Abstract
The major products of the trichothecene mycotoxin biosynthetic pathway produced in a species– and sometimes isolate–specific manner by cereal–pathogenic Fusarium fungi include T–2 toxin, diacetoxyscirpenol, deoxynivalenol and nivalenol. This paper briefly reviews the major effects of such trichothecenes on the gross morphology, cytology and molecular signalling within eukaryotic cells. The gross toxic effects of select trichothecenes on animals include growth retardation, reduced ovarian function and reproductive disorders, immuno–compromization, feed refusal and vomiting. The phytotoxic effects of deoxynivalenol on plants can be summarized as growth retardation, inhibition of seedling and green plant regeneration. Trichothecenes are now recognized as having multiple inhibitory effects on eukaryote cells, including inhibition of protein, DNA and RNA synthesis, inhibition of mitochondrial function, effects on cell division and membrane effects. In animal cells, they induce apoptosis, a programmed cell death response. Current knowledge about the eukaryotic signal transduction cascades and downstream gene products activated by trichothecenes is limited, especially in plants. In mammalian cells, certain trichothecenes trigger a ribotoxic stress response and activate mitogen–activated protein kinases. DON mediates the inflammatory response by modulating the binding activities of specific transcription factors and subsequently inducing cytokine gene expression. Several genes are up–regulated in wheat in response to trichothecene mycotoxins; the significance, if any, of these genes in the host response to trichothecenes has yet to be elucidated.
Introduction
Trichothecenes are secondary fungal metabolites that are harmful to human and animal health causing a range of acute and chronic symptoms (D’Mello and Macdonald Citation1997, D’Mello et al. Citation1999). This class of mycotoxins comprises a very large family of related chemicals produced by a number of taxonomically unrelated fungal genera, including Fusarium, Mycothecium, Trichoderma, Trichothecium, Stachybotrys, Verticimonosporium, and Cephalosporium (Ueno Citation1985). The first trichothecene type compound discovered was the antifungal trichothecin isolated from a culture of Tricothecium roseum (Ueno Citation1985). Fusarium species are probably the most commonly cited and amongst the most prolific of the trichothecene-producing fungi. Many trichothecene-producing Fusarium species are common causal organisms of Fusarium head blight (FHB, also known as ‘scab’ or head blight), foot rot and root rot disease of cereals (including F. culmorum, F. poae and F. graminearum). Consequently, infection and colonization of cereal heads by such fungi not only affects yield, but can also result in trichothecene contamination of grain.
Many of the known trichothecenes are toxic to cells and the gross effects of trichothecenes on eukaryotic organisms are a manifestation of their effect at the cellular level. The cellular effects of these toxins on eukaryotic cells are not yet fully understood, especially in plants. In this chapter, we will introduce the trichothecenes with respect to their diversity, chemistry and relative importance. We will briefly review the gross toxic effects of trichothecenes on eukaryotic cells, and thereafter concentrate our review on the known cytological and molecular impacts of such mycotoxins on eukaryotic cells.
Trichothecenes
Over 150 trichothecenes and trichothecene derivatives have been isolated and characterized (Gutleb et al. Citation2002). They are all non-volatile, low-molecular-weight sesquiterpene epoxides (Wannemacher et al. Citation2000). They share a tricyclic nucleus named trichothecene and usually contain an epoxide at C-12 and C-13, which are essential for toxicity (Desjardins et al. Citation1993). Most trichothecenes also have a C-9,10 double bond, which is important for their toxicity (Ehrlich and Daigle Citation1987). Their chemical structure varies in both the position and the number of hydroxylations, as well as in the position, number and complexity of esterifications (Desjardins et al. Citation1993).
The trichothecene skeleton is chemically stable and the 12,13-epoxide ring is stable to nucleophilic attack. Furthermore, trichothecenes are heat stable and are not degraded during normal food processing (Eriksen Citation2003) or autoclaving (Wannemacher et al. Citation2000). Trichothecenes are also stable at neutral and acidic pH (Ueno Citation1987) and consequently, they are not hydrolysed in the stomach after ingestion (Eriksen Citation2003).
Trichothecenes are divided into four groups (types A–D) according to both their chemical properties and their producer fungi (Ueno et al. Citation1973). Only a few of the known trichothecenes (all type A or B) seem to be of importance with respect to their presence in crops; for the majority of trichothecenes, there is little evidence of their occurrence under field conditions. Fusaria produce type A and B trichothecenes. Types A and B are distinguished by the presence of an oxygen or carbonyl functional group at the C-8 position, respectively. The major products of the trichothecene biosynthetic pathway are known as T-2 toxin and diacetoxyscirpenol (DAS) (type A) and deoxynivalenol (DON) and nivalenol (NIV) (type B) (Ueno Citation1985). Fusarium species differ in their trichothecene profiles; DON is one of the primary trichothecene metabolites found in wheat and is commonly produced by several phytopathogenic Fusarium species including F. graminearum and F. culmorum. Producers of type B trichothecenes can be grouped into two chemotypes based on whether they produce DON (or acetylated derivatives) or NIV (Lee et al. Citation2001, Chandler et al. Citation2003). Mycothecium verrucaria and other genera of fungi produce type C and D trichothecenes. The type C are characterized by a second epoxide function at C-7,8 or C-9,10 position of the pentane ring common to all trichothecenes, and include crotocin produced by Trichotheceum roseum and Cephalosporium crotocinigerum (Ueno Citation1985). Type D trichothecenes are those containing a macrocyclic ring between C-4 and C-5 position the pentane ring with two ester linkages, and include verrucarin produced by Myrothecium verrucaria, satratoxin produced by Stachybortys atra and roridin produced by Myrothecium roridum and Cylindrocarpon (Ueno Citation1985).
Gross toxic effects of trichothecenes on animals
The gross toxic effects of trichothecenes on animals have been extensively reviewed (Beasley Citation1989, Rotter et al. Citation1996). Trichothecenes are toxic to all tested animal species (SCF Citation2002), T-2 toxin generally being the most toxic. However, the sensitivity towards different trichothecenes varies considerably amongst organisms (as reviewed by SCF Citation2002).
The gross toxic effects of select trichothecenes on animals include growth retardation, reduced ovarian function and reproductive disorders, immuno-compromization, feed refusal and vomiting. These toxic effects are mediated via several mechanisms (SCF Citation2002). Trichothecenes such as T-2 toxin, HT-2 toxin, DON and NIV retard growth, albeit in a non-specific manner, and as a consequence of earlier toxic effects such as inhibition of protein synthesis and cell proliferation rather than as a direct mycotoxic effect. T-2 reduced body weight gain in pigs (Weaver et al. Citation1978) and DON and NIV caused growth retardation in mice (Ryu et al. Citation1988, Ohtsubo et al. Citation1989). Reproductive defects in mice caused by trichothecenes include embryo or foetal toxicity caused by T-2 toxin (Rousseaux et al. Citation1986), increased postnatal mortality caused by DON (Khera et al. Citation1984) and intrauterine growth retardation caused by NIV (Ito et al. 1988). DON increased the susceptibility of mice to infections (Tryphonas et al. Citation1986). DAS exposure caused suppression in chicken macrophage phagocitic function (Qureshi et al. Citation1998). Lymphocytes are more sensitive to T-2 toxin than other cell types such as kidney cells, and this corresponds well with the data from in vivo experiments that showed that trichothecenes act as immunosuppressive agents (Holladay et al. Citation1993). Lymphoid cells and fibroblasts were the most sensitive cell types to DON (Reubel et al. Citation1987, Charoenpornsook et al. Citation1998).
A special feature of DON toxicity is the characteristic induction of vomiting (DON is also called vomitoxin) and feed refusal, as seen in pigs, or delayed gastric emptying and feed refusal, as observed in rats and mice (SCF Citation1999). The emetic effect is thought to be mediated through affection of the serotonergic activity in the central nervous system or via peripheral actions on serotinin receptors (SCF Citation1999).
Gross toxic effects of trichothecenes on plants
Shimada and Otani (Citation1990) reported that NIV was 10–24-fold less toxic than DON to seedlings of seven Japanese wheat cultivars. Eudes et al. (Citation2000) demonstrated the phytotoxicity of the six trichothecenes DON, 3-acetyldesoxynivalenol (3-ADON), NIV, DAS, T-2 toxin and HT2 in four wheat cultivars Norseman, Katepwa, Toropi and Nyu-Bay. They found that DON and 3-ADON were more toxic than T-2 toxin, HT-2 and DAS, and that those toxins inhibited wheat coleoptile elongation at very low concentrations. Wakulinski (Citation1989) used seedlings of three varieties of winter wheat, Grana, Emika, and SMH-68-4 to assess the phytotoxic effects of six Fusarium metabolites. He showed that T-2 toxin was less phytotoxic than DON, but more toxic than DAS. He also found DON and 3-ADON to be the most potent inhibitors of germination, and of root and leaf mass increases. shows the effect of DON on germinating seeds of wheat.
Figure 1. Inhibitory effect of (A) 20 ppm DON as compared with (B) water on wheat cv. Avalon seedling growth (48 h post-treatment).
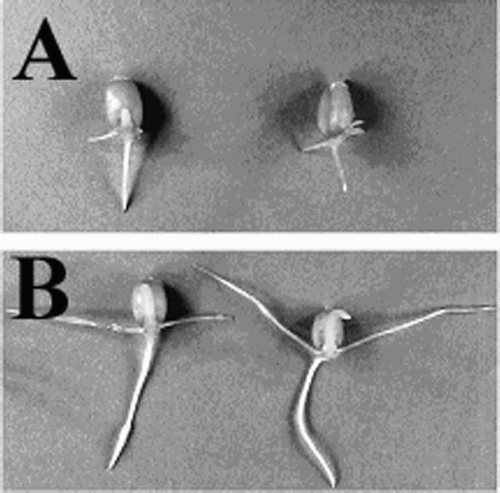
Trichothecenes are phytotoxic, and at very low level cause wilting, chlorosis, necrosis, and other symptoms in a wide variety of plants (Cutler Citation1988). The phytotoxic effects of DON on plants can be summarized as growth retardation, inhibition of seedling and green plant regeneration (Bruins et al. Citation1993, McLean Citation1996). Bruins et al. showed that DON inhibited growth of wheat seedlings, coleoptile segments, anther-derived callus, anther-derived embryos and green plant regeneration. DON also inhibited wheat root growth during germination (Shimada and Otani Citation1990). Wang and Miller (Citation1998) observed that DON strongly inhibited coleoptile growth of wheat cultivars with different levels of resistance to FHB, however they found cultivars resistant to FHB were more tolerant to DON and 3-ADON than those that were susceptible.
Little is known about the combined (additive or antagonistic) effects of trichothecenes on plant or indeed animal tissue. A few studies reported dose additivity and antagonism among T-2 toxin, DON and NIV in vitro (yeast, lymphocyte and Vero cells); in in vivo animal studies antagonism was only observed between DON and T-2 toxin (for a synopsis of this research, see SCF Citation2002).
Cellular effects of trichothecenes on eukaryotes
Trichothecenes are now recognized as having multiple inhibitory effects on eukaryote cells. lists some of the effects of trichothecene mycotoxins on animal and plant cell functions. These include inhibition of protein, DNA and RNA synthesis, inhibition of mitochondrial function, effects on cell division and membrane effects (McLean Citation1996, Miller and Ewen Citation1997). In animal cells, they induce apoptosis, a programmed cell death (PCD) response (Ihara et al. Citation1998, Shifrin and Anderson Citation1999, Yang, et al. Citation2000, Minervini et al. Citation2004).
Table I. Examples of the cellular effects of trichothecenes on animals and plants.
A number of cytotoxicity assays have been developed or adapted to study trichothecene toxicity at the cellular level. A neutral red (NR) (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride) cell-viability assay has been successfully used to determine the cytotoxic effect of trichothecenes (Babich and Borenfreund Citation1991, Shokri et al. Citation2000). This assay is based on the incorporation of neutral red, a supravital dye, into the lysosomes of viable cells. Compounds that injure the plasma or lysosomal membrane show decreased uptake and retention of the dye. Analysis of the incorporation of 3H-thymidine into DNA is a very sensitive method to study trichothecene-induced inhibition of DNA synthesis in cultured cells (Porcher et al. Citation1987, Charoenpornsook et al. Citation1998). However, this is a time consuming technique and unfortunately carries the usual risks associated with a radioactive method. 5-Bromo-2′-deoxyuridine (BrdU) has been used as a rapid and non-radioactive immunocytochemical assay to determine DNA synthesis in proliferating cells (Dolbeare Citation1995). Deoxynucleotidyl transferase (TdT)-mediated fluorescein-dUTP nick end-labelling (TUNEL) method highlights DNA cleavage (Gavrieli et al. Citation1992) and has been used to study the effect of trichothecenes on DNA fragmentation (Miura et al. Citation1998, Rachid et al. Citation2000). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is a yellow salt that is reduced to dark purple formazan crystals by mitochondrial enzymes in living cells (Slater et al. Citation1963). MTT has been used in several studies to assess the cytotoxicity of mycotoxins (Reubel et al. Citation1987, Holt et al. Citation1988, Visconti et al. Citation1991). Trichothecene induced alteration of the plasma membrane can be determined by quantifying enzymes released in the cell culture medium by damaged cells. Lactate dehydrogenase (LDH) is a ubiquitous cytoplasmic enzyme and the amount released into the cell culture medium or present in intact cells has been used to evaluate mycotoxin-mediated plasma membrane damage (Bunner and Morris, Citation1988, Charoenpornsook et al. Citation1998).
Inhibition of protein synthesis
Evidence suggests that the primary toxic effects of trichothecenes may be facilitated by their action as potent inhibitors of protein synthesis in most eukaryotes (McLaughlin et al. Citation1977); the biochemical basis of trichothecene toxicity is non-competitive inhibition of protein synthesis (Cole and Cox Citation1981) (). They interfere with the active site of peptidyl transferase on ribosomes, and inhibit the initiation, elongation or termination step of protein synthesis. From the data on polysomal breakdown, the trichothecenes were classified into two types: type I inhibited the initiation step of protein synthesis (T type) and type II inhibited the elongation-termination step (ET type) (McLaughlin et al. Citation1977). Type I includes T-2 toxin, NIV, verrucarin A and fusarenon-X, and the ET type includes DON, crotocin, verrucarol and fusarenon-X (Ueno Citation1985). One target organelle of trichothecenes has been identified as the 60S subunit of mammalian ribosomes, and a good correlation was observed between the inhibition of protein synthesis by trichothecenes and the affinity of trichodermin to ribosomes (Wei et al. Citation1974). Trichothecene-producing fungi have an altered ribosomal protein L3 (Rpl3) that protects the fungi from the primary effect of trichothecenes on protein synthesis, and a semidominant mutation in the Saccharomyces cerevisiae TCM1 gene (that encodes the Rpl3) conferred resistance to trichodermin (Fried and Warner Citation1981). An altered Rpl3 protein expressed in wheat and maize increased resistance to FHB and ear rot, respectively; Harris and Gleddie (Citation2001) found that the Rpl3 gene conferred reduced sensitivity to DON in cells, tissues, and protoplasts of transgenic tobacco.
Trichothecenes differ in their toxicity towards eukaryotic cells due to chemical features of the side chains (Ueno Citation1985). Thompson and Wannemacher (Citation1986) tested nineteen 12,13-epoxytrichothecene mycotoxins for their relative capabilities to inhibit protein synthesis in Vero cells and rat spleen lymphocytes. They showed a clear chemical structure dependence of the capacity of trichothecenes and trichothecenes derivatives to inhibit protein synthesis. Their study proved that the most potent protein synthesis inhibitors were T-2, verrucarin A and roridin A; HT-2, neosolaniol or DAS showed reduced protein synthesis inhibition; T-2 triol, T-2 tetraol, 15-monoacetyl DAS, scirpentriol, fusarenon X and DON further weakened their effect; Verrucarol and deoxyverrucarol, reduced their effectiveness by over a thousand-fold compared with the most potent mycotoxins. Addition of side groups resulted in reduced effectiveness only when an acetyl group was added to the carbon 3 position of T-2 (acetyl T-2) and DON (3-acetyl DON) or on substitution of an epoxide across the 9,10 carbons of DAS. When the in vitro effects of the mycotoxins were compared with results from whole animal lethality tests, several of the trichothecenes were weak inhibitors of protein synthesis in vitro but had in vivo toxicities similar to that of T-2 toxin. Thus, the in vitro cell response of a given trichothecene is not always an accurate predictor of toxicity in whole animals. In vivo, inhibition of protein synthesis has been demonstrated in cells from bone marrow, spleen and thymus (Rosenstein and Lafarge-Frayssinet Citation1983, Thompson and Wannemacher Citation1990). In plants, low levels of DON inhibited protein synthesis in wheat (Miller and Arnison Citation1986). Bandurska et al. (Citation1994) shown that the growth of Fusarium culmorum-susceptible wheat seedlings (winter, Alba, Almari, Begra, Kamila, Liwilla and Parada) was inhibited by DON. In this study DON caused an increase in the free proline levels in seedling leaves: this increase was dependent on cultivar and DON concentration, and the authors suggested that this increase in free proline levels may be attributed to decreased incorporation of the amino acid into protein. However, they added that increases in proline might reflect a plant defence mechanism in response to pathogen invasion or pathogen virulence factors.
Inhibition of RNA and DNA synthesis
In mammalian cells, trichothecenes inhibit both RNA and DNA synthesis (Ueno Citation1985) (). However, in vitro experiments revealed no direct effects of fusarenon-X on either DNA-dependent RNA polymerases of rat liver nuclei or on nuclear ribonuclease H of rat liver and the protozoa Tetrahymena pyriformis (Tashiro et al. Citation1979). Substantial inhibition of ribonucleic acid (RNA) synthesis (86% inhibition) by trichothecenes mycotoxins was observed in human (HeLa) cells, while T-2 toxin showed minor effects (15% inhibition) on RNA synthesis in Vero cells (Thompson and Wannemacher Citation1986).
DNA synthesis is strongly inhibited in various types of cells that are exposed to trichothecenes (McLauglin et al. 1977). In mice or rat treated with trichothecene mycotoxins, DNA synthesis was suppressed in all tissues studied, although to a lesser degree than protein synthesis (Thompson and Wannemacher Citation1990). The pattern by which DNA synthesis is inhibited by trichothecenes is consistent with the primary effect of these toxins on protein synthesis. In appropriate cell models, for the most part trichothecenes demonstrated neither mutagenic activity nor the capacity to damage DNA (Busby and Wogan Citation1981). Thus, the inhibition of nucleic acid synthesis is presumed to be a secondary effect of trichothecenes (Ueno Citation1985).
Inhibition of mitosis
Inhibition of DNA synthesis, cell growth and mitosis are consequences of the interference of trichothecenes in the cell growth cycle (). NIV and fusarenon-X affected HeLa cells in every phase of the growth cycle (Ohtsubo et al. Citation1968). NIV induced a G1 block before the beginning of the S phase of mitosis, and a G2 block just before mitosis (Ohtsubo et al. Citation1968).
Packa (Citation1991) studied the effect of DON and DAS on actively dividing cells of rye, wheat, triticale and field bean. In all plants, treatment with DON decreased the mitotic index (MI) of germinated roots; mitosis was strongly inhibited in wheat and field bean. Germinating caryopses of rye and field beans, and to a lesser extent, triticale and wheat, were sensitive to DAS, resulting in a decrease in the MI. A decline in the MI and relative division rate was also observed in dividing root tip cells of onion treated with DON or T-2 toxin (Rahman et al. Citation1993). Chromosome and nuclear abnormalities caused by DON and DAS included spiralization of metaphase chromosomes, retarded equatorial chromosome arrangement, asymmetric arrangement of daughter chromosomes and numerous ana- and telophase bridges (Packa Citation1991). Such cytogenetic changes reflect the disturbed functioning of the karyokinetic spindle which leads to the formation of cells containing various number of chromosomes; part of the genetic material may be eliminated in the form of micronuclei or chromosomes may be duplicated. These cytological effects may be caused by a reduction in the synthesis of proteins involved in microtubule formation (Packa Citation1991).
Effect on cell membrane and organelles
Since trichothecene mycotoxins are amphophilic molecules, they may exert their cytotoxicity by acting on the cell membranes (MacLean 1996) (). T-2 toxin alters various myoblast cell membrane functions, such as calcium efflux, rubidium uptake, or residual cellular lactate dehydrogenase activity (Bunner and Morris Citation1988).
Lipid peroxidation was increased in the liver, spleen, kidney, thymus and bone marrow of rats treated with a single oral dose of T-2 toxin (Suneja et al. Citation1989). These observations led to the suggestion that trichothecenes might induce some alterations in membrane structure, which consequently stimulates lipid peroxidation. Tsuchida et al. (Citation1984) suggested that in animal cells (rat liver cells) T-2 toxin may be taken up by the cell membrane as an analogue of cholesterol. Toxin-stimulated alteration in mitochondria membranes contributes to the effects on cellular energetics and cellular cytotoxicity.
Once trichothecenes cross the plasma membrane barrier, they enter the cell, where they can interact with a number of targets, including ribosome (McLaughlin et al. Citation1977) and mitochondria (Pace et al. Citation1988). Trichothecenes inhibit mitocondrial function in vivo and in vitro. In a bovine kidney cell line, the mitocondrial activity was reduced after treatment with T-2 toxin (Holt et al. Citation1988). In vitro, low concentrations of T-2 toxin resulted in a 50% inhibition of mitocondrial protein synthesis in rat liver cells (Pace et al. Citation1988). T-2 toxin also inhibit electron transport activity in rat liver cells (Pace et al. Citation1983) as suggested by the in vitro inhibition of succinic dehydrogenase activity and mitochondrial protein synthesis in rat hepatocytes (Trusal and O’Brien Citation1986). T-2 toxin also affected mitocondrial electron transport system in yeast by inhibiting succinic dehydrogenease (Khachatourians Citation1990). Yabe et al. (Citation1993) found that NIV increased cytochrome P-450 and glutathione S-transferase (GST) activities in rats. In mice and monkey, the hepatic cytochrome P-450 is responsible for catalysing the hydroxylation of the C-3 and C-4 positions on the isovaleryl side chain of the T-2 and HT-2 toxins (Yoshizawa et al. Citation1984). When oxygen is removed from the epoxide group of a trichothecene to produced the carbon–carbon bond, non-toxic de-epoxy metabolites are formed (Swanson et al. Citation1988). This indicates that trichothecene epoxide reduction is a single-step detoxification reaction. Cytochrome P-450 and GST of human liver cells were also found to be involved in the metabolism of other mycotoxins (e.g. aflatoxin B1) (Guengerich et al. Citation1996).
In corn, DON inhibited mitocondrial enzymes and caused electrolyte loss (Cosette and Miller 1995). Kang and Buchenauer (Citation2002) developed an immunogold-labelling method to study the subcellular localization of DON and 3-ADON in F. culmorum-infected wheat spikes and kernels. During penetration, toxins were found in the host cell wall around the infection peg and in the cytoplasm of the host cells. In host cells, these trichothecenes were localized in the cytoplasm, plasmalemma, and chloroplasts and sometimes associated with ribosomes and endoplasmatic reticula. Their association with the plasmalemma might result in the alteration of membrane permeability.
Induction of apoptosis
At the cellular level, certain trichothecenes have recently been shown to induce apoptosis in a variety of cell types via mitochondrial and non-mitochondrial mechanisms (Shifrin and Anderson Citation1999, Yang et al. Citation2000) (). Shifrin and Anderson (Citation1999) classified trichothecenes according to their ability to induce apoptosis in human Jurkat T-lymphoid (Jurkat T) cells. Strong inducers included DON, scirpentriol, and T-2 triol; intermediate inducers included NIV, DAS, HT-2 toxin; and weak inducers included 3-acetyl DON, verrucarin and T-2 toxin. The authors suggested that the ability of individual trichothecenes to induce rapid apoptosis might require both translational arrest and mitogen-activated protein kinase activity (as will be discussed later). Among the many biochemical changes commonly found in cells undergoing apoptosis, is the systematic degradation and fragmentation of DNA (Bortner et al. Citation1995). Thus, the induction of apoptosis in mice thymocytes by fusarenon-X (Miura et al. Citation1998) and in the lymphoid of the bursa of Fabricus in broiler chicks by T-2 (Rachid et al. Citation2000) was proved by in situ labelling of DNA fragmentation (TUNEL). They also used electrophoresis of DNA in agarose gel to show that DNA cleavage due to fusarenon-X or T-2 toxin treatment resulted in fragments that were multimers of about 180 base pairs (bp), a DNA profile typical of apoptotic cells (Wyllie et al. Citation1984). Trichothecene-mediated apoptosis was also shown by Yang et al. (Citation2000) using DNA fragmentation in two myeloid models, RAW 264.7 murine macrophage and U937 human leukaemic cells treated with satratoxin G, roridin A, verrucarin A, T-2 toxin, satratoxin F, H, NIV and DON.
Recently, we have found that DON negatively affects PCD morphology in Arabidiopsis thaliana cv. Landsberg erecta suspension cultures, as indicated by a reduction of the level of cells showing membrane blebbing and cytoplasmic shrinkage (Rocha et al., unpublished data).
Functional genomics of eukaryote–trichothecene interactions: Current knowledge
Current knowledge about the eukaryotic signal transduction cascades and downstream gene products activated by trichothecenes is limited, especially in plants. As stated earlier, plants may differ from animals in their trichothecene uptake or metabolism. Analysis of the functional genomics of animal and plant–mycotoxin interactions will help identify genes involved in proliferation, apoptosis (or PCD in plants) and/or plant disease resistance.
Activation of mammalian MAPKs and induction of cytokine gene expression
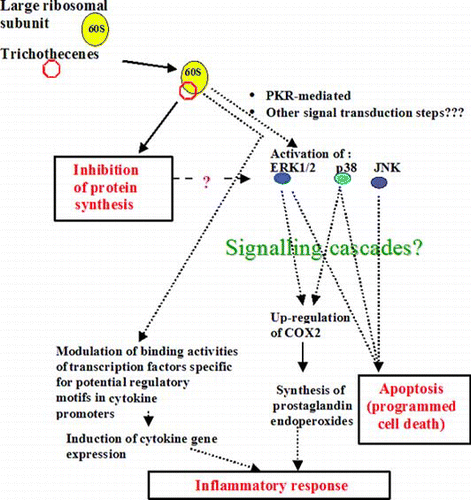
gives a basic outline of much of what is known to date regarding how trichothecenes affect signal transduction and downstream processes in mammalian cells. As mentioned above, trichothecenes inhibit protein synthesis by binding to the ribosomal peptidyltransferase. Inhibitors of the peptidyltransferase reaction can trigger a ribotoxic stress response that activates mitogen-activated protein kinase (MAPK) components of a signalling cascade that regulate cell survival in response to stress (Iordanov et al. Citation1997). In mammalian cells, certain trichothecenes triggered a ribotoxic stress response and activated MAPKs (Shifrin and Anderson Citation1999, Zhou et al. Citation2003b). In murine cells this induction was mediated in an unknown fashion by a dsRNA-activated protein kinase R (PKR) (Zhou et al. Citation2003b). Shifrin and Anderson (Citation1999) showed that translational arrest and MAPK activation cooperated in the induction of apoptosis by trichothecenes, but postulated that different parts of the trichothecene molecule are responsible for MAPK activation and translational arrest: trichothecene derivatives that did not significantly affect protein synthesis activated MAPKs. A conformational change in the ribosome can result in MAPK activation (Iordanov et al. Citation1997) and therefore MAPK activation by such trichothecene derivatives may be due to their interaction with unique ribosome target sites or their displacement of the binding of ribosome-associated molecules. Moon and Pestka (Citation2002) showed that, as a consequence of MAPK activation, DON induced expression and increased the transcript stability of cyclooxygenase-2 (COX-2) mRNA and hence COX-2 protein content in leukocytes, thus increasing the inducible synthesis of prostaglandin endoperoxides, critical components of the inflammatory response. Further evidence for DON mediation of the inflammatory response was provided by Zhou et al. (Citation2003a) who showed that this toxin modulated the binding activities of specific transcription factors and subsequently induced cytokine gene expression.
Figure 2. Trichothecene-mediated signal transduction and downstream processes in mammalian cells. Protein synthesis inhibition: trichothecenes interfere with the active site of peptidyl transferase on ribosomes. Mitogen-activated protein kinase (MAPK) activation: certain trichothecenes induce ERK1/2, JNK and p38 MAPKs (Laskin et al. Citation2002). A double-stranded RNA-activated protein kinase R (PKR) mediates DON-induced phosphorylation of ERK1/2, p38 and JNK MAPKs (Zhou et al. Citation2003b). DON activation of the proinflammatory response: it induces cytokine transcription and, as a consequence of MAPK activation, induces cyclooxygenase-2 (COX-2) and increases synthesis of prostaglandin endoperoxides. DON-induced apoptosis (Shifrin and Anderson Citation1999, Yang et al. Citation2000, Poapolathep et al. Citation2002): increases linearly with inhibition of protein synthesis and the peptidyl transferase site is postulated to be a regulator of both MAPK activation and apoptosis in Jurkat T cells (Shifrin and Anderson Citation1999).
Induction of wheat EF-1α, adenosine kinase and genes of unknown function
Recently, as part of a differential gene expression study, we have found translation elongation factor 1α (EF-1α), adenosine kinase (ADK), and several genes of unknown function were overexpressed in wheat roots in response to DON treatment (Ansari et al. unpublished data). We are now studying the expression profiles of these genes in plants in response to DON and DON producers. Retardation of growth by DON may not be accompanied by cell senescence since, in various eukaryotes, decreased EF-1α activity correlated with senescence and increased activity with longevity (Cavallius et al. Citation1986, Silar and Picard Citation1994).
Activation of wheat retrotransposon-like transcripts
Retrotransposons are mobile genetic elements found throughout the plant kingdom (Fedoroff Citation2000) and they move to new chromosomal locations via an RNA intermediate (Boeke and Corces Citation1989). In previous research, we found three mRNA transcript fragments, overexpressed in wheat roots treated with DON, with full or partial homology to retrotransposons (Ansari et al, unpublished data). The level of transcript activation was genotype-specific. This and other research (Ivashuta et al. Citation2002) implies that there may be genetic or epigenetic transposon activation in plant cells in response to stress and the degree of activation may be genotype-specific (perhaps due to genotype-specific sites of genomic integration). Even if they are transpositionally inactive, the transcription of redundant RNA could affect the expression of other genes through RNA–RNA interaction or RNA-directed DNA methylation (Grant Citation1999, Wolffe and Matzke Citation1999).
Conclusions
All trichothecenes and trichothecene derivatives isolated to date differ in their toxicity towards eukaryotic cells due to their chemical structure. The gross and cellular toxic effects of trichothecenes have been more extensively studied in animals than in plants. Regarding the relative toxicity of trichothecenes, it may not always be possible to extrapolate from animal to plant systems that may differ in their trichothecene uptake or metabolism. The common effects of trichothecenes on both animal and plant cells include inhibition of protein, DNA and RNA synthesis, inhibition of mitochondrial functions, cell division and membrane effects. In animal cells, trichothecenes induce apoptosis, a programmed cell death (PCD) response via mitochondrial and non-mitochondrial mechanisms. The effects of trichothecenes on plant PCD have not yet been extensively investigated. The amphophilic nature of trichothecenes facilitates their cytotoxic effect on cell membranes (McLean Citation1996). Once trichothecenes cross the plasma membrane barrier, they enter the cell, where they can interact with a number of targets, including ribosome (McLaughlin et al. Citation1977) and mitochondria (Pace et al. Citation1988). Protein synthesis inhibition is the primary toxic effect of trichothecenes. The dependence of all cell metabolic process on protein synthesis suggests that many of the other effects of trichothecenes may be secondary effects of protein synthesis inhibition.
Acknowledgements
The authors thank the European Union Framework Programme 5 Project QLRT-2000-02044, the Irish Department of Agriculture Research Stimulus Fund (National Development Plan), and Enterprise Ireland Basic Research awards (National Development Plan) for associated research funding.
References
References
- Babich , H and Borenfreund , E . 1991 . Cytotoxicity of T-2 toxin and its metabolites determined with the neutral red cell viability assay . Applied and Environmental Microbiology , 57 : 2101 – 2103 .
- Bandurska , H , Chelkowski , J and Wisniewska , H . 1994 . Free proline accumulation in wheat seedlings influenced by Fusarium culmorum infection and the pathogen metabolite deoxynivalenol . Acta Physiologiae Plantarum , 16 : 111 – 116 .
- Beasley VR editor 1989 Trichothecene mycotoxicosis: pathophysiological effects Boca Raton CRC Press Vols I and II
- Boeke , JD and Corces , VG . 1989 . Transcription and reverse transcription of retrotransposons . Annual Review of Microbiology , 43 : 403 – 434 .
- Bortner , CD , Oldenburg , NBE and Cidlowski , JA . 1995 . The role of DNA fragmentation in apoptosis . Trends in Cell Biology , 5 : 21 – 25 .
- Bruins , MBM , Karsai , I , Schepers , J and Snijders , CHA . 1993 . Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for Fusarium head blight resistance . Plant Science , 94 : 195 – 206 .
- Bunner , DL and Morris , ER . 1988 . Alteration of multiple cell membrane functions in L-6 myoblasts by T-2 toxin: an important mechanism of action . Toxicology and Applied Pharmacology , 92 : 113 – 121 .
- Busby WF Jr Wogan GN 1981 Trichothecenes In: Shank RC, editor Mycotoxins and N-nitroso compounds: environmental risks Boca Raton CRC Press pp 29–41
- Carter , C , Cannon , M and Smith , K . 1976 . Inhibition of protein synthesis in reticulocyte lysates by trichodermin . Biochemistry Journal , 154 : 171 – 178 .
- Casale , WL and Hart , LP . 1988 . Inhibition of 3H-leucine incorporation by trichothecene mycotoxins in maize and wheat tissue . Phytopathology , 78 : 1673 – 1677 .
- Cavallius , J , Rattan , SI and Clark , BF . 1986 . Changes in activity and amount of active elongation factor 1 alpha in aging and immortal human fibroblast cultures . Experimental Gerontology , 21 : 149 – 157 .
- Chandler , EA , Simpson , DR , Thomsett , MA and Nicholson , P . 2003 . Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis . Physiological and Molecular Plant Pathology , 62 : 355 – 367 .
- Charoenpornsook , K , Fitzpatrick , JL and Smith , JE . 1998 . The effect of four mycotoxins on the mitogen stimulated proliferation of bovine peripheral blood mononuclear cells in vitro . Mycopathologia , 143 : 105 – 111 .
- Cole RA Cox RH editors 1981 Handbook of toxic fungal metabolites New York Academic Press
- Cossette , F and Miller , JD . 1995 . Phytotoxic effect of deoxynivalenol and Gibberella ear rot resistance of corn . Natural Toxins , 3 : 383 – 388 .
- Cutler H 1988 Trichothecenes and their role in the expression of plant disease Biotechnology of Crop Protection, ACD Symposium Series 379 50 72
- D’Mello , JPF and MacDonald , AMC . 1997 . Mycotoxins . Animal Feed Science and Technology , 69 : 155 – 166 .
- D’Mello , JPF , Placinta , CM and McDonald , AMC . 1999 . Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity . Animal Feed Science and Technology , 80 : 183 – 205 .
- Desjardins , AE , Hohn , TM and McCormick , SP . 1993 . Trichothecenes biosynthesis in Fusarium species: Chemistry, genetics, and significance . Microbiological Reviews , 57 : 595 – 604 .
- Dolbeare , F . 1995 . Bromodeoxyuridine: a diagnostic tool in biology and medicine, Part I: Historical perspectives, histochemical methods and cell kinetics . Histochemical Journal , 27 : 339 – 369 .
- Dong , W , Azcona-Olivera , JI , Brooks , KH , Linz , JE and Pestka , JJ . 1994 . Elevated gene expression and production of interleukins 2, 4, 5, and 6 during exposure to vomitoxin (deoxynivalenol) and cycloheximide in the EL-4 thymoma . Toxicology and Applied Pharmacology , 127 : 282 – 290 .
- Ehrlich , K and Daigle , KW . 1987 . Protein synthesis inhibition by 8-oxo-12,13-epoxytrichothecenes . Biochimica and Biophysica Acta , 923 : 206 – 213 .
- Eriksen GS 2003 Metabolism and toxicity of trichothecenes Doctoral thesis Swedish University of Agricultural Science Uppsala available at:, http://diss-epsilon.slu.se/archive/00000287/01/Thesis.pdf
- Eudes , F , Comeau , A , Rioux , S and Collin , J . 2000 . Phytotoxicité de huit mycotoxines associées à la fusariose de l’épi chez le blé . Canadian Journal of Plant Pathology , 22 : 286 – 292 .
- Fedoroff , N . 2000 . How jumping genes were discovered . Nature Structural Biology , 8 : 300 – 301 .
- Fried , HM and Warner , JR . 1981 . Cloning of yeast gene for trichodermin resistance and ribosomal protein L3 . Proceedings of the National Academy of Sciences, USA , 78 : 238
- Gavrieli , Y , Sherman , Y and Bem-Sasson , B . 1992 . Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation . Journal of Cell Biology , 119 : 493 – 501 .
- Grant , SR . 1999 . Dissecting the mechanisms of post-transcriptional gene silencing: divide and conquer . Cell , 96 : 303 – 306 .
- Guengerich , FP , William , WJ , Ueng , YF , Yamazaki , H and Shimada , T . 1996 . Involvement of cytochrome P450, glutathione S-transferase, and epoxide hydrolase in the metabolism of aflatoxin B1 and relevance to human liver cancer . Environmental Health Perspectives , 104 : 557 – 562 .
- Gutleb , AC , Morrison , E and Murk , AJ . 2002 . Cytotoxicity assays for mycotoxins produced by Fusarium strains: a review . Environmental Toxicology and Pharmacology , 11 : 309 – 320 .
- Harris , LJ and Gleddie , SC . 2001 . A modified Rpl3 gene from rice confers tolerance of the Fusarium graminearum mycotoxin deoxynivalenol to transgenic tobacco . Physiological and Molecular Plant Pathology , 58 : 173 – 181 .
- Holladay , SD , Blaylock , BL , Comment , CE , Heindel , JJ and Luster , MI . 1993 . Fetal thymic atrophy after exposure to T-2 toxin: selectivity for lymphoid progenitor cells . Toxicology and Applied Pharmacology , 121 : 8 – 14 .
- Holt , PS , Buckley , S , Norman , JO and Deloach , JR . 1988 . Cytotoxic effect of T-2 mycotoxin on cells in culture as determined by a rapid colorimetric bioassay . Toxicon , 26 : 549 – 558 .
- Ihara , T , Yamamoto , T , Sugamata , M , Okumura , H and Ueno , Y . 1998 . The process of ultrastructural changes from nuclei to apoptotic body . Virchows Archive , 433 : 443 – 447 .
- Iordanov , MS , Pribnow , D , Magun , JL , Dinh , TH , Pearson , JA and Magun , BE . 1997 . Ribotoxic stress response: activation of the stress activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA . Molecular and Cellular Biology , 17 : 3373 – 3381 .
- Islam , Z and Pestka , JJ . 2003 . Role of IL-1ß in endotoxin potentiation of deoxynivalenol-induced corticosterone response and leukocyte apoptosis in mice . Toxicological Sciences , 74 : 93 – 102 .
- Ito , Y , Ohtsubo , K , Ishii , K and Ueno , Y . 1986 . Effects of nivalenol on pregnancy and fetal development in mice . Mycotoxin Research , 2 : 71 – 77 .
- Ivashuta , S , Naumkina , M , Gau , M , Uchiyama , K , Isobe , S , Mizukami , Y and Shimamoto , Y . 2002 . Genotype-dependent transcriptional activation of novel repetitive elements during cold acclimation of alfalfa (Medicago sativa) . Plant Journal , 31 : 615 – 627 .
- Kang , Z and Buchenauer , H . 1999 . Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum . Physiological and Molecular Plant Pathology , 55 : 275 – 288 .
- Kang , Z and Buchenauer , H . 2002 . Studies on the infection process of Fusarium culmorum in wheat spikes: degradation of host cell wall components and localization of trichothecenes toxins in infected tissue . European Journal of Plant Pathology , 108 : 653 – 660 .
- Khachatourians , GG . 1990 . Metabolic effects of trichothecene T-2 toxin . Canadian Journal of Physiology and Pharmacology , 68 : 1004 – 1008 .
- Khera , KS , Arnold , DL , Whalen , C , Angers , G and Scott , PM . 1984 . Vomitoxin (4-deoxynivalenol): effects on reproduction of mice and rats . Toxicology and Applied Pharmacology , 74 : 345 – 356 .
- Laskin , JD , Heck , DE and Laskin , DL . 2002 . The ribotoxic stress response as a potential mechanism for MAP kinase activation in xenobiotic toxicity . Toxicological Sciences , 69 : 289 – 291 .
- Lee , T , Oh , DW , Kim , HS , Lee , J , Kim , YH , Yun , SH and Lee , YH . 2001 . Identification of deoxynivalenol and nivalenol producing chemotypes of Gibberella zeae . Applied and Environmental Microbiology , 67 : 2966 – 2972 .
- McLaughlin CS Vaughan MH Campbell IM Wei CM Stafford ME Hansen BS 1977 Inhibition of protein synthesis by trichothecenes In: Rodericks JV, Hesseltine CW, Mehlman MA, editors Mycotoxins in human and health Park Forest South IL Pathotox pp 263–275
- McLean , M . 1996 . The phytotoxicity of Fusarium metabolites: an update since 1989 . Mycopathologia , 133 : 163 – 179 .
- Meky , FA , Hardie , LJ , Evans , SW and Wild , CP . 2001 . Deoxynivalenol-induced immunomodulation of human lymphocyte proliferation and cytokine production . Food and Chemical Toxicology , 39 : 827 – 836 .
- Miller , JD and Arnison , PG . 1986 . Degradation of deoxynivalenol by suspension cultures of Fusarium head blight resistant wheat cultivar Frontana . Canadian Journal of Plant Pathology , 8 : 147 – 150 .
- Miller , JD and Ewen , MA . 1997 . Toxic effects of deoxynivalenol on ribosomes and tissues of the spring wheat cultivar Frontana and Casavant . Natural Toxins , 5 : 234 – 237 .
- Minervini , F , Fornelli , F and Flynn , KM . 2004 . Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line . Toxicology in Vitro , 18 : 21 – 28 .
- Miura , K , Nakajima , Y , Yamanaka , N , Terao , K , Shibato , T and Ishino , S . 1998 . Induction of apoptosis with tubarenon-X mouse . Toxicology , 127 : 195 – 206 .
- Moon , Y and Pestka , JJ . 2002 . Vomitoxin-induced cyclooxigenase-2 gene expression in macrophages mediated by activation of ERK-Hymocytes and p38 but not JNK-mitogen-activated protein kinases . Toxicological Sciences , 69 : 373 – 382 .
- Ohtsubo , K , Ryu , LC , Nakamura , K , Izumiyama , N , Tanaka , T , Yamamura , H , Kobayashi , T and Ueno , Y . 1989 . Chronic toxicity of nivalenol in female mice: a 2 year feeding study with Fusarium nivale Fn 2b moulded rice . Food Chemistry and Toxicology , 27 : 591 – 598 .
- Ohtsubo , K , Yamada , M and Saito , M . 1968 . Inhibitory effect of nivalenol, a toxic metabolite of Fusarium nivale on the growth cycle and biopolymer synthesis of HeLa cells . Japanese Journal of Medical Science and Biology , 21 : 185 – 194 .
- Ouyang , YL , Azcona-Olivera , JI and Pestka , JJ . 1995 . Effects of trichothecene structure on cytokine secretion and gene expression in murine CD4+ T cells . Toxicology , 104 : 187 – 202 .
- Pace , JG . 1983 . Effect of T-2 mycotoxin on the rat liver mitochondria electron transport system . Toxicon , 21 : 675 – 680 .
- Pace , JG , Watts , MR and Canterbury , WJ . 1988 . T-2 mycotoxin inhibits mitochondrial protein synthesis . Toxicon , 26 : 77 – 85 .
- Packa , D . 1991 . Cytogenetic changes in plant cells as influenced by mycotoxins . Mycotoxin Research , 7 : 150 – 155 .
- Poapolathep , A , Ohtsuka , R , Kiatipattanasakul , W , Ishigami , N , Nakayama , H and Doi , K . 2002 . Nivalenol-induced apoptosis in thymus, spleen and Peyer's patches of mice . Experimental and Toxicologic Pathology , 53 : 441 – 446 .
- Porcher , JM , Lafarge-Frayssinet , C , Frayssinet , C , Nurie , A , Melcion , D and Richard-Molard , D . 1987 . Determination of cytotoxic trichothecenes in corn cell culture toxicity assay . Journal of the Association of Official Analytical Chemists , 70 : 844 – 849 .
- Qureshi , MA , Brundage , MA and Hamilton , PB . 1998 . 4-Beta, 15-diacetoxyscirpenol induces cytotoxicity and alterations in phagocytic and Fc-receptor expression functions in chicken macrophages in vitro . Immunopharmacology and Immunotoxicology , 20 : 541 – 553 .
- Rachid , MA , Vasconcelos , AC and Nunes , VA . 2000 . Apoptosis in the lymphoid depletion induced by T-2 toxin in broiler chicks. Histomophometry of the bursa of Fabricus . Arquivo Brasileiro de Medicina Veterin ia e Zootecnia , 52 : 592 – 598 .
- Rahman , MF , Bilgrami , KS and Masood , A . 1993 . Cytotoxic effects of DON and T-2 toxin on plant cells . Mycopathologia , 124 : 95 – 97 .
- Reubel , GH , Gareis , M and Amselgruber , WM . 1987 . Cytotoxicity evaluation of mycotoxins . Mycotoxin Research , 3 : 85 – 86 .
- Rosenstein , Y and Lafarge-Frayssinet , C . 1983 . Inhibitory effect of Fusarium T-2 toxin on lymphoid DNA and protein synthesis . Toxicology and Applied Pharmacology , 70 : 283 – 288 .
- Rotter , BA , Prelusky , DB and Pestka , JJ . 1996 . Toxicology of deoxynivalenol (vomitoxin) . Journal of Toxicology and Environmental Health , 48 : 1 – 34 .
- Rousseaux , CG , Schiefer , HB and Hancock , DS . 1986 . Reproductive and teratological effects of continuous low-level dietary T-2 toxin in female CD-1 mice for two generations . Journal of Applied Toxicology , 6 : 179 – 184 .
- Ryu , JC , Ohtsubo , K , Izurniyarna , N , Nakamura , K , Tanaka , T , Yamarnura , H and Ueno , Y . 1988 . The acute and chronic toxicities of nivalenol in mice . Fundamental and Applied Toxicology , 11 : 38 – 47 .
- SCF (Scientific Committee for Food) 1999 Opinion on Fusarium toxins. Part 1: Deoxynivalenol (DON) available at:, http://www.europa.eu.int/comm/food/fs/sc/scf/out44_en.pdf
- SCF (Scientific Committee for Food) 2002 Opinion on Fusarium toxins. Part 6: Group evaluation of T-2 toxin, HT-2 toxin, nivalenol and deoxynivalenol available at:, http://www.europa.eu.int/comm/food/fs/sc/scf/out123_en.pdf
- Shifrin , VI and Anderson , P . 1999 . Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N -terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis . Journal of Biological Chemistry , 274 : 13985 – 13992 .
- Shimada , T and Otani , M . 1990 . Effects of Fusarium mycotoxins on the growth of shoots and roots at germination in some Japanese wheat cultivars . Cereals Research Communication , 18 : 229 – 232 .
- Shokri , F , Heidari , M , Gharagozloo , S and Ghazi-Khansari , M . 2000 . In vitro inhibitory effects of antioxidants on cytotoxicity of T-2 toxin . Toxicology , 146 : 171 – 176 .
- Silar , P and Picard , M . 1994 . Increased longevity of EF-1 alpha high-fidelity mutants in Podospora anserina . Journal of Molecular Biology , 235 : 231 – 236 .
- Slater , TF , Sawyer , B and Strauli , U . 1963 . Studies on succinate-tetrazolium reductase systems. III. Points of coupling of four different tetrazolium salts . Biochimica and Biophysica Acta , 77 : 383 – 393 .
- Suneja , SK , Wagle , DS and Ram , GC . 1989 . Effects of oral administration of T-2 toxin on glutathione shuttle enzymes, microsomal reductase and lipid peroxidation in rat liver . Toxicon , 27 : 995 – 1001 .
- Swanson , SP , Helaszek , C , Buck , WB , Rood , HDJ and Haschek , WM . 1988 . The role of intestinal microfloral in the metabolism of trichothecenes mycotoxins . Food Chemistry and Toxicology , 26 : 823 – 830 .
- Tashiro , F , Hirai , K and Ueno , Y . 1979 . Inhibitory effects of carcinogenic mycotoxins on deoxyribonucleic acid-dependent ribonucleic acid polymerases and ribonuclease H . Applied and Environmental Microbiology , 38 : 191 – 196 .
- Thompson , WL and Wannemacher , RW Jr. 1986 . Structure–function relationship of 12,13-epoxytrichothecene mycotoxins in cell culture: comparison of whole animal lethality . Toxicon , 24 : 985 – 994 .
- Thompson , WL and Wannemacher , RW Jr. 1990 . In vivo effects of T-2 toxin on synthesis of proteins and DNA in rat tissues . Toxicology and Applied Pharmacology , 105 : 482 – 491 .
- Trusal , LR and O’Brien , JC . 1986 . Ultrastructural effects of T-2 toxin on rat hepatocytes in vitro . Toxicon , 24 : 481 – 488 .
- Tryphonas , H , Iverson , F , Ying So , EA , Mgguire , PF , O’Grady , L , Clayson , DB and Scott , PM . 1986 . Effects of deoxynivalenol (vomitoxin) on the humoral and cellular immunity of mice . Toxicology Letters , 30 : 137 – 150 .
- Tsuchida , M , Miura , T , Shimizu , T and Albara , K . 1984 . Elevation of thiobarbituric acid levels in the rat liver intoxicated by T-2 toxin . Biochemical Medicine , 31 : 147 – 166 .
- Ueno , Y . 1985 . The toxicology of mycotoxins . Critical Reviews in Toxicology , 14 : 99 – 133 .
- Ueno Y 1987 Trichothecenes in food In: Krogh P, editor Mycotoxins in food, food science and technology London Academic Press
- Ueno , Y , Ishii , K , Sato , N , Shimada , N , Tsunoda , H , Sawano , M and Enomoto , M . 1973 . Screening of trichothecenes producing fungi and the comparative toxicity of isolated mycotoxins . Japanese Journal of Pharmacology , 23 : 133
- Visconti , A , Minervini , F , Lucivero , G and Gambatesa , V . 1991 . Cytotoxic and immunotoxic effects of Fusarium mycotoxins using a rapid colorimetric bioassay . Mycopathologia , 113 : 181 – 186 .
- Wakulinski , W . 1989 . Phytotoxicity of the secondary metabolites of fungi causing wheat head fusariosis (head blight) . Acta Physiologiae Plantarum , 11 : 301 – 306 .
- Wang , YZ and Miller , JD . 1988 . Effects of Fusarium graminearum metabolites on wheat tissue in relation to FHB resistance . Journal of Phytopathology , 122 : 118 – 125 .
- Wannemacher RJ Stanley L Wiener MD 2000 Trichothecenes mycotoxins In: Zajtchuk R, editor Medical aspects of chemical and biological warfare Washington DC Department of the Army pp 656–676
- Weaver , GA , Kurtz , HJ , Bates , FY , Chi , MS , Mirocha , CJ , Behrens , JC and Robinson , TS . 1978 . Acute and chronic toxicity of T-2 mycotoxin in swine . Veterinary Research , 103 : 531 – 535 .
- Wei , CM , Campbell , IM , McLaughlin , CS and Maurice , H . 1974 . Binding of trichodermin to mammalian ribosomes and its inhibition by other 12,13-epoxytrichothecenes . Molecular and Cell Biochemistry , 3 : 215
- Wolffe , AP and Matzke , MA . 1999 . Epigenetics: regulation through repression . Science , 286 : 481 – 486 .
- Wyllie , AH , Morris , RG , Smith , AL and Dunlop , D . 1984 . Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis . Journal of Pathology , 142 : 67 – 77 .
- Yabe , T , Hashimoto , H , Sekijima , M , Degawa , M , Hashimoto , Y , Tashiro , F and Ueno , Y . 1993 . Effects of nivalenol on hepatic drug-metabolizing activity in rats . Food Chemistry and Toxicology , 31 : 573 – 581 .
- Yang , G-H , Jarvis , BB , Chung , Y-J and Pestka , JJ . 2000 . Apoptosis induction by satratoxins and other trichothecene mycotoxins: Relationship to ERK, p38 MAPK, and SAPK/JNK activation . Toxicology and Applied Pharmacology , 164 : 149 – 160 .
- Yoshizawa , T , Sakamoto , T and Okamkoto , K . 1984 . In vitro formation of 3-hydroxy T-2 and 3-hydroxy HT-2 toxins from T-2 toxin by liver homogenates from mice and monkeys . Applied and Environmental Microbiology , 47 : 130 – 134 .
- Zhou , HR , Islam , Z and Pestka , JJ . 2003a . Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleen of mice exposed to the trichothecene vomitoxin . Toxicological Sciences , 72 : 130 – 142 .
- Zhou , HR , Lau , AS and Pestka , JJ . 2003b . Role of double-stranded RNA-activated protein kinase R (PKR) in deoxynivalenol-induced ribotoxic stress response . Toxicological Sciences , 74 : 335 – 344 .