Abstract
Under conditions of heat stress and hyperosmotic dehydration, both animals and humans reduce thermoregulatory evaporation and regulate deep body temperature at elevated levels. Regarding the mechanisms, the main role in producing these thermoregulatory changes during dehydration is attributed to the increased osmolality of body fluids, although the role of the decreased plasma volume without changes in plasma osmolality (hypovolemia/isosmotic dehydration) has not been so far investigated. There are also controversial experimental results regarding the effects of dehydration on heat stress-induced cutaneous vasodilation. Therefore, this paper studied the effects of hypovolemia/isosmotic dehydration on cardiorespiratory responses to hyperthermia and its physical treatment in 17 anaesthetized adult rabbits. The animals were divided into two groups: normovolemic group (NV; n = 10) and hypovolemic group (HV; n = 7). In the HV group, hypovolemia/isosmotic dehydration (decrease in plasma volume by 16.1 ± 1.2%) was induced by furosemide (5 mg kg−1 i.v.) without change in measured plasma Na+ concentration. Hyperthermia (the rise in body temperature (BT) to 42°C by a gradual body surface heating) caused significant increase in minute ventilation (VE) in both groups. However, VE values were significantly higher in the HV rabbits compared to the NV animals despite the lower breathing frequency (p < 0.05). The panting was absent in the HV rabbits at the BT of 42°C, unlike the NV animals. From cardiovascular variables, the vasoconstrictor response in visceral (mesenteric) region during hyperthermia in hypovolemic/isosmotic animals was attenuated (p < 0.05), whereas the heat stress-induced cutaneous vasodilation was not influenced by hypovolemia. Recovery of the BT by body surface cooling was accompanied by further increase in VE in the NV group, whereas VE decreased (p < 0.05) in the HV animals. Cooling led to recovery of the cardiovascular parameters. There were found no significant cardiorespiratory differences between the groups (NV:HV) during cooling. The lower frequency of breathing and attenuation of the mesenteric vasoconstriction during exogenous hyperthermia are present not only during hyperosmotic dehydration induced by water deprivation, but they also occur under conditions of furosemide-induced isosmotic dehydration/hypovolemia in rabbits. The heat stress-induced cutaneous vasodilation regarding its biological importance was not influenced by hypovolemia/isosmotic dehydration. Therefore, it is suggested that hypovolemia alone is sufficient to produce described respiratory, thermoregulatory and cardiovascular changes in dehydrated rabbits during exogenous hyperthermia, whereas hyperosmolality is not a requisite.
Introduction
Hyperthermia induced by acute overheating results in marked respiratory and cardiovascular adjustments in humans or animals. The majority of them are thermal polypnoea—panting—and an increase in skin blood flow to enhance heat loss from the body surface. The compensating mechanisms such as splanchnic vasoconstriction Citation[1], Citation[2] and an increase in cardiac output primarily due to a rise in heart rate Citation[3], Citation[4] are concomitantly evoked to maintain arterial blood pressure and organ perfusion.
Dehydration can adversely affect the cardiovascular and thermoregulatory functions during hyperthermia. Under conditions of heat stress and hyperosmotic dehydration induced by water deprivation, both animals and humans reduce thermoregulatory evaporation and regulate deep body temperature at elevated levels Citation[5], Citation[6]. Both sweat rate and respiratory evaporative heat loss are altered in hyperosmotic dehydration. Heat-stressed animals respond to water deprivation by elevating panting threshold Citation[7–9]. Regarding the cardiovascular variables, it has been shown that dehydration attenuates the heat stress-induced cutaneous vasodilation in both humans Citation[5], Citation[10], Citation[11] and animals—the baboons and the rats Citation[12], Citation[13].
Regarding the mechanisms underlying changes in thermoregulation during dehydration, it has been suggested that alterations in the central processes regulating evaporation are mediated mainly by the increased osmolality of body fluids Citation[14–16]. Accordingly, also, the decreased plasma volume without changes in plasma osmolality (hypovolemia/isosmotic dehydration) is very important, although its role in producing the dehydration-induced thermoregulatory and respiratory changes during hyperthermia has not been so far investigated.
It was hypothesized that hypovolemia alone (i.e. the decreased plasma volume without plasma osmolality changes) could be sufficient to produce the thermoregulatory, respiratory and cardiovascular changes induced by dehydration in hyperthermia. Therefore, the aim of this study was to find out effects of diuretic-induced hypovolemia/isosmotic dehydration on respiratory, regional and systemic cardiovascular and thermoregulatory responses to hyperthermia and to its physical treatment in rabbits.
Materials and methods
Experimental animals and procedures
The experiments were performed on 17 adult rabbits (chinchilla) of either sex (eight males, nine females), mean body weight 2.2 ± 0.07 kg (x ± SEM) under general anaesthesia with a mixture of 20 mg kg−1 ketamine and 5 mg kg−1 xylazine i.m. in an introductory dose and the maintenance doses of 20 mg kg−1 h−1 ketamine by intravenous infusion. The animals were tracheotomized and they breathed air (FiO2 = 0.21) spontaneously through a tracheal cannula. The tidal volume (VT) was recorded through the Fleisch head of a pneumotachograph (ÚMMT SAV, Bratislava) connected with the tracheal cannula. The frequency of breathing (f) was calculated from the tidal volume record. Minute ventilation (VE) was calculated as a product of VT and f (VT f). End-tidal CO2 (ETCO2) was monitored by the mainstream sensor of a capnograph (Capnogard, Novametrix, USA) connected with the pneumotachograph in series. The pneumotachograph was closest to the airway. The capnograph displayed always the maximum ETCO2 value obtained during the last 10 s.
Bilateral femoral arterial catheters were inserted for blood samples withdrawal and arterial blood pressure (ABP) monitoring with the calibrated electromanometer LDP 102 (Tesla, Czech Republic). A catheter in the femoral vein was used for a continuous administration of the anaesthetic by the injection pump IPA 2050 (COMPACT Co., Czech Republic). ECG was recorded by means of needle subcutaneous electrodes and R-R intervals were used for evaluation of the heart rate (HR). Signals of the ECG, arterial blood pressure and tidal volume were recorded simultaneously by a multi-channel recorder 6 NEK 4 (RFT, Germany).
After a mid-line laparotomy, small segments of the right renal and superior mesenteric arteries were isolated. Electromagnetic probes (Type 1RB2006, Type 2.5SB379, Hugo Sachs Elektronik, Germany) were implanted around each artery to monitor renal (RBF) and mesenteric blood flow (MBF) using Transit Time Flowmeter Type 700 (Hugo Sachs Elektronik, Germany). The incision was closed with surgical clips. Skin blood flow (SBF) as laser Doppler flowmetry was measured in the middle part of the left auricula by laser flowmeter BRL-100 (Bio Research Center Co, Ltd, USA) using the plate probe Type BP-8L (Bio Research Center Co, Ltd, USA).
Arterial blood PO2, PCO2, pH and sodium concentration [Na+] were also monitored during experiments. Blood samples (0.2 ml for each sample, replaced by an equal volume of heparinized saline) were taken from the femoral artery and were evaluated using a blood gas analyser Rapidlab™ 348 (Bayer Diagnostics, UK). Blood gases and pH were corrected for actual body temperature.
Relative changes in plasma volume were determined by the changes in hematocrit (Hct) using the equation by van Beaumont Citation[17]. Hct was measured using a microhematocrit centrifuge (type 316, Mechanika precyzyjna, Poland). Rectal body temperature (BT) was measured with a digital thermometer (Microlife AG, Switzerland) at a depth of 6–7 cm. The digital thermometer used in the experiments was calibrated against a precise laboratory electronic thermometer with a precision of ±0.1°C.
Experimental protocol
The animals were divided into two groups: normovolemic group (NV; n = 10) and hypovolemic group (HV; n = 7). After induction of anaesthesia and surgical procedures, in the HV group furosemide (Hoechst Biotika) was administered in a dose of 5 mg kg−1 into the femoral vein to induce isosmotic dehydration/hypovolemia. After administration of furosemide, venous blood hematocrit as a marker of hydration state was measured every 15 min, until stabilization of its value was attained. Stabilization of Hct value appeared 60 min on average after administration of furosemide, when a decrease in plasma volume by 16.1 ± 1.2% was found.
In both the groups, the animal body temperature was gradually elevated to 42°C by body surface heating using a heating pad and radiant heat from an infrared lamp. This process took 58 ± 2 min on average. Subsequently, body surface cooling with a cooling pad and with wet cold wraps was used for recovery of BT to the initial value (38°C). Mean cooling time was 56 ± 2 min. Cardiorespiratory variables were recorded and evaluated at each 1°C change in BT during body surface heating or cooling.
The rabbits were killed by overdosing with the anaesthetic drug at the end of the experiment. Experiments were performed according to Helsinki Declaration.
Statistical analysis
Data were analysed using a Wilcoxon test to evaluate changes of the individual variables within each group. Mann-Whitney U-test was used to compare changes of the individual variables between the two groups. The results are expressed as means ± SEM. Differences were considered significant when p < 0.05.
Results
Administration of furosemide caused significant increases in minute ventilation (VE, due to non-significant tendency of breathing frequency to tachypnoea) and HR and decreases in ETCO2, MBF, RBF and SBF (). However, VT, PaO2, PaCO2, pH, sodium concentration and mean arterial pressure (MAP) were not significantly changed by furosemide-induced hypovolemia.
Table I. Respiratory and cardiovascular variables before and after induction of hypovolemia by administration of furosemide.
Respiratory and cardiovascular effects of hyperthermia and its physical treatment in normovolemic (NV) and hypovolemic (HV) animals are summarized in . Hyperthermia evoked a significant increase in VE in both groups. However, VE values were significantly higher in the HV group compared to the NV group (). In the course of recovery of BT by body surface cooling, a tendency to further increase in VE was found in the NV group, whereas VE decreased (p < 0.05) in the HV animals (). There were no significant differences in absolute values of VE between the groups (NV:HV) during recovery of BT.
Figure 1. Changes in minute ventilation (VE) during hyperthermia and its physical treatment in the normovolemic (solid line) and hypovolemic (dashed line) groups. Each point represents mean ± SEM. *p < 0.05 significantly different from the normovolemic group at the same body temperature. 38a: initial level of animal body temperature after induction of hypovolemia/isosmotic dehydration.
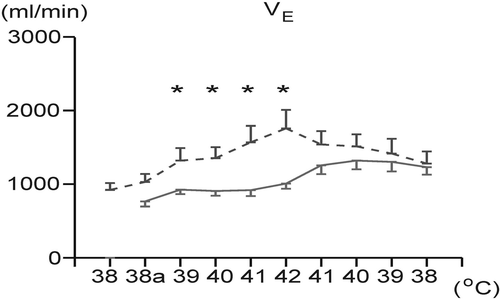
Table II. Respiratory and cardiovascular effects of hyperthermia and its physical treatment in normovolemic (NV) and hypovolemic (HV) groups.
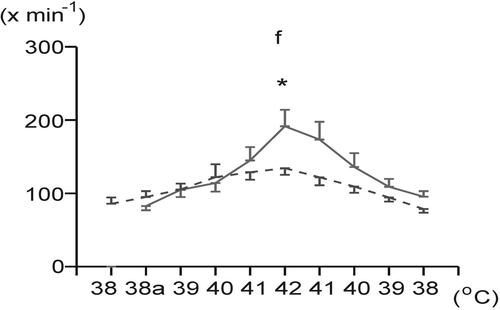
Frequency of breathing (f) significantly increased during overheating in both groups. However, at maximum BT (42°C) it was significantly lower in the HV group (), in which panting was absent at 42°C, unlike the NV group. Cooling led to a decrease (p < 0.05) of this variable in both groups. No significant differences were found in absolute values of breathing frequency between the groups (NV:HV) during cooling.
Figure 2. Changes in frequency of breathing (f) during body surface heating and cooling in the normovolemic (solid line) and hypovolemic (dashed line) groups. Each point represents mean ± SEM. *p < 0.05 significantly different from the normovolemic group at the same body temperature. 38a: initial level of animal body temperature after induction of hypovolemia/isosmotic dehydration.
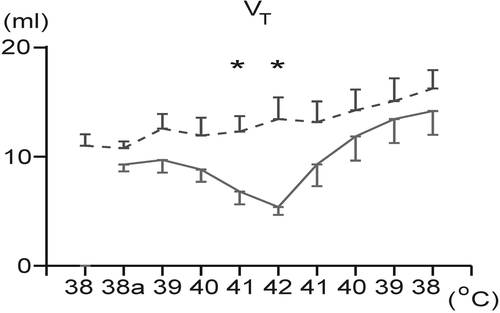
In the course of overheating, VT significantly decreased only in the NV animals. At the end of heating (BT of 41 and 42°C) it was significantly higher in the HV animals compared to the NV rabbits (). In both groups cooling led to an increase in VT, but this increase was statistically significant only in the NV group. There were found no significant differences in absolute values of VT between the two groups (NV:HV) during cooling.
Figure 3. Changes in tidal volume (VT) during overheating to 42°C and during body temperature recovery in the normovolemic (solid line) and hypovolemic (dashed line) groups. Each point represents mean ± SEM. *p < 0.05 significantly different from the normovolemic group at the same body temperature. 38a: initial level of animal body temperature after induction of hypovolemia/isosmotic dehydration.
Blood gas tensions and pH changes due to hyperthermia and its physical therapy in both groups are summarized in . PaO2 significantly decreased during hyperthermia in both groups. During cooling it rose significantly in both, the HV and NV groups.
During hyperthermia, ETCO2 decreased significantly in both groups. PaCO2 was decreasing during hyperthermia in both groups, however, the significant decrease in this variable was observed only in the NV animals. During recovery of BT, there was found further decrease in PaCO2 in both groups, but only in the NV group the decrease was significant. During heating, pH did not show any significant change in both groups.
Hyperthermia caused a uniform significant increase in heart rate (HR) in both groups. Recovery of BT led to a decrease (p < 0.05) in this variable without significant differences between the two groups.
In both groups, mean arterial pressure (MAP) did not significantly change by heating or cooling (). No significant blood pressure differences were found between the two groups throughout the experiment, although MAP tended to be higher in absolute values during hyperthermia in the HV rabbits compared to the NV animals.
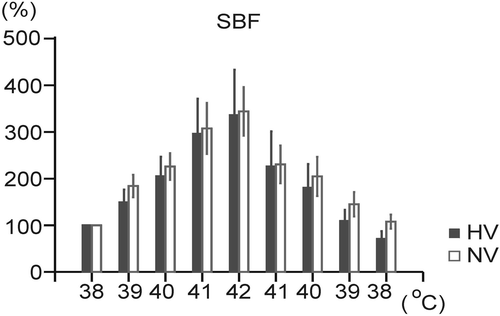
In both groups, skin blood flow (SBF) as laser Doppler flowmetry increased significantly during hyperthermia (), in the NV group by 244.2 ± 48.5% and in the HV group by 236.2 ± 87.6%. During recovery phase, SBF decreased (p < 0.05) in both groups (), in the NV group to 107.9 ± 14.3% of the initial value and in the HV group to 70.8 ± 15.2% of the initial value. No significant differences in SBF were found between the two groups throughout the experiment ().
Figure 4. Changes in ear skin blood flow (SBF), as laser Doppler flowmetry during hyperthermia and its physical treatment in the normovolemic (NV) and hypovolemic (HV) groups. Each point represents mean ± SEM.
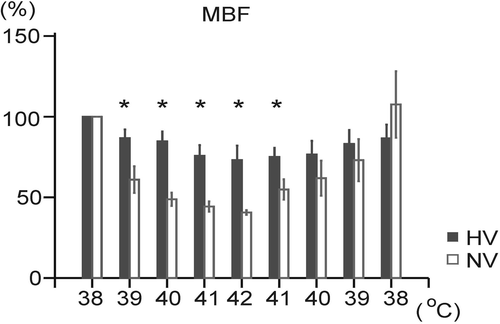
In the course of heating, mesenteric blood flow (MBF) decreased significantly in both groups (), in the NV group by 59.4 ± 1.2% and in the HV group by 27.0 ± 8.2%. Percentual values of the decreases in MBF during hyperthermia were significantly lower in the HV animals compared to the NV animals (). During cooling, MBF increased (p < 0.05) in both groups (), in the NV group to 107.6 ± 18.1% of the initial value and in the HV group to 86.5 ± 7.7% of the initial value. Only at the beginning of cooling (BT of 41°C) was there found a significant difference in MBF between the NV and HV animals.
Figure 5. Changes in mesenteric blood flow (MBF) during body surface heating and cooling in the normovolemic (NV) and hypovolemic (HV) groups. Each point represents mean ± SEM. *p < 0.05 significantly different from the normovolemic group at the same body temperature.
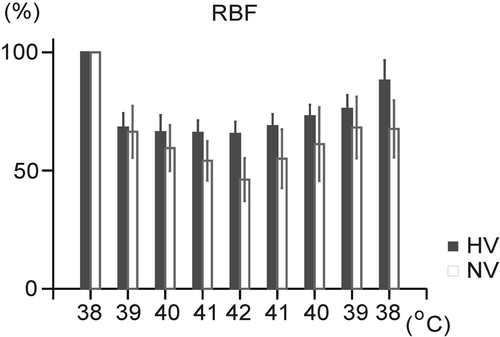
Hyperthermia caused a significant decrease in renal blood flow (RBF) in both groups (NV: by 53.8 ± 8.2%, HV: by 34.5 ± 4.7%). During recovery of BT, RBF increased (p < 0.05) in both groups (), in the NV group to 67.6 ± 10.9% of the initial value and in the HV group to 88.1 ± 7.9% of the initial value. No significant differences in RBF were found between the two groups throughout the experiment ().
Discussion
In this study, the respiratory and cardiovascular responses to hyperthermia and its physical treatment were studied in hypovolemic/isosmotically dehydrated anaesthetized rabbits and compared to the normovolemic controls.
Respiratory variables
Administration of furosemide, leading to the decrease in plasma volume without changing the plasma Na+, influenced the minute ventilation (VE) and end-tidal CO2. VE significantly increased and ETCO2 consequently decreased (p < 0.05) by furosemide-induced hypovolemia.
Hyperthermia in the normovolemic rabbits evoked the increases in minute ventilation, breathing frequency and a marked decrease in VT characteristic for panting. End-tidal CO2, PaCO2 and PaO2 decreased significantly during panting. An increase in VE was found also in the hypovolemic animals during hyperthermia; however, values of VE were significantly higher in the hypovolemic/isosmotically dehydrated animals compared to the controls. The higher VE values under hypovolemic conditions during hyperthermia seem to be a result of the significantly higher VT, since the breathing frequency in the hypovolemic/dehydrated animals was during hyperthermia below the normovolemic level.
In the present study, changes in minute ventilation during hyperthermia were found to be much stronger in the hypovolemic rabbits compared to those normovolemic. Similar results were observed also in acutely haemodiluted/anaemic rabbits exposed to heat stress Citation[18]. Therefore, it was supposed that combined stress of hyperthermia with acute haemodilution/anaemia or with hypovolemia even in the isosmotic conditions could be responsible for higher VE values, indicating thus a greater activation of the respiratory system. At maximum BT (42°C), the hypovolemic/dehydrated animals, unlike those normovolemic, were not able to reach such high breathing frequencies with small tidal volumes typical for panting.
During hyperthermia, the significantly lower frequency of breathing observed in the hypovolemic/dehydrated animals is in accordance with other experimental results Citation[14], Citation[19]. It has been shown that the frequency of breathing in heat-stressed animals correlated highly with evaporative water loss Citation[20]. Thus, reduction in the breathing frequency appears to be the principal mechanism for reducing evaporative water loss in dehydrated animals during heat stress, confirming that control of evaporation in dehydrated animals depends largely on control of breathing frequency Citation[7], Citation[20].
Dehydration can cause a change in the sensitivity of the thermoregulatory system to increased ambient or body temperature. In Taylor's Citation[21] experiments on African ungulates, dehydration was associated with an increase in the threshold air temperature for panting or sweating and a decrease in the rate of panting or sweating at a given deep BT. Also other authors described elevation of the panting threshold in dehydrated animals exposed to heat stress Citation[7], Citation[9], Citation[22]. Several investigators have found that under conditions of dehydration and ambient or exercise heat load, also humans reduce thermoregulatory evaporation (mainly sweat rate) and regulate deep body temperature at elevated levels Citation[6], Citation[23].
Therefore, it is supposed that an absence of the panting at 42°C under hypovolemic conditions in these experiments could be associated with a delay in the onset of panting—with an upward shift of the threshold body temperature for panting, thus supporting the idea of dehydration-changed sensitivity of the thermoregulatory system to increased body temperature.
Mechanisms of the panting absence in dehydrated animals are unclear. It has been suggested that dehydration-induced alterations in the central processes regulating evaporation are mediated mainly by the increased osmolality of body fluids, because intra-carotid or intra-cerebroventricular (icv) infusions of hypertonic saline produced a significant reduction in breathing frequency and a rise in body temperature in hydrated animals exposed to heat load Citation[14–16]. Since arginine vasopressin (AVP) is released in response to both reduced blood volume and elevated body fluid osmolality, it has frequently been suggested that the thermoregulatory effects of dehydration might be mediated by AVP.
A role of the decreased circulating plasma volume without changes in plasma osmolality in producing of the thermoregulatory and respiratory changes during dehydration and exogenous hyperthermia has not been so far investigated.
In this study, dehydration was induced by furosemide which is known to produce only a decrease in plasma volume without a significant change in plasma osmolality Citation[13], Citation[24]. The unchanged plasma osmolality was confirmed by unchanged sodium concentration in the rabbits. Therefore, it is suggested that the lower frequency of breathing during hyperthermia in dehydrated animals occurs not only during hyperosmotic dehydration induced by water deprivation, but also under conditions of furosemide-induced isosmotic dehydration/hypovolemia, possibly as a means to reduce respiratory evaporative heat loss. Hypovolemia alone seems to be sufficient to produce the thermoregulatory changes or to elevate the panting threshold in dehydrated rabbits during exogenous hyperthermia; hyperosmolality is not a requisite.
In the course of the BT recovery, the minute ventilation increased further in the normovolemic/euhydrated rabbits, thus affecting ETCO2 and blood gases more markedly. In the hypovolemic/dehydrated animals VE decreased during cooling. In accordance with this response, ETCO2 significantly increased and hypocapnia was not intensified more in the hypovolemic rabbits during cooling. Respiratory variables did not reveal significant differences between the hypovolemic/isosmotically dehydrated and normovolemic/euhydrated animals during recovery of the BT.
Cardiovascular variables
In these experiments, isosmotic dehydration under normothermic conditions—the reduction in plasma volume approximately by 16%—caused a significant increase in heart rate and significant decreases in all studied regional blood flows (SBF, MBF, RBF). The arterial pressure was not changed significantly by hypovolemia.
It is known that skin blood flow increases when mammals are exposed to the heat stress. Heat stress-induced cutaneous vasodilation is mediated mainly by a reduction in sympathetic vasoconstrictor activity and in nonacral regions also by an activation of the sympathetic cutaneous active vasodilator system Citation[25]. Local effects of temperature are also involved Citation[26].
The state of body hydration profoundly affects both the ability to regulate deep body temperature and the ability to maintain circulatory stability during prolonged heat exposure Citation[27]. Under conditions of dehydration and heat stress mammals regulate deep body temperature at elevated levels due to a reduction of the sweating response Citation[6], Citation[23] and a redistribution of blood flow away from regional (cutaneous) circulations to maintain central blood volume Citation[10], Citation[11].
Dehydration or hypovolemia attenuates the heat stress-induced cutaneous vasodilation in both humans Citation[5], Citation[11], Citation[28], Citation[29] and animals—the baboons and the rats Citation[12], Citation[13], Citation[30], Citation[31].
Regarding the mechanisms producing vasoconstrictor bias on the heat stress-induced cutaneous vasodilation during dehydration, it was suggested that both hypovolemia and hyperosmolality triggers attenuation of cutaneous vasodilation during environmental heating Citation[10], Citation[32]. Activity of the sympathetic nervous system, plasma vasopressin level and activity of the renin-angiotensin system are all elevated during dehydration. It is possible that these vasoconstrictor mechanisms are also involved in producing vasoconstrictor bias on the cutaneous circulation during dehydration, induced mainly by fluid deprivation (during hyperosmotic dehydration). They could also be involved in producing of MAP tendency to be higher during hyperthermia in the hypovolemic/dehydrated rabbits compared to the normovolemic animals. However, Thornton and Proppe Citation[33] have found that pharmacological blockade of these vasoconstrictor systems does not reverse the dehydration-produced attenuation of the increase in skin blood flow during exogenous over-heating. This finding raises the possibility that the body hydration alterations can act directly on vascular smooth muscle in addition to or instead of acting as stimuli for activation of neurohumoral effector mechanisms.
On the other hand, Turlejska-Stelmasiak Citation[34] has shown in the experiments on rabbits that the increase in ear skin blood flow elicited by local pre-optic-anterior hypothalamic warming was not influenced by dehydration. This finding was also confirmed by the results, in which the increase in ear skin blood flow during exogenous hyperthermia was not markedly changed by isosmotic hypovolemia/dehydration. Therefore, attenuation of the heat stress-induced cutaneous vasodilation during dehydration does not appear to occur in rabbits, based on Turlejska-Stelmasiak's Citation[34] and these results, indicating thus a great biological value of the cutaneous vasodilation in rabbits during hyperthermia.
During hyperthermia, the vasoconstriction in mesenteric and renal circulations occurs Citation[2], Citation[35], Citation[36]. This regional vasoconstriction compensates the cutaneous vasodilation and, thus, plays an important role in maintaining of the arterial blood pressure under hyperthermic conditions. Several experimental studies Citation[37–39] have indicated that elevation of sympathetic nervous activity is the major, if not the sole, mechanism in the producing of the visceral vasoconstriction.
Also in these experiments on anaesthetized rabbits, the mesenteric and renal blood flows decreased during body surface heating, indicating the vasoconstriction in visceral region during hyperthermia. However, the mesenteric vasoconstriction was significantly attenuated in the hypovolemic animals. The renal vasoconstriction found during body surface heating in these rabbits only tended to be attenuated in the hypovolemic state.
Similar findings were described by Massett et al. Citation[40] who studied the regional and systemic haemodynamic and sympathoadrenal responses to heating in dehydrated anaesthetized rats. In their study, dehydration was induced by 24 and 48-h water deprivation (hyperosmotic dehydration). Compared with euhydrated controls, dehydrated rats in their experiments exhibited attenuated visceral (mesenteric) vasoconstrictor responses during heating. Tail-skin blood flow estimated from tail temperature was similar across groups during heating, in accordance with the results on anaesthetized rabbits. Therefore, it is suggested that the attenuation of the mesenteric vasoconstrictor response observed during heating in dehydrated animals occurs not only during hyperosmotic dehydration, but it is present also under conditions of the isosmotic dehydration/hypovolemia, producing only changes in volumoreceptor activity without changes in osmoreceptor activity.
Although the mechanism of this attenuated vasoconstrictor response in mesenteric region under hypovolemic conditions was not elucidated in the study, one potential explanation could be accepted, based on the results of Massett et al. Citation[40], Citation[41]. They found exaggerated levels of plasma catecholamines despite the attenuation of the pressor and regional vasoconstrictor responses during heating in dehydrated rats. They have supposed that one possible factor contributing to the reduced pressor and vasoconstrictor responses in dehydrated rats is a reduction in adrenergic receptor sensitivity. However, further experiments would be necessary to confirm the possibility that adrenergic receptor sensitivity is altered with dehydration.
Regarding the biological role of the reduced vasoconstrictor response in mesenteric region, it was supposed that the attenuation of the mesenteric vasoconstriction during hyperthermia in the hypovolemic/dehydrated rabbits is aimed at decreasing a danger of splanchnic ischaemia.
Similarly to other studies, also in these experiments, the arterial blood pressure during heating was not significantly affected by hypovolemic/dehydrated state Citation[5], Citation[27], although it tended to be higher compared to the normovolemic animals. Similar findings were described by Thornton and Proppe Citation[12], who observed that arterial blood pressure during environmental heating in dehydrated baboons was regulated at 15–16 mmHg above the euhydrated level. An elevation in arterial blood pressure also occured in Long-Evans rats that were dehydrated for 48 h Citation[42]. Why arterial blood pressure is elevated during dehydration is unknown. The vasoconstrictor mechanisms mentioned above could play a crucial role in this response.
It is well documented that heart rate (HR) increases as hyperthermia developes during environmental heat stress. Also in the present study, a corresponding increase in HR was observed during body surface heating. HR responses to the increasing BT were similar between the hypovolemic and normovolemic groups.
Recovery of the body temperature by body surface cooling was accompanied by return of the cardiovascular variables approximately to the initial values. The regional and systemic cardiovascular variables did not reveal significant differences between the hypovolemic/dehydrated and normovolemic/euhydrated animals during physical treatment of the experimental hyperthermia.
Conclusion
During exogenous hyperthermia, the hypovolemic/isosmotically dehydrated rabbits had lower frequency of breathing compared to the controls. Panting was absent in the hypovolemic rabbits, unlike the controls.
The vasoconstrictor response in visceral (mesenteric) region during hyperthermia was attenuated, whereas the heat stress-induced cutaneous vasodilation regarding its biological importance was not influenced by hypovolemia/isosmotic dehydration. Therefore, it was suggested that hypovolemia alone is sufficient to produce described respiratory, thermoregulatory and cardiovascular changes in dehydrated rabbits during exogenous hyperthermia, whereas hyperosmolality is not a requisite.
During physical treatment of the experimental hyperthermia, respiratory and cardiovascular variables did not reveal significant differences between the hypovolemic/isosmotically dehydrated and normovolemic/euhydrated rabbits.
References
- Kullmann R, Schönung W, Simon E. Antagonistic changes of blood flow and sympathetic activity in different vascular beds following central thermal stimulation. I. Blood flow in skin, muscle and intestine during spinal cord heating and cooling in anesthetized dogs. Pflügers Archives 1970; 319: 146–161
- Kregel KC, Overton JM, Johnson DG, Tipton CM, Seals DR. Mechanism for pressor response to nonexertional heating in the conscious rat. Journal of Applied Physiology 1991; 71: 192–196
- Gorman AJ, Proppe DW. Mechanisms producing tachycardia in conscious baboons during environmental heat stress. Journal of Applied Physiology: Respiratory Environmental & Exercise Physiology 1984; 56: 441–446
- Javorka K, Čalkovská A, Petrášková M, Gecelovská V. Cardiorespiratory parameters and respiratory reflexes in rabbits during hyperthermia. Physiological Research 1996; 45: 439–447
- Horstman DH, Horvath SM. Cardiovascular and temperature regulatory changes during progressive dehydration and euhydration. Journal of Applied Physiology 1972; 33: 446–450
- Takamata A, Mack GW, Gillen CM, Jozsi AC, Nadel ER. Osmoregulatory modulation of thermal sweating in humans: reflex effects of drinking. American Journal of Physiology 1995; 268: R414–R422
- Doris PA, Baker MA. Hypothalamic control of thermoregulation during dehydration. Brain Research 1981a; 206: 219–222
- Dupre RK, Crawford EC, Jr. Control of panting in the desert iguana: roles for peripheral temperatures and the effect of dehydration. Journal of Experimental Zoology 1985; 235: 341–347
- Maloney SK, Dawson TJ. Changes in pattern of heat loss at high ambient temperature caused by water deprivation in a large flightless bird, the emu. Physiological Zoology 1998; 71: 712–719
- Nadel ER, Fortney SM, Wenger CB. Effect of hydration state on circulatory and thermal regulations. Journal of Applied Physiology: Respiratory Environmental & Exercise Physiology 1980; 49: 715–721
- González-Alonso J. Separate and combined influences of dehydration and hyperthermia on cardiovascular responses to exercise. International Journal of Sports Medicine 1998; 19: S111–S114
- Thornton RM, Proppe DW. Attenuation of hindlimb vasodilation in heat-stressed baboons during dehydration. American Journal of Physiology: Regulatory Integrative and Comparative Physiology 1986; 250: R30–R35
- Nakajima Y, Nose H, Takamata A. Plasma hyperosmolality and arterial pressure regulation during heating in dehydrated and awake rats. American Journal of Physiology: Regulatory Integrative and Comparative Physiology 1998; 275: R1703–R1711
- Turlejska E, Baker MA. Elevated CSF osmolality inhibits thermoregulatory heat loss responses. American Journal of Physiology 1986; 251: R749–R754
- Baker MA, Dawson DD. Inhibition of thermal panting by intracarotid infusion of hypertonic saline in dogs. American Journal of Physiology 1985; 249: R787–R791
- Nijland MJ, Baker MA. Effects of hydration state on exercise thermoregulation in goats. American Journal of Physiology 1992; 263: R201–R205
- Van Beaumont W. Evaluation of hemoconcentration from hematocrit measurements. Journal of Applied Physiology 1972; 32: 712–713
- Brozmanova A, Zila I, Javorka K, Porubcan J, Kapsova J. Effects of acute normovolemic haemodilution on cardiorespiratory changes in hyperthermia and its physical treatment. International Journal of Hyperthermia 2004; 20: 851–864
- Itsaki-Glucklich S, Arad Z. The effect of dehydration on brain temperature regulation in Japanese quail (Coturnix coturnix japonica). Computational Biochemistry & Physiology: Comparative Physiology 1992; 101: 583–588
- Doris PA, Baker MA. Effect of dehydration on thermoregulation in cats exposed to high ambient temperatures. Journal of Applied Physiology: Respiratory Environment & Exercise Physiology 1981b; 51: 46–54
- Taylor CR. Dehydration and heat: effects on temperature regulation of East African ungulates. American Journal of Physiology 1970; 219: 1136–1139
- Baker MA, Doris PA. Effect of dehydration on hypothalamic control of evaporation in the cat. Journal of Physiology 1982; 322: 457–468
- Sawka MN, Young AJ, Francesconi RP, Muza SR, Pandolf KB. Thermoregulatory and blood responses during exercise at graded hypohydration levels. Journal of Applied Physiology 1985; 59: 1394–1401
- Thenuwara K, Todd MM, Brian JE. Effect of manitol and furosemide on plasma osmolality and brain water. Anesthesiology 2002; 96: 416–421
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. Handbook of Physiology. Environmental Physiology, A Brozmanova. American Physiology Society, Bethesda, MD 1996; 1: 215–243, Sect. 4, Chapt. 11
- Taylor WF, Johnson JM, O’Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. Journal of Applied Physiology: Respiratory Environmental & Exercise Physiology 1984; 7: 191–196
- Takamata A, Nose H, Mack GW, Morimoti T. Control of total peripheral resistance during hyperthermia in rats. Journal of Applied Physiology 1990; 69: 1087–1092
- Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of acute alterations of blood volume on circulatory performance in humans. Journal of Applied Physiology: Respiratory Environmental & Exercise Physiology 1981; 50: 292–298
- González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. Journal of Applied Physiology 1995; 79: 1487–1496
- Ryan KL, Proppe DW. Effect of water or saline intake on heat-induced limb vasodilation in dehydrated baboons. American Journal of Physiology 1990a; 258: R318–R324
- Ryan KL, Proppe DW. Effects of compartmental fluid repletion on heat-induced limb vasodilation in dehydrated baboons. American Journal of Physiology 1990b; 259: R1139–R1147
- Proppe DW. Effects of hyperosmolarity and diuretics on heat-induced limb vasodilation in baboons. American Journal of Physiology 1990; 258: R309–R317
- Thornton RM, Proppe DW. Influence of vasoconstrictor systems on leg vasodilation during heating of dehydrated baboons. American Journal of Physiology 1988; 254: H11–H19
- Turlejska-Stelmasiak E. The influence of dehydration on heat dissipation mechanisms in the rabbit. Journal of Physiology, Paris 1974; 68: 5–15
- Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. Journal of Applied Physiology 1970; 28: 415–420
- Kenney MJ, Musch KI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. American Journal of Physiology: Heart Circulation Physiology 2001; 280: H2868–H2875
- Iriki M, Riedel W, Simon E. Regional differentiation of sympathetic activity during hypothalamic heating and cooling in anesthetized rabbits. Pflügers Archives 1971; 328: 320–331
- Gisolfi CV, Matthes RD, Kregel KC, Oppliger R. Splanchnic sympathetic nerve activity and circulating catecholamines in the hyperthermic rat. Journal of Applied Physiology 1991; 70: 1821–1826
- Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. Journal of Applied Physiology 1995; 78: 881–889
- Massett MP, Johnson DG, Kregel KC. Cardiovascular and sympathoadrenal responses to heat stress following water deprivation in rats. American Journal of Physiology: Regulatory Integrative and Comparative Physiology 1996; 270: R652–R659
- Massett MP, Lewis SJ, Kregel KC. Effect of heating on the hemodynamic responses to vasoactive agents. American Journal of Physiology 1998; 275: R844–R853
- Rockhold RW, Share L, Crofton JT, Brooks DP. Cardiovascular response to vasopressin vasopressor antagonist administration during water deprivation in the rat. Neuroendocrinology 1984; 38: 139–144