298.2004
- Gu, L., Fullam, A., Brennan, R., & Schroder, M. (2013). Human DEAD box helicase 3 couples IkappaB kinase epsilon to interferon regulatory factor 3 activation. Molecular and Cellular Biology, 33(10), 2004–2015. doi:10.1128/mcb.01603-12.
- Guo, X., Ma, J., Sun, J., & Gao, G. (2007). The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proceedings of the National Academy of Sciences, 104(1), 151–156. doi:10.1073/pnas.0607063104.
- Han, C., Liu, Y., Wan, G., Choi, H. J., Zhao, L., Ivan, C., … Lu, X. (2014). The RNA-binding protein DDX1 promotes primary microRNA maturation and inhibits ovarian tumor progression. Cell Reports, 8(5), 1447–1460. doi:10.1016/j.celrep.2014.07.058.
- Harris, M. E., & Hope, T. J. (2000). RNA export: Insights from viral models. Essays In Biochemistry, 36, 115–127. doi:10.1042/bse0360115.10.1042/bse0360115
- Harris, D., Zhang, Z., Chaubey, B., & Pandey, V. N. (2006). Identification of cellular factors associated with the 3′-nontranslated region of the hepatitis C virus genome. Molecular & Cellular Proteomics, 5(6), 1006–1018. doi:10.1074/mcp.M500429-MCP200.
- He, L., Chen, Y., Wu, Y., Xu, Y., Zhang, Z., & Liu, Z. (2017). Nucleic acid sensing pattern recognition receptors in the development of colorectal cancer and colitis. Cellular and Molecular Life Sciences, 74(13), 2395–2411. doi:10.1007/s00018-017-2477-1.
- Heerma van Voss, M. R., Vesuna, F., Trumpi, K., Brilliant, J., Berlinicke, C., de Leng, W., … Raman, V. (2015). Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget, 6(29), 28312–28326. doi:10.18632/oncotarget.4873.
- Heerma van Voss, M. R., Schrijver, W. A., Ter Hoeve, N. D., Hoefnagel, L. D., Manson, Q. F., van der Wall, E., … Dutch Distant Breast Cancer Metastases, C. (2017). The prognostic effect of DDX3 upregulation in distant breast cancer metastases. Clinical & Experimental Metastasis, 34(1), 85–92. doi:10.1007/s10585-016-9832-8.
- Hilbert, M., Karow, A. R., & Klostermeier, D. (2009). The mechanism of ATP-dependent RNA unwinding by DEAD box proteins. Biological Chemistry , 390(12), 1237–1250. doi:10.1515/bc.2009.135
- Hirling, H., Scheffner, M., Restle, T., & Stahl, H. (1989). RNA helicase activity associated with the human p68 protein. Nature, 339(6225), 562–564. doi:10.1038/339562a0
- Hope, T., & Pomerantz, R.J. (1995). The human immunodeficiency virus type 1 Rev protein: A pivotal protein in the viral life cycle. Current Topics in Microbiology and Immunology, 193, 91–105. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7648880
- Huang, H., Shiffman, M. L., Cheung, R. C., Layden, T. J., Friedman, S., Abar, O. T., … Wright, T. L. (2006). Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology, 130(6), 1679–1687. doi:10.1053/j.gastro.2006.02.032
- Huang, J. S., Chao, C. C., Su, T. L., Yeh, S. H., Chen, D. S., Chen, C. T., … Jou, Y. S. (2004). Diverse cellular transformation capability of overexpressed genes in human hepatocellular carcinoma. Biochemical and Biophysical Research Communications, 315(4), 950–958. doi:10.1016/j.bbrc.2004.01.151
- Huang, W., Thomas, B., Flynn, R. A., Gavzy, S. J., Wu, L., Kim, S. V., … Littman, D. R. (2015). DDX5 and its associated lncRNA Rmrp modulate TH17 cell effector functions. Nature, 528(7583), 517–522. doi:10.1038/nature16193.
- Ishaq, M., Ma, L., Wu, X., Mu, Y., Pan, J., Hu, J., … Guo, D. (2009). The DEAD-box RNA helicase DDX1 interacts with RelA and enhances nuclear factor kappaB-mediated transcription. Journal of Cellular Biochemistry, 106(2), 296–305. doi:10.1002/jcb.22004
- Iyer, R. S., Nicol, S. M., Quinlan, P. R., Thompson, A. M., Meek, D. W., & Fuller-Pace, F. V. (2014). The RNA helicase/transcriptional co-regulator, p68 (DDX5), stimulates expression of oncogenic protein kinase, Polo-like kinase-1 (PLK1), and is associated with elevated PLK1 levels in human breast cancers. Cell Cycle, 13(9), 1413–1423. doi:10.4161/cc.28415.
- Jalal, C., Uhlmann-Schiffler, H., & Stahl, H. (2007). Redundant role of DEAD box proteins p68 (Ddx5) and p72/p82 (Ddx17) in ribosome biogenesis and cell proliferation. Nucleic Acids Research, 35(11), 3590–3601. doi:10.1093/nar/gkm058.
- Janknecht, R. (2010). Multi-talented DEAD-box proteins and potential tumor promoters: P68 RNA helicase (DDX5) and its paralog, p72 RNA helicase (DDX17). American Journal of Translational Research, 2(3), 223–234. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2892403/
- Jankowsky, E., & Fairman, M.E. (2007). RNA helicases–one fold for many functions. Current Opinion in Structural Biology, 17(3), 316–324. doi:10.1016/j.sbi.2007.05.007
- Jiang, X., Kanda, T., Wu, S., Nakamura, M., Miyamura, T., Nakamoto, S., … Yokosuka, O. (2014). Regulation of microRNA by hepatitis B virus infection and their possible association with control of innate immunity. World Journal of Gastroenterology, 20(23), 7197–7206. doi:10.3748/wjg.v20.i23.7197.
- Kato, H., Takeuchi, O., Sato, S., Yoneyama, M., Yamamoto, M., Matsui, K., … Akira, S. (2006). Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature, 441(7089), 101–105. doi:10.1038/nature04734
- Kellner, J. N., & Meinhart, A. (2015). Structure of the SPRY domain of the human RNA helicase DDX1, a putative interaction platform within a DEAD-box protein. Acta Crystallographica Section F Structural Biology Communications, 71(9), 1176–1188. doi:10.1107/S2053230X15013709
- Khadivjam, B., Stegen, C., Hogue-Racine, M. A., El Bilali, N., Dohner, K., Sodeik, B., & Lippe, R. (2017). The ATP-dependent RNA helicase DDX3X modulates herpes simplex virus 1 gene expression. Journal of Virology , 91(8), doi:10.1128/jvi.02411-16.
- Ko, C., Lee, S., Windisch, M. P., & Ryu, W. S. (2014). DDX3 DEAD-box RNA helicase is a host factor that restricts hepatitis B virus replication at the transcriptional level. Journal of Virology, 88(23), 13689–13698. doi:10.1128/JVI.02035-14.
- Krishnan, V., & Zeichner, S.L. (2004). Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. Journal of Virology, 78(17), 9458–9473. doi:10.1128/jvi.78.17.9458-9473.2004.
- Kubota, S., & Pomerantz, R. J. (2000). The nuclear function of the nuclear diffusion inhibitory signal of human immunodeficiency virus type 1: Critical roles in dominant nuclear localization and intracellular stability. Journal of Human Virology, 3(4), 173–181. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10990165
- Lagos, D., Pollara, G., Henderson, S., Gratrix, F., Fabani, M., Milne, R. S., … Boshoff, C. (2010). miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nature Cell Biology, 12(5), 513–519. doi:10.1038/ncb2054.
- Lamm, G. M., Nicol, S. M., Fuller-Pace, F. V., & Lamond, A. I. (1996). p72: A human nuclear DEAD box protein highly related to p68. Nucleic Acids Research, 24(19), 3739–3747. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC146168/, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC146168/pdf/243739.pdf10.1093/nar/24.19.3739
- Lee, C.G. (2002). RH70, a bidirectional RNA helicase, co-purifies with U1snRNP. Journal of Biological Chemistry, 277(42), 39679–39683. doi:10.1074/jbc.C200337200
- Li, C., Ge, L.L., Li, P. P., Wang, Y., Sun, M. X., Huang, L., … Mao, X. (2013). The DEAD-box RNA helicase DDX5 acts as a positive regulator of Japanese encephalitis virus replication by binding to viral 3′ UTR. Antiviral Research, 100(2), 487–499. doi:10.1016/j.antiviral.2013.09.002.
- Li, C., Ge, L. L., Li, P. P., Wang, Y., Dai, J. J., Sun, M. X., … Mao, X. (2014). Cellular DDX3 regulates Japanese encephalitis virus replication by interacting with viral un-translated regions. Virology, 449, 70–81. doi:10.1016/j.virol.2013.11.008.
- Li, Q., Pene, V., Krishnamurthy, S., Cha, H., & Liang, T. J. (2013). Hepatitis C virus infection activates an innate pathway involving IKK-alpha in lipogenesis and viral assembly. Nature Medicine, 19(6), 722–729. doi:10.1038/nm.3190.
- Lin, S., Tian, L., Shen, H., Gu, Y., Li, J.L., Chen, Z., … Wu, L. (2013). DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene, 32(40), 4845–4853. doi:10.1038/onc.2012.482.
- Lin, M.H., Sivakumaran, H., Jones, A., Li, D., Harper, C., Wei, T., … Harrich, D. (2014). A HIV-1 Tat mutant protein disrupts HIV-1 Rev function by targeting the DEAD-box RNA helicase DDX1. Retrovirology, 11, 491. doi:10.1186/s12977-014-0121-9.
- Linder, P., & Jankowsky, E. (2011). From unwinding to clamping – The DEAD box RNA helicase family. Nature Reviews Molecular Cell Biology, 12(8), 505–516. doi:10.1038/nrm3154.
- Linder, P., Lasko, P. F., Ashburner, M., Leroy, P., Nielsen, P. J., Nishi, K., … Slonimski, P. P. (1989). Birth of the D-E-A-D box. Nature, 337(6203), 121–122. doi:10.1038/337121a0
- Liu, F., Putnam, A., & Jankowsky, E. (2008). ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proceedings of the National Academy of Sciences, 105(51), 20209–20214. doi:10.1073/pnas.0811115106.
- Long, J. S., Howard, W. A., Nunez, A., Moncorge, O., Lycett, S., Banks, J., & Barclay, W. S. (2013). The effect of the PB2 mutation 627K on highly pathogenic H5N1 avian influenza virus is dependent on the virus lineage. Journal of Virology, 87(18), 9983–9996. doi:10.1128/jvi.01399-13
- Loret, S., Guay, G., & Lippe, R. (2008). Comprehensive characterization of extracellular herpes simplex virus type 1 virions. Journal of Virology, 82(17), 8605–8618. doi:10.1128/jvi.00904-08.
- Lorgeoux, R. P., Pan, Q., Le Duff, Y., & Liang, C. (2013). DDX17 promotes the production of infectious HIV-1 particles through modulating viral RNA packaging and translation frameshift. Virology, 443(2), 384–392. doi:10.1016/j.virol.2013.05.026.
- Ma, Z., Feng, J., Guo, Y., Kong, R., Ma, Y., Sun, L., … Liu, S. (2017). Knockdown of DDX5 Inhibits the Proliferation and Tumorigenesis in Esophageal Cancer. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics, 25(6), 887–895. doi:10.3727/096504016x14817158982636
- Maga, G., Falchi, F., Radi, M., Botta, L., Casaluce, G., Bernardini, M., … Botta, M. (2011). Toward the discovery of novel anti-HIV drugs. Second-generation inhibitors of the cellular ATPase DDX3 with improved anti-HIV activity: Synthesis, structure–activity relationship analysis, cytotoxicity studies, and target validation. ChemMedChem, 6(8), 1371–1389. doi:10.1002/cmdc.201100166.
- Mallam, A. L., Campo, M. D., Gilman, B., Sidote, D. J., & Lambowitz, A. M. (2012). Structural basis for RNA duplex recognition and unwinding by the DEAD-box helicase Mss116p. Nature, 490(7418), 121–125. doi:10.1038/nature11402.
- Mallam, A. L., Jarmoskaite, I., Tijerina, P., Del Campo, M., Seifert, S., Guo, L., … Lambowitz, A. M. (2011). Solution structures of DEAD-box RNA chaperones reveal conformational changes and nucleic acid tethering by a basic tail. Proceedings of the National Academy of Sciences, 108(30), 12254–12259. doi:10.1073/pnas.1109566108. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3145681/, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3145681/pdf/pnas.1109566108.pdf
- Mamiya, N., & Worman, H. J. (1999). Hepatitis C virus core protein binds to a DEAD box RNA helicase. Journal of Biological Chemistry, 274(22), 15751–15756. Retrieved from http://www.jbc.org/content/274/22/15751.full.pdf10.1074/jbc.274.22.15751
- Mazurek, A., Park, Y., Miething, C., Wilkinson, J. E., Gillis, J., Lowe, S. W., … Stillman, B. (2014). Acquired dependence of acute myeloid leukemia on the DEAD-box RNA helicase DDX5. Cell Reports, 7(6), 1887–1899. doi:10.1016/j.celrep.2014.05.019. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24910429, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4100070/pdf/nihms599050.pdf
- McCormick, C., & Khaperskyy, D.A. (2017). Translation inhibition and stress granules in the antiviral immune response. Nature Reviews Immunology. doi:10.1038/nri.2017.63. Retrieved from http://www.nature.com/articles/nri.2017.63
- Meyerhans, A., Martinez De La Sierra, M. A., Brai, A., Fazi, R., Tintori, C., Botta, M., … Martinez, J. (2018). Human helicase ddx3 inhibitors as therapeutic agents. U. S. Patent No. 20180016243 Retrieved from https://patents.google.com/patent/US20180016243A1/en
- Moy, R. H., Cole, B. S., Yasunaga, A., Gold, B., Shankarling, G., Varble, A., … Cherry, S. (2014). Stem-loop recognition by DDX17 facilitates miRNA processing and antiviral defense. Cell, 158(4), 764–777. doi:10.1016/j.cell.2014.06.023.
- Müller, S., Möller, P., Bick, M. J., Wurr, S., Becker, S., Günther, S., & Kümmerer, B. M. (2007). Inhibition of filovirus replication by the zinc finger antiviral protein. Journal of Virology, 81(5), 2391–2400. doi:10.1128/JVI.01601-06.
- Naji, S., Ambrus, G., Cimermancic, P., Reyes, J. R., Johnson, J. R., Filbrandt, R., … Gerace, L. (2012). Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Molecular & Cellular Proteomics, 11(4), M111.015313. doi:10.1074/mcp.M111.015313.
- Nicol, S. M., & Fuller-Pace, F. V. (2010). Analysis of the RNA helicase p68 (Ddx5) as a transcriptional regulator. Methods in Molecular Biology, 587, 265–279. doi:10.1007/978-1-60327-355-8_19.
- Owsianka, A. M., & Patel, A. H. (1999). Hepatitis C virus core protein interacts with a human DEAD box protein DDX3. Virology, 257(2), 330–340. doi:10.1006/viro.1999.9659.
- Parker, R., & Sheth, U. (2007). P bodies and the control of mRNA translation and degradation. Molecular Cell, 25(5), 635–646. doi:10.1016/j.molcel.2007.02.011
- Pene, V., Li, Q., Sodroski, C., Hsu, C. S., & Liang, T. J. (2015). Dynamic interaction of stress granules, DDX3X, and IKK-alpha mediates multiple functions in hepatitis C virus infection. Journal of Virology, 89(10), 5462–5477. doi:10.1128/jvi.03197-14.
- PyMOL. The PyMOL Molecular Graphics System (Version 1.8): Schrödinger, LLC. Retrieved from https://pymol.org/2/
- Radi, M., Botta, M., Falchi, F., Maga, G., Baldanti, F., & Paolucci, S. (2011). Compounds with ddx3 inhibitory activity and uses thereof. Retrieved from https://patents.google.com/patent/WO2011039735A2/enIt
- Radi, M., Botta, M., Falchi, F., Maga, G., Baldanti, F., & Paolucci, S. (2012). Retrieved from https://patents.google.com/patent/US20120202814A1/en
- Radi, M., Falchi, F., Garbelli, A., Samuele, A., Bernardo, V., Paolucci, S., … Botta, M. (2012). Discovery of the first small molecule inhibitor of human DDX3 specifically designed to target the RNA binding site: Towards the next generation HIV-1 inhibitors. Bioorganic & Medicinal Chemistry Letters, 22(5), 2094–2098. doi:10.1016/j.bmcl.2011.12.135.
- Remenyi, J., Bajan, S., Fuller-Pace, F. V., Arthur, J. S., & Hutvagner, G. (2016). The loop structure and the RNA helicase p72/DDX17 influence the processing efficiency of the mice miR-132. Scientific Reports, 6, 415. doi:10.1038/srep22848.
- Rössler, O. G., Straka, A., & Stahl, H. (2001). Rearrangement of structured RNA via branch migration structures catalysed by the highly related DEAD-box proteins p68 and p72. Nucleic Acids Research, 29(10), 2088–2096. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC55448/
- Saban, M. R., Hellmich, H. L., Turner, M., Nguyen, N.-B., Vadigepalli, R., Dyer, D. W., … Saban, R. (2006). The inflammatory and normal transcriptome of mouse bladder detrusor and mucosa. BMC Physiology, 6, 1–1. doi:10.1186/1472-6793-6-1.
- Sathish, N., & Yuan, Y. (2011). Evasion and subversion of interferon-mediated antiviral immunity by Kaposi’s Sarcoma-associated herpesvirus: An overview. Journal of Virology, 85(21), 10934–10944. doi:10.1128/JVI.00687-11.
- Sato, S., Fukasawa, M., Yamakawa, Y., Natsume, T., Suzuki, T., Shoji, I., … Nishijima, M. (2006). Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. The Journal of Biochemistry, 139(5), 921–930. doi:10.1093/jb/mvj104.
- Schroder, M. (2011). Viruses and the human DEAD-box helicase DDX3: Inhibition or exploitation? Biochemical Society Transactions, 39(2), 679–683. doi:10.1042/BST0390679.
- Schroder, M., Baran, M., & Bowie, A. G. (2008). Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. The EMBO Journal, 27(15), 2147–2157. doi:10.1038/emboj.2008.143.
- Schutz, P., Karlberg, T., van den Berg, S., Collins, R., Lehtio, L., Hogbom, M., … Schuler, H. (2010). Comparative structural analysis of human DEAD-box RNA helicases. PLoS ONE, 5(9). doi:10.1371/journal.pone.0012791.
- Sharma, D., & Jankowsky, E. (2014). The Ded1/DDX3 subfamily of DEAD-box RNA helicases. Critical Reviews in Biochemistry and Molecular Biology, 49(4), 343–360. doi:10.3109/10409238.2014.931339.
- Shih, J. W., Wang, W. T., Tsai, T. Y., Kuo, C. Y., Li, H. K., & Wu Lee, Y. H. (2012). Critical roles of RNA helicase DDX3 and its interactions with eIF4E/PABP1 in stress granule assembly and stress response. Biochemical Journal, 441(1), 119–129. doi:10.1042/bj20110739.
- Shin, S., Rossow, K. L., Grande, J. P., & Janknecht, R. (2007). Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Research, 67(16), 7572–7578. doi:10.1158/0008-5472.CAN-06-4652.
- Soulat, D., Burckstummer, T., Westermayer, S., Goncalves, A., Bauch, A., Stefanovic, A., … Superti-Furga, G. (2008). The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. The EMBO Journal, 27(15), 2135–2146. doi:10.1038/emboj.2008.126.
- Stegen, C., Yakova, Y., Henaff, D., Nadjar, J., Duron, J., & Lippe, R. (2013). Analysis of virion-incorporated host proteins required for herpes simplex virus type 1 infection through a RNA interference screen. PLoS ONE, 8(1), e53276. doi:10.1371/journal.pone.0053276.
- Steimer, L., & Klostermeier, D. (2012). RNA helicases in infection and disease. RNA Biology, 9(6), 751–771. doi:10.4161/rna.20090.
- Su, C. Y., Lin, T. C., Lin, Y. F., Chen, M. H., Lee, C. H., Wang, H. Y., … Hsiao, M. (2015). DDX3 as a strongest prognosis marker and its downregulation promotes metastasis in colorectal cancer. Oncotarget, 6(21), 18602–18612. doi:10.18632/oncotarget.4329.
- Sun, C., Pager, C. T., Luo, G., Sarnow, P., & Cate, J. H. (2010). Hepatitis C virus core-derived peptides inhibit genotype 1b viral genome replication via interaction with DDX3X. PLoS ONE, 5(9), doi:10.1371/journal.pone.0012826.
- Sunden, Y., Semba, S., Suzuki, T., Okada, Y., Orba, Y., Nagashima, K., … Sawa, H. (2007a). DDX1 promotes proliferation of the JC virus through transactivation of its promoter. Microbiology and Immunology, 51(3), 339–347. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17380054, http://onlinelibrary.wiley.com/store/10.1111/j.1348-0421.2007.tb03907.x/asset/mim03907.pdf?v=1&t=iplgs1e0&s=9c64859a5d6952f55f28d81e56653b1cb144b04810.1111/mim.2007.51.issue-3
- Sunden, Y., Semba, S., Suzuki, T., Okada, Y., Orba, Y., Nagashima, K., … Sawa, H. (2007). Identification of DDX1 as a JC virus transcriptional control region-binding protein. Microbiology and Immunology, 51(3), 327–337. Retrieved from http://onlinelibrary.wiley.com/store/10.1111/j.1348-0421.2007.tb03915.x/asset/mim03915.pdf?v=1&t=j72s86mi&s=ad447cb3c2e2473405bd485041633db21adc7abb10.1111/mim.2007.51.issue-3
- Tanaka, K., Okamoto, S., Ishikawa, Y., Tamura, H., & Hara, T. (2009). DDX1 is required for testicular tumorigenesis, partially through the transcriptional activation of 12p stem cell genes. Oncogene, 28(21), 2142–2151. doi:10.1038/onc.2009.89.
- Taniguchi, T., Iizumi, Y., Watanabe, M., Masuda, M., Morita, M., Aono, Y., … Sakai, T. (2016). Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death & Disease, 7, e2211. doi:10.1038/cddis.2016.114
- Theissen, B., Karow, A.R., Köhler, J., Gubaev, A., & Klostermeier, D. (2008). Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proceedings of the National Academy of Sciences, 105(2), 548–553. doi:10.1073/pnas.0705488105.
- Thulasi Raman, S. N., Liu, G., Pyo, H. M., Cui, Y. C., Xu, F., Ayalew, L. E., … Zhou, Y. (2016). DDX3 interacts with influenza A virus NS1 and NP proteins and exerts antiviral function through regulation of stress granule formation. Journal of Virology, 90(7), 3661–3675. doi:10.1128/jvi.03010-15.
- Tingting, P., Caiyun, F., Zhigang, Y., Pengyuan, Y., & Zhenghong, Y. (2006). Subproteomic analysis of the cellular proteins associated with the 3′ untranslated region of the hepatitis C virus genome in human liver cells. Biochemical and Biophysical Research Communications, 347(3), 683–691. doi:10.1016/j.bbrc.2006.06.144.
- Uhlmann-Schiffler, H., Rossler, O. G., & Stahl, H. (2002). The mRNA of DEAD box protein p72 is alternatively translated into an 82-kDa RNA helicase. Journal of Biological Chemistry, 277(2), 1066–1075. doi:10.1074/jbc.M107535200.
- Upadya, M. H., Aweya, J. J., & Tan, Y. J. (2014). Understanding the interaction of hepatitis C virus with host DEAD-box RNA helicases. World Journal of Gastroenterology, 20(11), 2913–2926. doi:10.3748/wjg.v20.i11.2913.
- Varnum, S.M., Streblow, D.N., Monroe, M.E., Smith, P., Auberry, K.J., Pasa-Tolic, L., … Wang, D. (2004). Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. Journal of Virology, 78(20), 10960–10966. doi:10.1128/jvi.78.20.10960-10966.2004
- Vashist, S., Urena, L., Chaudhry, Y., & Goodfellow, I. (2012). Identification of RNA-protein interaction networks involved in the norovirus life cycle. Journal of Virology, 86(22), 11977–11990. doi:10.1128/jvi.00432-12.
- Wang, H., Gao, X., Huang, Y., Yang, J., & Liu, Z.-R. (2009). P68 RNA helicase is a nucleocytoplasm shuttling protein. Cell Research, 19(12), 1388–1400. doi:10.1038/cr.2009.113.
- Wang, D., Huang, J., & Hu, Z. (2012). RNA helicase DDX5 regulates microRNA expression and contributes to cytoskeletal reorganization in basal breast cancer cells. Molecular & Cellular Proteomics, 11(2), M111.011932. doi:10.1074/mcp.M111.011932.
- Wang, H., Kim, S., & Ryu, W. S. (2009). DDX3 DEAD-Box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids. Journal of Virology, 83(11), 5815–5824. doi:10.1128/JVI.00011-09.
- Wang, H., & Ryu, W. S. (2010). Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: Implications for immune evasion. PLoS Pathogens, 6(7), e1000986. doi:10.1371/journal.ppat.1000986.
- Wilson, B. J., Bates, G. J., Nicol, S. M., Gregory, D. J., Perkins, N. D., & Fuller-Pace, F. V. (2004). The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Molecular Biology, 5, doi:10.1186/1471-2199-5-11.
- Wortham, N. C., Ahamed, E., Nicol, S. M., Thomas, R. S., Periyasamy, M., Jiang, J., … Fuller-Pace, F. V. (2009). The DEAD-box protein p72 regulates ERalpha-/oestrogen-dependent transcription and cell growth, and is associated with improved survival in ERalpha-positive breast cancer. Oncogene, 28, doi:10.1038/onc.2009.261.
- Wu, C. H., Chen, P. J., & Yeh, S. H. (2014). Nucleocapsid phosphorylation and RNA helicase DDX1 recruitment enables coronavirus transition from discontinuous to continuous transcription. Cell Host & Microbe, 16(4), 462–472. doi:10.1016/j.chom.2014.09.009.
- Xu, L., Khadijah, S., Fang, S., Wang, L., Tay, F. P., & Liu, D. X. (2010). The cellular RNA helicase DDX1 interacts with coronavirus nonstructural protein 14 and enhances viral replication. Journal of Virology, 84(17), 8571–8583. doi:10.1128/JVI.00392-10.
- Yamada, S., Hatta, M., Staker, B. L., Watanabe, S., Imai, M., Shinya, K., … Kawaoka, Y. (2010). Biological and structural characterization of a host-adapting amino acid in influenza virus. PLoS Pathogens, 6(8), e1001034. doi:10.1371/journal.ppat.1001034
- Yasuda-Inoue, M., Kuroki, M., & Ariumi, Y. (2013). DDX3 RNA helicase is required for HIV-1 Tat function. Biochemical and Biophysical Research Communications, 441(3), 607–611. doi:10.1016/j.bbrc.2013.10.107.
- Yedavalli, V. S., Neuveut, C., Chi, Y. H., Kleiman, L., & Jeang, K. T. (2004). Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell, 119(3), 381–392. doi:10.1016/j.cell.2004.09.029.
- You, L. R., Chen, C. M., Yeh, T. S., Tsai, T. Y., Mai, R. T., Lin, C. H., & Lee, Y. H. (1999). Hepatitis C virus core protein interacts with cellular putative RNA helicase. Journal of Virology, 73(4), 2841–2853. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10074132, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC104042/pdf/jv002841.pdf
- Zhao, S., Ge, X., Wang, X., Liu, A., Guo, X., Zhou, L., … Yang, H. (2015). The DEAD-box RNA helicase 5 positively regulates the replication of porcine reproductive and respiratory syndrome virus by interacting with viral Nsp9 in vitro. Virus Research, 195, 217–224. doi:10.1016/j.virusres.2014.10.021.
- Zhou, X., Luo, J., Mills, L., Wu, S., Pan, T., Geng, G., … Zhang, H. (2013). DDX5 facilitates HIV-1 replication as a cellular co-factor of Rev. PLoS ONE, 8(5), e65040. doi:10.1371/journal.pone.0065040.
- Zhou, Y., Wu, W., Xie, L., Wang, D., Ke, Q., Hou, Z., … Fang, L. (2017). Cellular RNA helicase DDX1 is involved in transmissible gastroenteritis virus nsp14-induced interferon-beta production. [Original Research]. Frontiers in Immunology , 8(940), doi:10.3389/fimmu.2017.00940.
- Zhu, Y., Chen, G., Lv, F., Wang, X., Ji, X., Xu, Y., … Gao, G. (2011). Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proceedings of the National Academy of Sciences, 108(38), 15834–15839. doi:10.1073/pnas.1101676108.