Abstract
In recent years, both hyperthermia and gene-therapy have been evaluated as approaches to improve cancer radiotherapy. In addition, potential exists to combine these approaches to increase the overall therapeutic efficacy. For example, it has been reported that adenovirus-mediated heat-inducible gene expression may reduce the normal tissue toxicity associated with constitutively controlled expression of therapeutic genes. In our laboratory, we have shown that adenovirus-mediated, heat-activated antisense Ku70 expression radiosensitizes tumor cells in vitro and in vivo, suggesting a novel approach to use heat-activated gene-radiotherapy to radiosensitize human tumors.
However, to optimize the application of heat-activated gene-radiotherapy in the clinic, we need to develop techniques to improve the delivery of the therapeutic adenovirus and to verify/monitor the delivery non-invasively. In an ongoing study we test the effect of mild hyperthermia in improving adenovirus-medicated vector delivery in a mouse tumor model. In addition, we evaluate the use of non-invasive microPET imaging to monitor the spread of the adenoviral vector. Our preliminary results show that (1) microPET imaging can be used to monitor non-invasively the viral vector delivery and dissemination, and (2) mild heat shock leads to significantly improved viral vector distribution, in other words, a wider spatial spread, in vivo.
Here, we will present a short review on the current status of hyperthermia and heat-activated gene-radiotherapy, and the potential use of PET imaging in gene therapy.
Hyperthermia as an adjuvant of radiotherapy
The use of hyperthermia as an adjuvant in cancer radiotherapy, based on the potentiation of radiation effect by heat treatment, has been the subject of clinical investigation for some time Citation[1–3]. Thus far, full realization of this potential has been hindered by an inability to deliver sufficient heat dose in a spatially- and temporally-controlled manner. This is especially true for deep-seated tumors, for which improvement is needed in the equipment used for planning, implementing, and monitoring hyperthermic treatments. Recently, a randomized trial of hyperthermia and radiation for superficial tumors demonstrated that adjuvant hyperthermia with a thermal dose more than 10CEM 43°C T90 confers a significant local control benefit Citation[4]. Alternatively, improvement may be possible through better understanding of the biology of heat shock in combination with radiation. In this regard, basic science investigation has unraveled important mechanistic knowledge of the heat shock phenomenon, including transcription control, protein trafficking, chaperon function, and the role of heat shock proteins in cancer immunotherapy Citation[5].
The heat shock response, a highly conserved process, involves the rapid and transient increases in transcription of a number of heat shock genes when cells are subjected to heat stress. In eukaryotes, the response is mediated by a sequence-specific DNA binding protein termed HSF1 Citation[6]. In unstressed mammalian cells, HSF1 is maintained in a monomeric, non-DNA binding form. Upon heat shock, HSF1 assembles into trimers, binds tightly to the heat shock elements (HSEs) located in the promoter region of heat shock genes and trans-activates the heat shock gene expression Citation[7], Citation[8]. This heat-induced transactivation can be several orders of magnitude, thus making the heat shock promoter, specifically, the hsp70 promoter one of the most efficient inducible regulatory sequences Citation[5], Citation[6], Citation[9].
Heat-activated gene-radiotherapy targeting DNA repair
Altering the genetic makeup of a cancer cell by gene transfer is a potentially powerful strategy for treating human cancer. However, the relative low efficiency of gene delivery in vivo and relatively poor tumor specificity has prevented the widespread implementation of this technology in the clinic. Despite these formidable obstacles, successful application of gene therapy in the treatment of cancer may be possible when it is combined with localized modalities such as ionizing radiation (for review see Citation[10] and references within). In the past decade, the vast knowledge of the molecular defects that drive the cancer process, coupled with the rapidly expanding information of the genes responsible for tumor cell radioresistance, have led to the development of rational, targeted gene therapy strategy designed to increase tumor cell radiosensitivity.
The repair of DNA double-strand break (DSB) induced by ionizing radiation is essential for the survival of irradiated mammalian cells. DNA DSB repair involves two mechanistically distinct, but sometimes overlapping pathways: homologous recombination (HR) and non-homologous end-joining (NHEJ). Recent genetic and biochemical studies firmly established that DNA-dependent protein kinase (DNA-PK) activated by DNA ends play a central role in the NHEJ pathway of DSB repair. DNA-PK is a serine/threonine kinase that consists of a large catalytic subunit of 465-kDa (DNA-PKcs) and a DNA-targeting component Ku, which itself is a heterodimer of 70-kDa and 86-kDa polypeptides (Ku70 and Ku80, respectively) Citation[11–13]. Recently, studies using Ku80-/-, Ku70-/- and DNA-PKcs-/- knockout mouse model systems, have clearly shown that deficiency in any of the three subunits of DNA-PK leads to decreased DNA DSB repair and increased radiosensitivity, both in vitro and in vivo Citation[14–16]. In particular, absence of Ku70 or Ku80 led to a ∼2.5-fold increase in the slope of the radiation survival curve. These findings motivated investigators to design methods to down-regulate the Ku level in tumors, and to evaluate whether this approach will enhance the response of tumors to ionizing radiation.
A plausible approach to radiosensitize cells is to reduce the cellular level or activity of Ku70, using gene delivery systems to express antisense Ku70 Citation[17]. This idea is similar in principle to the work by Marangoni et al., who showed that constitutive expression of antisense Ku80 radiosensitizes human fibroblasts Citation[18]. To induce the overexpression of antisense Ku70, the use of the heat shock process was proposed, as the heat shock promoter is one of the most effective inducers Citation[1], Citation[9], Citation[17], Citation[19]. The use of a heat-inducible system for the activation has other advantages as well; it provides geometrical definition by focal heat delivery in the in vivo situation, and it may circumvent unexpected normal tissue toxicity associated with a constitutive high-level expression of antisense Ku70 RNA or other therapeutic target genes.
In terms of the gene delivery system, although both viral and non-viral delivery systems have been exploited, the former are the most advanced in terms of preclinical development and clinical potential Citation[20]. In the past decades, adenovirus vectors have been developed as a tool for gene transfer into mammalian cells and for gene therapy applications Citation[1], Citation[9], Citation[10], Citation[19], Citation[21–26]. These vectors have many advantages: the recombinant adenoviruses can infect a wide variety of dividing and non-dividing cells; they can be purified to high titers; the strains commonly used to construct recombinant viruses are well characterized; their genome rarely integrates into the host chromosome, thus making them suitable in applications that require efficient and transient expression of vector-borne genes. The recombinant viruses are propagated in special cell lines that express the deleted viral genes in trans, so that viral particles can be made and isolated, but are not themselves capable of replication when infecting non-permissive cells. Furthermore, the well characterized adenovirus shuttle vectors and the 293 cells used to produce non-replicating adenovirus vectors are commercially available.
In the study by Li et al., the potential of a heat-activated gene-therapy approach to enhance the response of tumors to ionizing radiation was tested in in vitro (cell culture) and in vivo (rodent) systems using adenoviral-mediated transfer of an antisense Ku70 under the control of the heat-inducible hsp70 promoter, followed by heat and radiation treatment. These data clearly demonstrated that heat-induced expression of antisense Ku70 RNA attenuates Ku70 protein level, and significantly sensitizes the FSa-II tumors to ionizing radiation both in vitro and in vivo Citation[17]. These results suggest that adenovirus-mediated, heat-activated antisense Ku70 expression may provide a novel approach to radiosensitize human tumors and improve clinical outcome.
The study described above is consistent with earlier reports that demonstrated the feasibility of heat-activated, target gene and radiation therapy strategy Citation[1], Citation[9]. Huang et al. showed that intralesion injection of the interleukin-12 (IL-12) carrying adenovirus vector in a mouse melanoma tumor model caused significant tumor growth delay only with hyperthermia treatment Citation[19]. Lohr et al. from the same group further demonstrated that adenovirus mediated intratumoral expression of IL-12 under the control of a heat-inducible promoter in combination with hyperthermia is almost as effective as that under the control of a constitutively activated cytomegalovirus (CMV) promoter, whereas systemic transgene levels are substantially reduced with the heat-inducible promoter. Importantly, these investigators showed that the tumor response to radiotherapy is considerably improved when combined with heat-inducible gene therapy without apparent systemic toxicity Citation[27]. To further examine the effect of heat shock, the systemic leakage and transgene expression by intratumorally injected recombinant adenoviral vectors, an inducible hsp70 promoter-driven IL-12 strategy was tested Citation[27]. It was shown that after localized hyperthermia at 42.5°C, high intratumoral levels of a therapeutic transgene can be obtained while systemic expression is reduced to a minimum, thus increasing the safety of adenovirus-based tumor gene therapy Citation[27].
Hyperthermia improves viral vector distribution: Potential use of PET imaging
To optimize the application of heat-activated, adenovirus-mediated gene and radiation therapy to the clinic, one needs to develop techniques to improve the delivery of adenovirus and to verify the vector delivery non-invasively. It is well documented that physical treatment such as mild hyperthermia increases tumor vascular permeability, blood flow and perfusion, leading to faster diffusion Citation[17], Citation[28–38]. Thus, in a pilot study we investigate the effect of mild hyperthermia on the diffusion of adenoviral vectors in a rodent model. To visualize and quantify the improvement due to hyperthermic application we use a molecular imaging approach with a dedicated animal positron emission tomography system (microPET).
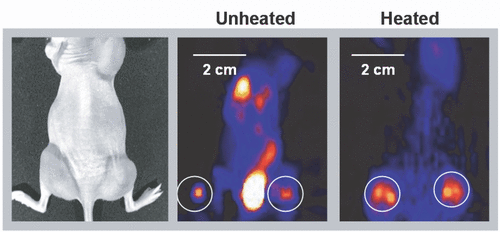
For this pilot study, we generated replication-defective adenoviruses rAd(CMV-TKeGFP) containing a CMV promoter-driven tk gene, the expression of which can be imaged by the trapping of 18F-FEAU inside adenovirus-infected tumor cells. Dunning rat prostate tumor R3327-AT cells were implanted subcutaneously into the thighs of nude mice. When the tumors reach ∼1 cm in diameter, adenoviruses were injected into the tumors. Six hour later, the tumors were heated at 42°C for 30 minutes or sham-treated. The virus-mediated tk gene expression was monitored at 24 hr and 48 hr post-viral injection by microPET imaging using 18F-FEAU as the marker/reporter substrate Citation[39]. The serial microPET images of heated and control (injected with viruses but not heated) tumors were analyzed to determine the 18F-FEAU distribution, as a surrogate of the dissemination of the adenoviral vectors. Our preliminary data () clearly shows that (i) microPET imaging can be used to monitor non-invasively the viral vector delivery and dissemination, and (ii) mild heat shock leads to significantly improved viral vector distribution, in other words, a wider spread, in vivo.
Discussion and summary
Although only preliminary results are presented, they demonstrate the potential of using molecular imaging methods to monitor the delivery of adenoviral vectors in gene-therapy. In this study the replication-defective adenovirus rAd(CMV-TKeGFP) was used as a proof of principle. We believe that if adenovirus containing a therapeutic gene is co-injected, the microPET images obtained due the expression of the CMV-TKeGFP serve as surrogates for the spread of the therapeutic adenovirus. Of course, it would be more desirable to image the therapeutic gene directly, however, that is difficult or impossible to do. An alternative would be to construct an adenoviral vector that contains both the imaging marker gene and the therapeutic gene. Even in that scenario there is still the caveat that the images are the results of enzymatic reactions of the HSV1-tk protein with the associated amplification. Nevertheless, PET imaging of gene expression provides a promising approach that may have applications in the treatment of cancer and other diseases based on the gene therapy paradigm.
One advantage of PET imaging is that it is non-invasive. But that could also be a disadvantage in that the detailed information in the tumor is not available. Thus, validation of studies need to be performed to relate PET images to analysis of tumor specimens. We are currently performing such studies comparing PET images with tumor sections. Our study endpoints of tumor sections include H&E, immunohistochemistry of endogenous proteins, and autoradiographs of 18F-FEAU and 124F-FIAU (an analog of FEAU radiolabeled with a longer-lived radioisotope). Through these correlative studies we hope to calibrate the PET images and provide the biological underpinning.
Positive results from preclinical feasibility studies, in increasing the sensitivity of radioresistant cells to ionizing radiation, may lead to future clinical trials targeting disease sites that can benefit from radiosensitization. Advances in conformal and intensity-modulated radiation therapy have provided a powerful tool in the treatment of cancer by locally irradiating the tumors with minimal damage to healthy tissues. However, a number of tumors, such as glioblastomas, are resistant to radiation. It is conceivable to develop an image-guided therapeutic strategy combining (i) image-guided delivery of vectors that express a heat-inducible radiosensitizing effector gene and a marker gene, (ii) application of hyperthermia followed by verification of vector delivery to tumor cells by molecular imaging, and (iii) tumor eradication by ionizing radiation. If tumor cells in general and radioresistant tumor cells in particular could be sensitized by heat-activated expression of genes which antagonize the DNA repair mechanism, novel approaches could be developed as an adjuvant to radiation therapy.
Acknowledgements
This work is supported in part by research grants from the National Institute of Health (CA56909 and CA109772).
The material in this paper was presented in an invited lecture in the 2005 Conference of the European Society of Hyperthermic Oncology, held in Graz, Austria. This report is prepared as a summary of that lecture, to be part of a special issue of the International Journal of Hyperthermia that highlights the proceedings of the conference.
References
- Blackburn RV, Galoforo SS, Corry PM, Lee YJ. Adenoviral-mediated transfer of a heat-inducible double suicide gene into prostate carcinoma cells. Cancer Research 1998; 58: 1358–1362
- Leibel S, Phillips T. Textbook of radiation oncology. W. B. Saunders, Philadelphia, PA 1998, editors
- Oleson JR, Calderwood SK, Coughlin CT, Dewhirst MW, Gerweck LE, Gibbs FA, Kapp DS. Biological and clinical aspects of hyperthermia in cancer therapy. Am J Clin Oncol 1988; 11: 368–380
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23(13)3079–85
- Morimoto RI, Tissieres A, Georgopoulos C. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Plainview 1994, editors
- Morimoto RI, Tissieres A, Georgopoulos C. Stress proteins in biology and medicine. Cold Spring Harbor Laboratory Press, Plainview 1990, editors
- Maresca B, Lindquist S. Heat shock. Springer-Verlag, New York 1991, editors
- Wu C, Zimarino V, Tsai C, Walker B, Wilson S. Transcriptional regulation of heat shock genes. Stress proteins in biology and medicine, RI Morimoto, A Tissieres, C Georgopoulos. Cold Spring Harbor Laboratory Press, Plainview 1990; 429–442
- Borrelli MJ, Schoenherr DM, Wong A, Bernock LJ, Corry PM. Heat-activated transgene expression from adenovirus vectors infected into human prostate cancer cells. Cancer Research 2001; 61: 1113–1121
- Freytag SO, Kim JH, Brown SL, Barton K, Lu M, Chung M. Gene therapy strategies to improve the effectiveness of cancer radiotherapy. Expert Opin Biol Ther 2004; 4(11)1757–70
- Anderson CW. DNA damage and the DNA-activated protein kinase. Trends in Biochemical Science 1993; 18: 433–437
- Featherstone C, Jackson SP. Ku, a DNA repair protein with multiple cellular functions?. Mutation Research 1999; 434: 3–15
- Tuteja R, Tuteja N. Ku autoantigen: A multifunctional DNA-binding protein. Critical Reviews in Biochemistry and Molecular Biology 2000; 35: 1–33
- Kurimasa A, Ouyang H, Dong L-J, Wang S, Li X, Cordon-Cardo C, Chen DJ, Li GC. Catalytic subunit of DNA-dependent protein kinase: Impact on lymphocyte development and tumorigenesis. Proceedings of the National Academy of Sciences. USA 1999; 96: 1403–1408
- Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, Ling CC, Fuks Z, Cordon-Cardo C. Ku70: A candidate tumor suppressor gene for murine T cell lymphoma. Molecular Cell 1998; 2: 1–8
- Nussenzweig A, Sokol K, Burgman P, Li L, Li GC. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage: The effects of ionizing radiation on growth, survival, and development. Proceedings of the National Academy of Sciences. USA 1997; 94: 13588–13593
- Li GC, He F, Shao X, Urano M, Shen L, Kim D, Borrelli M, Leibel SA, Gutin PH, Ling CC. Adenoviral-mediated heat-activated antisense Ku70 RNA radiosensitizes tumor cells in vitro and in vivo. Cancer Research 2003; 63: 3268–3274
- Marangoni E, Le Romancer M, Foray N, Muller C, Douc-Rasy S, Vaganay S, Abdulkarim B, Barrois M, Calsou P, Bernier J, Salles B, Bourhis J. Transfer of Ku86 RNA antisense decreases the radioresistance of human fibroblasts. Cancer Gene Therapy 2000; 7: 339–346
- Huang Q, Hu JK, Lohr F, Zhang L, Braun R, Lanzen J, Little JB, Dewhirst MW, Li C-Y. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Research 2000; 60: 3435–3439
- Gupta N. Current status of viral gene therapy for brain tumors. Exp Opin Invest Drugs 2000; 9: 713–726
- Danthinne X, Imperiale MJ. Production of first generation adenovirus vectors: A review. Gene Therapy 2000; 7: 1707–1714
- Fisher KJ, Choi H, Burda J, Chen SJ, Wilson JM. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology 1996; 217: 11–22
- Le Gal LaSalle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet L, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science 1993; 259: 988–990
- Wang Q, Finer MH. Second-generation adenovirus vectors. Nature Medicine 1996; 2: 714–716
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proceedings of the National Academy of Sciences. USA 1994; 91: 4407–4411
- Zhang Y, Schneider R. Adenovirus inhibition of cell translation facilitates release of virus particles and enhances degradation of the cytokeratin network. Journal of Virology 1994; 68: 2544–2555
- Lohr F, Huang Q, Hu K, Dewhirst M, Li C. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clinical Cancer Research 2001; 7: 3625–3628
- Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990; 50(3 Suppl)814s–819s
- Kong G, Anyarambhatla G, Petros WP, Braun RD, Colvin M, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Research 2000; 60: 6950–6957
- Schuster J, Zalusky M, Noska M, Dodge R, Friedman H, Bigner d, Dewhirst MW. Hyperthermic modulation of radiolabelled antibody uptake in a human glioma xenograft and normal tissues. International Journal of Hyperthermia 1995; 11: 59–72
- Hauck M, Coffin D, Dodge R, Dewhirst MW, Mitchell M, Zalusky M. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumor interstitial fluid pressure. International Journal of Hyperthermia 1997; 13: 307–316
- Fujiwara K, Watanabe T. Effects of hyperthermia, radiotherapy and thermoradiotherapy on tumor microvascular permeability. Acta Pathol Jpn 1990; 40: 79–84
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155(4)515–528
- Okajima K, Griffin RJ, Iwata K, Shakil A, Song CW. Tumor oxygenation after mild-temperature hyperthermia in combination with carbogen breathing: Dependence on heat dose and tumor type. Radiat Res 1998; 149(3)294–299
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 1996; 12(3)367–373
- Lyons JC, Song CW. Killing of hypoxic cells by lowering the intracellular pH in combination with hyperthermia. Radiat Res 1995; 141(2)216–218
- Lin JC, Song CW. Influence of vascular thermotolerance on the heat-induced changes in blood flow, pO2, and cell survival in tumors. Cancer Res 1993; 53(9)2076–2080
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984; 44(10 Suppl)4721s–4730s
- Blasberg R, Gelovani J. Molecular-genetic imaging: A nuclear medicine-based perspective. Molecular Imaging 2002; 1: 160–180