ABSTRACT
The endosphere represents intracellular regions within plant tissues colonize by microbial endophytes without causing disease symptoms to host plants. Plants harbor one or two endophytic microbes capable of synthesizing metabolite compounds. Environmental factors determine the plant growth and survival as well as the kind of microorganisms associated with them. Some fungal endophytes that symbiotically colonize the endosphere of medicinal plants with the potential of producing biological products have been employed in traditional and modern medicine. The bioactive resources from endophytic fungi are promising; biotechnologically to produce cheap and affordable commercial bioactive products as alternatives to chemical drugs and other compounds. The exploration of bioactive metabolites from fungal endophytes has been found applicable in agriculture, pharmaceutical, and industries. Thus, fungal endophytes can be engineered to produce a substantive quantity of pharmacological drugs through the biotransformation process. Hence, this review shall provide an overview of fungal endophytes, ecology, their bioactive compounds, and exploration with the biosystematics approach.
1. Introduction
Many plants predominantly occupy larger niches in the biosphere based on their classification and relative medicinal value in addressing diseases affecting humans (Hosseinzadeh et al., Citation2015; Michel et al., Citation2020). In the world today, the use of medicinal plants in herbal drug preparations is on the increase; with more than 80% of the world population currently attesting to its efficacy in phytomedicine; as it is readily available, cost-effective, and nontoxic (Oyenihi & Smith, Citation2019). Medicinal plants grow in terrestrial and aquatic habitats; although with perpetual challenges of attacks by human activities and pathogens (Bahadur et al., Citation2020). It is evident that increase in the world population and the use of plants with medicinal value in the synthesis of drugs can endanger plant distribution and biodiversity in the future, if not properly managed (Ghosh et al., Citation2019). However, finding an alternative source of bioactive metabolites from microbes associated with medicinal plants rather than from the plant itself can reduce the extinction of the most important agricultural plants (Ancheeva et al., Citation2020).
Notably, research findings into natural bioactive compounds with antimicrobial, antioxidant, and anti-diabetic properties are less studied and have evoked scientists in ethnobotany and phytomedicine fields to investigate possible natural compounds in medicinal plants to avert multiple drug-resistant syndromes by pathogens (Marquez & Quave, Citation2020). Limited information on the relevance of the uniqueness of medicinal plants has been a restraint to possible ideas and opportunities in pharmacological research. Apart from plants, studies have revealed possible exploitation of many bioactive compounds from microbial endophytes as a new dawn in pharmacological research in the discovery of notable drugs; potentially with antioxidant, antimicrobial, anticancer, and antidiabetic properties (Adeleke & Babalola, Citation2020b, Citation2020b; Praptiwi et al., Citation2018; Tanapichatsakul et al., Citation2018).
Various bioactive molecules have been explored from medicinal plants, but these compounds from their associated microbes have been underexplored, thus masking vital information of the bioactive properties for various agricultural and industrial applications (Shimoyama et al., Citation2018). Interestingly, the prospects of exploring natural biomolecules from microbes associating with diverse plants in various microhabitats are promising to the present and future studies. Previous findings have established the potential of endophytic fungi in the synthesis of bioactive compounds important in pharmaceutical industries (Adeleke & Babalola, Citation2021b; Ancheeva et al., Citation2020; Gupta et al., Citation2020). The bioactive metabolites from microbes; interestingly, can serve as an alternative to plant-based metabolites produced by the host plants, which can mirror the endophytic microbe’s bioactivity. Manipulation of the microbial endophyte communities and their interactions with the host plants can successfully enhance the synthesis of biological products and novel metabolites from medicinal plants under various environmental conditions (Maqsood et al., Citation2020).
The medicinal plants, such as sunflower (Helianthus annuus) are known as drought-tolerant plants, a native of American, and presently cultivated throughout the world (Babalola et al., Citation2021b; Torimiro et al., Citation2014). For example, the growing of sunflowers in Turkey, Nigeria, South Africa, China, Argentina, Ukraine, etc. with interest in terms of production (tons), and acreage (hectare) is on the increase (Adeleke & Babalola, Citation2020b; Konyalı, Citation2017). Research on endophytic microbiota from sunflower has not yet been fully studied; although, sunflower has various medicinal values with the possible exploration of bioactive compounds from the associated endophytes (Urooj et al., Citation2018). The leaves, petals, and roots of sunflowers have been used in herbal medicine for treating various human diseases (Kaur et al., Citation2020). Despite the medicinal values attributed to sunflower, a few reports have been documented on the bioactive metabolites of the endomycorrhiza community in sunflower plants (Caruso et al., Citation2020).
Despite the discoveries of endophytic microbes in most medicinal plants in the Southern part of Africa; specifically in South Africa with plant-growth-promoting and antibacterial properties (Adeleke et al., Citation2021a; Babalola et al., Citation2021a; Manganyi et al., Citation2018), many medicinal plants and associated microorganisms are largely unexplored. Reports have shown that the plant to fungal diversity ranged from 1 to 6 and the classical range can increase the probable measures in the discovery of bioactive metabolites from fungal domains (Manganyi et al., Citation2019). The clinical importance of bioactive compounds of endophytic fungi isolated from Pelargonium sidoides (African Geranium), a medicinal plant native to South Africa has been documented by Manganyi et al. (Citation2019). In essence, the quest for novel drugs of microbial origin can be mainly due to resistant tendencies of pathogens to chemical drugs for treating major human diseases (Lu et al., Citation2020). Hence, this review aims at providing information on fungal endophytes associated with medicinal plants and optimization processes for bioactive metabolites synthesis.
2. Historical conspectus of the endophyte community
Microorganisms are ubiquitous. They are found in many environments, such as water, air, soil, human skin, deep sea, plants, foods, and other surfaces and several studies have extolled their roles in medicine, pharmaceutical, and agriculture (Bamisile et al., Citation2018; Morales-Cedeño et al., Citation2020; Wolde-meskel et al., Citation2018). The roles of endophytic microbes in agriculture cannot be overemphasized, as endosphere science has widened the scope of research focusing on the characterization, diversity, and functions of microbial endophytes isolated from plant tissues (Babalola et al., Citation2021b, Citation2021c). The study of microbes in the endosphere i.e. specialized regions within the plant organs has revealed that some microorganisms colonize the sterile zones within plant tissues, hence making research into plant microbial communities more interesting than history foretold (Pelo et al., Citation2020). Except for the rhizobacteria and endomycorrhiza that enter the plant through the root rhizosphere, endophytes are naturally present in the stem, roots, and flowers of the host plants (Babalola et al., Citation2021b; Wolfe & Ballhorn, Citation2020). Endophytes occupy both aboveground and belowground parts of plants. The aboveground that comprises the leaves, stems, flowers, and mature seeds naturally contain fewer endophytes than the corresponding belowground root region (Adeleke & Babalola, Citation2020a).
Microbial endophytes colonizing the internal tissues of plant roots are often regarded as a subset of rhizosphere microbes, as high root exudates serve as chemo-attractants make the rhizosphere zone a hotspot for the high microbial population, which enable free access and infiltration from the rhizoplane into plant roots (Santoyo et al., Citation2016). It is evident that because of high rhizodeposits and root exudation, the microbial population in the root zones is usually higher in number than in other parts of the plant (Yuan et al., Citation2021). The characterization of endophytic microbes from some economic plants; namely wheat, rice, corn, sunflower, tomato, chickpeas, cowpea, pearl millet, maize, sugarcane, soybean, citrus, etc. with the potential of enhancing crop yield has been reported (Adeleke & Babalola, Citation2021a; Chowdhary et al., Citation2015; Hiruma et al., Citation2018). Hence, looking into the genesis and history of plant endophytes can create awareness of where and how endophytes originated.
Historical records showed that endophytes were first described by the German botanist Johann Heinrich Friedrich Link in 1809. Also, A French scientist, Béchamp classified endophytes to be a plant-parasitic fungus, which was later termed ‘microzymas’. In 1866, Heinrich Anton De Bary, a German researcher in the field of botany, mycology, microbiology, and surgery defined the word ‘endophyte’ as the microbial community found inhabiting the internal tissues of healthy plants without any pathological or disease symptoms (Stone et al., Citation2000). Furthermore, there was a belief that plants were healthy under sterile conditions and it was not until 1887 that Victor Galippe discovered bacteria normally occurring inside plant tissues (Compant et al., Citation2012). In the research conducted by Heinrich Anton De Bary on the potato crops, he observed a disease symptom ‘potato blight’ caused by Phytophthora infestans, and affirmed that pathogenic fungi were symbiotically associated with plants can be responsible for the pathological changes in the diseased plant. In that era, the origin of plant diseases or possible research into the endosphere was unknown. Subsequently, after 10 decades, Carroll (Citation1986) attributed the term ‘endophyte’ to only microorganisms that show no symptomatic infections on or in plants. Petrini and Müller (Citation1979) elucidated the commensalism association of endophytes with plants. Furthermore, Wilson Dennis gave more insights by grouping fungal endophytes as commensals (Wilson, Citation1993, Citation1995). Till today, the term endophytes is used as microbial domains, including bacteria, fungi, archaea, actinomycetes, etc. that intracellularly inhabit the internal tissues of plants for their entire life cycle (Adeleke & Babalola, Citation2021c; Fouda et al., Citation2021; Khalil et al., Citation2021).
Endophytes importantly occupy plant econiches by promoting plant growth and enhancing plant immunity (dos Santos Souza et al., Citation2019). In recent times, diverse endophytic microbes have been identified (Dastogeer et al., Citation2020; Dubey et al., Citation2021; A. A. Fouda et al., Citation2021), while several others are yet to be cultured. The isolation of endophytic fungi from various plant parts with bioprospecting in agricultural biotechnology has been reported (Shah et al., Citation2019; Yang et al., Citation2018). Endophytes symbiotically colonize the internal tissues of plants and exhibit asymptomatically features in sustaining plant growth and health (Mantzoukas & Eliopoulos, Citation2020). More than 1 million identifiable endophytic fungal species are known with plant specificity. The endophytic community varied from plant to plant due to diverse environmental factors influencing their survival and adaptation. Some of these factors include plant species, age, genotype, developmental stages, and soil parameters (Turrini et al., Citation2015). Also, other factors, such as plant habitat, genetic composition, and soil nutrients, determine the quality and quantity of chemical compounds in various medicinal plants (Adeleke & Babalola, Citation2020b; Ncube et al., Citation2012). Most endophytic fungi are grouped as either ascomycetes or fungi imperfecti. The mutual association of endophytes with their host plants is known to be beneficial in the synthesis of certain metabolites with enormous applications in agricultural biotechnology (Adeleke & Babalola, Citation2021a; Bhatti et al., Citation2017). Hence, various biotechnological applications of endophytes can increase the possibilities of exploring endophytic resources from diverse plant species. The plant’s internal environment is said to be sterile but harbors invisible microbial communities with multifaceted potentialities in ensuring higher productivity. Research has shown that virtually all plants with well-organized supporting tissues harbor endophytic microbes (Cope-Selby et al., Citation2017).
The association of endophytes with plants can be either mutual or pathogenic. The beneficial endophytes regarded as plant growth-promoting endophytes, which promote plant growth by enhancing plant nutrient acquisition, secretion of certain growth-promoting hormones (gibberellins, cytokinins, indole-3-acetic acid, etc.), and act as biocontrol agents against phytopathogens, which enabled them to stimulate plant growth, resistance to environmental stresses and accumulation of synthesized bioactive metabolites in building microbial networking below ground level (Adeleke & Babalola, Citation2021c). Till today, the importance of secondary metabolites produced by endophytes in an ecosystem has not yet been fully explored.
Despite the estimate that there are more than 3 million types of plants in the world, each plant contains varied endophytic communities (Baltrus, Citation2017). It is evident that based on colonization patterns, different endophyte species can be isolated from their host plants, thus providing an opportunity to harness new and targeted natural compounds from the microbial endophytes from different plants in ecological niches (Foito & Stewart, Citation2018). Based on the limited documentation on endophytic research, it is necessary to critically elucidate their potential application in various biotechnological processes with priority on bioactive compound synthesis.
The ability of endophytes to produce bioactive compounds can be proportional to their coevolution with the host plants by copying plant genetic information which may allow them to exercise adaptive mechanisms within the plant, and functionally, by protecting plants from pathogen attack and insect infestation (Hartmann et al., Citation2019). Despite the biological nature of endophytes, they potentially secrete chemicals within the plants i.e. the plant plays an important role in mediating endophyte selection for the synthesis of nontoxic bioactive metabolites to the higher organisms. The exploitation of bioactive compounds from endophytes is promising, relative to the human health and pharmaceutical industry (Sandargo et al., Citation2019); nevertheless, due to the methodology constraints, processing, and economic reasons, these have caused a higher demand for synthetic drugs. Hence, finding proactive and long-lasting measures in obtaining bioactive products from endophytes would effectively proffer curative solutions to human-related diseases caused by pathogenic microbes with antibiotic resistance genes/tendencies (Salehi et al., Citation2019).
3. Endophytic ecology in medicinal plants
Research into endophytic communities in plants has been under-focused, despite their possible reservoir of various bioactive and chemical compounds that can easily be explored in medicine and agriculture (Palanichamy et al., Citation2018). Taxonomically, fungal endophytes are classified into coelomycetes, hyphomycetes, and ascomycetes. Plant organs house a hundred thousand microorganisms with similar functions (Eke et al., Citation2019; Pelo et al., Citation2020). The colonization of endophytic fungi can be organ-specific, such as stems, roots, leaves, flowers, petiole, and seeds (Lata et al., Citation2019). Endophyte survival relies on the adaptation mechanisms of host plants that influence plant habitat; hence, there can be possible similarities in fungal composition in plants growing in the same location (Yin et al., Citation2020). The co-evolution of fungal endophytes can be informed through transmission (vertical or horizontal), nutrition, infection symptoms, site of colonization, and mechanisms of interactions (Adeleke et al., Citation2021b). Vertical transmission means can be achieved by seed inoculation, while the horizontal means of transmission can be achieved through soil-root interactions of fungal reproductive structures (spores) (Müller & Krauss, Citation2005).
The endophytic fungi establish microbial communities within the plant tissues by forming direct host-linkage, thus establishing cell biomass equilibrium between the plant and the associated microbes (Fernandez & Burch-Smith, Citation2019). The presence of these microorganisms has contributed to plant growth in enhancing crop productivity as well as the protection of plants from pathogens (Fadiji & Babalola, Citation2020). Fungal endophytes can survive plant niches without eliciting any symptoms of disease, due to their ability to secrete certain bioactive metabolites that can be explored as biocontrol agents against pathogenic microorganisms. The mutual interactions between the plants and endophytic fungi can remain without disease symptoms, and by circumventing the stimulation of host defense mechanisms (Xu et al., Citation2019). Under unfavorable or harsh conditions, beneficial endophytes can be pathogenic due to the expression of certain genes (Hiruma et al., Citation2018). The interactions between plant and endophytes can be extended beyond maintaining antagonistic equilibrium, rather, secretion of bioactive metabolites to further bridge the interactions between fungal endophytes and the host plants (Baltrus, Citation2017). The endophyte interactions with plants involve multi-species communications that account for partial or complete synthesis of secondary metabolites that vary from endophyte to endophyte and plant to plant (Sahu et al., Citation2019).
The bioactive attributes of plants can mirror the biological activity of their associated microbes, especially those colonizing the internal tissues called ‘endophytes’ (Mohotti et al., Citation2020). Many researchers have identified various bioactive compounds from plants and microorganisms, which include phenols, tannins, flavonoids, steroids, amides, terpenoids, quinones, glycosides, and saponins (Sugiyama, Citation2019), with antimicrobial (Egamberdieva et al., Citation2017), antioxidant (Vieitez et al., Citation2018), and anticancer properties (Chen et al., Citation2016), thus making them a suitable candidate for treating human diseases, such as cancer, malaria, tuberculosis, viral pathogens, diabetes, inflammation, and arthritis. As such, most plant microbiomes can be a source of bioactive compounds needed in ethnobotany research and the pharmaceutical industry (Ghosh et al., Citation2019).
Medicinal plants are like other plants harboring diverse microbes within their tissues or organs based on the bioactive metabolites embedded in the plant that make endophyte characterization from them promising (Hassan & Ullah, Citation2019). Hence, research into medicinal plants can readily make information available on their usage as optional sources of medicine and endophytes. The exploitation of the endophytic community from medicinal plants makes the plant a promising source of unidentified or novel bioactive compounds with much premium in pharmacology and agriculture (Nivetha & Kharwar, Citation2019). Fungal endophytes produce certain metabolites with anticancer compounds (Taxol), antimicrobial, and industrial enzymes. Sometimes, endophytes can be promising for use in the synthesis of silver and other metal nanoparticles with much significance in medicine and agriculture (Pandey, Citation2018). Currently, the need to characterize biologically active endophytic molecules from medicinal plants is advancing through research; to understand their structural diversity and novel functional profiling for future recommendation to ensure agricultural productivity sustainably.
Endophytes exhibit interdependence with the host plants in the synthesis of metabolites or as a result of genetic mimicry with the host plants over a long period (Audipudi et al., Citation2017). The endophytic competence in the synthesis of bioactive compounds enhances their co-metabolism with other endophytes, host plants, pathogens, and nutrition. Some examples of structural bioactive compounds from endophytes with therapeutic potential include alkaloids, steroids, isoprenoids, flavonoids, benzoquinone’s phenols, depsipeptides, phenols, lipids, proteins, shikimates, terpenoids, benzopyranones, xanthones, isocoumarines, perylene derivatives, quinones, furandiones, cytochalasin, polyketides, peptides and glycosides (Nisa et al., Citation2015). These compounds have been reported to stimulate antibacterial, antitumor, antiviral, antifungal, antidiabetic, antimalarial, cytotoxic, hypocholesterolemic, and immunomodulatory responses in living organisms (Martinez-Klimova et al., Citation2017).
Many researchers have described and documented a large number of natural products obtained from endophytic fungi as an alternative therapy to infectious diseases, as well as a source of antioxidants that safeguard cells from oxidative stress/damage (Manganyi et al., Citation2019; Palanichamy et al., Citation2018; Sandargo et al., Citation2019). The synthesis of metabolites from endophytes can be achieved through various metabolic pathways after obtaining the mycelia-free culture by filtration (Palanichamy et al., Citation2018). The synthesis of bioactive compounds, such as cichorine, xanthones, tetralones, benzopyranones, tetralones, zinnimide, dihydroaltersetin, macrosporin, alternariol, altersetin, homodetruxin, equisetin, alternariol 5-O-sulfate, and desmethyldestruxin B from fungi species Alternaria has been reported (Lou et al., Citation2013, Citation2016; Palanichamy et al., Citation2018).
The extraction of bioactive metabolites from natural sources can be achieved by the fermentation process to increase cell biomass in a culture production medium (Bier et al., Citation2017). The extraction of bioactive metabolites from plants can be influenced by seasonal changes, climatic and ecological problems (Ekiz et al., Citation2018). Hence, the need to find economical and modern approaches in obtaining bioactive compounds from natural sources became important. Fermentation technology that involves plant preparation, fermentation, culture production, separation, and harvesting of endophytes cell-free culture is promising as alternative methods in obtaining cell-biomass production, cost-effective bio-resources, and high-potent bioactive products and other compounds (Bier et al., Citation2017). The biotransformation involving the use of endophytes has been explored for the synthesis of volatile chemical compounds with desirable properties, such as antioxidant, antifungal, antibacterial, anti-inflammatory, antiviral, and blood pressure regulation.
For endophytes to successfully colonize the host plants, the first mechanism is to overcome both the physical and chemical barriers. The hypothesis about balance antagonism intends to perorate on the mechanisms employed by plant endophytes in circumventing activation of host defenses, protect themselves from host toxic metabolites and survival within host plants without conspicuous visibility of any disease symptoms (). This assumption about the non-symptomatic correlation between plants and endophytes is called ‘balance antagonisms’. Biotic and abiotic factors play a key role in maintaining effective interactions between plants and microbes (Shrivastava et al., Citation2019). The virulence factors properties of pathogens and endophytes do counter by host plants stimulating defense responses.
Table 1. Medicinal plant-fungal endophyte mechanisms and survival under various ecological stress adaptors
In the tropical and temperate regions of the world, approximately 391,000 species of vascular plants are currently known to science, of which about 369,000 species are flowering plants, according to a report by the Royal Gardens, Kew, in the United Kingdom (Audah, Citation2019). Medicinal plants can easily grow in any environment, but a lack of scientific evidence in their identification before use is still a major challenge in most countries where herbal drugs form the frontline in health care for treating diseases in most rural communities, especially in Africa (Ghosh et al., Citation2019). In most cases, the use of medicinal plants traditionally can be based on assumptions and beliefs from previous knowledge inherited from herbal practitioners without any laboratory screening for the bioactive compounds present in those plants. Hence, the advancement in science in the identification of bioactive metabolites in medicinal plants for various pharmacological studies in drug delivery systems remains fundamental in modern science. Notably, despite the exploration of medicinal plants over a long time as therapy for various diseases, information about the chemical compounds and identification of the bioactive metabolites in some plants are unknown and yet to be fully explored.
As stated by the World Health Organization (WHO), medicinal plants are classified as plants with one or more specialized organs containing curative substances or alternative therapy. From the WHO estimate, it has been documented that approximately 80% of the human population in the world explores medicinal plants, either in wet or dry form. The researchers in the ethnopharmacology field have shown more interest due to the indispensability of plants in the synthesis of important biological products (Krishnaiah et al., Citation2011).
4. Production, exploration of bio-resources from endophytic fungi
Fungi associated with plants are naturally endowed with the potential of producing diverse bioactive compounds against nematodes, herbivores, and other pathogens (Al-Ani, Citation2019). These compounds can be screened/exploited for various biological activities; including their application as curative bioproducts for treating various diseases (Gupta et al., Citation2020). Fungal endophytes have been isolated from different plant species across different eco-geographical locations (Adeleke & Babalola, Citation2021b; Fouda et al., Citation2015). The novel structural bioactive metabolites produced by endophytic fungi have an impression of it as a reservoir of different bioactive compounds (Toghueo, Citation2020). The fungal endophytes producing bioactive substances can be subjected to fermentation on a commercial scale to produce unlimited bioactive metabolites by harnessing active metabolites with important functions.
The bioactive metabolites synthesized by endophytic fungi in host plants can saliently boost plant adaptation and endophytic fungi survival in a given microhabitat (Martínez-Diz et al., Citation2019). Endophytic fungi can indirectly improve plant tolerance to environmental stress adaptors and induce excess production of novel bioactive metabolites that can be conceivably exploited as crucial plant resources. From the coevolution perspective, endophyte interactions with plants improve plant resistance to withstand unfavorable harsh environmental conditions by synthesizing secondary metabolites (Eljounaidi et al., Citation2016). Hence, harnessing endophytic resources from medicinal plants can be promising in enhancing plant resistance to insects, protozoans, and other attacks.
Endophytic fungi and their derivable natural products as sources of novel bioactive metabolites have been explored with much application in modern medicine (Naik, Citation2019). These derivable compounds are essential and have accounted for approximately 68% and 34% of antibacterial and anticancer compounds, respectively. Based on the aforementioned premise, it is imperative to explore novel bioactive compounds from endophytic fungi to produce pharmaceutical drugs to meet the demand for basic health care in the human populace (Ke & Yoshikuni, Citation2020). Findings have shown that endophytic fungi potentially synthesize more secondary metabolites than bacterial or archaeal endophytes (Adeleke & Babalola, Citation2021b; Li et al., Citation2018; Nisa et al., Citation2015). The succinct elucidation of endophytic fungi and their interactions with plants can further inform the medicinal contents of host plants as devisable means to explore plant potential as applied in drug production (Salehi et al., Citation2019).
Besides the function of endophytic fungi in determining the number of metabolites from medicinal plants, they also contribute to plant performance, in terms of quantity and quality (Adeleke & Babalola, Citation2021a). The mechanism of endosymbiotic association that exists between fungal communities and their host plants is still not well studied and limited documentation is available in this regard. Hence, understanding various endophyte mechanisms in promoting plant growth can significantly pinpoint easy techniques in the exploitation of bioactive compounds from medicinal plants for drug production (Fadiji & Babalola, Citation2020).
To maximally explore bioactive compounds from endophytic fungi, the culture or growing medium needs to be manipulated, following the inclusion of certain substrates/chemicals into the fermentation medium. This method can serve as an alternative to conventional means of synthesizing drugs from medicinal plants, using cell-free culture of endophytic fungi under optimized cultural conditions. The isolation of notable bioactive metabolites, such as xanthones, steroids, xanthones, furadiones, isocoumarines, and depsipeptides from endophytic fungi synthesized through a polyketide pathway has been documented (Song et al., Citation2017). From the literature, reports have shown that endophytic fungi potentially produce higher bioactive compounds (51%) than the soil-inhabiting fungi, thus rejuvenating the recruitment of the plant endophytic community as a major source of bioactive metabolites (Nisa et al., Citation2015). Some researchers have also established the discovery of invaluable microbial products from endophytic fungi by recommending them as alternative sources of active biological molecules in the discovery of new drugs (Mani et al., Citation2015; Nalini & Prakash, Citation2017; Yang et al., Citation2018). Hence, the bioprospecting of endophytic fungi is advancing and has attracted urgent attention among researchers in the fields of ecology, pharmacology, microbiology, agronomy, and chemistry.
5. Biosystems approach and synthesis of bioactive metabolite compounds
The biological activities of endophytic fungi are not unique in their ability to produce novel bioactive metabolites, but also in the transformation of structural and biological entities into natural products (Bier et al., Citation2017). The use of a biological process that brings about chemical changes for the synthesis of natural products or other compounds different from the natural substrates is called biotransformation. The biotransformation process has great advantages over chemical synthesis based on the resultant efficacy in the production of novel and important products that cannot be synthesized by chemical methods, and this method depends on the free transformation of functional groups of synthetic products without altering the carbon structure (Bianchini et al., Citation2015).
It is important to test for the bio-transformed metabolites with feeble activity on target extracellular enzymes to produce bioactive analogs. The use of microorganisms as a precursor in the transformation process can be efficient and economical for easy assessment of bioactive compounds and new therapeutic drugs with bioactivities in the medicine and pharmaceutical industries (Torres-Mendoza et al., Citation2020). The biotransformation process of natural products can be initiated as assisted by enzyme (reductases, hydrolases, and oxidases) synthesis to facilitate endophytic fungi involvement in drug synthesis through metabolic pathways. The ability of endophytic fungi to modify chemical structures with specific stereoisomers and synthesize novel enzymes can speed up the production of compounds of interest.
The biotransformation process performed under controlled conditions requires adequate regulation time, temperature, pH, agitation, aeration, and substrate concentration; thus, reducing the intrusion of contaminants, cost, and energy (Fernandes et al., Citation2021). The chemical processes allow the synthesis of enantiomer pure compounds and reduce difficulties in the separation and purification of the synthesized compounds. The biochemical processes that occur under controlled conditions reduce the rate of industrial emission and production of high accumulated organic residues and environmental problems. Usually, the resulting products from the biotransformation process are pure, natural, and safer than chemical products that generate several undesirable reaction mixtures due to nonselective substrates that reduce product efficiency and hike in the downstream production process (He et al., Citation2019). Hence, the biotransformation technique is required for the synthesis of new and unknown bioactive entities, massive production of desirable and quality compounds, to tame the chemical analysis-related problems, and provide insights into the biosynthetic pathways (Passari et al., Citation2017).
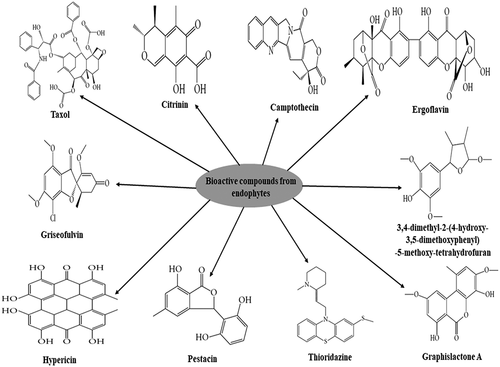
Furthermore, biotransformation that involves the use of microbial cultures or its products, such as enzymes, has gained attention in recent studies as an efficient technique in the bioconversion of essential bioactive molecules, such as steroids, lipids, lignans, alkaloids, triterpenes, monoterpenes, and diterpenes (Segaran & Sathiavelu, Citation2019). Consequently, the synthetic chemicals used can be specific or selective to stereo reactions in the synthesis of new products applicable in pharmaceutical industries. Some of the important screened bioactive compounds from the endophytes culture filtrates include Taxol, camptothecin, ergoflavin, griseofulvin, hypericin, citricin, pestacin, graphislactone A, thioridazine, and 3,4-dimethyl-2-(4-hydroxy-3,5-dimethoxyphenyl)-5-methoxy-tetrahydrofuran are structurally presented in . These compounds are said to possess trypanocidal activity against Trypanosoma cruzi causing Chagas disease.
The production of limonene (terpene) in a submerged fermentation medium inoculated with the spores of endophytic fungus Diaporthe spp. isolated from Pinus taeda has been reported (Bier et al., Citation2017), with a high concentration of limonene derivatives, such as carvone, limoneno-1,2-diol, and α-terpineol. The bioconversion of cyclocanthogenol from an endophytic fungus, Alternaria eureka 1E1BL1 isolated from Astragalus angustifolius in the synthesis of certain metabolites via oxidation, hydroxylation, O-methylation, ring-expansion, epoxidation and methyl migration reactions on the triterpenoid skeleton has been reported (Ekiz et al., Citation2018). The possibility of obtaining novel bioactive compounds in a fermenting medium containing fungal endophyte spores may depend on many factors, which include, culture medium, optimization conditions, tissue type, plant age, genetic make-up for the mycobionts and photobionts (Morales-Sánchez et al., Citation2020). In recent studies, different authors have reported the modification of culture medium containing fungal endophytes under different growing conditions in the synthesis of new bioactive compounds (Bier et al., Citation2017; Deepika et al., Citation2016; Gouda et al., Citation2016).
6. Conclusion and future prospects
The biotransformation of suitable substrates in developing cheap and affordable bioactive compounds can offer new hope and opportunities in deriving novel bioactive metabolites from culturable endophytic fungi that can be employed in the synthesis of drugs, as a baseline in the healthcare delivery system to circumvent various diseases challenging human health. The use of the fermentation technique is advantageous and has provided a platform for a sustainable production system of novel bioactive compounds on an inexpensive easily metabolizable substrate.
Fungal endophytes have emerged as a promising bioactive reservoir and many studies have established this fact, although not all fungi colonizing the internal tissues of plants have been properly screened. Fermentation technology can be employed to manipulate microbial resources important to human health sustainability. Furthermore, the discovery of novel compounds from fungal endophytes associated with medicinal plants through fermentation techniques can serve as a precursor in the synthesis of new drugs. Hence, exploration of plant endosphere-hidden treasures can play vital roles in the synthesis of novel drugs and ensure safe healthy living.
Authors’ Contributions
B.S.A had the idea and suggested for the article, while O.O.B moderated the review topic. B.S.A. performed the literature search, and wrote the first draft. O.O.B made substantial technical and intellectual contributions to the structure of the various drafts of the manuscript. Both authors approved the article for publication.
Acknowledgments
B.S.A. thanked the National Research Foundation of South Africa and The World Academy of Science (TWAS) for the NRF-TWAS African Renaissance Doctoral scholarship (UID116100). O.O.B. acknowledges the National Research Foundation of South Africa for the grants, numbers: UID123634; UID132595, supporting research in her laboratory.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Bartholomew Saanu Adeleke
Bartholomew Saanu Adeleke is a seasoned researcher and academic with a high predilection toward research. He is currently undergoing his Doctoral studies in the Department of Microbiology, North-West University, South Africa. His research interests are on microbial biotechnology, next-generation sequencing, plant-microbe interactions, endosphere biology, endophytic microbiome, molecular biology, food security, and agricultural sustainability. His present research focuses on the use of culture-dependent and culture-independent approaches in the understanding of endophytic bacteria community structure in sunflower sourced from the North West Province of South Africa. He is a member of the American Society for Microbiology and an awardee of NRF-TWAS African Renaissance Doctoral scholarship.
Olubukola Oluranti Babalola
Prof Olubukola Oluranti Babalola (Pr. Sci. Nat, MASSAF) holds BSc, MSc, Ph.D. (Microbiology), and an MBA in Science Leadership. She is a product of the International Institute of Tropical Agriculture (IITA), the Organization for Women in Science for the Developing World (OWSD), the Weizmann Institute of Science, Israel, and the University of Western Cape (UWC), South Africa. She is the Research Director of Food Security and Safety Niche Area at North-West University (NWU) South Africa, the Vice President of OWSD, and without reservation leading, as the Principal Investigator, a Microbial Biotechnology laboratory. Her laboratory is a mini united nation, with students from within and outside Africa. She has graduated with 19 PhDs, 22 masters, and 54 Honors students. Babalola is a prolific author with ~200 publications. She has over 40 professional certificates from the University of California, Berkeley, USA; University of Mauritius, Reduit; NWU, South Africa and Bradford University, United Kingdom to mention a few. Her wealth of international experience spans through Americas, Asia, Europe, and Oceania.
References
- Adeleke, B. S., Ayangbenro, A. S., & Babalola, O. O. (2021a). Genomic analysis of endophytic Bacillus cereus T4S and its plant growth-promoting traits. Plants, 10(9), 1776. https://doi.org/https://doi.org/10.3390/plants10091776
- Adeleke, B. S., Ayangbenro, A. S., & Babalola, O. O. (2021b). Genomic assessment of Stenotrophomonas indicatrix for improved sunflower plant. Current Genetics, 1–17. https://doi.org/https://doi.org/10.1007/s00294-00021-01199-00298.
- Adeleke, B. S., & Babalola, O. O. (2020a). The endosphere microbial communities, a great promise in agriculture. International Microbiology, 24, 1–17. https://doi.org/https://doi.org/10.1007/s10123-020-00140-21
- Adeleke, B. S., & Babalola, O. O. (2020b). Oilseed crop sunflower (Helianthus annuus) as a source of food: nutritional and health benefits. Food Science and Nutrition, 8(9), 4666–4684. Article fsn3.1783. https://doi.org/https://doi.org/10.1002/fsn3.1783
- Adeleke, B. S., & Babalola, O. O. (2021a). Biotechnological overview of agriculturally important endophytic fungi. Horticulture, Environment, and Biotechnology, 62(4), 507–520. https://doi.org/https://doi.org/10.1007/s13580-021-00334-1
- Adeleke, B. S., & Babalola, O. O. (2021b). Pharmacological potential of fungal endophytes associated with medicinal plants: A review. Journal of Fungi, 7(2), 147. https://doi.org/https://doi.org/10.3390/jof7020147
- Adeleke, B. S., & Babalola, O. O. (2021c). Roles of plant endosphere microbes in agriculture - a review. In : EgamberdievaDilfuza and TiezziAntonio (Eds). Journal of Plant Growth Regulation (pp. 1–18). https://doi.org/https://doi.org/10.1007/s00344-021-10406–2.
- Al-Ani, L. K. T. (2019). The importance of endophytic fungi from the medicinal plant: Diversity, natural bioactive compounds, and control of plant pathogens. In Medically Important Plant Biomes: Source of Secondary Metabolites (pp. 189–238). Springer.
- Ancheeva, E., Daletos, G., & Proksch, P. (2020). Bioactive secondary metabolites from endophytic fungi. In Shafiquzzaman Siddiquee, Gan Jet Hong Melvin and Md. Mizanur Rahman (Eds). Current Medicinal Chemistry, (27(11), pp. 1836–1854. Springer. 10.2174/0929867326666190916144709.
- Audah, K. A. (2019). Drug discovery: A biodiversity perspective. In Nanotechnology: Applications in energy, drug and food (pp. 249–265).
- Audipudi, A., Chakicherla, B., & Bhore, S. (2017). Bacterial endophytes as biofertilizers and biocontrol agents for sustainable agriculture. Biotechnology and Sustainability, 1, 223–247.
- Babalola, O. O., Adeleke, B. S., & Ayangbenro, A. S. (2021b). Whole genome sequencing of sunflower root-associated Bacillus cereus. Evolutionary Bioinformatics, 17, 1–6. https://doi.org/https://doi.org/10.1177/11769343211038948
- Babalola, O. O., Adeleke, B. S., & Ayangbenro, A. S. (2021a). Draft genome sequencing of Stenotrophomonas indicatrix BOVIS40 and Stenotrophomonas maltophilia JVB5, two strains with identifiable genes involved in plant growth promotion. Microbiology Resource Announcements, 10(28), 482. https://doi.org/https://doi.org/10.1128/MRA.00482-21
- Babalola, O. O., Emmanuel, O. C., Adeleke, B. S., Odelade, K. A., Nwachukwu, B. C., Ayiti, O. E., Adegboyega, T. T., & Igiehon, N. O. (2021c). Rhizosphere microbiome cooperations: Strategies for sustainable crop production. Current Microbiology, 78(4), 1069–1085. https://doi.org/https://doi.org/10.1007/s00284-021-02375-2
- Bahadur, S., Khan, M. S., Shah, M., Shuaib, M., Ahmad, M., Zafar, M., Begum, N., Gul, S., Ashfaq, S., Mujahid, I., & Hussain, F. (2020). Traditional usage of medicinal plants among the local communities of Peshawar valley, Pakistan. Acta Ecologica Sinica, 40(1), 1–29. https://doi.org/https://doi.org/10.1016/j.chnaes.2018.12.006
- Baltrus, D. A. (2017). Adaptation, specialization, and coevolution within phytobiomes. Current Opinion in Plant Biology, 38, 109–116. https://doi.org/https://doi.org/10.1016/j.pbi.2017.04.023
- Bamisile, B. S., Dash, C. K., Akutse, K. S., Keppanan, R., Afolabi, O. G., Hussain, M., Qasim, M., & Wang, L. (2018). Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiological Research, 217, 34–50. https://doi.org/https://doi.org/10.1016/j.micres.2018.08.016
- Bhatti, A. A., Haq, S., & Bhat, R. A. (2017). Actinomycetes benefaction role in soil and plant health. Microbial Pathogenesis, 111, 458–467. https://doi.org/https://doi.org/10.1016/j.micpath.2017.09.036
- Bianchini, L. F., Arruda, M. F., Vieira, S. R., Campelo, P., Grégio, A. M., & Rosa, E. A. (2015). Microbial biotransformation to obtain new antifungals. Frontiers in Microbiology, 6, 1433. https://doi.org/https://doi.org/10.3389/fmicb.2015.01433
- Bier, M. C. J., Medeiros, A. B. P., & Soccol, C. R. (2017). Biotransformation of limonene by an endophytic fungus using synthetic and Orange residue-based media. Fungal Biology, 121(2), 137–144. https://doi.org/https://doi.org/10.1016/j.funbio.2016.11.003
- Carroll, G. (1986). The biology of endophytism in plants with particular reference to woody ornamentals. In N. J. Fokkema & J. van den Heuvel (Eds.), Microbiology of the Phyllosphere (pp. 205–222). Cambridge University Press. https://ci.nii.ac.jp/naid/20001355511/#cit
- Caruso, G., Abdelhamid, M. T., Kalisz, A., & Sekara, A. (2020). Linking endophytic fungi to medicinal plants therapeutic activity. A case study on asteraceae. Agriculture, 10(7), 286. https://doi.org/https://doi.org/10.3390/agriculture10070286
- Chen, L., Zhang, Q.-Y., Jia, M., Ming, Q.-L., Yue, W., Rahman, K., Qin, L.-P., & Han, T. (2016). Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Critical Reviews in Microbiology, 42(3), 454–473. https://doi.org/https://doi.org/10.3109/1040841X.2014.959892
- Chowdhary, K., Kaushik, N., & Ganapathi, T. R. (2015). Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PloS One, 10(11), e0141444. https://doi.org/https://doi.org/10.1371/journal.pone.0141444
- Compant, S., Sessitsch, A., & Mathieu, F. (2012). The 125th anniversary of the first postulation of the soil origin of endophytic bacteria–a tribute to MLV Galippe. Plant and Soil, 356(1), 299–301. https://doi.org/https://doi.org/10.1007/s11104-012-1204-9
- Cope-Selby, N., Cookson, A., Squance, M., Donnison, I., Flavell, R., & Farrar, K. (2017). Endophytic bacteria in Miscanthus seed: Implications for germination, vertical inheritance of endophytes, plant evolution and breeding. Gcb Bioenergy, 9(1), 57–77. https://doi.org/https://doi.org/10.1111/gcbb.12364
- Dastogeer, K., Oshita, Y., Yasuda, M., Kanasugi, M., Matsuura, E., Xu, Q., & Okazaki, S. (2020). Host specificity of endophytic fungi from stem tissue of nature farming tomato (Solanum lycopersicum Mill.) in Japan. Agronomy, 10(7), 1019. https://doi.org/https://doi.org/10.3390/agronomy10071019
- Deepika, V. B., Murali, T. S., & Satyamoorthy, K. (2016). Modulation of genetic clusters for synthesis of bioactive molecules in fungal endophytes: A review. Microbiological Research, 182, 125–140. https://doi.org/https://doi.org/10.1016/j.micres.2015.10.009
- dos Santos Souza, C. R., de Oliveira Barbosa, A. C., Fortes Ferreira, C., F, V. D. S., de Souza Rocha, L., de Souza, E. H., & de Oliveira, S. A. S. (2019). Diversity of microorganisms associated to Ananas spp. from natural environment, cultivated and ex situ conservation areas. Scientia Horticulturae, 243, 544–551. https://doi.org/https://doi.org/10.1016/j.scienta.2018.09.015
- Dubey, A., Saiyam, D., Kumar, A., Hashem, A., Abd_Allah, E. F., & Khan, M. L. (2021). Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. International Journal of Environmental Research and Public Health, 18(3), 931. https://doi.org/https://doi.org/10.3390/ijerph18030931
- Egamberdieva, D., Wirth, S., Behrendt, U., Ahmad, P., & Berg, G. (2017). Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Frontiers in Microbiology, 8, 199. https://doi.org/https://doi.org/10.3389/fmicb.2017.00199
- Eke, P., Kumar, A., Sahu, K. P., Wakam, L. N., Sheoran, N., Ashajyothi, M., Patel, A., & Fekam, F. B. (2019). Endophytic bacteria of desert cactus (Euphorbia trigonas Mill) confer drought tolerance and induce growth promotion in tomato (Solanum lycopersicum L.). Microbiological Research, 228, 126302. https://doi.org/https://doi.org/10.1016/j.micres.2019.126302
- Ekiz, G., Duman, S., & Bedir, E. (2018). Biotransformation of cyclocanthogenol by the endophytic fungus Alternaria eureka 1E1BL1. Phytochemistry, 151, 91–98. https://doi.org/https://doi.org/10.1016/j.phytochem.2018.04.006
- Eljounaidi, K., Lee, S. K., & Bae, H. (2016). Bacterial endophytes as potential biocontrol agents of vascular wilt diseases–review and future prospects. Biological Control, 103, 62–68. https://doi.org/https://doi.org/10.1016/j.biocontrol.2016.07.013
- Fadiji, A. E., & Babalola, O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Frontiers in Bioengineering and Biotechnology, 8, 467. https://doi.org/https://doi.org/10.3389/fbioe.2020.00467
- Fernandes, S., Belo, I., & Lopes, M. (2021). Highly aerated cultures boost gluconic acid production by the yeast-like fungus Aureobasidium pullulans. Biochemical Engineering Journal, 175, 108133. https://doi.org/https://doi.org/10.1016/j.bej.2021.108133
- Fernandez, J. C., & Burch-Smith, T. M. (2019). Chloroplasts as mediators of plant biotic interactions over short and long distances. Current Opinion in Plant Biology, 50, 148–155. https://doi.org/https://doi.org/10.1016/j.pbi.2019.06.002
- Foito, A., & Stewart, D. (2018). Metabolomics: A high-throughput screen for biochemical and bioactivity diversity in plants and crops. Current Pharmaceutical Design, 24(19), 2043–2054. https://doi.org/https://doi.org/10.2174/1381612824666180515125926
- Fouda, A., Eid, A. M., Elsaied, A., El-Belely, E. F., Barghoth, M. G., Azab, E., Gobouri, A. A., & Hassan, S. E.-D. (2021). Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC Inherent to arid regions. Plants, 10(1), 76. https://doi.org/https://doi.org/10.3390/plants10010076
- Fouda, A. H., Hassan, S. E.-D., Eid, A. M., & Ewais, E. E.-D. (2015). Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Annals of Agricultural Sciences, 60(1), 95–104. https://doi.org/https://doi.org/10.1016/j.aoas.2015.04.001
- Ghosh, P., Chatterjee, S., Das, P., Karmakar, S., & Mahapatra, S. (2019). Natural habitat, phytochemistry and pharmacological properties of a medicinal weed - Cleome rutidosperma DC. (Cleomaceae): A comprehensive review. International Journal of Pharmaceutical Sciences and Research, 10(4), 1605–1612.
- Gouda, S., Das, G., Sen, S. K., Shin, H.-S., & Patra, J. K. (2016). Endophytes: A treasure house of bioactive compounds of medicinal importance. Frontiers in Microbiology, 7, 1538. https://doi.org/https://doi.org/10.3389/fmicb.2016.01538
- Gupta, S., Chaturvedi, P., Kulkarni, M. G., & Van Staden, J. (2020). A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnology Advances, 39, 107462. https://doi.org/https://doi.org/10.1016/j.biotechadv.2019.107462
- Hartmann, A., Fischer, D., Kinzel, L., Chowdhury, S. P., Hofmann, A., Baldani, J. I., & Rothballer, M. (2019). Assessment of the structural and functional diversities of plant microbiota: Achievements and challenges - A review. Journal of Advanced Research, 19, 3–13. https://doi.org/https://doi.org/10.1016/j.jare.2019.04.007
- Hassan, A., & Ullah, H. (2019). Antibacterial and antifungal activities of the medicinal plant Veronica biloba. Journal of Chemistry, 5264943. https://doi.org/https://doi.org/10.1155/2019/5264943
- He, Y., Wang, X., Ma, B., & Xu, J. (2019). Ramanome technology platform for label-free screening and sorting of microbial cell factories at single-cell resolution. Biotechnology Advances, 37(6), 107388. https://doi.org/https://doi.org/10.1016/j.biotechadv.2019.04.010
- Hiruma, K., Kobae, Y., & Toju, H. (2018). Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: Evolutionary origins and host–symbiont molecular mechanisms. Current Opinion in Plant Biology, 44, 145–154. https://doi.org/https://doi.org/10.1016/j.pbi.2018.04.009
- Hosseinzadeh, S., Jafarikukhdan, A., Hosseini, A., & Armand, R. (2015). The application of medicinal plants in traditional and modern medicine: A review of thymus vulgaris. International Journal of Clinical Medicine, 6(9), 635. https://doi.org/https://doi.org/10.4236/ijcm.2015.69084
- Jia, M., Chen, L., Xin, H.-L., Zheng, C.-J., Rahman, K., Han, T., & Qin, L.-P. (2016). A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Frontiers in Microbiology, 7, 906. https://doi.org/https://doi.org/10.3389/fmicb.2016.00906
- Kaur, J., Kaur, R., & Nagpal, A. (2020). Traditional use of ethnomedicinal plants among people of Kapurthala District, Punjab, India. Pharmacognosy Magazine, 16(68), 69–80. https://doi.org/https://doi.org/10.4103/pm.pm_311_19
- Ke, J., & Yoshikuni, Y. (2020). Multi-chassis engineering for heterologous production of microbial natural products. Current Opinion in Biotechnology, 62, 88–97. https://doi.org/https://doi.org/10.1016/j.copbio.2019.09.005
- Khalil, A. M. A., Hassan, S. E.-D., Alsharif, S. M., Eid, A. M., Ewais, E. E.-D., Azab, E., Gobouri, A. A., Elkelish, A., & Fouda, A. (2021). Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules, 11(2), 140. https://doi.org/https://doi.org/10.3390/biom11020140
- Khan, A. L., Kang, S.-M., Dhakal, K. H., Hussain, J., Adnan, M., Kim, J.-G., & Lee, I.-J. (2013). Flavonoids and amino acid regulation in Capsicum annuum L. by endophytic fungi under different heat stress regimes. Scientia Horticulturae, 155, 1–7. https://doi.org/https://doi.org/10.1016/j.scienta.2013.02.028
- Konyalı, S. (2017). Sunflower production and agricultural policies in Turkey. Social Sciences Research Journal, 6(4), 11–19. https://dergipark.org.tr/en/pub/ssrj/issue/32264/343313
- Krishnaiah, D., Sarbatly, R., & Nithyanandam, R. (2011). A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing, 89(3), 217–233. https://doi.org/https://doi.org/10.1016/j.fbp.2010.04.008
- Lata, R. K., Divjot, K., & Nath, Y. A. (2019). Endophytic microbiomes: Biodiversity, ecological significance and biotechnological applications. Research Journal of Biotechnology 14, 10.
- Li, S.-J., Zhang, X., Wang, X.-H., & Zhao, C.-Q. (2018). Novel natural compounds from endophytic fungi with anticancer activity. European Journal of Medicinal Chemistry, 156, 316–343. https://doi.org/https://doi.org/10.1016/j.ejmech.2018.07.015
- Lou, J., Fu, L., Peng, Y., & Zhou, L. (2013). Metabolites from Alternaria fungi and their bioactivities. Molecules, 18(5), 5891–5935. https://doi.org/https://doi.org/10.3390/molecules18055891
- Lou, J., Yu, R., Wang, X., Mao, Z., Fu, L., Liu, Y., & Zhou, L. (2016). Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivities. Brazilian Journal of Microbiology, 47(1), 96–101. https://doi.org/https://doi.org/10.1016/j.bjm.2015.11.004
- Lu, S., Qiu, Y., Ni, D., He, X., Pu, J., & Zhang, J. (2020). Emergence of allosteric drug-resistance mutations: New challenges for allosteric drug discovery. Drug Discovery Today, 25(1), 177–184. https://doi.org/https://doi.org/10.1016/j.drudis.2019.10.006
- Manganyi, M., Regnier, T., Kumar, A., Bezuidenhout, C., & Ateba, C. (2018). Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. South African Journal of Botany, 116, 192–199. https://doi.org/https://doi.org/10.1016/j.sajb.2018.03.016
- Manganyi, M. C., Tchatchouang, C.-D. K., Regnier, T., Bezuidenhout, C. C., & Ateba, C. N. (2019). Bioactive Compound Produced by Endophytic Fungi Isolated From Pelargonium sidoides Against Selected Bacteria of Clinical Importance. Mycobiology, 47(3), 335–339. https://doi.org/https://doi.org/10.1080/12298093.2019.1631121
- Mani, V. M., Gnana Soundari, A. P., Karthiyaini, D., & Preethi, K. (2015). Bioprospecting endophytic fungi and their metabolites from medicinal tree aegle marmelos in Western Ghats, India. Mycobiology, 43(3), 303–310. https://doi.org/https://doi.org/10.5941/MYCO.2015.43.3.303
- Mantzoukas, S., & Eliopoulos, P. A. (2020). Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Applied Sciences, 10(1), 360. https://doi.org/https://doi.org/10.3390/app10010360
- Maqsood, S., Adiamo, O., Ahmad, M., & Mudgil, P. (2020). Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chemistry, 308, 125522. https://doi.org/https://doi.org/10.1016/j.foodchem.2019.125522
- Marquez, L., & Quave, C. L. (2020). Prevalence and therapeutic challenges of fungal drug resistance: Role for plants in drug discovery. Antibiotics, 9(4), 150. https://doi.org/https://doi.org/10.3390/antibiotics9040150
- Martínez-Diz, M. D. P., Andrés-Sodupe, M., Bujanda, R., Díaz-Losada, E., Eichmeier, A., & Gramaje, D. (2019). Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecology, 41, 234–244. https://doi.org/https://doi.org/10.1016/j.funeco.2019.07.003
- Martinez-Klimova, E., Rodríguez-Peña, K., & Sánchez, S. (2017). Endophytes as sources of antibiotics. Biochemical Pharmacology, 134, 1–17. https://doi.org/https://doi.org/10.1016/j.bcp.2016.10.010
- Michel, J., Abd Rani, N. Z., & Husain, K. (2020). A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Frontiers in Pharmacology, 11, 852. https://doi.org/https://doi.org/10.3389/fphar.2020.00852
- Mohotti, S., Rajendran, S., Muhammad, T., Strömstedt, A. A., Adhikari, A., Burman, R., De Silva, E. D., Göransson, U., Hettiarachchi, C. M., & Gunasekera, S. (2020). Screening for bioactive secondary metabolites in Sri Lankan medicinal plants by microfractionation and targeted isolation of antimicrobial flavonoids from Derris scandens. Journal of Ethnopharmacology, 246, 112158. https://doi.org/https://doi.org/10.1016/j.jep.2019.112158
- Morales-Cedeño, L. R., del Carmen Orozco-Mosqueda, M., Loeza-Lara, P. D., Parra-Cota, F. I., de Los Santos-Villalobos, S., & Santoyo, G. (2020). Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiological Research, 242, 126612. https://doi.org/https://doi.org/10.1016/j.micres.2020.126612
- Morales-Sánchez, V., Fe Andrés, M., Díaz, C. E., & González-Coloma, A. (2020). Factors affecting the metabolite productions in endophytes: Biotechnological approaches for production of metabolites. Current Medicinal Chemistry, 27(11), 1855–1873. https://doi.org/https://doi.org/10.2174/0929867326666190626154421
- Müller, C. B., & Krauss, J. (2005). Symbiosis between grasses and asexual fungal endophytes. Current Opinion in Plant Biology, 8(4), 450–456. https://doi.org/https://doi.org/10.1016/j.pbi.2005.05.007
- Naik, B. S. (2019). Developments in taxol production through endophytic fungal biotechnology: A review. Oriental Pharmacy and Experimental Medicine, 19(1), 1–13. https://doi.org/https://doi.org/10.1007/s13596-018-0352-8
- Nalini, M., & Prakash, H. (2017). Diversity and bioprospecting of actinomycete endophytes from the medicinal plants. Letters in Applied Microbiology, 64(4), 261–270. https://doi.org/https://doi.org/10.1111/lam.12718
- Ncube, B., Finnie, J., & Van Staden, J. (2012). Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. South African Journal of Botany, 82, 11–20. https://doi.org/https://doi.org/10.1016/j.sajb.2012.05.009
- Nisa, H., Kamili, A. N., Nawchoo, I. A., Shafi, S., Shameem, N., & Bandh, S. A. (2015). Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microbial Pathogenesis, 82, 50–59. https://doi.org/https://doi.org/10.1016/j.micpath.2015.04.001
- Nivetha, L., & Kharwar, R. (2019). Antimicrobial and antioxidant activities of endophytes from Citrus Limon. Journal of Medicinal Plants, 7(6), 10–13. https://www.plantsjournal.com/archives/2019/vol7issue6/PartA/7-5-15-996.pdf
- Oyenihi, A., & Smith, C. (2019). Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? Journal of Ethnopharmacology, 229, 54–72. https://doi.org/https://doi.org/10.1016/j.jep.2018.09.037
- Palanichamy, P., Krishnamoorthy, G., Kannan, S., & Marudhamuthu, M. (2018). Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egyptian Journal of Basic and Applied Sciences, 5(4), 303–312. https://doi.org/https://doi.org/10.1016/j.ejbas.2018.07.002
- Pandey, G. (2018). Challenges and future prospects of agri-nanotechnology for sustainable agriculture in India. Environmental Technology and Innovation, 11, 299–307. https://doi.org/https://doi.org/10.1016/j.eti.2018.06.012
- Passari, A. K., Mishra, V. K., Singh, G., Singh, P., Kumar, B., Gupta, V. K., Sarma, R. K., Saikia, R., Singh, B. P., & Singh, B. P. (2017). Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Scientific Reports, 7(1), 1–17. https://doi.org/https://doi.org/10.1038/s41598-017-12235-4
- Pelo, S., Mavumengwana, V., & Green, E. (2020). Diversity and antimicrobial activity of culturable fungal endophytes in Solanum mauritianum. International Journal of Environmental Research and Public Health, 17(2), 439. https://doi.org/https://doi.org/10.3390/ijerph17020439
- Petrini, O., & Müller, E. (1979). Endophyte, am Beispiel von Juniperus communis L. Sydowia, 32, 224–251. https://www.zobodat.at/pdf/Sydowia_32_0224-0251.pdf
- Praptiwi, M. R., Wulansari, D., Fathoni, A., & Agusta, A. (2018). Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. Journal of Applied Pharmaceutical Science, 8(8), 069–074. https://doi.org/https://doi.org/10.7324/JAPS.2018.8811
- Sahu, P. K., Singh, S., Gupta, A., Singh, U. B., Brahmaprakash, G., & Saxena, A. K. (2019). Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium rolfsii in tomato. Biological Control, 137, 104014. https://doi.org/https://doi.org/10.1016/j.biocontrol.2019.104014
- Salehi, M., Naghavi, M. R., & Bahmankar, M. (2019). A review of Ferula species: Biochemical characteristics, pharmaceutical and industrial applications, and suggestions for biotechnologists. Industrial Crops and Products, 139, 111511. https://doi.org/https://doi.org/10.1016/j.indcrop.2019.111511
- Sandargo, B., Chepkirui, C., Cheng, T., Chaverra-Muñoz, L., Thongbai, B., Stadler, M., & Hüttel, S. (2019). Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnology Advances, 37(6), 107344. https://doi.org/https://doi.org/10.1016/j.biotechadv.2019.01.011
- Santoyo, G., Moreno-Hagelsieb, G., del Carmen Orozco-Mosqueda, M., & Glick, B. R. (2016). Plant growth-promoting bacterial endophytes. Microbiological Research, 183, 92–99. https://doi.org/https://doi.org/10.1016/j.micres.2015.11.008
- Segaran, G., & Sathiavelu, M. (2019). Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatalysis and Agricultural Biotechnology, 21, 101284. https://doi.org/https://doi.org/10.1016/j.bcab.2019.101284
- Shah, S., Shrestha, R., Maharjan, S., Selosse, M.-A., & Pant, B. (2019). Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants, 8(1), 5. https://doi.org/https://doi.org/10.3390/plants8010005
- Shimoyama, T., Miyoshi, M., Nehira, T., Motojima, A., Oikawa, T., Laurence, O., & Igarashi, Y. (2018). Two new secondary metabolites from a fungus of the genus Robillarda. The Journal of Antibiotics, 71(4), 432–437. https://doi.org/https://doi.org/10.1038/s41429-017-0015-x
- Shrivastava, M., Srivastav, A., Gandhi, S., Rao, S., Roychoudhury, A., Kumar, A., Singhal, R. K., Jha, S. K., & Singh, S. D. (2019). Monitoring of engineered nanoparticles in soil-plant system: A review. Environmental Nanotechnology, Monitoring and Management, 11, 100218. https://doi.org/https://doi.org/10.1016/j.enmm.2019.100218
- song, R.-Y., Wang, X.-B., Yin, G.-P., Liu, R.-H., Kong, L.-Y., & Yang, M.-H. (2017). Isocoumarin derivatives from the endophytic fungus, Pestalotiopsis sp. Fitoterapia, 122, 115–118. https://doi.org/https://doi.org/10.1016/j.fitote.2017.08.012
- Stone, J. K., Bacon, C. W., & White, J. F., Jr. (2000). An overview of endophytic microbes: Endophytism defined. Microbial Endophytes (1st ed., pp. 28). CRC Press. https://doi.org/https://doi.org/10.1201/9781482277302-1
- Sugiyama, A. (2019). The soybean rhizosphere: Metabolites, microbes, and beyond—A review. Journal of Advanced Research, 19, 67–73. https://doi.org/https://doi.org/10.1016/j.jare.2019.03.005
- Tanapichatsakul, C., Monggoot, S., Gentekaki, E., & Pripdeevech, P. (2018). Antibacterial and Antioxidant Metabolites of Diaporthe spp. Isolated from Flowers of Melodorum fruticosum. Current Microbiology, 75(4), 476–483. https://doi.org/https://doi.org/10.1007/s00284-017-1405-9
- Toghueo, R. M. K. (2020). Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology, 11(1), 1–21. https://doi.org/https://doi.org/10.1080/21501203.2019.1645053
- Torimiro, D., Yusuf, O., Subair, S., Amujoyegbe, B., Tselaesele, N., & Ayinde, J. (2014). Utilisation of sunflower crop among smallholder farmers in sub-Saharan Africa: Evidence from Nigeria and Botswana. Journal of Agricultural Extension and Rural Development, 6(9), 298–304. https://doi.org/https://doi.org/10.5897/JAERD2014.0579
- Torres-Mendoza, D., Ortega, H. E., & Cubilla-Rios, L. (2020). Patents on endophytic fungi related to secondary metabolites and biotransformation applications. Journal of Fungi, 6(2), 58. https://doi.org/https://doi.org/10.3390/jof6020058
- Turrini, A., Sbrana, C., & Giovannetti, M. (2015). Belowground environmental effects of transgenic crops: A soil microbial perspective. Research in Microbiology, 166(3), 121–131. https://doi.org/https://doi.org/10.1016/j.resmic.2015.02.006
- Urooj, F., Farhat, H., Ali, S. A., Ahmed, M., Sultana, V., Shams, Z. I., Ara, J., & Ehteshamul-Haque, S. (2018). Role of endophytic Penicillium species in suppressing the root rotting fungi of sunflower. Pakistan Journal of Botany, 50(4), 1621–1628. https://www.pakbs.org/pjbot/papers/1524267982.pdf
- Vieitez, I., Maceiras, L., Jachmanián, I., & Alborés, S. (2018). Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. The Journal of Supercritical Fluids, 133, 58–64. https://doi.org/https://doi.org/10.1016/j.supflu.2017.09.025
- Wilson, D. (1993). Fungal endophytes: Out of sight but should not be out of mind. Oikos, 68(2), 379–384. https://doi.org/https://doi.org/10.2307/3544856
- Wilson, D. (1995). Endophyte: The evolution of a term, and clarification of its use and definition. Oikos, 73(2), 274–276. https://doi.org/https://doi.org/10.2307/3545919
- Wolde-meskel, E., van Heerwaarden, J., Abdulkadir, B., Kassa, S., Aliyi, I., Degefu, T., Wakweya, K., Kanampiu, F., & Giller, K. E. (2018). Additive yield response of chickpea (Cicer arietinum L.) to Rhizobium inoculation and phosphorus fertilizer across smallholder farms in Ethiopia. Agriculture, Ecosystems & Environment, 261, 144–152. https://doi.org/https://doi.org/10.1016/j.agee.2018.01.035
- Wolfe, E. R., & Ballhorn, D. J. (2020). Do foliar endophytes matter in litter decomposition? Microorganisms, 8(3), 446. https://doi.org/https://doi.org/10.3390/microorganisms8030446
- Xu, T., Cao, L., Zeng, J., Franco, C. M., Yang, Y., Hu, X., Liu, Y., Wang, X., Gao, Y., Bu, Z., Shi, L., Zhou, G., Zhou, Q., Liu, X., & Zhu, Y. (2019). The antifungal action mode of the rice endophyte Streptomyces hygroscopicus OsiSh-2 as a potential biocontrol agent against the rice blast pathogen. Pesticide Biochemistry and Physiology, 160, 58–69. https://doi.org/https://doi.org/10.1016/j.pestbp.2019.06.015
- Yang, B., Huang, J., Zhou, X., Lin, X., Liu, J., Liao, S., Wang, J., Liu, F. A., Tao, H., & Liu, Y. (2018). The fungal metabolites with potential antiplasmodial activity. Current Medicinal Chemistry, 25(31), 3796–3825. https://doi.org/https://doi.org/10.2174/0929867325666180313105406
- Yang, H., Ye, W., Ma, J., Zeng, D., Rong, Z., Xu, M., Wang, Y., & Zheng, X. (2018). Endophytic fungal communities associated with field-grown soybean roots and seeds in the Huang-Huai region of China. PeerJ, 6, e4713. https://doi.org/https://doi.org/10.7717/peerj.4713
- Yin, X., Lu, J., Wang, Y., Liu, G., Hua, Y., Wan, X., Zhao, J., & Zhu, D. (2020). The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere, 240, 124903. https://doi.org/https://doi.org/10.1016/j.chemosphere.2019.124903
- Yuan, Z.-S., Liu, F., Liu, Z.-Y., Huang, Q.-L., Zhang, G.-F., & Pan, H. (2021). Structural variability and differentiation of niches in the rhizosphere and endosphere bacterial microbiome of moso bamboo (Phyllostachys edulis). Scientific Reports, 11(1), 1574. https://doi.org/https://doi.org/10.1038/s41598-021-80971-9