Abstract
Purpose: The purpose of this study was to assess the pain relief in patients with unresectable and recurrent colorectal cancer treated with radiation plus 8 MHz radiofrequency-capacitive regional hyperthermia and to identify predictors of the good outcome.
Methods: Between February 1986–May 2003, 41 patients with primarily unresectable and recurrent colorectal cancer that caused pain were treated with thermoradiotherapy at the hospital and retrospectively analysed. Radiotherapy was administered with a mean total radiation dose of 56 Gy. Hyperthermia was usually applied within 30 min after radiotherapy once or twice a week. For cooling of the skin surface, the overlay boluses were applied in addition to regular boluses. The external cooling unit has been used to reinforce the cooling ability of the overlay bolus and achieve strong surface cooling to reduce the preferential heating of the subcutaneous fat tissue and treat with more RF-output in 17 patients since January 1997.
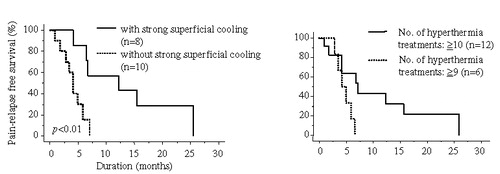
Results: Pain relief was obtained in 83% of the patients. Multi-variate analysis by logistic regression to evaluate the effects of certain factors on pain relief (complete response + good response) was strongly correlated with the presence of radiating pain to leg(s) (p < 0.05). The median follow-up was 18 months. The median duration of pain relief was 7.0 months. For the 27 patients in whom the tumour temperature was estimated, the median duration of pain relief was 14.6 months for the patients with a mean average tumour temperature of >42.5°C and 5.7 months for those of <42.5°C (p < 0.05). In the 18 patients with radiating pain to leg(s), use of strong superficial cooling and the higher numbers of hyperthermia treatments were better prognostic factors for the duration of pain relief (p < 0.01 and p < 0.05, respectively).
Conclusions: Radiotherapy with 8 MHz radiofrequency-capacitive regional hyperthermia provided an efficient, effective means on pain relief of treating unresectable and recurrent colorectal cancer. The duration of pain relief can be prolonged, if an adequate heating is achieved, especially in the patients with radiating pain to the leg(s).
Introduction
Approximately 10% of patients with colorectal cancer present with locally advanced disease that frequently compromises the ability to perform a complete surgical resection and, therefore, adversely affects survival Citation[1–6]. In addition, 30–40% of all patients originally resected for cure will develop recurrent disease. Most patients with unresectable and recurrent colorectal cancer have intractable pain. Palliative radiotherapy (RT) is one of the most commonly used therapies to relieve this symptom within this clinical context Citation[7], Citation[8]. However, the median duration of symptom control after RT for local recurrence is short, ranging from 3–6 months, which is not satisfactory for the patients Citation[9], Citation[10].
Nishimura et al. Citation[11] reported that the efficacy of hyperthermia including 8 MHz radiofrequency (RF)-capacitive regional hyperthermia in conjunction with RT in patients with unresectable and recurrent colorectal cancer; the incidence of freedom from local tumour regression at 6 months after the treatment was 59% for RT plus hyperthermia and 37% for RT alone. On the authors’ previous results, 8 MHz RF-capacitive regional hyperthermia with strong superficial cooling was potentially useful for the improvement of treatment results as for local tumour response and survival rate in unresectable and recurrent colorectal cancers Citation[12]. However, reports as for pain relief of thermoradiotherapy were few and the potential prognostic factors have been scarcely evaluated Citation[13–15]. At present, it is unclear whether it would be possible to select patients who have a better chance of benefiting as pain relief and its duration from this combined treatment. The purpose of this study was to assess the pain relief in patients with unresectable and recurrent colorectal cancer treated with 8 MHz RF-capacitive regional hyperthermia plus RT and to identify predictors of the good outcome.
Materials and methods
Patients
Between February 1986–May 2003, 41 patients with primarily unresectable or locally recurrent colorectal cancer that caused pain were treated with thermoradiotherapy at the hospital. Their medical records were evaluated retrospectively. During the same periods, 18 cases with primarily unresectable or locally recurrent colorectal cancer that caused pain were treated with radiotherapy alone because of the following reasons: obesity as subcutaneous fat thickness more than 3 cm in 10 cases, higher age in three, poor general condition in three and patients’ refusal in two. Treatments and characteristics of patients are given in . Four patients had newly diagnosed unresectable colorectal cancer (T4N2M0 in two patients, T3N1M1 in two) and 37 had recurrent disease. The primary lesion consisted of three sigmoid colons and 34 rectums. Histological diagnosis was all adenocarcinoma. The extra-pelvic distant metastasis was recognized in 16 patients. Liver metastasis was found in seven, lung metastasis in six and the lymph node distant metastasis in six. The ECOG performance status, the tumour size and the extra-pelvic distant metastasis were evaluated at the start of the treatment. To categorize the intensity of pain, a 5-point pain scale was used to classify the intensity of pain as follows: mild, moderate, severe, very severe and excruciating Citation[15], Citation[16]. Severity of pain and pain medication at the start of combined treatment are summarized in . In 18 patients, the pain was radiating to one leg or both legs, indicating nerve invasion or compression. In 15 patients, there was clear evidence of bone invasion evaluated with CT and/or MRI. Six patients were treated with concomitant chemotherapy during the course of RT, although no specific chemotherapy protocol existed. They received the following chemotherapy: 5-Fluorurscil in combination with Mitomycin C in two patients; 5-Fluorurscil in combination with Cisplatin in one patient; 5-Fluorurscil in combination with Levofolinate calcium in one patient; 5-Fluorurscil in combination with Leucovorin in one patient; Mitomycin C alone in one patient.
Table I. Characteristis of patients and treatment.
Table II. Pain score and pain medication at the start of combined treatment.
Toxicity was scored according to the criteria of the radiation therapy oncology group, except hyperthermia-related toxicity of skin burn Citation[17]. Criteria for acute dermatitis were as follows; grade 2: tender or bright erythema, patchy moist desquamation/moderate oedema; grade 3: confluent, moist desquamation other than skin folds, pitting oedema; and grade 4: ulceration, haemorrhage or necrosis. Criteria for cystitis were as follows: grade 2: frequency of urination or nocturia that is less frequent than every hour, dysuria, urgency or bladder spasm requiring local anaesthetic; grade 3: frequency with urgency and nocturia hourly or more frequently/dysuria, pelvis pain or bladder spasm requiring regular, frequent narcotic/gross haematuria with/without clot passage; and grade 4: haematuria requiring transfusion, acute bladder obstruction not secondary to clot passage, ulceration or necrosis. Criteria for gastrointestinal toxicity were as follows: grade 2: diarrhoea requiring parasympatholytic drugs, rectal or abdominal pain requiring analgesics; grade 3: diarrhoea requiring parenteral support, abdominal distention (flat plate radiograph demonstrates distended bowel loops); and grade 4: acute or sub-acute obstruction, fistula or perforation, gastrointestinal bleeding requiring transfusion, abdominal pain or tenesmus requiring tube decompression or bowel diversion. Grade 1 toxicity, which is not clinically problematic, was not evaluated. The hyperthermia-related skin burn was defined as a subcutaneous induration.
Hyperthermia
Hyperthermia was usually applied within 30 min after RT once or twice a week and started on the median of 8 days (range 0–22 days) after the initial date of radiation therapy. In some early cases or cases with relatively less radiofrequency output or heating time, hyperthermia was applied twice weekly. Heating duration was 40–60 min (mean 46 min). Number of treatments ranged from 3–38 times (mean 10 ± 6.8). Patients were treated with concomitant hyperthermia during the course of RT in principle. In six cases, however, hyperthermia was continued 5–21 times (median 14) after the completion of radiotherapy because of the following reasons; insufficient tumour response in three cases, expectation of enhancement effect for continuing chemotherapy in two and treatment of incontinence which was refractory with any other treatments but markedly improved with hyperthermia in one. The physical features of the RF-8 clinical hyperthermia machine (Thermotron RF-8, Yamamoto Vinita Co., Osaka, Japan) and thermal distribution characteristics in phantom as well as in the human body when heating with this device have been reported previously Citation[18], Citation[19]. In most cases, upper electrodes was 25 cm, lower was 30 cm in diameter, placed on opposite sides of the pelvic region. The output power ranged from 500–1500 W. A treatment posture of all cases was prone position. For the reduction of the preferential heating of the subcutaneous fat tissue, the overlay boluses were applied in addition to regular boluses attached in front of the metal electrodes. Before December 1996, the liquid of overlay boluses had been cooled only by the circulatory system of the RF-8 during heating. The external cooling unit (manufactured by Yamamoto Viniter Co., Osaka, Japan) has been used to reinforce the cooling ability of the overlay bolus and achieve strong surface cooling in 17 patients since January 1997. The features of the device and its usefulness have been reported previously Citation[12], Citation[20].
The temperature was measured in the 27 patients using a 4-point micro-thermocouple-sensor, which was inserted into the tumour through a 21-gauge catheter. In clinically impossible cases, temperature in a tumour was estimated by detaining a 4-point micro-thermocouple-sensor in the rectum, bladder or vagina. The maximum intra-tumour temperature (Tmax) was defined as the maximum tumour temperature obtained in the tumour during the steady state and at the end of treatment. The steady state was defined 20 min after the start of heating. The minimum intra-tumour temperature (Tmin) was defined as the minimum tumour temperature obtained by the same method. The averages of these parameters (Tave) were calculated over the steady state for a given tumour. The thermometric parameters were measured every time of hyperthermia treatment in seven patients, while in the remaining 20 patients the number of measurements ranged from one-to-five times. The median of all 27 cases was three times.
Radiation
All patients were treated with external pelvic RT using a 10-MV linear accelerator. A mean total radiation dose was 56 ± 10.5 Gy. The fractions were 1.6–2.0 Gy daily, given 5 days per week. In the majority of the patients, an irradiation dose of 40–50 Gy was given through anterior-posterior portals encompassing the tumour with a margin and 10–20 Gy boost dose delivered to the tumour with various techniques. Only three of the 37 recurrent tumours had been treated with prior radiotherapy and the total dose was 50.4–70 Gy. Re-irradiation was limited to gross disease with various techniques to minimize the dose to normal tissues.
Evaluation of pain relief
A complete response was defined as the complete disappearance of pain without any pain medication for at least 1 month. A good response was defined as either a pain reduction (with equal pain medication) by at least 2-points on the 5-point pain scale or a reduction by at least 1-point if accompanied by a worthwhile decrease (in potency and/or quantity) in pain medication, both for at least 1 month. A minor response was defined as a decrease of pain and/or pain medication for at least 1 month, but less than the decrease in pain defined as a good response. No change meant that there was no decrease in pain or pain medication or that the pain relief was <1 month Citation[15]. The term duration of pain relief was defined as the interval between the initial date of radiation therapy and the date of the first documentation of increased pain or increased dosage of the analgesic after the best response of pain relief. These response and duration of pain relief were assessed using clinical records described by attending physicians or radiation oncologists.
Evaluation of tumour response
The change in tumour volume was evaluated mainly by CT scan, which was performed before and every 1–3 months after treatment and the degree of tumour response was determined when the maximum regression of the tumour continued at least for 4 weeks. Grading of tumour response was as follows: complete tumour regression was designated as complete response (CR), ≥50% regression in volume as partial response (PR), <50% response or <25% increase as no change (NC) and ≥25% regression as progressive disease (PD).
Statistical analysis
Logistic regression analyses were used to compare pain relief (complete response + good response) with tumour size, radiating pain to leg(s), bone invasion, performance score, metastatic disease, radiation total dose, number of hyperthermia treatments, strong superficial cooling and chemotherapy. Kaplan-Meier methods were used to estimate the duration of pain relief. To identify prognostic factors for the duration of pain relief, univariate and multi-variate analyses were performed. Log-rank tests were used to determine which covariates were univariately predictive of time to pain relapse. Cox's proportional hazards regression model was used for multi-variate analysis. A value of p < 0.05 was considered as significant difference.
Results
Complete response for pain relief was achieved in nine patients (22%), a good response in 15 patients (37%), a minor response in 10 patients (24%), no change in six patients (15%) and progressive pain in one patient (2%) (Tables ). The initial and the maximum response of pain relief were achieved a median of 17.6 days (range 2–64 days) and 34.4 days (range 2–83 days) after the initiation of radiation therapy, respectively. shows multi-variate analysis by logistic regression to evaluate effects of certain factors on pain relief (complete response + good response). Pain relief was strongly correlated with presence of radiating pain to leg(s) (p < 0.05). The tumour response was CR of two patients, PR of 14 and NC of 25.
Table III. Results of pain relief.
Table IV. Separate data of pain score (a) and pain medication (b) for pre- and post-treatment.
Table V. Multi-variate analysis by logistic regression to evaluate effects of certain factors on pain relief (complete response + good response) in all patients.
The follow-up periods were 5–42 (median 18) months. The median duration of pain relief was 7.0 months (range 0.8–29.1). summarizes the univariate and multi-variate analysis for the duration of pain relief. None of the potentially prognostic factors analysed were statistically significant for the achievement of duration of pain relief. For the 27 patients who could be estimated, the tumour temperature, Tave ≥ 42.5°C, was a statistically significant superior prognostic factor for the duration of pain relief (). The median duration of pain relief was 14.6 months (range 2.5–29.1) for patients with Tave ≥ 42.5°C and 5.7 months (range 1.8–11.3) for those with Tave<42.5°C (p < 0.05) (). In the 18 patients with radiating pain to leg(s), strong superficial cooling and higher number of hyperthermia treatments were superior prognostic factor for the duration of pain relief (, ). The median durations of pain relief were 9.4 (range 1.0–25.6) vs. 3.8 (range 0.8–7.0) months for with and without strong superficial cooling (p < 0.01), respectively, and were 6.8 (range 0.8–25.6) vs. 4.1 (range 2.8–6.5) months for number of hyperthermia treatments ≥10 and ≤9 (p < 0.05), respectively.
Table VI. Univariate and multi-variate analysis of certain factors on duration of pain relief in all patients.
Table VII. Univariate analysis of certain factors on duration of pain relief in patients who performed measurement of tumour temperature.
Table VIII. Univariate analysis of certain factors on duration of pain relief in patients with radiating pain to leg(s).
Figure 1. Duration of pain relief of patients with Tave ≥ 42.5°C compared with that of patients with Tave < 42.5°C using the Kaplan-Meier method and analysed with the log-rank test. The duration of patients with Tave ≥ 42.5°C was significantly better than that of patients with Tave < 42.5°C (p < 0.05).
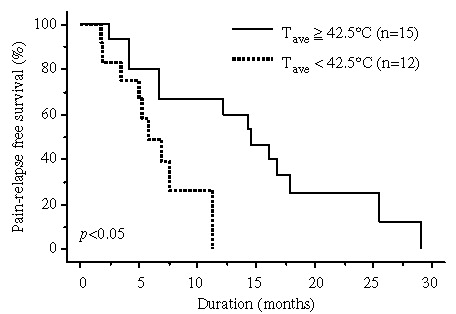
Figure 2. As for the 18 patients with radiating pain to leg(s), strong superficial cooling (a) and higher number of hyperthermia treatments (b) were superior prognostic factor for the duration of pain relief using the Kaplan-Meier method and analysed with the log-rank test.
Regarding the correlation between tumour temperature and tumour response in the 27 patients, all 10 patients with tumour response of CR or PR showed Tmax ≥ 43°C and Tave ≥ 42.5°C, while Tmax ≥ 43°C and Tave ≥ 42.5°C was recorded in 12 of the 17 patients with tumour response of NC. Six of 10 patients with tumour response of CR or PR showed Tmin ≥ 42°C, while Tmin ≥ 42°C was achieved in only two of 17 patients with tumour response of NC.
Acute toxicities greater than grade 2 included acute dermatitis in two patients (grade 3) and cystitis in one (grade 3). Acute gastrointestinal toxicities greater than grade 2 were not recognized. Acute toxicities of grade 4 were not observed. Skin burn as a subcutaneous induration was seen in three patients and disappeared spontaneously after completion of combined therapy. In the chronic phase, obstructive ileus was observed in four patients and intestinal fistula in one. Three of four obstructive ileus cases and an intestinal fistula case occurred in the patients with re-recurrent colon cancer.
Discussion
This study investigated the efficacy of thermoradiotherapy for unresectable and recurrent colorectal cancer for the purpose of pain relief and showed that pain relief was obtained in 83% of patients and the median duration was 7 months. The results published on a pain relief from RT either with or without hyperthermia in patients with colorectal cancer are summarized in . There have already been two randomized studies on pain relief of advanced colorectal cancer Citation[13], Citation[14], which have demonstrated the efficacy of radiation plus hyperthermia. First, Berdov and Menteshashvili Citation[13] enforced in 56 patients with locally advanced rectal cancer as a pre-operative treatment using intra-luminal microwave hyperthermia and found pain relief in 32% of patients, who were treated with RT alone, compared with 86% of patients who were treated with RT plus hyperthermia. Second, van der Zee and Gonzalez Gonzalez Citation[14] observed a difference, mainly in the duration of a palliative effect for patients with rectal carcinoma (mostly recurrences); after RT alone, palliative effect was achieved in 59% of patients for a median of 7 months, while 68% of patients experienced palliative effect for a median of 17 months after RT with RF-capacitive regional hyperthermia. The results of the previous studies as well as these results suggest that the duration of pain relief may be longer for RT with hyperthermia than for RT alone.
Table IX. Pain relief, dulation of pain relief and tumour response after radiotherapy with or without hyperthermia for colorectal cancer in previous reports.
The existence of a relationship between thermal parameters and tumour response or control following combined hyperthermia and RT has been reported by several authors Citation[21–26]. Nishimura and Hiraoka Citation[24] reported that the relationship between Tave and tumour response for advanced colorectal cancer treated by thermoradiotherapy; a local tumour response late (CR + PR) of 67% was obtained in the tumour with Tave > 42°C. The authors previously developed RF-capacitive regional hyperthermia with their own external cooling unit and achieved strong superficial cooling for the reduction of the preferential heating of the subcutaneous fat tissue and reported its usefulness of possibility to treat with more RF outputs and obtain significantly improving temperature rise of the tumour, local tumour response and survival rate in unresectable and recurrent colorectal cancers Citation[12], Citation[20]. In this study, the tumour with Tave > 42.5°C were statistically significant superior prognostic factors for the duration of pain relief. It was confirmed that thermoradiotherapy, if achieved tumour temperature rise of therapeutic range, is useful for pain relief as well as local tumour control in unresectable and recurrent colorectal cancers.
The lumbosacral plexus, situated at the upper lumbar region down to the sacrum, is particularly prone to injury. Pain from neural injury is a deafferentation pain and it often is poorly tolerated and difficult to control by morphinomimetics. Malignant neoplasm is one of the disease conditions that cause secondary lumbosacral neuropathy Citation[27]. Major clinical manifestation of carcinomatous lumbosacral neuropathy includes radiating pain to leg(s) with or without accompanying unilateral or bilateral paresis/paralysis. Some papers reported the treatment results of RT for carcinomatous lumbosacral neuropathy, pain relief was good and obtained in the patients of 77.4–100% and the median duration of response was 4.1–6.5 months Citation[28–30]. Russi et al. Citation[29] reported that complete remission of pain relief was obtained 1 month after the initial date of RT in all the 13 patients with lumbosacral carcinomatous neuropathy, the median duration of response was 6.5 months. In this study, pain relief was strongly correlated with presence of radiating pain to leg(s) and the median duration of pain relief was significantly prolonged by adding strong superficial cooling or higher number of hyperthermia treatments for the patients with radiating pain to leg(s). It seems that an effect of thermoradiotherapy, particularly if an adequate heating was undergone, is favourable for this kind of pain.
As for RT, the dose-response relationship for symptom relief is controversial Citation[7], Citation[8], Citation[10], Citation[31]. Several studies reported that there was a dose-response relationship with RT in the management of recurrent rectal cancer Citation[7], Citation[10]. However, Wong et al. Citation[8] performed a systematic study and reported that there was no significant difference between patients receiving a dose of 45.5 Gy and patients receiving <45 Gy for pain relief. In this study, although many cases had been irradiated with more than 50 Gy with various techniques, the symptomatic response rates were not dose-related.
A randomized study by Moertel et al. Citation[32] reported that the addition of 5-fluorouracil showed significantly longer survival rates than RT alone for locally unresectable colon and rectal cancer, although several other randomized studies reported no benefit of the addition of chemotherapy in overall survival and symptom control and showed that there was an increase in toxicity on the addition of chemotherapy Citation[33–35]. Recently, chemoradiotherapy using a continuous intravenous infusion of 5-fluorouracil was shown to be effective on pain relief and had a low incidence of severe toxicity in patients with recurrent rectal cancer Citation[36]. In the present study, the improvement of outcomes by chemotherapy was not confirmed. However, since only six patients were treated with concomitant chemotherapy and no specific chemotherapy protocol existed, it was not evaluated adequately. It is recognized that pre-operative chemoradiotherapy with hyperthermia for rectal cancer can increase resectability and decrease local recurrence Citation[37–39]. Further studies as for pain relief using chemoradiotherapy plus hyperthermia are necessary in patients with unresectable and recurrent colorectal cancer.
A Dutch phase III trial failed to show improvement of tumour response and survival in patients with rectal cancer by adding hyperthermia to RT, although a major survival benefit was obtained in patients with cervical cancer Citation[40]. As discussed elsewhere, the value of hyperthermia in these patients might have been under-estimated for various reasons such as lack of thermal data analysis and the problem of patients who received insufficient hyperthermia treatment Citation[14]. Gonzalez Gonzalez et al. Citation[41] evaluated 72 patients with unresectable or recurrent colorectal cancer treated with RT with hyperthermia and also described that the most disappointing fact was an insufficient increase in intra-tumoural temperature with the available hyperthermia equipment. Although this study was a retrospective study in a single institution and did not include a control group of patients who were treated with RT alone, one can still suggest that if adequate heating such as tumour temperature rise of therapeutic range and use of strong superficial cooling was achieved, the duration of pain relief were prolonged significantly, particularly for the patients with radiating pain to leg(s). It is believed that future clinical research of hyperthermia requires more precise evaluation of temperature data. Recently, feasibility of non-invasive thermography in a hyperthermia/magnetic resonance hybrid system is reported and further development for clinical applications is expected Citation[42], Citation[43]. In addition, clinical studies using RF-8 with the reinforced superficial cooling may improve the treatment outcome of deep-seated tumours.
Currently, standardized reporting systems to measure pain or quality of life (QoL) in colorectal cancers, such as the Brief Pain Inventory (BPI), Functional Assessment of Cancer Therapy-colorectal (FACT-C) questionnaire and European Organization for Research and Treatment of Cancer QoL questionnaire (EORTC) have been proposed and used in several studies Citation[50–54], although they were not applied to this study. In a study of post-treatment pain control for locally recurrent rectal cancers Citation[51], not only BPI but also each patient's reported level of pain with the most potent analgesic prescribed was evaluated using the pain-management index to assess the adequacy of pain management more objectively. Since increase or decrease in quantity and/or change of analgesic was often recognized after the start of treatment, this study also evaluated alteration of pain medication, response and duration of pain relief, which have not been included in the evaluation of most previous reports as for radiotherapy with or without hyperthermia. Future clinical trials of hyperthermia for pain relief should evaluate pain medication more objectively like this study, in addition to use of standardized scales for QoL and pain.
In conclusion, the results of this study have confirmed that RT with 8-MHz radiofrequency-capacitive regional hyperthermia provided an efficient, effective means on pain relief of treating unresectable and recurrent colorectal cancer. If an adequate heating such as tumour temperature rise of therapeutic range, use of strong superficial cooling and higher number of hyperthermia treatments was achieved, the duration of pain relief may be prolonged, particularly for the patients with radiating pain to leg(s).
References
- Curley SA, Carlson GW, Shumate CR, Wishnow KI, Ames FC. Extended resection for locally advanced colorectal carcinoma. American Journal of Surgery 1992; 163: 553–559
- Sugarbaker CD. Coincident removal of additional structures in resections for carcinoma of the colon and rectum. Annals of Surgery 1946; 123: 1036–1046
- Polk HC, Jr. Extended resection for selected adenocarcinomas of the large bowel. Annals of Surgery 1972; 175: 892–899
- Eldar S, Kemeny MM, Terz JJ. Extended resections for carcinoma of the colon and rectum. Surgical Gynecology & Obstetrics 1985; 161: 319–322
- Davies GC, Ellis H. Radical surgery in locally advanced cancer of the large bowel. Clinical Oncology 1975; 1: 21–26
- Bonfanti G, Bozzetti F, Doci R, Baticci F, Marolda R, Bignami P, Gennari L. Results of extended surgery for cancer of the rectum and sigmoid. British Journal of Surgery 1982; 69: 305–307
- Knol HP, Hanssens PE, Rutten HJ, Wiggers T. Effect of radiation therapy alone or in combination with surgery and/or chemotherapy on tumor and symptom control of recurrent rectal cancer. Strahlentherapie Onkologie 1997; 173: 43–49
- Wong R, Thomas G, Cummings B, Froud P, Shelley W, Withers R, Williams J. In search of a dose-response relationship with radiotherapy in the management of recurrent rectal carcinoma in the pelvis: A systematic review. International Journal of Radiation Oncology, Biology & Physics 1998; 40: 437–446
- Allum WH, Mack P, Priestman TJ, Fielding JW. Radiotherapy for pain relief in locally recurrent colorectal cancer. Annals of the Royal College of Surgery, England 1987; 69: 220–221
- Overgaard M, Overgaard J, Sell A. Dose–response relationship for radiation therapy of recurrent, residual and primarily inoperable colorectal cancer. Radiotherapy & Oncology 1984; 1: 217–225
- Nishimura Y, Hiraoka M, Akuta K, Jo S, Nagata Y, Masunaga S, Takahashi M, Abe M. Hyperthermia combined with radiation therapy for primarily unresectable and recurrent colorectal cancer. International Journal of Radiation Oncology, Biology & Physics 1992; 23: 759–768
- Ohguri T, Imada H, Yahara K, Kakeda S, Tomimatsu A, Kato F, Nomoto S, Terashima H, Korogi Y. Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. International Journal of Hyperthermia 2004; 20: 465–475
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. International Journal of Hyperthermia 1990; 6: 881–890
- van der Zee J, Gonzalez Gonzalez D. Regional hyperthermia for rectal cancer. Lancet 2000; 356: 771–772
- Juffermans JH, Hanssens PE, van Putten WL, van Rhoon GC, van Der Zee J. Reirradiation and hyperthermia in rectal carcinoma: A retrospective study on palliative effect. Cancer 2003; 98: 1759–1766
- Twycross RG, Fairfield S. Pain in far-advanced cancer. Pain 1982; 14: 303–310
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). International Journal of Radiation Oncology, Biology & Physics 1995; 31: 1341–1346
- Song CW, Rhee JG, Lee CK, Levitt SH. Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). International Journal of Radiation Oncology, Biology & Physics 1986; 12: 365–372
- Lee CK, Song CW, Rhee JG, Foy JA, Levitt SH. Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: Results of a phase I/II study. International Journal of Radiation Oncology, Biology & Physics 1995; 32: 733–745
- Tomimatsu A, Imada H, Kosaka K, Nomoto S, Kusano S, Ostapenko VV, Terashima H. Advantage of external cooling unit in deep hyperthermia using an 8 MHz RF capacitive heating device. Japanese Journal of Hyperthermic Oncology 1999; 15: 65–70
- Valdagni R, Liu FF, Kapp DS. Important prognostic factors influencing outcome of combined radiation and hyperthermia. International Journal of Radiation Oncology, Biology & Physics 1988; 15: 959–972
- Leopold KA, Dewhirst M, Samulski T, Harrelson J, Tucker JA, George SL, Dodge RK, Grant W, Clegg S, Prosnitz LR. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. International Journal of Radiation Oncology, Biology & Physics 1992; 22: 989–998
- Oleson JR, Sim DA, Manning MR. Analysis of prognostic variables in hyperthermia treatment of 161 patients. International Journal of Radiation Oncology, Biology & Physics 1984; 10: 2231–2239
- Nishimura Y, Hiraoka M. Relationship between thermometry results and tumor response in thermoradiotherapy. Japanese Journal of Hyperthermic Oncology 1998; 14: 162–169
- Takeshita N, Tanaka Y, Matsuda T. Thermoradiotherapy for adenocarcinoma of the rectum and sigmoid—application to primarily inoperable and recurrent cases. Nippon Igaku Hoshasen Gakkai Zasshi 1992; 52: 472–482
- Shimizu T, Tanaka Y, Nishimura Y, Hiraoka M, Wada S, Terashima H, Tamura H, Hashida I, Suyama S, Muramoto H, Sueyama H. Thermoradiotherapy for colorectal carcinoma. Journal of the Japanese Society of Therapy in Radiology & Oncology 1995; 7: 143–150
- Jaeckle KA, Young DF, Foley KM. The natural history of lumbosacral plexopathy in cancer. Neurology 1985; 35: 8–15
- Ampil FL. Palliative irradiation of carcinomatous lumbosacral plexus neuropathy. International Journal of Radiation Oncology, Biology & Physics 1986; 12: 1681–1686
- Russi EG, Pergolizzi S, Gaeta M, Mesiti M, D’Aquino A, Delia P. Palliative-radiotherapy in lumbosacral carcinomatous neuropathy. Radiotherapy & Oncology 1993; 26: 172–173
- Taniguchi M, Kaneyasu Y, Karasawa K, Fukuhara N, Tanaka M, Kita-Okawa M, Okawa T. Analysis of radiotherapy for lumbosacral carcinomatous neuropathy. Journal of the Japanese Society of Therapy in Radiology & Oncology 1996; 8: 195–204
- Ciatto S, Pacini P. Radiation therapy of recurrences of carcinoma of the rectum and sigmoid after surgery. Acta Radiologica Oncologia 1982; 21: 105–109
- Moertel CG, Childs DS, Jr, Reitemeier RJ, Colby MY, Jr, Holbrook MA. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969; 2: 865–867
- Danjoux CE, Gelber RD, Catton GE, Klaassen DJ. Combination chemoradiotherapy for residual, recurrent or inoperable carcinoma of the rectum: E.C.O.G. study (EST 3276). International Journal of Radiation Oncology, Biology & Physics 1985; 11: 765–771
- Dunst J. Radiotherapy versus radiotherapy plus 5-FU in inoperable or recurrent rectal cancer. Strahlentherapie Onkologie 1994; 170: 245–246
- Wong CS, Cummings BJ, Keane TJ, Dobrowsky W, O'Sullivan B, Catton CN. Combined radiation therapy, mitomycin C and 5-fluorouracil for locally recurrent rectal carcinoma: Results of a pilot study. International Journal of Radiation Oncology, Biology & Physics 1991; 21: 1291–1296
- Ito Y, Ohtsu A, Ishikura S, Boku N, Nihei K, Ogino T, Ikeda H. Efficacy of chemoradiotherapy on pain relief in patients with intrapelvic recurrence of rectal cancer. Japanese Journal of Clinical Oncology 2003; 33: 180–185
- Rau B, Wust P, Hohenberger P, Loffel J, Hunerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, Felix R, Schlag PM. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: A phase II clinical trial. Annals of Surgery 1998; 227: 380–389
- Riess H, Loffel J, Wust P, Rau B, Gremmler M, Speidel A, Schlag P. A pilot study of a new therapeutic approach in the treatment of locally advanced stages of rectal cancer: Neoadjuvant radiation, chemotherapy and regional hyperthermia. European Journal of Cancer 1995; 31: 1356–1360
- Ohno S, Tomoda M, Tomisaki S, Kitamura K, Mori M, Maehara Y, Sugimachi K. Improved surgical results after combining preoperative hyperthermia with chemotherapy and radiotherapy for patients with carcinoma of the rectum. Diseases of the Colon & Rectum 1997; 40: 401–406
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Gonzalez Gonzalez D, van Dijk JD, Blank LE. Radiotherapy and hyperthermia. European Journal of Cancer 1995; 3: 1351–1355
- Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, Felix R, Wust P. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. International Journal of Radiation Oncology, Biology & Physics 2005; 61: 267–277
- Peller M, Kurze V, Loeffler R, Pahernik S, Dellian M, Goetz AE, Issels R, Reiser M. Hyperthermia induces T1 relaxation and blood flow changes in tumors. A MRI thermometry study in vivo. Magnetic Resonance Imaging 2003; 21: 545–551
- Williams IG, Shulman IM, Todd IP. The treatment of recurrent carcinoma of the rectum by supervoltage x-ray therapy. British Journal of Surgery 1957; 44: 506–508
- Smedal MI, Wright KA, Siber FJ. The palliative treatment of recurrent carcinoma of rectum and rectosigmoid with 2 mv radiation. Some results and description of a technique. American Journal of Roentgenology & Radium Therapy in Nuclear Medicine 1967; 100: 904–908
- Ciatto S, Pacini P. Radiation therapy of recurrences of carcinoma of the rectum and sigmoid after surgery. Acta Radiologica Oncologica 1982; 21: 105–109
- James RD, Johnson RJ, Eddleston B, Zheng GL, Jones JM. Prognostic factors in locally recurrent rectal carcinoma treated by radiotherapy. British Journal of Surgery 1983; 70: 469–472
- Guiney MJ, Smith JG, Worotniuk V, Ngan S, Blakey D. Radiotherapy treatment for isolated loco-regional recurrence of rectosigmoid cancer following definitive surgery: Peter MacCallum Cancer Institute experience, 1981–1990. International Journal of Radiation Oncology, Biology & Physics 1997; 38: 1019–1025
- Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, Nakatsugawa S. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. International Journal of Hyperthermia 1990; 6: 719–740
- Cleeland CS, Gonin R, Hatfield AK, Edmonson JH, Blum RH, Stewart JA, Pandya KJ. Pain and its treatment in outpatients with metastatic cancer. New England Journal of Medicine 1994; 330: 592–596
- Esnaola NF, Cantor SB, Johnson ML, Mirza AN, Miller AR, Curley SA, Crane CH, Cleeland CS, Janjan NA, Skibber JM. Pain and quality of life after treatment in patients with locally recurrent rectal cancer. Journal of Clinical Oncology 2002; 20: 4361–4367
- Allal AS, Gervaz P, Gertsch P, Bernier J, Roth AD, Morel P, Bieri S. Assessment of quality of life in patients with rectal cancer treated by preoperative radiotherapy: A longitudinal prospective study. International Journal of Radiation Oncology, Biology & Physics 2005; 61: 1129–1135
- Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. Quality of life among disease-free survivors of rectal cancer. Journal of Clinical Oncology 2004; 22: 354–360
- Camilleri-Brennan J, Steele RJ. Related articles, objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Disease 2002; 4: 61–66