Abstract
The main source of ochratoxin A (OTA) in the wine food chain is the infection of grapes by “black aspergilli” in the field. OTA-producing black aspergilli include principally Aspergillus carbonarius, followed by A. niger and possibly A. tubingensis. They are opportunistic fungi that develop particularly on damaged berries at ripening, although they may occur and form OTA on grapes from veraison to harvest. Climatic conditions (high humidity and temperature) and geographical location are important factors favouring OTA accumulation in grape berries. The severity of aspergillus rot is influenced by excessive irrigation and rainfall prior to harvest, which causes berry splitting. In addition, berry wounds caused by insect attack provide preferential entries for black aspergilli. High OTA levels occur in grapes severely damaged by the grape moth, Lobesia botrana, particularly in Mediterranean areas. Some grape varieties display greater susceptibility to aspergillus rot due to intrinsic genetic characteristics and bunch conformation (i.e. compact > sparse). Control measures for toxigenic mycoflora in the vineyards must consider these critical control points. Proper fungicidal and insecticidal treatments can reduce OTA contamination. Nevertheless, knowledge about the fate of OTA and its distribution in wine and winery by-products is important to manage OTA risk in contaminated stock. In our wine-making experiments, only 4% of the OTA present in grapes remained in the wine–the majority is retained in pressed grape pomaces. OTA concentration remained unchanged in wine after a 1-year aging as well as in all liquid fractions collected during vinification (i.e. must, free run wine, and wine after first and second decantation). Activated carbon can reduce OTA levels in wine but negatively affects wine quality.
Introduction
Ochratoxin A (OTA) is a major mycotoxin, produced by several species of Aspergillus and Penicillium, naturally occurring in a variety of food commodities prior to harvest or more commonly during storage. Numerous animal studies have shown that OTA is a potent nephrotoxin with the degree of renal injury depending on toxin, dose and exposure time; decreasing nephrotoxic sensitivity was observed from pig to rat to mice. OTA is immunotoxic, neurotoxic in vitro and in vivo in rats, teratogenic in mice, rats and rabbits (JECFA Citation2001). Based on renal carcinogenicity shown in rats and mice, the International Agency for Research on Cancer (IARC) has classified OTA as a Group 2B carcinogen, i.e. carcinogenic to animals and possible carcinogenic to human (IARC Citation1993). Studies on the genotoxicity of OTA remain controversial. Recent scientific evidence indicates that the site-specific renal toxicity as well as the DNA damage and genotoxic effects of OTA, measured in various in vivo and in vitro studies, are most likely attributable to cellular oxidative damage (EFSA Citation2006). Various studies in humans have associated OTA with an endemic kidney disease observed in the Balkans (Balkan Endemic Nephropathy and related Urinary Tract Tumors), but convincing epidemiological evidence associated with OTA exposure is currently lacking. It has been frequently found in human blood, urine and milk and a widespread individual exposure to low levels of OTA in Europe and other continents has been demonstrated (EFSA Citation2006).
The widespread human exposure to OTA is well documented by a number of surveys reporting the occurrence of OTA in a variety of food products. An assessment of the dietary intake of OTA by the population of the European Community has been performed showing that the main contributors to OTA exposure are cereals and cereal products (European Commission Citation2002). Wine, coffee and beer were identified as significant contributors to human OTA exposure. Dried vine fruit and grape juice contribute to a significant extent to the OTA exposure of vulnerable groups of consumers, such as children. The EFSA Scientific Panel on contaminants in the food chain has recently adopted an updated scientific opinion relating to OTA in food, taking into account new scientific information and derived a tolerable weekly intake (TWI) of 120 ng kg−1 body weight (EFSA Citation2006).
Based on the available scientific toxicological and exposure data, the European Union established maximum permitted limits for OTA in a variety of food products that have been updated with the EC Regulation 1881/2006 (European Commission Citation2006). Maximum levels have been set at 2 µg kg−1 for wine, fruit wine, grape juice, grape nectar and grape must intended for direct human consumption, and at 10 µg kg−1 for dried vine fruit (currants, raisins and sultanas). OTA was detected in wine for the first time in 1996 (Zimmerli and Dick Citation1996). Thereafter, several surveys have been conducted, mainly in Europe, on the occurrence of the toxin in wine and related products, showing it as a problem mainly for Southern Europe. The results of several reports from different countries are reported in for a total of 3512 samples. A high incidence of contamination (from 40 to 87%) was reported in all surveys–with the exception of the Australian survey having an incidence of 15% (Hocking et al. Citation2003); the maximum OTA level was recorded in Italy at 15.6 µg kg−1 (European Commission Citation2002). OTA levels showed a decreasing gradient from red to rosé to white wines, and the same trend was observed for grape juice. Wines from southern and warmer regions of Europe showed incidence and levels of contamination (72.3%, mean value 0.64 µg kg−1, n = 635) higher than those from northern European areas (incidence 50.3%, average 0.18 µg kg−1, n = 835). Wines produced in Southern Italy, where climatic conditions favour the growth of OTA-producing fungi in grapes, generally show incidence and levels of contamination higher than wines produced in Northern and Central Italy. Recently, a survey of retailed wine samples from Southern Italy showed a positive correlation between high levels of OTA and resveratrol-related compounds (Perrone et al. Citation2007b). Considering that a significant reduction of hepatic and renal damage caused by OTA was reported in mice fed with grape juice contaminated with OTA (Jeswal Citation1998), there may be some possibility that toxic levels of OTA in red wine are, to some extent, counterbalanced by the beneficial effects of resveratrol derivatives.
Table I. Occurrence of ochratoxin A in wine.
In the following sections, different aspects of OTA formation in grapes and strategies for prevention and control in the field are reported together with OTA distribution and possible corrective actions during wine processing. Good agriculture and manufacturing practices are currently under discussion by Codex Alimentarius for the adoption of a code of practice for the prevention and reduction of OTA contamination in wine, based on a code of sound vinicultural practices adopted by the Organisation Internationale de la Vigne et du Vin (OIV) in 2005 (FAO/WHO Citation2007).
Fungi responsible for OTA accumulation in grapes: Taxonomy and ecology
Fungi responsible for OTA accumulation in cereals, i.e. Aspergillus ochraceus and Penicillium verrucosum, were initially thought to be involved in OTA formation in grapes. However, a number of studies performed over the last decade have provided evidence showing that all fungi responsible for OTA in grapes belong to Aspergillus section Nigri, the so called “black aspergilli” (Battilani et al. Citation2003a). Most epidemiological surveys, performed in Mediterranean, Australian and South American countries, have shown that, within the black aspergilli, the biseriate species, Aspergillus carbonarius and A. niger “aggregate”, and the uniseriate species, A. aculeatus and A. japonicus, are prevalent on grapes (Da Rocha Rosa et al. Citation2002; Battilani et al. Citation2003b; Leong et al. Citation2006a).
The taxonomy of this Section is still not completely resolved, especially within the A. niger “aggregate” (a group of morphologically indistinguishable species), often leading to misidentification of the species distribution in food. A comprehensive molecular characterization of the black aspergilli occurring in grape in Europe was performed within the EU project Wine-Ochra Risk (QLK1-CT-2001-01761) using representative strains isolated from 107 vineyards of the Mediterranean basin (Bau et al. Citation2006; Perrone et al.Citation 2006a,Citationb . These studies led to the identification of four main populations, namely A. carbonarius, A. tubingensis, A. niger and a group of Aspergillus “uniseriate”, which could be separated using molecular methods, including amplified fragment length polymorphism (AFLP), restriction fragment length polymorphism (RFLP) and sequences analysis. The Aspergillus “uniseriate” group was clearly separated from A. japonicus and A. aculeatus by molecular techniques but was morphologically indistinguishable (Perrone et al. Citation2006a,b). Ecological and morphological differences between these species are summarized below.
A. carbonarius was easily distinguished from other biseriates species due to its large, spiny conidia; a high percentage of strains of this species (98–100%) have been shown to produce OTA. Spore germination of A. carbonarius is very rapid, occurring within 24 h at water activity (a w) 0.90–0.99 and temperature 25–35°C. Optimal growth is at 32–35°C and a w 0.95–0.98 (min 10°C and max 45°C). Optimal conditions for OTA production by A. carbonarius are at 20–25°C and a w 0.95/0.98 (Belli et al. Citation2005).
The A. niger “aggregate” comprise four different species indistinguishable by morphological characteristics. The most frequent species isolated from grapes are A. niger and A. tubingensis, while A. foetidus and A. brasiliensis have been detected to a minor extent (Perrone et al. Citation2007c). A. niger aggregate optimal growth conditions are 35–37°C and a w 0.93–0.98 (min 6–8°C and max 47°C). This is one of the most common species group found in a wide range of fresh and dry fruits, cereals, etc., and is seen in food processing as “GRAS” (generally regarded as safe). OTA production by A. niger “aggregate” normally occurs at 20–25°C and a w 0.95/0.98 (Esteban et al. Citation2004). A low percentage of OTA-producing strains (5–10%) were detected among the A. niger “aggregate” (Perrone et al., Citation2006a).
Among the uniseriate group, A. aculeatus and A. japonicus are often isolated from grapes, but have not been proven to produce OTA although they grow under similar conditions to A. carbonarius. Recently, the Aspergillus “uniseriate” population from grapes in Europe was characterized molecularly as a population quite different from A. japonicus and A. aculeatus (Perrone et al. Citation2006b). This population is being described as a new species to be called A. uvarum (Perrone et al. Citation2007d).
Several reports from South America, claiming production of OTA by strains of A. japonicus or that A. niger is the major culprit for OTA accumulation in grapes, are based on morphological identification of the producing strains (Dalcero et al. Citation2002; Chulze et al. Citation2006; Ponsone et al. Citation2007). This evidence has not been confirmed by molecular identification of the species and should be regarded judiciously to avoid confusion in the literature. In our survey of about 600 strains of black aspergilli, representative of a 3-year sampling, 5% of A. niger “aggregate” strains (360) were OTA producers, while all A. carbonarius strains (200) and none of the A. “uniseriate” strains (50) were positive for OTA production (Cozzi et al. Citation2007).
Black aspergilli and OTA occurrence in vineyards: Role of environmental, ecological and agronomical factors
Black aspergilli cause a black-rot disease of grape due to high fungal sporulation on berries which renders them completely shrunken and dry. The incidence of colonised berries is more closely related to seasonal conditions during the year of cultivation than the grape growth stage (Battilani et al. Citation2003b; Leong et al. Citation2006a; Cozzi et al. Citation2007). Fungal conidia are usually present on berry skins from setting and increase in number from early veraison to harvest, with a peak at ripening. Black aspergilli overwinter in soil and frequent soil cultivation can favour fungal infection in vineyard. The severity of aspergillus rot is influenced by excessive irrigation prior to ripening, which causes berry splitting. Rain prior to harvest is a common cause of berry damage, favouring Aspergillus infection (Leong et al. Citation2006a; Cozzi et al. Citation2007). Berry damage caused by insects, birds or other fungal infection is the primary factor affecting disease development and OTA accumulation in berries.
OTA is produced in vineyards and is normally absent up to early veraison. Bunches without visible symptoms can also contain OTA although berries with visible black mould normally show higher contamination levels. The absence of OTA at early growth stages can be explained by major difficulties encountered by fungi in berry penetration (Cozzi et al. Citation2007).
The distribution of black aspergilli in vineyards can be summarized as follows: (i) A. niger “aggregate” is the principal group at all growth stages; (ii) A. carbonarius incidence is 2–3 times less than A. niger “aggregate” and increases from ripening to harvest; (iii) Aspergillus uniseriate is the least represented group, sporadically occurring in Portugal, Greece and Spain, and more frequently in Italy, France and Israel (Battilani et al. Citation2006).
Based on the results of the Wine-Ochra Risk project carried out in six Mediterranean countries, the incidence of berries infected by black aspergilli at harvesting is significantly correlated with latitude and longitude, with a positive West–East and North–South gradient (Battilani et al. Citation2006). Following the geostatistical approach described by Battilani et al. (Citation2006), data on the incidence of A. carbonarius were run with ArcView and a predictive map was drawn. The incidence of A. carbonarius was significantly correlated with geographic coordinates showing a positive gradient going towards the South of Europe. Based on the combination of degree-day and rainfall parameters in late August–early September in several countries of the Mediterranean basin, discriminant analysis gave promising perspectives for predicting OTA presence in vineyards by the development of thermo-wetness maps (Battilani et al. Citation2006).
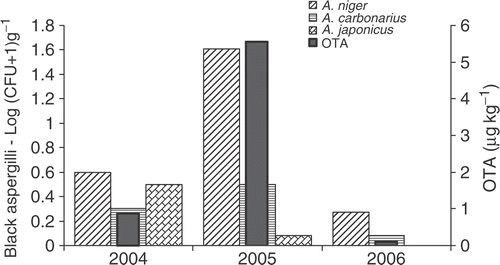
Meteorological conditions as well as closeness to the sea have been shown to play a major role in determining OTA occurrence in grapes (Cozzi et al. Citation2007). A 3-year survey (2004–2006), performed in eight vineyards located in the Salento peninsula of Southern Italy, showed a wide variability of OTA levels between different cultivation years. In particular, the 2005 crop was the most conducive to black aspergilli contamination due to the higher relative humidity and rainfall levels associated with hot temperatures at ripening and harvest time (late August–September). shows the occurrence of black aspergilli and OTA in eight vineyards of the two major grape varieties (Negroamaro and Primitivo) cultivated in this area during the three harvest seasons (Cozzi et al. Citation2007). Aspergillus niger aggregate was predominant from early veraison to ripening, representing 80–85% of contamination. A. carbonarius increased from veraison to ripening. OTA contamination of processed berries was assessed and results were correlated with the incidence of black aspergilli population, in particular with the increasing colony-forming unit (CFU) values of A. carbonarius. The incidence of A. carbonarius increased from ripening to harvest, when vineyard relative humidity usually increased, with high risk of OTA accumulation always associated with hot temperatures (Cozzi et al. Citation2007).
Figure 1. Occurrence of black aspergilli and OTA in eight vineyards of Primitivo and Negroamaro varieties in Apulia over three grape-harvest seasons (2004–2006).
Despite the widespread occurrence of OTA in various types of wine, there is limited information on the ability of black aspergilli to infect berries and produce OTA in different grape varieties (Battilani et al. Citation2004). In in vitro experiments, grape variety was shown to affect the incidence of black aspergilli and the level of OTA contamination. Three of the 12 tested varieties, namely “Bianco di Alessano”, “Pampanuto” and “Uva di Troia”, showed low OTA contamination after artificial infection with a mixture of five OTA-producing strains, whereas the most susceptible variety (Cabernet Sauvignon) contained over 200 µg kg−1 OTA and ∼80% incidence of colonized berries (Battilani et al. Citation2004).
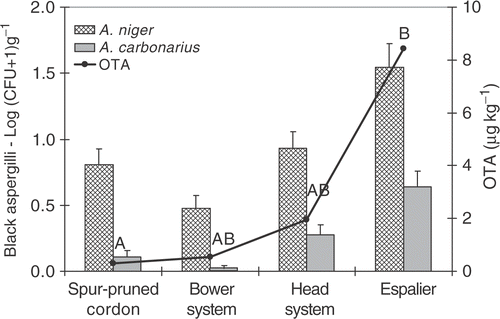
The role of the cropping system was monitored in a 2-year survey carried out on four different systems, namely spur-pruned cordon, bower system, head (or small tree) system and espalier, in eight vineyards in Apulia. In both years, the espalier cropping system produced the most contaminated grapes in terms of A. carbonarius infection and OTA accumulation, as shown in . This can be explained by the closeness of bunches to the soil, which is the most important source of A. carbonarius inocula, compared to spur pruned cordon and bower system. The higher humidity occurring in the espalier cropping system compared to the head system can explain the different contamination level, despite the similar distance of bunches from the soil (Cozzi et al. Citation2007).
Figure 2. Influence of the training systems on the epiphitic black aspergilli in CFU g−1 of grape berries samples and the OTA contamination in the Primitivo variety during 2004/2005. OTA levels with same letters are not significantly different according to the Duncan test (p < 0.01).
All the above ecoagronomical factors play individual roles in developing A. carbonarius on grapes and consequent OTA accumulation, although the final result in relation to OTA risk is better represented by a combination of all these factors. Developing risk maps based on critical control points can help to prevent and control OTA accumulation in grapes.
Lobesia botrana: An OTA risk factor in grapevine management
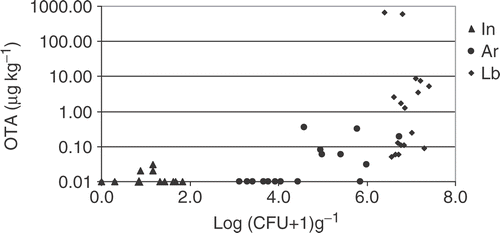
Black aspergilli are opportunistic fungi (saprophytes), mainly responsible for secondary rot of grape berries, that develop through entry sites favoured by berry wounds or splitting caused by either biotic or abiotic factors (insects, fungi, birds, rainfall, hail). Lobesia botrana (Lepidoptera: Tortricidae) is the principal grape-berry moth in the vineyards of Southern Europe and can complete three to four generations a year, depending on weather conditions during late summer. Generally, the first generation larvae of L. botrana damage flowers, while the following generations damage berries at different ripening stages. A good correlation between pest damage and OTA content has been found in grape berries, due to the contribution of L. botrana to berry wounds and fungal spore dissemination (Cozzi et al. Citation2006). Larvae can either contribute to spore dispersal or act as spore vectors, by trapping conidia in the cuticle ornamentation, then facilitating a rapid fungal penetration by tunnelling into berries, as demonstrated for Botrytis cinerea (Fermaud et al. Citation1989; Cozzi et al. Citation2006). Grape berry damage by L. botrana has been shown to considerably increase the contamination level of black aspergilli and consequent OTA accumulation in grapes. In , a comparison between groups of intact berries, black rot berries with and without L. botrana damages is reported. All samples of berries damaged by L. botrana showed considerable levels of OTA contamination (up to 1000 µg kg−1 OTA) and black aspergilli infection higher than 106 CFU. OTA was not detected in the group of intact berries, while it was found at levels below 1 µg kg−1 in ∼42% of black-rot berries without grape moth damages (Cozzi et al. Citation2006). Field trials, performed in 2004 and 2005 using both biological and conventional insecticidal treatments, confirmed that a successful control of the third generation of L. botrana reduced the Aspergilli inocula and the formation of OTA in grapes (Kappes et al. Citation2005; Perrone et al. Citation2007a). It is, therefore, important to ensure adequate insect control in combination with fungicide treatment to realize effective pest management.
Chemical and biological control of OTA-producing fungi
The following chemicals have been shown to be active in reducing, to varying degrees, both fungal growth and OTA levels in grape bunches: mepanipyrim, pyrimethanil, fluazinam, iprodione and the mixture cyprodinil/fludioxonil. The latter mixture was confirmed as effective in a number of field trials carried out in several Mediterranean countries, including France, Spain, Greece and Italy (Tjamos et al. Citation2004; Kappes et al. Citation2005; Bellì et al. Citation2007). The most effective treatment was observed at 21 days before harvesting and a previous treatment at veraison was suggested in high-risk conditions. This mixture of active ingredients applied against black aspergilli at the same combination and schedule, both in dosage and timing, is effective against grey mould, caused by Botrytis cinerea. Moreover, the insecticide treatment against L. botrana in combination with the fungicide contributed significantly to a reduction of OTA levels in the field, particularly in crop-years at high contamination risk (Kappes et al. Citation2005). Promising results were also obtained using yeast as a biological control agent, isolated from grapes in Greece and in Italy. In particular, good results were obtained with two strains, Cryptococcus laurentii and Aureobasidium pullulans, in Greece (Dimakopoulou et al. Citation2005) and with a strain of Hanseniaspora uvarum in Italy using weekly or two-weekly treatments.
Distribution of OTA in wine and winery by-products and its fate during vinification of red grapes
OTA in grapes is transferred to wine and relevant by-products during vinification. Therefore, the availability of reliable analytical methods for OTA determination in must, wine and relevant by-products is important for the risk management of OTA contamination in the wine food chain. To take prompt corrective action, the availability of rapid methods is necessary in wineries for screening the whole production. Several rapid methods, available for OTA analysis in food products, need to be adapted to wine and by-products (Visconti and De Girolamo Citation2005). The AOAC official method 2001.01 for OTA determination by HPLC in wine (Visconti et al. Citation2001) can also be used for must, if the solid fraction is previously separated by centrifugation (Solfrizzo et al. Citation2006). The fate of OTA during vinification has been studied, with contrasting results. Fernandes et al. (Citation2003) observed an increase of OTA concentration in must during maceration of crushed grapes and a consistent reduction in OTA during pomaces and lees separations. Grazioli et al. (Citation2006) found little or no reduction in OTA concentration in wine after the first racking (separation of lees), while a significant reduction in OTA was observed after spontaneous malo-lactic fermentation occurring between the first and the second racking. In contrast, Rousseau (Citation2004) reported that OTA content in must increased after grape crushing and reached maximum levels during malo-lactic fermentation. Leong et al. (Citation2006b) reported that 24% of OTA originally present in crushed red grape passed into free run wine (must) and a 72% OTA reduction was recorded in wine after the first racking.
The differences in approach by these authors could explain the conflicting results. Due to unavailability of naturally contaminated grape some of these studies were performed either by artificially inoculating grape with toxigenic A. carbonarius (Leong et al. Citation2006b) or by spiking uncontaminated grapes with OTA (Fernandes et al. Citation2003). These materials differ from naturally contaminated grapes, which comprise both contaminated and uncontaminated berries. Spiking uncontaminated grapes with OTA produces an apparent reduction in OTA concentration in the resulting wine since most of the spiked OTA is adsorbed by the grape pomaces and biomass (grape skins, pulp, yeasts released in must) (Fernandes et al. Citation2003). On the other hand, when using naturally contaminated berries for vinification, the grape pomaces and solid biomass have a high OTA concentration and represent the source of OTA in wine (Solfrizzo et al. Citation2007). Moreover, in most of these studies, OTA was only monitored in the liquid fractions and no measurements were recorded for pressed pomaces and lees; thus, the distribution of OTA between solid and liquid fractions during vinification was not established (Fernandes et al. Citation2003; Rousseau Citation2004; Grazioli et al. Citation2006). Another critical point is sample preparation of the liquid fractions (must and wines before racking), which contain suspended biomass, before OTA analysis. The separation or inclusion of the biomass in the sample to be analysed has a significant effect on the measured OTA concentration due to the high amount of OTA reversibly bound to the biomass. Indeed, Leong et al. (Citation2006b) included the biomass when unsedimented liquid fractions (must and wines before racking) were analysed for OTA. Consequently, the OTA concentrations found in these fractions were much higher than those found in the same liquid fraction analysed after spontaneous sedimentation of biomass (first racking).
The fate of OTA and its distribution in wine and winery by-products during vinification of naturally contaminated Negroamaro and Primitivo grapes has been recently reinvestigated at laboratory (microvinification) and industrial level by Solfrizzo et al. (Citation2007). Samples of must (before and after maceration), grape pomaces, wine and lees (after the first and second racking) were analysed for OTA to evaluate the levels at each step of vinification. Before analysis, the liquid fractions were centrifuged to separate the biomass and measure soluble OTA. Results of microvinification experiments showed that only 4% of the OTA present in grapes remains in the wine, whereas 95% of the originally OTA is retained on pressed grape pomaces (98% in the skin and 2% in the seeds) and 1% is retained on the lees. Leong et al (Citation2006b) found that 9% of OTA originally present in grapes passed into wine. Therefore, the use of these wine by-products as food ingredients should be avoided or checked for OTA contamination. OTA concentration in must remained nearly constant after maceration, pressing, juice clarification, alcoholic fermentation and lees separations (after first and second racking). The same OTA concentrations were found in wine samples analysed after 1 year. The results obtained with the microvinification were also confirmed at an industrial level. An increase of OTA concentration in must was observed during maceration of Primitivo crushed grapes highly contaminated with OTA. This increase could be explained by the high concentration of OTA in the grapes, which required a longer time for the toxin to equilibrate between must and grape pomaces.
Removal of OTA
Several fining agents have been tested for their ability to remove OTA from contaminated must/wines (Castellari et al. Citation2001; Leong et al. Citation2006c). Oenological decolourising carbon has been reported to remove the highest amount of OTA, although carbon also removes anthocyanins and other coloured polyphenols from wine. The effectiveness of the treatment with oak wood fragments depended upon the quantity of wood chips and powder used (Savino et al. Citation2007). Removal of OTA from grape juice, must and wine using oenological yeast strains has been reported (Bejaoui et al. Citation2004; Garcia Moruno et al. Citation2005; Cecchini et al. Citation2006). The removal of OTA during fermentation is based on adsorption mechanism other than degradation; however, the efficacy of yeasts for OTA reduction at industrial level, as well as their impact on wine quality parameters (phenol compounds), is unclear. Our laboratory has confirmed OTA reduction by yeasts or inactivated yeast walls, with a consistent reduction in colour index (expressed in terms of the Folin Ciocalteu index).
The results obtained in our laboratory on the efficacy of selected adsorbent materials to remove OTA from contaminated red wine are reported in . The best results, in terms of OTA removal, were obtained with carbon or commercial preparations containing carbon (i.e. Mikofree, Myco AD A-Z, Standard Q/FIS). On the other hand, the efficacy in OTA removal was proportional to the reduction in polyphenol content of treated wines.
Table II. Percentage removal of ochratoxin A (OTA) from red wine containing 10 µg l–1 OTA and treated with different amounts of adsorbent.
Conclusions
The main source of OTA in the wine production chain is infection by “black aspergilli” in the field. A. carbonarius is the principal species responsible for OTA accumulation in grape berries from early veraison to ripening. OTA production is influenced by climatic conditions/geographic areas, grape varieties/crop systems and berries damage caused by insects, fungal infection or excessive irrigation/rainfall. Fungicidal and insecticidal treatments can reduce OTA contamination and susceptibility to infection can vary between year and region. Developing of risk maps, based on critical control points, can help to prevent and control OTA accumulation in grapes. Availability of rapid methods for OTA analysis is also important for preventive and corrective intervention at critical control points. After maceration of (red) grapes, OTA remains stable during vinification and after a 1-year aging. During vinification of (red) grapes only 4% OTA remains dissolved in the wine, while 96% is retained by solid winery by-products (grape pomace and lees). Carbon reduces OTA concentrations in wines, but negatively affects quality. Good Agriculture Practices (balanced soil tillage, irrigation, nitrogen fertilization, pruning) and Good Manufacturing Practices (reduced harvest to vinification time, segregation of rot bunches) help considerably to reduce OTA contamination risk. The main critical control points, as well as preventive and corrective actions, are summarized in .
Table III. Main critical control points plus suggested preventive and corrective measures to reduce ochratoxin A contamination in grapes and wine.
Acknowledgements
Work partially supported by the Italian Ministry of Education, University and Research, MIUR Project n. 12818 “SIVINA” (D.M. 593/200).
References
- Battilani , P , Giorni , P and Pietri , A . 2003a . Epidemiology of toxin producing fungi and ochratoxin A occurrence in grape . European Journal of Plant Pathology , 109 : 715 – 722 .
- Battilani , P , Pietri , A , Bertuzzi , T , Languasco , L , Giorni , P and Kozakiewicz , Z . 2003b . Occurrence of ochratoxin A-producing fungi in grapes grown in Italy . Journal of Food Protection , 66 : 633 – 636 .
- Battilani , P , Logrieco , A , Giorni , P , Cozzi , G , Bertuzzi , T and Pietri , A . 2004 . Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in Italy . Journal of the Science of Food and Agriculture , 84 : 1736 – 1740 .
- Battilani , P , Barbano , C , Marin , S , Sanchis , V , Kozakiewicz , Z and Magan , N . 2006 . Mapping of Aspergillus Section Nigri in Southern Europe and Israel based on geostatistical analysis . International Journal of Food Microbiology , 111S1 : S72 – S82 .
- Bau , M , Castellá , G , Bragulat , MR and Cabañes , FJ . 2006 . RFLP characterization of Aspergillus niger aggregate species from grapes from Europe and Israel . International Journal of Food Microbiology , 111S1 : S18 – S21 .
- Bejaoui , H , Mathieu , F , Taillandier , P and Lebrihi , A . 2004 . Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains . Journal of Applied Microbiology , 97 : 1083 – 1044 .
- Bellí , N , Ramos , AJ , Coronas , I , Sanchis , V and Marín , S . 2005 . Aspergillus carbonarius growth and ochratoxin A production on a synthetic grape medium in relation to environmental factors . Journal of Applied Microbiology , 98 : 839 – 844 .
- Bellí , N , Marín , S , Argiles , E , Ramos , AJ and Sanchis , V . 2007 . Effect of chemical treatments on ochratoxigenic fungi and common mycobiota of grapes (Vitis vinifera) . Journal of Food Protection , 70 : 157 – 163 .
- Burdaspal , PA and Legarda , TM . 1999 . Ocratoxina a en vinos, mostos y zumos de uva elaborados en Espana y en otros paises eropeos . Alimentaria , 299 : 107 – 113 .
- Castellari , M , Versari , A , Fabiani , A , Parpinello , GP and Galassi , S . 2001 . Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents . Journal of Agricultural and Food Chemistry , 49 : 3917 – 3921 .
- Cecchini , F , Morassut , M , Garcia Moruno , E and Di Stefano , R . 2006 . Influence of yeast strain on ochratoxin A content during fermentation of white and red must . Food Microbiology , 23 : 411 – 417 .
- Chulze , SN , Magnoli , CE and Dalcero , AM . 2006 . Occurrence of ochratoxin a in wine and ochratoxigenic mycoflora in grape and dried vine fruits in South America . International Journal of Food Microbiology , 111S1 : S5 – S9 .
- Cozzi , G , Pascale , M , Perrone , G , Visconti , A and Logrieco , A . 2006 . Effect of Lobesia botrana damages on black aspergilli rot and ochratoxin A content in grapes . International Journal of Food Microbiology , 111S1 : S88 – S92 .
- Cozzi , G , Perrone , G , Epifani , F , Pascale , M and Visconti , A . May 21–25 2007 . “ Epidemiology of ochratoxin A producing fungi in Apulian vineyards ” . In Poster 1422r presented at XII International IUPAC Symposium on Mycotoxins and Phycotoxins, 2007 May 21–25 , Istanbul
- Da Rocha Rosa , CA , Palacios , V , Combina , M , Fraga , ME , De Oliveira Rekson , A , Magnoli , CE and Dalcero , AM . 2002 . Potential ochratoxin A producers from wine grapes in Argentina and Brazil . Food Additives and Contaminants , 19 : 408 – 414 .
- Dalcero , A , Magnoli , C , Hallak , C , Chiacchiera , SM , Palacio , G and Rosa , CAR . 2002 . Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin by Aspergillus section Nigri in Argentina . Food Additives and Contaminants , 19 : 1065 – 1072 .
- Dimakopoulou , M , Tjamos , SE , Tjamos , EC and Antoniou , PP . 2005 . Chemical and biological control of sour rot caused by black aspergilli in the grapevine variety agiorgitico of Korinth region , 77 Poster presented at the International Workshop on Ochratoxin A in Grapes and Wine; Prevention and Control; Marsala (TP) Italy, 2005 October 20–21. .
- Esteban , A , Abarca , ML , Bragulat , MR and Cabañes , FJ . 2004 . Effects of temperature and incubation time on production of ochratoxin A by black aspergilli . Research in Microbiology , 155 : 861 – 866 .
- EFSA . 2006 . European Food Safety Authority. Opinion of the Scientific Panel on contaminants in the Food Chain of the EFSA on a request from the Commission related to ochratoxin A in food . EFSA J , 365 : 1 – 56 . Available: http://www.efsa.europa.eu/etc/medialib/efsa/science/contam/contam_opinions/1521.Par.0001.File.dat/contam_op_ej365_ochratoxin_a_food_en1.pdf. Accessed 5 September 2007
- European Commission . 2002 . SCOOP EC Directorate-General Health and Consumer Protection . Assessment of dietary intake of ochratoxin A by the population of EU Member States. Reports on tasks for scientific cooperation 153 pages. Available from: http://ec.europa.eu/food/fs/scoop/3.2.7_en.pdf. Accessed 5 September 2007
- European Commission . 2006 . Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs . Official Journal of the European Union , 364 : 5 – 24 .
- FAO/WHO Codex Alimentarius Commission . 2007 . Proposed draft code of practice for the prevention and reduction of ochratoxin A contamination in wine . Codex Committee on Contaminants in Foods. ALINORM 07/30/41 Session 1: 57–60. Available from: http://www.codexalimentarius.net/download/report/691/al30_41e[1].pdf. Accessed 5 September 2007
- Fermaud , M and Le Menn , R . 1989 . Association of Botrytis cinerea with Grape Berry Moth Larvae . Phytopatology , 79 : 651 – 656 .
- Fernandes , A , Venancio , A , Moura , F , Garrido , J and Cerdeira , A . 2003 . Fate of ochratoxin A during a vinification trial . Aspects of Applied Biology , 68 : 73 – 80 .
- Finoli , C , Vecchio , A , Scarpellini , M and Burruano , S . 2004 . Ochratoxin A occurrence in Italian wines of different origins . Rivista di Viticoltura e di Enologia , 57 : 63 – 77 .
- Garcia Moruno , E , Sanlorenzo , C , Boccacino , B and Di Stefano , R . 2005 . Treatment with yeast to reduce the concentration of ochratoxin A in red wine . American Journal of Enology and Viticulture , 56 : 73 – 76 .
- Grazioli , B , Fumi , MD and Silva , A . 2006 . The role of processing on ochratoxin A content in Italian must and wine: A study on naturally contaminated grapes . International Journal of Food Microbiology , 111S1 : S93 – S96 .
- Hocking , AD , Varelis , P , Pitt , JI , Cameron , S and Leong , S . 2003 . Occurrence of ochratoxin A in Australian wine . Australian Journal of Grape and Wine Research , 9 : 72 – 78 .
- IARC . 1993 . Monographs on the evaluation of carcinogenic risks to humans. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Lyon . France: International agency for research on cancer , 56 : 489 – 521 .
- JECFA . 6–15 February 2001 2001 . “ Joint FAO/WHO Expert Committee on Food Additives; 56th Meeting ” . 6–15 February 2001 , Geneva
- Jeswal , P . 1998 . Antidotal effect of grape juice (Vitis vinifera) on ochratoxin A caused hepatorenal carcinogenesis in mice (Mus musculus) . Cytobios , 93 : 123 – 128 .
- Kappes , ME , Serrati , L , Drouillard , JB , Cantus , JM and Kazantzidou , M . 2005 . “ Paper presented at the International Workshop on Ochratoxin A in Grapes and Wine ” . In A crop protection approach to Aspergillus and OTA management in Southern European vineyards , 24 Marsala (TP), , Italy : Prevention and Control . 2005 October 20–21.
- Leong , SL , Hocking , AD , Pitt , JI , Kazi , BA , Emmett , RW and Scott , ES . 2006a . Australian research on ochratoxigenic fungi and ochratoxin A . International Journal of Food Microbiology , 111S1 : S10 – S17 .
- Leong , SL , Hocking , AD , Varelis , P , Giannikopoulos , G and Scott , ES . 2006b . Fate of ochratoxin A during vinification of Semillon and Shiraz grapes . Journal of Agricultural and Food Chemistry , 54 : 6460 – 6464 .
- Leong , SL , Hocking , AD and Scott , ES . 2006c . The effect of juice clarification, static or rotary fermentation and fining on ochratoxin A in wine . Australian Journal of Grape and Wine Research , 12 : 245 – 251 .
- MAFF . 1997 . Ministry for agriculture, fisheries and food. Survey of aflatoxins and ochratoxin A in cereals and retail products , London : HMSO .
- MAFF . 1998 . Ministry for agriculture, fisheries and food. Survey of retail products for ochratoxin A , London : HMSO .
- Majerus , P and Otteneder , H . 1996 . Nachweis und Vorkommen von Ochratoxin A in Wein und Traubsaft . Dtsch Lebensm-Rundsch , 92 : 388 – 390 .
- Ospital , M , Cazabeil , JM , Betberder , AM , Tricard , C , Creppy , E and Medina , B . 1998 . L’Ochratoxine A dans les vins . Revue Française d’Oenologie , 169 : 16 – 18 .
- Otteneder , H and Majerus , P . 2000 . Occurrence of ochratoxin A (OTA) in wines: influence of the type of wine and its geographical origin . Food Additives and Contaminants , 17 : 793 – 798 .
- Perrone , G , Mulè , G , Susca , A , Battilani , P , Pietri , A and Logrieco , A . 2006a . Ochratoxin A production and AFLP analysis of Aspergillus carbonarius, Aspergillus tubingensis, and Aspergillus niger strains isolated from grapes in Italy . Applied and Environmental Microbiology , 72 : 680 – 685 .
- Perrone , G , Susca , A , Epifani , F and Mulè , G . 2006b . AFLP characterization of Southern Europe population of Aspergillus Section Nigri from grapes . International Journal of Food Microbiology , 111S1 : S22 – S27 .
- Perrone , G , Cozzi , G , Pascale , M , Logrieco , A and Visconti , A . May 21–25; 2007a . “ Lobesia botrana–an ochratoxin A risk factor in grapevine management ” . In presented at the XII International IUPAC Symposium on Mycotoxins and Phycotoxins; 2007 May 21–25; , Istanbul
- Perrone , G , Nicoletti , I , Pascale , M , De Rossi , A , De Girolamo , A and Visconti , A . 2007b . Positive correlation between high levels of ochratoxin A and resveratrol related compounds in red wines . Journal of Agricultural and Food Chemistry , 55 : 6807 – 6812 .
- Perrone , G , Susca , A , Epifani , F , Mulè , G and Logrieco , A . April 12–14; 2007c . “ Biodiversity of Aspergillus Section Nigri from grapes in Europe. Book of Abstracts of the Aspergillus International Workshop: “Aspergillus systematics in the genomic era”; 2007 ” . April 12–14; , 39 – 41 . Utrecht, , The Netherlands : CBS .
- Perrone , G , Varga , J , Susca , A , Frisvad , JC , Stea , G , Kocsubé , S , Tóth , B , Kozakiewicz , Z and Samson , RA . 2007d . Aspergillus uvarum sp. nov. an uniseriate black Aspergillus species isolated from grapes in Europe . International Journal of Systematic and Evolutionary Microbiology , In press
- Pietri , A , Bertuzzi , T , Pallaroni , L and Piva , G . 2001 . Occurrence of ochratoxin A in Italian wines . Food Additives and Contaminants , 18 : 647 – 654 .
- Ponsone , ML , Combina , M , Dalcero , A and Chulze , S . 2007 . Ochratoxin a and ochratoxigenic Aspergillus species in Argentinian wine trapes cultivated under organic and non-organic systems . International Journal of Food Microbiology , 114 : 131 – 135 .
- Rousseau , J . 2004 . Ochratoxin A in wines: current knowledge. Vinidea.net . Wine Internet Technical Journal (5) , Available: http://www.infowine.com/default.asp?scheda=1038. Accessed 5 September 2007
- Savino , M , Limosani , P and Garcia-Moruno , E . 2007 . Reduction of ochratoxin A contamination in red wines by oak wood fragments . American Journal of Enology and Viticulture , 58 ( 1 ) : 97 – 101 .
- Soufleros , EH , Tricard , C and Bouloumpasi , EC . 2003 . Occurrence of ochratoxin A in Greek wines . Journal of the Science of Food and Agriculture , 83 : 173 – 179 .
- Solfrizzo , M , Panzarini , G , Pascale , M and Visconti , A . 2006 . “ Determination of ochratoxin A in grape and winery by-products by immunoaffinity column cleanup and HPLC/fluorescence detection ” . San Francisco, CA Paper presented at the 232nd American Chemical Society National Meeting & Exposition. September 10–14, 2006, Section D, no. 140
- Solfrizzo , M , Panzarini , G and Visconti , A . May 21–25 2007 . “ Fate of ochratoxin A during vinification of naturally contaminated primitivo and negroamaro grapes ” . In Paper presented at the XIIth International IUPAC Symposium on Mycotoxins and Phycotoxins May 21–25 , 1425 Istanbul, , Turkey
- Tateo , F and Bononi , M . Survey on ochratoxin A in wine. More data concerning bottled red wines . Le Bulletin de l’OIV , 74 766 – 778 .
- Tjamos , SE , Antoniou , PP , Kazantzidou , A , Antonopoulos , DF , Papageorgiou , I and Tjamos , EC . 2004 . Aspergillus niger and Aspergillus carbonarius in Corinth raisin and wine-producing vineyards in Greece: population composition, ochratoxin A production and chemical control . Journal of Phytopatology , 152 : 250 – 255 .
- Ueno , Y . 1998 . Residue and risk of ochratoxin A in human plasma and beverages in Japan . Mycotoxins , 47 : 25 – 32 .
- Visconti , A and De Girolamo , A . 2005 . Fitness for purpose–Ochratoxin A analytical developments . Food Additives and Contaminants Suppl , 1 : 37 – 44 .
- Visconti , A , Pascale , M and Centonze , G . 1999 . Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and high-performance liquid chromatography . Journal of Chromatography A , 864 : 89 – 101 .
- Visconti , A , Pascale , M and Centonze , G . 2001 . Determination of ochratoxin A in wine and beer by immunoaffinity column cleanup and liquid chromatographic analysis with fluorometric detection: collaborative study . Journal of the Association of Official Analytical Chemists International , 84 : 1818 – 1827 .
- Zimmerli , B and Dick , R . 1996 . Ochratoxin A in table wine and grape juice: occurrence and risk assessment . Food Additives and Contaminants , 13 : 655 – 668 .