Abstract
Because infants are more susceptible to the adverse effects of mycotoxins, this work was carried out to determine aflatoxin M1 (AFM1) and ochratoxin A (OA) in milk from the Human Milk Bank of the Southern Regional Hospital, São Paulo, Brazil. Analytical methods were first established and evaluated. The methods involved the extraction of AFM1 with methanol and OA with 1% aqueous sodium bicarbonate solution and methanol, clean-up with immunoaffinity columns having antibodies specific for each mycotoxin and quantification by high performance liquid chromatography (HPLC) with fluorescence detection. The method established for AFM1 had mean recovery percentages of 94, 77 and 82% and coefficients of variation of 17.5, 3.4 and 4.2% at 0.01, 0.03 and 0.05 ng ml−1, respectively. For the OA method, the corresponding values were 84, 84 and 75% for recovery and 14.1, 3.7 and 4.0% for the coefficient of variation. The limit of quantification for both methods was 0.01 ng ml−1. Of a total of 50 samples analysed, only one was contaminated with AFM1, at 0.024 ng ml−1, and two with OA, at 0.011 and 0.024 ng ml−1. Although the incidence observed was low, it is recommended that the study be extended to other milk banks of the city of São Paulo.
Introduction
Aflatoxin M1 (AFM1) is the principal hydroxylated aflatoxin metabolite in the milk of dairy cows fed a diet contaminated with aflatoxin B1 (AFB1). It is also present in the milk of nursing mothers who consumed foodstuffs with AFB1 (Neal et al. Citation1998). The fact that milk, the basic food of infants, can be contaminated with AFM1, a highly toxic substance, has been a matter of considerable concern. There are few papers on the occurrence of AFM1 in human milk in the world (Wild et al. Citation1987; El-Nezami et al. Citation1995; Jonsyn et al. Citation1995; Saad et al. Citation1995; Alla et al. Citation2002; Abdulrazzaq et al. Citation2003; Turconi et al. Citation2004). While the percentages of contaminated samples in France, Italy, Australia and Zimbabwe were 0 (0/42), 0.4% (1/231; 0.194 ng ml−1), 15% (11/73; range, 0.028–1.031 ng ml−1) and 11% (6/54; range 0.014–0.05 ng ml−1), respectively, in Thailand and Sierra Leone AFM1 was found in 45% (5/11; range, 0.039–1.736 ng ml−1) and 31% (35/113; range, 0.2–99 ng ml−1), respectively, of the samples. Egypt and the United Arab Emirates also had high incidence and levels, 55% (66/120; range, 0.02–2.09 ng ml−1) and 91% (127/140; range, 0.053–3.4 ng ml−1), respectively.
Ochratoxin A (OA) is a nephrotoxic and nephrocarcinogenic mycotoxin produced by Penicillium verrucosum in temperate or cold climates and a number of species of Aspergillus in warmer and tropical parts of the world (Pittet Citation1998). OA has been reported as a contaminant in human milk (Gareis et al. Citation1988; Breitholtz-Emanuelsson et al. Citation1993; Jonsyn et al. Citation1995; Micco et al. Citation1995; Miraglia et al. Citation1995, Citation1998; Zimmerli & Dick Citation1995; Apostolou et al. Citation1998; Skaug et al. Citation1998; Brera et al. Citation2001; Skaug et al. Citation2001; Alla et al. Citation2002; Turconi et al. Citation2004), the surveys showing marked differences in OA incidence and levels in the different countries.
The contamination percentage in Italy varied from 20–87% (22/111, 9/33, 294/465, 74/85, 198/231), the levels being generally low (range, 0.001–12.0 ng ml−1). OA was also found at varying incidence but at low levels in Norway (33%; 38/115; range, 0.01–0.13 ng ml−1 and 21%; 17/80; range, 0.01–0.182 ng ml−1), Germany (11%; 4/36; range, 0.017–0.03 ng ml−1), Australia (2%; 2/100; range, 3–3.6 ng ml−1), Switzerland (10%; 4/40; range, 0.005–0.014 ng ml−1) and Sweden (58%; 23/40; range, 0.01–0.04 ng ml−1). Sierra Leone and Egypt had the highest levels of contamination (35%; 40/113; range, 0.2–337 ng ml−1 and 36%; 43/120; range 5.07–45.01 ng ml−1, respectively).
Children are considered to be more susceptible than adults to the effects of mycotoxins because of their lower body weight, higher metabolic rate, lower ability to detoxify and incomplete development of some organs and tissues, notably the central nervous system (Kuiper-Goodman Citation1989; Galvano et al. Citation1996).
The objectives of this study were:
| 1. | To standardize and validate analytical methods for the determination of AFM1 and OA in human milk. | ||||
| 2. | Determine AFM1 and OA in human milk samples from the Human Milk Bank of the Southern Regional Hospital in São Paulo, Brazil. | ||||
The evaluation of these mycotoxins in human milk has not been studied in this country.
Materials and methods
Samples
A total of 50 samples of human milk, 22 samples collected in the winter (2001–2002) and 28 samples in the summer (2002), were obtained from the Human Milk Bank of the Southern Regional Hospital in São Paulo, Brazil. The samples (about 60 ml each) were kept in glass bottles at −20°C until analysed. The mothers signed a written permission for the donation of the samples and the use of information about their diets obtained through a questionnaire.
Reagents and materials
HPLC solvents were of HPLC grade and other chemicals were of analytical reagent grade. The immunoaffinity clean-up columns, specific for AFM1 and OA, were obtained from Rhône-Diagnostics Technologies Ltd. (Glasgow, UK). Immunoaffinity columns were kept under refrigeration at 2–8°C and brought to room temperature before analysis. Standards of AFM1 and OA were purchased from Sigma Chemical Co. (St Louis, MO, USA). AFM1 stock solution was prepared in benzene-acetonitrile (90:10 v/v) and for OA was prepared in toluene-acetic acid (99:1 v/v). The concentrations were 0.16 µg ml−1 and 9.72 µg ml−1, respectively, and were determined according to AOAC International (Citation1995), checked with UV spectrofotometry in 350 nm for AFM1 and 333 nm for OA, using ε = 5440 m2 mol−1 (OA). Working solution (0.02 µg ml−1) for AFM1 and OA were prepared together by appropriate dilution in methanol. The solutions were stored below −20°C and protected from light.
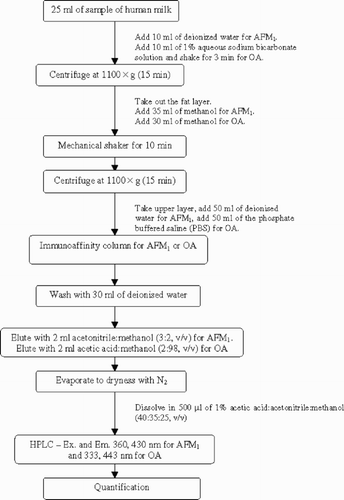
Determination of AFM1
The samples were extracted and submitted to clean-up according to instructions from the producer of the immunoaffinity columns, with minor modifications. The analytical procedure is shown schematically in . For AFM1, an aliquot (25 ml) of the sample of human milk was diluted with 10 ml deionized water and centrifuged at 1100 × g for 15 min. The thin fat layer was removed, 35 ml of methanol were added, and the mixture was mechanically shaken for 10 min and centrifuged at 1100 × g for 15 min. The upper layer, after addition of 50 ml of deionized water, was passed through the immunoaffinity column for AFM1 at a slow steady flow rate of ca 2–3 ml min−1. The column was washed with 30 ml of deionized water and dried by passing air through it. AFM1 was slowly eluted from the column with 2 ml acetonitrile:methanol (3:2, v/v). Acetonitrile:methanol was allowed to remain in contact with the column for at least 60 s to ensure complete removal of the bound toxin from the antibody. The eluate was brought to dryness under a gentle stream of N2. The residue was dissolved in 500 µl of 1% acetic acid:acetonitrile:methanol (40:35:25, v/v) and a 20 µl aliquot was injected into the HPLC equipment.
Although the immunoaffinity column was expected to be highly specific, the identity of AFM1 was confirmed by derivatization with trifluoroacetic acid (AOAC International Citation1995).
Determination of OA
An aliquot (25 ml) of the sample of human milk was mixed with 10 ml of 1% aqueous sodium bicarbonate solution and the mixture was shaken for 3 min and centrifuged at 1100 × g for 15 min. The thin fat layer was removed and 30 ml of methanol were added. After mechanical shaking for 10 min and centrifugation at 1100 × g for 15 min, the upper layer was taken, 50 ml of phosphate buffered saline (PBS) were added and the total volume passed through the immunoaffinity column for OA at a slow steady flow rate of ca 2–3 ml min−1. The column was washed with 30 ml deionized water and dried by passing air through it. OA was slowly eluted from the column with 2 ml acetic acid: methanol (2:98, v/v). Acetic acid:methanol was allowed to be in contact with the column for at least 60 s to completely remove the bound toxin from the antibody. The eluate was brought to dryness with a gentle stream of N2. The residue was dissolved in 500 µl of 1% acetic acid:acetonitrile:methanol (40:35:25, v/v) and an aliquot of 20 µl was injected into the HPLC chromatograph. The identity of OA was confirmed by derivatization with concentrated HCl according to Zimmerli and Dick (Citation1995) and Skaug et al. (Citation2001).
HPLC equipment and operating conditions
All analyses were carried out using a Shimadzu (Kyoto, Japan) SCL-10AVP LC system equipped with an on-line degasser, automatic injector, quaternary pump LC-10ADVP, a CTO-10ASVP column oven kept at 35°C, fluorescence detector model RF-10AXL and CLASS-VP 5.02. The analytical column was a LiChrosorb C18 column (250 × 4 mm, 10 µm, Merck, Germany) and the mobile phase consisted of 2% acetic acid:acetonitrile:methanol (40:35:25, v/v) pumped at a flow rate of 1 ml min−1. For fluorescence detection, excitation was set at 360 nm and emission at 430 nm for AFM1, and at 333 nm and 443 nm, respectively, for OA. The same mobile phase was used for determinations of AFM1 and OA, but was carried out in separation injections.
Evaluation of the methods
A pool of samples of human milk was analysed repeatedly to verify if AFM1 and OA were detected (uncontaminated samples). To evaluate recoveries, the methods were applied to the analysis of uncontaminated samples spiked with 0.01, 0.03 and 0.05 ng ml−1 of AFM1 and OA. For both mycotoxins, the recovery test was carried out five times at the concentration of 0.01 ng ml−1 and four times at 0.03 and 0.05 ng ml−1.
The limit of quantification was determined by the addition of decreasing amounts of the mycotoxin standards to an uncontaminated sample.
Standard curves for the two mycotoxins, bracketing the expected levels in the samples, were constructed with seven points (from 0.5–1.0 ng ml−1), six injections being made for each point. The curves passed through the origin and demonstrated linearity within the concentration range, the coefficients of correlation being 0.9997 for AFM1 and 0.9983 for OA and the coefficients of variation being 1.68 and 3.20%, respectively.
Results and discussion
The method established for AFM1 had mean recovery percentages of 94; 77 and 82% with coefficients of variation of 17.5; 3.4 and 4.2% at concentrations of 0.01, 0.03 and 0.05 ng ml−1, respectively (see ). The method for OA had mean recovery percentages of 84, 84 and 75% and coefficients of variation of 14.1; 3.7 and 4.0% at concentrations 0.01, 0.03 and 0.05 ng ml−1, respectively (). Therefore the limit of quantification for both methods was 0.01 ng ml−1 defined as the minimum level at which the analyte can be quantified with acceptable accuracy and precision (Garfield et al. Citation2000).
Table I. Recovery of AFM1 and OA added to samples of human milk.
Of a total of 50 samples analysed, only one was contaminated with AFM1, at 0.02 ng ml−1, and two with OA, at 0.01 and 0.02 ng ml−1 (). Chromatograms of AFM1 and OA contaminated samples are shown in .
Figure 2. HPLC-FD chromatograms of (a) sample spiked with standards of AFM1 and OA at 0.5 ng ml−1; (b) sample of human milk contaminated with AFM1 at 0.02 ng ml−1 and (c) sample of human milk contaminated with OA at 0.02 ng ml−1.
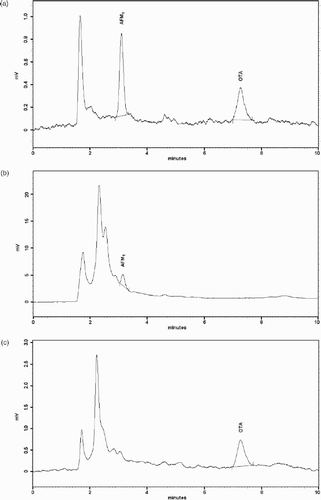
Table II. Incidence of AFM1 and OA in Milk Bank.
The contamination levels obtained in the present study of AFM1 and OA were lower than Abdulrazzaq et al. (Citation2003) and Micco et al. (Citation1995). In our evaluation a body weight of 4 kg assumed as a basis for the exposure assessment of the babies was below the estimated Tolerable Daily Intake (TDI). With a TDI of 5 ng kg−1 bw/day suggested by the Scientific Committee for Food at European level (European Commission Citation1998), the maximum tolerable OA daily intake for babies should be 20 ng.
The levels found in our study by comparing the legal limits for OA in milk and baby food that exist in some countries e.g., Italy, France and Switzerland, were not significant, however in one sample, the level of AFM1 was above or equal to the legal limit (FAO Citation2004).
We expected a difference between summer and winter in the samples of human milk because in our country in winter the eating of peanuts and peanut products is greater than in summer indicating the possibility of contamination from aflatoxins. The samples of human milk contaminated with OA were collected from two mothers who consumed rice bran, beer and coffee in the summer.
Although the incidence of AFM1 and OA in the human milk samples from the Milk Bank of the Southern Regional Hospital was low, it is recommended that the study be extended to other milk banks of the city of São Paulo. The fact that three samples were contaminated indicated the possibility of contamination, exposing infants to these two mycotoxins.
There was an intention to correlate the incidence of the mycotoxins to the mothers’ diets, with information obtained through a questionnaire. The low incidence observed prevented such a correlation being made.
Acknowledgements
The authors would like to thank the employees of the Human Milk Bank of the Southern Regional Hospital, city of São Paulo, for their collaboration in collecting the samples of human milk and for filling out the questionnaires to obtain information about the mothers’ diets.
References
References
- Abdulrazzaq , YM , Osman , N , Yousif , ZM and Al-Falahi , S . 2003 . Aflatoxin M1 in breast-milk of UAE women . Annals of Tropical Paediatrics , 23 : 173 – 179 .
- Alla , EAMA , Aly , SE and Neamat-Allah , AA . 2002 . Human exposure to mycotoxins in Egypt . Mycotoxin Research , 18 : 23 – 30 .
- AOAC International. 1995 Official methods of analysis 16th ed. Gaithersburg MD Association of Official Analytical Chemists Chapter 49, pp 4; 34 35 38
- Apostolou , E , El-Nezami , HS , Ahokas , JT and Donohue , DC . 1998 . The evaluation of ochratoxin A in breast milk in Victoria (Australia) . Revue de Médecine Vétérinaire , 149 : 709
- Breitholtz-Emanuelsson , A , Olsen , M , Oskarsson , A , Palminger , I and Hult , K . 1993 . Ochratoxin A in cow's milk and in human milk with corresponding human blood samples . Journal of AOAC International , 76 : 842 – 846 .
- Brera C Miraglia M Ambruzzi MA Calfapietra FR Pazzaglini B Grossi S Yazdanpanah H 2001 Ochratoxin A in human milk: Exposure assessment of babies In: WJ de Koe, RA Samson, HP Van Egmond, J Gilbert, M Sabino, editors. Mycotoxins and Phycotoxins in Perspective at the Turn of the Millennium Wageningen Ponsen & Looyen pp 201–207
- El-Nezami , HS , Nicoletti , G , Neal , GE , Donohue , DC and Ahokas , JT . 1995 . Aflatoxin M1 in human breast milk samples from Victoria, Australia and Thailand . Food Chemistry Toxicology , 33 : 173 – 179 .
- European Commission 18 September 1998 Scientific Committee for Food. Opinion on Ochratoxin A CS/CNTM/MYC/14
- FAO 2004 Worldwide regulations for mycotoxins in food and feed in 2003 FAO Food and Nutrition Paper 81 Rome
- Galvano , F , Galofaro , V and Galvano , G . 1996 . Occurrence and stability of Aflatoxin M1 in milk and milk products: A worldwide review . Journal of Food Protection , 59 : 1079 – 1090 .
- Gareis , M , Maertlbauer , E , Bauer , J and Gedek , B . 1988 . Determination of Ochratoxin A in human milk . Zeitschrift fuer Lebensmittel Untersuchung und Forschung , 186 : 114 – 117 .
- Garfield FM Klesta E Hirsch J 2000 Quality Assurance Principles for Analytical Laboratories AOAC International. 3rd ed Gaithersburg MD Association of Official Analytical Chemists Chapter 9 p 122
- Jonsyn , FE , Maxwell , SM and Hendrickse , RG . 1995 . Ochratoxin A and aflatoxins in breast milk samples from Sierra Leone . Mycopathologia , 131 : 121 – 126 .
- Kuiper-Goodman T 1989 In: S Natori, K Hashimoto, Y Ueno, editors Risk assessment of mycotoxins Mycotoxins and Phycotoxins 1988 Amsterdam Elsevier Science Publishers B.V. pp 257–264
- Micco , C , Miraglia , M , Brera , C , Corneli , S and Ambruzzi , A . 1995 . Evaluation of ochratoxin A level in human milk in Italy . Food Additives and Contaminants , 12 : 351 – 354 .
- Miraglia , M , De Dominicis , A , Brera , C , Corneli , S , Cava , E , Menghetti , E and Miraglia , E . 1995 . Ochratoxin A levels in human milk and related food samples: An exposure assessment . Natural Toxins , 3 : 436 – 444 .
- Miraglia , M , Brera , C , Cava , E and Calfapietra , FR . 1998 . The evaluation of major sources of ochratoxin A (OA) intake through the analysis of OA in biological fluids in Italy . Revue de Médecine Vétérinaire , 149 : 711
- Neal , GE , Eaton , DL , Judah , DJ and Verma , A . 1998 . Metabolism and toxicity of aflatoxins M1 and B1 in human-derived in vitro systems . Toxicology and Applied Pharmacology , 151 : 152 – 158 .
- Pittet , A . 1998 . Natural occurrence of mycotoxins in foods and feeds – an updated review . Revue de Médecine Vétérinaire , 149 : 479 – 492 .
- Saad , AM , Abdelgadir , AM and Moss , MO . 1995 . Exposure of infants to aflatoxin M1 from mothers’ breast milk in Abu Dhabi, UAE . Food Additives and Contaminants , 12 : 255 – 261 .
- Skaug , MA , Stormer , FC and Saugstad , OD . 1998 . Ochratoxin A: A naturally occurring mycotoxin found in human milk samples from Norway . Acta Paediatrica , 87 ( 12 ) : 1275 – 1278 .
- Skaug , MA , Helland , I , Solvoll , K and Saugstad , OD . 2001 . Presence of ochratoxin A in human milk in relation to dietary intake . Food Additives and Contaminants , 18 : 321 – 327 .
- Turconi , G , Guarcello , M , Livieri , C , Comizzoli , S , Maccarini , L , Castellazzi , AM , Pietri , A , Piva , G and Roggi , C . 2004 . Evaluation of xenobiotics in human milk and ingestion by the newborn . An epidemiological survey in Lombardy (Northern Italy). European Journal of Nutrition , 43 : 191 – 197 .
- Wild , CP , Pionneau , FA , Montesano , R , Mutiro , CF and Chetsanga , CJ . 1987 . Aflatoxin detected in human breast milk by immunoassay . International Journal of Cancer , 40 : 328 – 333 .
- Zimmerli , B and Dick , R . 1995 . Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: Methodology and Swiss data . Journal of Chromatography B , 666 : 85 – 99 .