Abstract
The experimental validation of a model-based, thermal therapy control system which automatically and simultaneously achieves the specified efficacy and safety objectives of the treatment is reported. MR-thermometry measurements are used in real-time to control the power of a stationary, focused ultrasound transducer in order to achieve the desired treatment outcome in minimum time without violating the imposed safety constraints. Treatment efficacy is quantified in terms of the thermal dose delivered to the target. Normal tissue safety is ensured by automatically maintaining normal tissue temperature below the imposed limit in the user-specified locations. To reflect hardware limitations, constraints on the maximum applied power are also imposed. At the pretreatment stage, MR imaging and thermometry are used to localize the treatment target and identify thermal and actuation models. The results of phantom and canine experiments demonstrate that spatially-distributed, real-time MR temperature measurements enhance one's ability to robustly achieve the desired treatment outcome in minimum time without violating safety constraints. Post-treatment evaluation of the outcome using T2-weighted images of canine muscle showed good spatial correlation between the sonicated area and thermally damaged tissue.
Introduction
Non-invasive thermal therapies continue to show promise in treating various sites, including prostate, breast, liver, kidney and brain tumours. The feasibility of MR-guided ultrasound ablation was demonstrated clinically for breast fibroadenomas Citation[1] and breast carcinomas Citation[2–4]. Tempany et al. Citation[5] and Bergamini et al. Citation[6] have respectively reported clinical results on the feasibility of non-invasive ultrasound and radiofrequency heating for thermal surgery of uterine fibroids. The benefits of thermal therapy have also been reported as an adjuvant modality in conjunction with radiation and chemotherapy (see review of multiple Phase III trials by Dewhirst and Sneed Citation[7]).
The clinical goal of thermal therapies is to achieve an efficacious treatment outcome without damaging critical normal tissues. A recently proposed feedback control approach Citation[8] allows for automatic and simultaneous control of treatment efficacy and safety, as was validated in phantoms and in-vivo Citation[9] using temperature feedback provided by a thermocouple array. The present paper demonstrates that the developed control system can take full advantage of real-time MR thermometry feedback and evaluates its closed-loop performance in-vitro and in-vivo using focused ultrasound heating. The results of Smith et al. Citation[10], Palussiere et al. Citation[11] and the current study are the only reported experimental demonstrations of automatic ultrasound treatment control based on real-time MR-thermometry feedback. Compared to prior work, where the temperature control was the objective, this paper is the first to demonstrate automatic control of thermal dose with MR-thermometry feedback.
The developed control system is designed to achieve user-specified efficacy and normal tissue safety of the treatment. The treatment efficacy is quantified in terms of thermal dose delivered to the target, while safety is maintained by satisfying temperature constraints in the selected normal tissue locations. Compared to previously reported thermal therapy controller designs Citation[10–12], this control approach offers the following significant advantages:
When both efficacy and safety objectives are explicitly addressed by a feedback controller, the treatment duration can be significantly shortened. Conversely, a control system which cannot explicitly ensure normal tissue safety must address treatment safety in an implicit way. For instance, normal tissue damage can be reduced by using pulsed application of highly focused heating patterns. The dissipation of thermal build-up during power-off periods implicitly addresses safety by allowing the surrounding normal tissue to cool. Although an adequate solution, as demonstrated in several clinical trials, its conservatism results in rather long treatment times (e.g. longer than 2 h for a 279 mm3 tumour Citation[3]).
The proposed MRI-based feedback control system allows systematic and direct incorporation of safety objectives, which creates a potential for the safe treatment of a wide range of tumour sites using the existing transducers, applicators and heating modalities. On the other hand, in the absence of any robust technique to prevent normal tissue damage, the treatable sites become limited to those located at a safe distance away from critical normal tissue Citation[3].
The direct thermal dose control approach is equally applicable to all therapies in which time varying temperatures are involved, including thermal surgery and lower-dose, adjuvant thermal therapies.
MR thermometry, used for real time temperature feedback, enhances one's ability to robustly achieve the desired treatment outcome without violating safety constraints. The spatially-distributed, real-time temperature information in and around the target is exploited in the controller design to realize the specified efficacy in the target and safety in the normal tissue. The MR imaging the thermometry are also useful in pre- and post-treatment phases Citation[13] of thermal therapies. In the present work, during pre-treatment planning, MR images and temperature maps are used to localize the target and identify thermal response and ultrasound actuation models, subsequently used in the model-predictive treatment control system. Post-treatment, T2-weighted MR images are used to assess the damage in the treated region.
Methods
Thermal dose controller
Thermal dose quantifies the relationship between treatment efficacy and target temperature evolution, T(x, t), t ∈ [t0, t], during the therapy. A commonly used definition of the thermal dose in the number of cumulative equivalent minutes (CEM) at 43°C Citation[14], Citation[15]:where T90 is the 10th percentile of the measured temperatures and R = 0 for T90 < 2, R = 0.25 for 2 < T90 < 6 and R = 0.5 for T90 ≥ 6 Citation[16].
The thermal dose controller, evaluated in this paper, has the cascade structure depicted in and is briefly described below. The detailed description can be found in Arora et al. Citation[8], where the results of controller evaluations using computer simulations are presented. Experimental validation with temperature feedback provided by a thermocouple array was reported in Arora et al. Citation[9] for a phantom and canine model.
Figure 1. Feedback control of the thermal dose: KD = dose controller; KT = temperature controller; P = patient; S(·) = T90 selector and H = dose estimator.
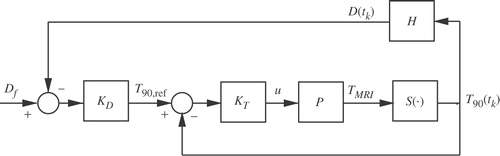
The main non-linear dose controller, KD, continuously generates a reference temperature trajectory, T90,ref, for the secondary temperature controller, KT. The thermal dose controller is designed to quickly deliver the desired thermal dose without consideration of normal tissue and hardware constraints. It maps the difference between the desired final dose, Df, and the thermal dose, D(tk), that has been already derived, into the reference 10th percentile temperature trajectory, T90,ref:where α depends on the error between the desired and the delivered thermal dose and the selected final treatment time, tf = tk + tTH:
The tuning parameter tTH is the moving treatment horizon. The vector T(t) (TMRI in ) of temperatures in different spatial locations inside the subject, P, is measured at the MRI scan rate. Using the last measured temperature distribution, T(tk), the selection operator
(·) selects the corresponding T90(tk), which is then used in Equation (1) to calculate the delivered thermal dose, D(tk). The thermal dose error is updated each time a new temperature measurement becomes available, followed by the corresponding update of the reference, T90,ref(t). The control law, given by Equation (2), assumes a linear evolution of the thermal dose over the treatment horizon tTH until the desired dose, Df, is reached.
The calculated T90,ref(t) is the reference trajectory for the inner temperature controller. To reduce the treatment time, KD is designed to generate an aggressive reference (by choosing a short treatment horizon, tTH), which often cannot be achieved without violating the imposed normal tissue or hardware constraints. The role of KT is to find an ultrasound power, u, such that the difference between T90,ref(t) and the model-predicted T90(t) is minimized without violating normal tissue and hardware constraints. KT is implemented as a linear, constrained, model predictive controller, which finds m future control moves, u = [u(tk), …, u(tk+m−1)], by solving, in real time, the following minimization problem:subject to normal tissue and actuation constraints:
where weights wy and wu penalize the error between the desired and the predicted T90 and the control effort, respectively. The normal tissue constraints, Tmax, are imposed in terms of the maximum allowable temperature in the selected normal tissue location with the temperature Tcons. The hardware limitation on the maximum possible transducer power is given by umax. The tuning parameters p and m are the prediction and control horizons. The prediction horizon, p, is chosen long enough to predict the thermal dose accrued during tissue cooling after the power is turned off. The control horizon, m, determines the number of future control moves that are calculated each time a new measurement becomes available. Only the first component, u(k), of the calculated vector u of m sequential power levels is sent to the transducer. The process is repeated at tk+1, when the next MRI temperature measurement becomes available.
The prediction of T90(t) is calculated as S(T(t)), where the vector of predicted temperature, T(t), satisfies the thermal response model:In the current study, the predictive model (Equation 7) along a line of interest (e.g. the axis of symmetry of a focused ultrasound beam) is obtained by finite difference approximation of a one-dimensional Pennes bioheat transfer equation Citation[17]:
where C and Cb are the specific heat of tissue and blood [J/kg°C)] and We[kg/(m2s)] is the effective blood perfusion parameter. The one-dimensional effective perfusion parameter, We, is used to characterize the energy removed by both blood flow and conduction in a plane perpendicular to the axis of beam symmetry. Consequently, We is non-zero even in the unperfused phantom. Ta is the arterial temperature (in phantoms, Ta is the temperature before heating is initiated), which was assumed to be constant for the duration of experiments. All results are reported as the deviation of the subject's temperature from the baseline, which was set equal to Ta. Q is the power deposition density in W m−3. At the boundaries of the domain, T = Ta is assumed. Handbook values Citation[18] for conductivity, k = 0.7 W/(m°C), and specific heat, C = Cb = 4186 J/(kg°C), were used. The values of patient-specific perfusion and power deposition in the Pennes model were identified experimentally, following the procedure described elsewhere Citation[19].
The predictive model (Equation 7) is used internally by the control system. Matrix A depends on both conduction and perfusion. The term Bu approximates the power deposition term, Q, where u is the applied ultrasound power in Watts. The state T is a vector of deviation temperatures above Ta along the one-dimensional treatment domain. The position of the ultrasound transducer was fixed and the magnitude of the applied ultrasound power, u, was the only manipulated variable.
Tissue phantom
The phantom experiments were performed with an 11 × 11 × 7 cm agarose phantom. The T2 relaxation time of the phantom was modified by adding 1 mM per-litre of copper sulphate to the recipe of Madsen et al. Citation[20]. After preparation, the tissue-mimicking phantom was allowed to solidity inside of an acrylic box with a Mylar membrane on the bottom surface.
Animal model
A 29 kg male Labrador was used in the study. The animal protocol was approved by the institutional animal care and use committee at the University of Utah. The animal was given 75 mg (1.5 ml) of Telazol (Lederle Pharmaceuticals, Carolina, Puerto Rico) by IM injection. When the animal was recumbent, the trachea was intubated. For the duration of the experiment the dog was mechanically ventilated with isoflurane in oxygen, keeping the end tidal CO2 at ∼38 ± 2 mmHg. An intravenous drip was started in the cephalic vein with lactated Ringer's (LR) solution. The dog received ∼15 ml of LR per kg of body weight per hour of anaesthesia. The dog was given pancuronium bromide at a rate of 1 mg h−1 to inhibit leg motion. Blood pressure was measured with a non-invasive cuff placed on the forelimb. Isoflurane concentration was adjusted to keep the mean blood pressure at ∼90 ± 10 mmHg. The SpO2 was monitored and maintained at ∼98% for the duration of araesthesia. Throughout the experiments, the rate of respiration was controlled by a mechanical ventilator. The rate of respiration was set to allow a breathing cycle of 6 s. In order to minimize MRI artifacts due to canine breathing motion and the associated change in susceptibility, the MRI scanner began acquiring an image immediately after each exhalation and completed the scan before the following inhalation. To improve the ultrasound coupling, prior to the experiments the hair on the dog's thigh was removed with clippers and hair removal cream.
Ultrasound heating and MR imaging
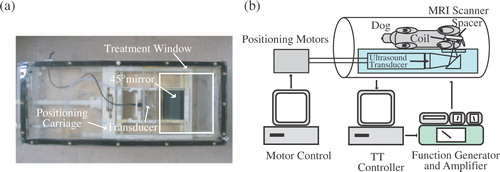
Both phantom and canine experiments were performed using an in-house manufactured Magnetic Resonance Compatible Ultrasound Positioning System (MaRCUPS), depicted in . The ultrasound field was generated by a single, stationary, spherically focused, air-backed transducer, resonating at 1.5 MHz, with a diameter and radius of curvature of 10 and 18 cm, respectively. Further details of driving circuitry and MaRCUPS design are given in Arora et al. Citation[9] and Perry Citation[21].
Figure 2. (a) MaRCUPS showing the Mylar-covered treatment window, transducer positioning components and 45° reflecting ultrasound mirror. (b) Thermal treatment control system during in-vivo experiments inside MRI.
MR imaging was performed using a Siemens Trio 3T Magnetom scanner. To improve the signal-to-noise ratio (SNR) and, thus, allow for a faster scan rate, prior to the canine and phantom experiments a custom built receive-only surface coil was tuned and matched to the desired imaging location. The temperature change in the dog's thigh and the phantom were measured using the proton resonance frequency (PRF) shift method Citation[22] with a temperature coefficient of 0.01 ppm/°C−1. Image data were gathered using a gradient echo (GRE) pulse sequence with a spoiled gradient. The following parameters were used for temperature measurements in the phantom during control experiments: TR = 14 ms, TE = 10, voxel size = 2.0 × 4.0 × 3.0 mm, FOV = 256 mm, matrix size = 128 × 64, flip angle = 25° and scan time of 1.15 s with a phase resolution of 50% to reduce acquisition time. During model identification step tests, the parameters were kept the same except that the scan was taken with the repetition time of TR = 30 ms and the corresponding overall scan rate of 2.45 s. The data were zero-filled in the phase encoding direction to a matrix of 128 × 128.
In the canine thigh, the temperature during model identification step tests and closed-loop controller runs was imaged using TR = 40 ms, TE = 10 ms, 1.6 × 3.2 × 3.0 mm voxel size, 200 mm FOV, 128 × 64 matrix, 25° flip angle and 2.56 s scan time with a phase resolution of 50%. The data were zero-filled in the phase encoding direction to a matrix of 128 × 128. Fat saturation was applied to the GRE sequence to suppress the fat signal and improve the SNR. A delay of 3.4 s was added to the data acquisition sequence, which made the overall rate at which data were acquired equal to 5.96 s. This synchronized temperature imaging with the breathing cycle and, thus, reduced motion artifacts.
The subject (phantom or dog thigh) was positioned on MaRCUPS in the centre of the Mylar treatment window () with the receive coil in the sagittal plane. Ultrasound gel was used to couple the subject to the Mylar window. The focal zone of the transducer was located by applying a step input of ultrasound power while phase images were acquired in the coronal plane, approximately halfway through the subject. A sagittal MR thermal image corresponding to maximum temperature location was chosen through the centre of the heated region. To ensure that the centre of the ultrasound beam was located in the chosen slice, the step input of power and phase image subtraction were repeated while slightly adjusting the position of the sagittal slice.
Results
The experiments were carried out with the efficacy objective of delivering the specified CEM43° T90 to the designated tumour region while maintaining normal tissue temperature in the selected location below the specified maximum allowable value (safety objective). A number of phantom and canine experimental runs were performed to analyse the effect of the turning parameters on controller performance. Only one set of in-vitro and in-vivo results is presented in this paper. It was found that the effect of controller turning qualitatively agrees with the previous conclusions, obtained using computer simulations Citation[8] and experiments with thermocouple-based feedback Citation[9].
Phantom results
The objective was to deliver Df = 10 CEM43° T90 to the selected target region while limiting the temperature at the constraint location to below 6.5°C. A low thermal dose was selected to reduce time and the associated cost required to complete multiple experimental runs. The ultrasound power was constrained (umax = 11 W) to reflect hardware limitations and to avoid cavitation.
The controller tuning parameters were set as follows: prediction horizon, p = 4.6; control horizon, m = 2.3 s; and the moving treatment horizon, tTH = 4.6 s. The treatment horizon was selected to: (a) force the activation of the transducer power constraint at the beginning of the treatment when the normal tissue temperature constraint was not active and (b) towards the end of the treatment, to ensure that the thermal dose controller, KD, generates an almost attainable reference temperature trajectory to minimize over-dosing of the target.
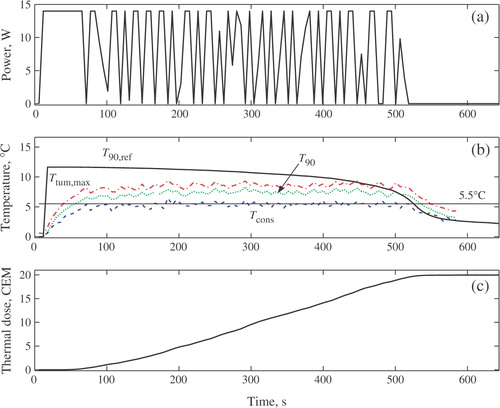
depicts the evolution of the controlled power input, u; maximum temperature increase inside the target, Ttum,max; T90 of the target; the temperature at the constraint location, Tcons; the reference temperature generated by the outer controller, T90,ref, and the thermal dose in the target. All temperature are plotted as deviations above the baseline value.
Figure 3. Thermal dose control in phantom. (a) Control input. (b) Increase in T90, T90,ref, Tcons and Ttum,max, (c) Tumour thermal dose.
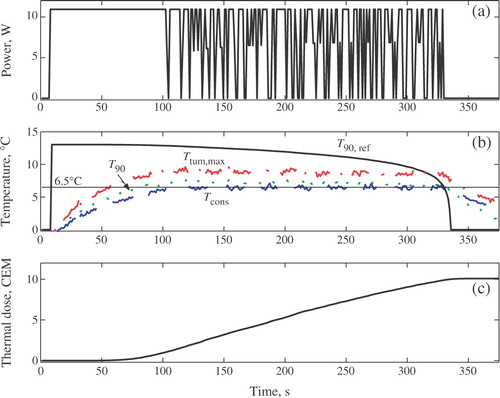
indicates that, at t = 103 s, the temperature constraint became active. The constrained model predictive controller, KT, automatically reduced the ultrasound power () in such a way that temperature in the constraint location is maintained near the maximum permitted value of 6.5°C.
The selected small value of the moving treatment horizon (tTH = 4.6 s) resulted in an aggressive thermal dose controller, KD. The KD-generated reference trajectory (T90,ref in ) is aggressive. In an attempt to follow T90,ref as closely as possible, the constrained temperature controller, KT, causes the saturation of ultrasound power at the beginning of the treatment. When the temperature in the constrained location reaches the maximum allowable level, the temperature controller begins to automatically modulate the ultrasound power so that the treatment progresses very close to the normal tissue constant. The progression of the treatment close to a constraint is necessary to achieve the efficacy objective in minimum time. Towards the end of the treatment, as the delivered thermal dose approaches the desired value, Df, the reference T90,ref(t) drops to zero as the error Df − D(tk) → 0. After the power is switched off, the residual thermal dose is delivered during tissue cooling. The desired thermal dose of 10CEM43° T90 was achieved in the target at ∼t = 330 s. Either the temperature or the power constraint is active throughout the treatment, which for the case of a single stationary transducer is the necessary and sufficient condition for minimum-time treatment.
In-vivo results
The in-vivo results were obtained with the ultrasound power constrained to umax = 14 W. The desired final thermal dose was set to 20 CEM. Compared to the phantom case, a tighter and clinically more realistic normal tissue constraint of 5.5°C was imposed at x = 8.3 cm to avoid damage to the muscle outside the target. By minimizing tissue damage, it was possible to perform multiple tests with the same subject and evaluate the effect of various tuning parameters on the controller performance.
depicts the controller-generated power, MR temperature measurements and the resulting thermal dose for one of the test runs. The controller tuning parameters p and m were set to 24 and 12 s, respectively. The value of the moving treatment horizon, tTH, was set to 24 s, which forced the activation of the transducer constraint at the beginning of the treatment. Because of a slower sampling of MR-thermometry measurements during in-vivo experiments (5.96 s vs 1.15 s in phantom case), the treatment controller was tuned less aggressively compared to the phantom case by selecting larger values of the treatment, control and prediction horizons. shows that at t = 70 s the temperature constraint became active. To avoid constraint violation, the temperature controller, KT, changed the ultrasound power in such a way that temperature in the constraint location was maintained close to the maximum allowed value of 5.5°C, which is necessary to minimize the treatment time. The highest safe temperature in the selected normal tissue location was maintained with active modulation of the applied power (), which is an expected behaviour for an aggressively-tuned controller. Tcons(t) is shown in , where the reference, T90,ref, and the measured T90 are also shown. indicates that the desired thermal dose of 20 CEM was achieved in the target in ∼515 s.
Figure 4. Thermal dose control in in-vivo canine. (a) Control input. (b) Increase in T90, T90,ref, Tcons and Ttum,max. (c) Tumour thermal dose.
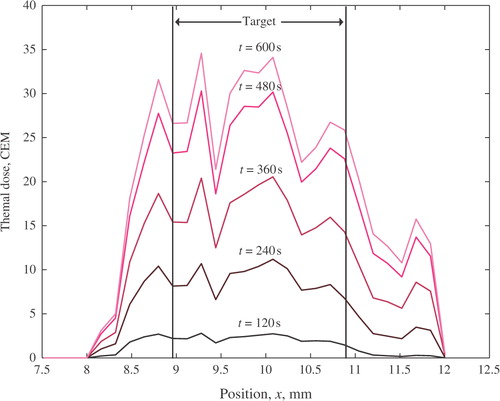
depicts the spatial distribution of thermal dose, D(x), on the line of beam symmetry of focused ultrasound transducer at various times during the treatment. A sharp thermal dose delineation in the target and normal tissue at the constraint location, x = 8.3 cm, is evident.
Figure 5. Spatial distribution of the delivered thermal dose in the treatment domain at various times. The target spans from 9.0–10.9 cm. The normal tissue constraint was placed at 8.3 cm.
During additional experiment runs (results not shown), an even lower value of normal tissue constraint (4°C) was used. This further reduced the thermal dose delivered at the constraint location, but at the expense of considerable lengthening of the treatment time. Such correlation between the treatment duration and the imposed normal tissue constraints is an expression of the trade-off between efficacy and safety objectives: when safety requirements are relaxed, the efficacy can be achieved with a more aggressive and shorter treatment. The treatment time was also longer when the controller was de-tuned (i.e. made less aggressive) by using larger values of the treatment horizon, tTH, which is the most important tuning parameter of the implemented control system.
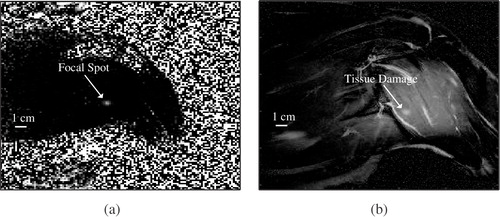
The last experiment in the series was performed to validate the ability of the control system to create an MRI-visible lesion. The maximum allowable power was set to umax = 28 W and the normal tissue constraints were not introduced. Ten minutes after the last treatment was concluded, the tissue damage, caused by cumulative thermal effect of all experiments, was evaluated by acquiring several T2-weighted Turbo Spin Echo (TSE) coronal images at various depths in the dog's thigh. The scan parameters were: TR = 580 ms, TE = 12 ms, 0.8 × 0.8 × 3.0 mm voxel size, 256 × 256 matrix, FOV 200 mm, average number = 2, slice number = 7. An image taken near the centre of the treated region is shown in . The location of the damaged region shown in correlates well with the position of the focal zone, clearly visible in the thermal image of .
Discussion
The reported in-vitro and in-vivo results demonstrate the feasibility of automatic, MRI-based control of safety and efficacy of thermal therapies. The designed control system simultaneously achieves the specified efficacy and safety objectives, expressed in terms of the desired thermal dose in the target and the maximum allowed normal tissue temperatures in the clinician-selected locations. The developed approach, validated using stationary ultrasound actuation, can be used without modifications with different stationary actuation modalities, including radiofrequency, microwave and laser treatments, performed with non-invasive, interstitial or intracavitary applicators.
The developed control system automatically delivers the desired target thermal dose in the presence of temporally and spatially varying temperatures. Since the control problem is formulated in terms of thermal dose, the controller does not try to create a uniform temperature distribution in the target, as is often attempted in standard hyperthermia by utilizing highly specialized, site- and patient-specific applicators. Instead, the treatment is controlled by directly and automatically controlling the thermal dose delivered to the target, subject to normal tissue constraints. This approach is equally applicable to moderate-temperature hyperthermia and high-temperature thermal ablation Citation[23], as long as the appropriate thermal dose is specified by the user. In thermal ablations, the automatic handling of normal tissue constraints allows one to minimize the treatment time by implementing the most aggressive treatment that does not violate the safety objectives.
The capability of the developed control system to safely deliver the specified thermal dose is enhanced by the use of MR thermometry, which provides spatially distributed measurements of temperatures, reducing treatment uncertainty compared to the case when sparse, pointwise temperature measurements are used. In particular, the availability of distributed MR-temperatures provide a better estimate of T90 of the target region as compared to the case when temperature measurements are limited. Another benefit of MR-thermometry is the improved accuracy of the identified model used internally by the treatment control. Comprehensive MR measurements of temperature distribution eliminate the need for temperature estimation (generated, for example, by the Kalman filter, as in Arora et al. Citation[8]), thus reducing the uncertainty in real-time assessment of treatment progression.
The model predictive capability of the developed control system allows it to assess the effect and interaction of m control actions over p steps into the future (p ≥ m). The thermal dose delivered during tissue cooling is also taken into account by the designed model-based controller, which minimizes target over-dosing and the active heating time. Note that the predictive thermal model, internally used by the control system, is updated each time a new MR temperature measurement becomes available. The continuous model adaptation decreases the sensitivity of the control system to modelling errors and changing target properties including blood perfusion Citation[24] and ultrasound absorption Citation[25].
The results of and show that the treatment evolves with either normal tissue or power constraints active at all times, which is the necessary condition for minimum time treatment Citation[26]. The normal tissue constraint is kept close to the maximum allowed value with active power modulation. The observed rapid change in the manipulated variable is typical of aggressive, minimum-time, controllers when time-varying disturbances affect the treatment. Earlier simulations Citation[8] showed that in an ideal case of no plant-model mismatch and with time-invariant disturbances, the controller was able to arrive at the exact power level that maintains the normal tissue at the constrained value, completely eliminating the rapid change of ultrasound power observed in the experiments. Further note that rapidly changing power causes relatively small temperature variations. If desired, the rapid power change can be impeded by using a larger value of the tuning parameter wu in objective function J (Equation 4). However, a higher control penalty will generally lead to a longer than time-optimal treatment. In order to obtain near minimum-time results wu was set to zero during the experiments.
The spatial distribution of the thermal dose in the treatment region, , shows a sharp delineation between the thermal dose in the target and the constraint location in normal tissue. This effectively demonstrates that by imposing temperature constraints, one can limit the dose delivered to the surrounding normal tissue.
During all experimental runs, including the case shown in , the CEM43° T90 thermal dose delivered to the target was almost exactly equal to the specified reference value, Df. The corresponding pointwise thermal dose distribution exceeds Df in most spatial locations (such as in the case of ). A pointwise over-dosing inside the target is usually acceptable from the clinical perspective and is to be expected when the treatment objective is formulated in terms of T90 temperature. A more uniform spatial dose profile and further reduction in treatment times are possible only when additional degrees of freedom are available for automatic control, as in the case of either a phased array or a single transducer with the controlled ultrasound intensity and the position of the focal zone.
With little modification, the developed approach can be extended to the treatment of large tumors with scanning or phased power fields by sub-dividing the tumour into sub-regions, determined by the size and shape of the heating pattern and their sequential treatment to the desired thermal dose under the control of the described system. The sequence of sub-regions can be obtained as a result of pre-treatment optimization or by following a pre-selected focal zone trajectory (e.g. rastering, as in Hynynen et al. Citation[1]). The heating interaction in different sub-regions can be accounted for by adapting the thermal dose set-point to reflect the already delivered dose due to SAR overlap and heat transfer between sub-regions. During the entire treatment, the controller would automatically adjust the power to ensure that normal tissue constraints are not violated, no matter the location of the focal zone. A more advanced implementation will require the development of a control system which automatically manipulates the focal zone location, rather than relying on pre-specified sequence of positions or trajectories, selected prior to the treatment.
The performance of the developed treatment control system depends on accurate and frequent MRI temperature measurements used in the feedback. This is especially true in the case of short-duration, high-temperature (e.g. HIFU) treatments, which may take 20 s or less to treat a sub region. Customized surface coils, used in the described phantom and dog experiments, ensured a relatively fast MR sampling (1.15 s and 5.96 s in phantom and animal experiments, respectively) and low measurement noise (below ±0.5°C). A slower sampling rate during canine experiments was necessary to reduce measurement artifacts due to motion, which suggests that motion-compensating algorithms Citation[27] will be necessary to fully exploit the advantages of non-invasive MR thermometry in providing real-time, spatially-distributed temperature feedback for advanced thermal therapy controllers.
The individual experimental runs reported in this study were no longer than 10 min. During longer treatments, characteristic of the traditional hyperthermia, the PRF-based MR-thermometry is susceptible to temporal and spatial variations due to drift of the Bo field. Uncertainties in the MR temperature measurements, including those caused by inhomogeneity of susceptibility, can have a negative effect on the ability to achieve safety and efficacy objectives of the treatment. In such cases, the signal correction techniques, utilizing direct measurements at regions with defined phase under ideal conditions (e.g. in a water bolus), as illustrated in Gellermann et al. Citation[28], can be used to improve the accuracy of MR thermometry.
Acknowledgements
The authors thank Scott McJames, Department of Anesthesiology, University of Utah, for help with animal experiments. The financial support from the grant NIH-1-RO1-CA87785 is gratefully acknowledged.
References
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach JK, Baum J, Singer S, Jolesz FA. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 2001; 219: 176–185
- Gianfelice D, Khiat A, Boulanger Y, Amara M, Belblidia A. Feasibility of magnetic resonance imaging-guided focused ultrasound surgery as-an adjunct to tamoxifen therapy in high-risk surgical patients with breast carcinoma. Journal of Vascular International Radiology 2003; 14: 1275–1282
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused ultrasound surgery of breast cancer: histopathologic assessment of efficacy — initial experience. Radiology 2003; 227: 849–855
- Huber PE, Jenne JW, Rastert R, Simiantonakis I, Sinn HP, Strittmatter HJ, von D, Wannenmacher MF, Debus J. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Research 2001; 61: 8441–8447
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology 2003; 226: 897–905
- Bergamini V, Ghezzi F, Cromi A, Bellini G, Zanconato G, Scarperi S, Franchi M. Laparoscopic radiofrequency thermal abalation: a new approach to symptomatic uterine myomas. American Journal of Obstetrics & Gynecology 2005; 192: 768–773
- Dewhirst MW, Sneed PK. Those in gene therapy should pay closer attention to lesions from hyperthermia. International Journal of Radiation Oncology Biology & Physics 2003; 57: 597–599
- Arora D, Skliar M, Roemer RB. Minimum time control of thermal therapies. IEEE transactions of Biomedical Engineering 2005; 52: 191–200
- Arora D, Cooley D, Perry T, Skliar M, Roemer RB. Direct thermal dose control of constrained focused ultrasound treatments; phantom and in-vivo evaluation. Physical Medicine Biology 2005; 50: 1919–1935
- Smith NB, Meriless NK, Hynynen K, Dahleh M. Control system for MRI compatible intracavitary ultrasound array for thermal treatment of prostate disease. International Journal of Hyperthermia 2001; 17: 271–282
- Palussiere J, Salomir R, Le Bail B, Fawaz R, Quesson B, Grenier N, Moonen CT. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magnetic Resonance Medicine 2003; 49: 89–98
- Vanne A, Hynynen K. MRI feedback temperature control for focused ultrasound surgery. Physical Medicine Biology 2003; 48: 31–43
- Kangasniemi M, Stafford RJ, Price RE, Jackson EF, Hazle JD. Dynamic gadolinium uptake in thermally treated canine brain tissue and experimental cerebral tumors. Investigative Radiology 2003; 38: 102–107
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. International Journal of Radiation Oncology, Biology & Physics 1984; 10: 787–800
- Leopold KA, Dewhirst MW, Samulski TV, Dodge RK, George SL, Blivin JL, Prosnitz LR, Oleson JR. Cumulative minutes with T90 greater than Tempindex is predictive of response of hyperthermia and radiation. International Journal of Radiation Oncology, Biology & Physics 1993; 25: 841–847
- Thrall DE, Rosner GL, Azuma C, Larue SM, Case BC, Samulski T, Dewhirst MW. Using units of CEM 43°C T90, local hyperthermia thermal dose can be delivered as prescribed. International Journal of Hyperthermia 2000; 16: 415–428
- Pennes HH. Analysis of tissues and arterial blood temperatures in resting human forearm. Journal of Applied Physiology 1948; 1: 93–122
- Heat and mass transfer in living systems, RK Diller, New York 1998, The New York Academy of Sciences
- Arora D, Cooley D, Perry T, Guo J, Parker D, Skliar M, Roemer RB. Thermal dose control of ultrasound therapies using MR thermometry images: an in-vitro phantom study. American Control Conference. Portland, ORUSA, 405–410, 2005
- Madsen EL, Frank GR, Dong F. Liquid or solid ultrasonically tissue-mimicking materials with very low scatter. Ultrasound in Medicine & Biology 1998; 24: 535–542
- Perry T. Use of a magnetic resonance compatible ultrasound positioning system for SAR field estimation. University of Utah. 2005, Master Thesis
- Chung AH, Hynynen K, Cline HE, Colucci V, Oshio K, Jolesz F. Optimization of spoiled gradient-echo phase imaging for in vivo location of a focused ultrasound beam. Magnetic Resonance Medicine 1996; 36: 745–752
- Stauffer PR, Goldberg SN. Introduction: thermal ablation therapy. International Journal of Hyperthermia 2004; 20: 671–677
- Akyurekli D, Gerig LH, Raaphorst GP. Changes in muscle blood flow distribution during hyperthermia. International Journal of Hyperthermia 1997; 13: 481–136
- Worthington AE, Sherar MD. Changes in the ultrasound properties of porcine kidney during heating. Ultrasound in Medicine and Biology 2001; 27: 673–682
- Pontryagin LS, Boltyanskii VG, Gamkrelidze RV, Mishchenko EF. The mathematical theory of optimal processes. Wiley, New York 1962
- de Zwart JA, Vimeux FC, Palussiere J, Salomir R, Quesson B, Delalande C, Moonen CT. On-line correction and visualization of motion during MRI-controlled hyperthermia. Magnetic Resonance Medicare 2001; 45: 128–137
- Gellermann J, Wlodarczyk W, Ganter H, Nadobny J, Fahling H, Seebass M, Felix R, Wust R. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: validation in a heterogeneous phantom. International Journal of Oncology, Biology & Physics 2004; 61: 267–277