Abstract
Tumour oxygenation was measured in seven canine soft tissue sarcomas being treated with a fractionated course of radiation and hyperthermia. Measurements obtained during treatment were compared to pre-treatment measurements. The most important finding was an increase in oxygenation in tumours with low pre-treatment oxygenation that persisted throughout treatment. This is an advantageous hyperthermia effect as it may lead to increased radiation cell killing at each fraction. In other tumours, potentially less advantageous changes in oxygenation may be hyperthermia fractionation related and this deserves further investigation.
Introduction
There have been numerous positive phase III trials of hyperthermia that support the clinical use of hyperthermia in combination with radiation. However, after decades of pre-clinical assessment and successful clinical trials, the mechanism whereby hyperthermia and radiation lead to improved tumour control compared to radiation alone is still unknown.
Much of the initial excitement regarding the use of hyperthermia was focused on its cytotoxic properties, especially the enhanced sensitivity of cells at low pH and cells in the S phase of the cell cycle. However, as thermal distributions in spontaneous tumours became more completely characterized, it was obvious that most of the tumour volume would never be heated to levels resulting in clinically significant cytotoxicity. As an example, the cytotoxicity of hyperthermia has been modelled in the setting of non-uniform temperature distributions using real SAR data. The simulated cell killing, based on in vitro derived CHO hyperthermia survival data, predicted that a typical clinical hyperthermia treatment might kill no more than 10–20% of cells Citation[1]. Considering that cells from human spontaneous tumours are probably more heat resistant than rodent cells, the impact of thermal cytotoxicity on outcome is almost certainly less than modelled for tumours based on the heat response of rodent cells.
Another characteristic of hyperthermia that contributed to the initial excitement was that of heat radiosensitization. However, taking advantage of heat radiosensitization for routine clinical use is also problematic due to maximal thermal enhancement requiring simultaneous application of radiation and hyperthermia; the thermal enhancement ratio decreases rapidly when time separates the two modalities, regardless of whether radiation is given before or after hyperthermia Citation[2].
If neither cytotoxicity nor thermal radiosensitization are the basis of the proven benefits of hyperthermia, what is? Clearly, there are molecular effects of tumour heating that have not been completely characterized and these phenomena may play an important role Citation[3], Citation[4]. However, at this time, the contributions of heat-induced molecular alterations to the beneficial effects of hyperthermia are unknown.
Focusing on changes that occur at the level of the tumour micro-environment, alterations in tumour oxygenation as a result of hyperthermia have been proposed as one mechanism of hyperthermia's beneficial effects Citation[5]. Hyperthermia has been shown to affect tumour oxygenation in rodent tumours and this has been known since the early 1980s Citation[6], Citation[7]. Additionally, evidence for a hyperthermia dose response on tumour oxygenation, with decreased oxygenation at higher temperatures, was also suggested based on studies of rodent tumours Citation[8], Citation[9]. Measurements of oxygenation in canine and human solid tumours undergoing thermoradiotherapy verify the concept of improved oxygenation in larger spontaneous tumours Citation[10–12]. However, these measurements in spontaneous tumours were snapshots in time, typically made before and after a hyperthermia treatment, but not throughout a course of fractionated treatment. The temporal change in oxygenation over the entire treatment may be more influential than changes over a few days.
Some have suggested that hyperthermia will confer radiation resistance by causing decreases in tumour oxygenation as a result of vascular injury. Heat associated intra-tumoural vasculopathy has been identified in mice Citation[13] but, just as with thermal cytotoxicity, murine tumour vessels are arguably more heat sensitive than vessels in spontaneous tumours in dogs and humans. For example, it was found that tumour oxygenation may decrease in canine tumours following hyperthermia, but only at high T50 values, e.g. T50 > 44°C Citation[12]. Also, thermal washout data from human tumours support there being no deleterious effect of hyperthermia on tumour blood flow and, thus, oxygenation Citation[14].
Clearly, hyperthermic modification of tumour oxygenation may be a critical factor with regard to tumour response in combination with radiation. It is important that more information is obtained on temporal changes of tumour oxygenation as a consequence of hyperthermia, rather than snapshots as a single time point. This study reports early results of extensive measurements of oxygenation during a highly fractionated thermoradiotherapy protocol.
Materials and methods
Pet dogs with a histologically proven spontaneous soft tissue sarcoma were studied. This study was approved by the North Carolina State University Institutional Animal Care and Use Committee. Tumours were located on the trunk or an extremity and tumour volume was estimated by multiplying the product of three orthogonal diameters by π/6; the maximum allowable volume was 400 cm3.
Dogs were part of a pilot study to identify a thermal dose to be used in a prospective study of hyperthermia fractionation on tumour oxygenation. Two thermal doses were assessed but thermal dose was not randomly assigned. The first three dogs were prescribed a total dose of 10 CEM43°CT90 and the next four a total dose of 40 CEM43°CT90. The total thermal dose was divided into five weekly hyperthermia fractions, with ∼20% of the prescribed total thermal dose given at each fraction.
CEM43°T90 is an isoeffective thermal dose descriptor Citation[15]. By definition it is the Cumulative Equivalent Minutes that T90 is equal to 43°C. It is used to describe a heterogeneous temperature distribution, taking into account the duration of the heat treatment. The cumulative CEM43°CT90 dose over all hyperthermia treatments is determined by the individual temperature measurements during each treatment (applied power), the overall number of treatments and the duration of each treatment. How the biologic effect of any given thermal dose, quantified as CEM43°CT90, is affected by each of the three parameters contributing to it is unknown.
For hyperthermia, dogs were under general anaesthesia maintained with isoflurane in 100% oxygen. Tumours were imaged using computed tomography and thermometry catheters were placed according to RTOG guidelines Citation[16], Citation[17]. Hyperthermia was induced using scanning spiral or annular array microwave applicators operating between 140–433 mHz. Upper temperature limits of 43°C and 48°C were placed on normal tissue and tumour, respectively. If these limits were exceeded, power was reduced until temperature decreased into the acceptable range. Temperatures, recorded along the path of the catheters by use of an automated translation device or by manual pullback, were examined manually and the T90 estimated by identifying the temperature representing the 10th percentile. This method has been shown to be valid for delivering a prescribed thermal dose Citation[18]. Continuous monitoring of T90 was used to determine the time needed to administer the daily prescribed thermal dose. Thermometry catheters were removed following each hyperthermia treatment and there was no attempt to reinsert them into the same location for subsequent hyperthermia treatments. Temperature data were analysed as previously described Citation[19].
Dogs also received radiation therapy using 60Co photons. Dogs were under general anaesthesia for radiation therapy; anaesthesia was maintained with isoflurane in 100% oxygen. The prescribed total dose was 56.25 Gy given in 25 daily fractions of 2.25 Gy. On days where hyperthermia was administered, the radiation treatment was given before the hyperthermia procedure with approximately a 2 h interval between the modalities.
Tumour oxygenation was measured before and multiple times after initiation of the treatment protocol. Oxygenation was always measured before the day's radiation fraction; oxygen was not measured on days the dog received hyperthermia. For oxygen measurement, dogs were under general anaesthesia but they did not breathe 100% oxygen. Rather, anaesthesia was maintained with a mixture of oxygen and air to maintain arterial normoxia. The Oxford-Optronix Oxylite system was used for oxygen measurement (http://www.oxford-optronix.com/ptissuemonitoring.htm). The flexible probes, 250–500 µ in diameter, were inserted through a pre-placed catheter and measurements recorded in 5 mm increments or less in small tumours, as the probe was withdrawn. A minimum of 20 oxygen determinations were sampled from four tracks as this has been shown to be representative of overall tumour oxygenation Citation[20]. Tumour oxygenation was expressed as the median of the measured values for each day.
Unfortunately, there are no outcome data in dogs in this study so the biologic effect of the observed changes in oxygenation cannot be related to a clinical end-point.
Results
Hyperthermia dose was delivered as prescribed in all dogs (). All dogs received five hyperthermia treatments, except dog 4 where four treatments were administered due to equipment malfunction. Oxygen was measured 12 or 13 times in each dog.
Table I. Tumour volume, prescribed hyperthermia dose and delivered hyperthermia dose for dogs in this study.
Prescribed CEM43°CT90 = 10 min ()
Dog 1 had a very hypoxic tumour (median pre-treatment pO2 = 0 mm Hg). Oxygenation increased following the first hyperthermia treatment and remained elevated throughout treatment.
Figure 1. Oxygen measurements during a course of fractionated thermoradiotherapy in three dogs where 10 CEM43°CT90 were prescribed. Tumour volume and total hyperthermia dose administered are given at the top of each panel. Filled squares are the oxygen measurements. The filled triangles along the abscissa represent hyperthermia treatments while the Xs represent radiation fractions. Fractional hyperthermia doses are given at the bottom of each panel. Note equality of ordinate scale in each panel. The line connecting the points is for visual reference only and does not imply that oxygenation status is known between measurements.
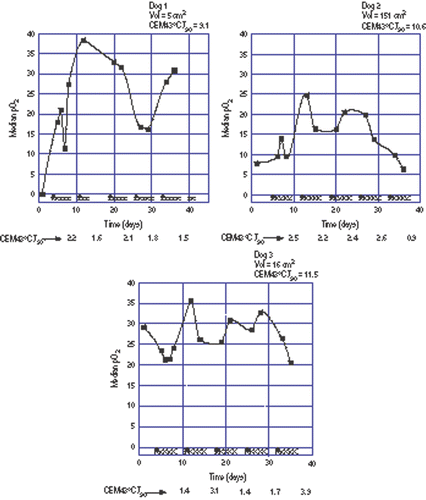
Dog 2 had a mildly hypoxic tumour (median pre-treatment pO2 = 7.2 mm Hg). Oxygenation increased during treatment then tended to decrease to a level nearly equal to the pre-treatment level.
Dog 3 had an oxygenated tumour (median pre-treatment pO2 = 29.3 mm Hg). Tumour oxygenation in this dog remained essentially unchanged during treatment.
Prescribed CEM43°C T90 = 40 min ()
Dog 4 had a moderately oxygenated tumour (median pre-treatment pO2 = 9.6 mm Hg). Oxygenation in this tumour decreased following the first hyperthermia treatment and remained low throughout the entire treatment course.
Figure 2. Oxygen measurements during a course of fractionated thermoradiotherapy in four dogs where 40 CEM43°CT90 were prescribed. Tumour volume and total hyperthermia dose administered are given at the top of each panel. Filled squares are the oxygen measurements. The filled triangles along the abscissa represent hyperthermia treatments while the Xs represent radiation fractions. Fractional hyperthermia doses are given at the bottom of each panel. Note expanded ordinate scale for Dogs 6 and 7. The line connecting the points is for visual reference only and does not imply that oxygenation status is known between measurements.
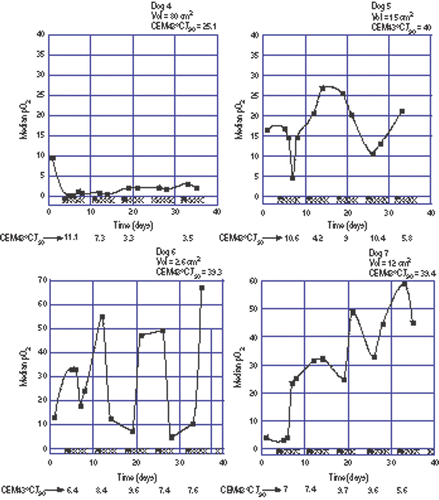
Dogs 5 and 6 had moderately to well oxygenated tumours (median pre-treatment pO2 = 16.5 mm Hg and 13.1 mm Hg, respectively). In dog 5, tumour oxygenation vacillated during treatment but a lasting upward or downward trend was not seen. In dog 6, tumour oxygenation fluctuated markedly during treatment. The tumour in dog 6 was very small which may have had a bearing on the degree of fluctuation.
Dog 7 had a poorly oxygenated tumour (median pre-treatment pO2 = 4.1 mm Hg). Oxygenation in this tumour increased following the first hyperthermia treatment and remained elevated throughout the treatment course.
There are too few dogs to assess the effect of thermal dose on oxygenation changes but it is of interest that the dog experiencing the most prolonged decrease in oxygenation had the highest overall mean T10 value and received the highest thermal dose quantified as CEM43°CT10 (Dog 4, , ).
Discussion
Results from this study must be interpreted with caution due to the small number of dogs studied and the non-randomized nature of hyperthermia dose prescription. Nevertheless, the results shed new light on the effects of hyperthermia on tumour oxygenation. The real value of the information from these dogs is the multiplicity of oxygen measurements, giving a more comprehensive assessment of the potential effects of hyperthermia over time. In many studies only pre-treatment and one post-treatment measurement were possible and the patterns seen here illustrate how this may not be an accurate representation of the micro-environmental changes that occur in response to a fractionated radiation/hyperthermia protocol.
Two distinct patterns of change were identified in tumour oxygenation following hyperthermia. First, in the two dogs with the lowest pre-treatment tumour oxygen measurements, hyperthermia led to improved oxygenation that persisted throughout the protracted course of radiation therapy (Dog 1, ; Dog 7, ). This improved oxygenation creates an environment where the efficacy of each daily radiation fraction may be enhanced. The cause for such an increase in oxygenation is not known, but this could be related to changes in perfusion or interstitial pressure; more detailed investigations are needed. The authors do not have detailed oxygen measurements during a course of fractionated radiation, as given in this study, without concurrent hyperthermia. However, others have assessed oxygenation in canine tumours before and during fractionated radiotherapy and it either remained at pre-treatment values or decreased, but increases in oxygenation such as seen here were not found with radiation alone Citation[21]. That hyperthermia may augment tumour oxygenation in hypoxic tumours is a noteworthy attribute.
Secondly, in one dog with a moderately oxygenated tumour, a rapid and persistent decrease in tumour oxygenation was observed following the first hyperthermia treatment (Dog 4, ). Considering this dog received the highest thermal dose, based on CEM43°CT10, and also had the highest overall mean T10 (), this observation is relevant to a recently completed Phase III assessment of thermal dose in canine sarcomas Citation[22]. That study identified a significant positive association between total thermal dose and duration of local tumour control and also found an independent inverse effect of the total duration of heating on local control duration. The longer it took to administer the prescribed thermal dose, the greater the chance for local failure; this was true within each thermal dose group. Another relevant finding was a lack of correlation between T90 and T10 values Citation[22]. Thus, it was reasoned that prolonged heating necessitated by low T90 values, especially if T10 values were high, might be leading to adverse heat effects on the tumour micro-environment, perhaps as occurred in this study in Dog 4. Unfortunately, the authors do not have tumour control data on dogs in this study so the actual effect of the relatively high temperatures at the upper end of the distribution, and also the effect of the decrease in oxygenation in Dog 4 is not known.
Quantification of thermal dose is typically based on T90 and in general the upper end of the temperature distribution has been given less attention, except for the setting of maximal limits to avoid complications. However, in a setting where T90 and T10 are not related and T90 is low, delivering large amounts of power to maintain T90 may be associated with undesirable temperatures in the upper range, e.g. T10, leading to vasculopathy or decreases in oxygenation from other mechanisms Citation[12]. Thus, the effect of hyperthermia on tumour oxygenation may be linked to the hyperthermia fractionation scheme. Perhaps a more finely fractionated hyperthermia prescription, with intentionally lower T90 values, will be associated with more favourable changes in oxygenation in a larger number of patients. These hypotheses need to be tested prospectively and provide an opportunity for even further improvements in the efficacy of hyperthermia when combined with radiation.
In the other four dogs (Dogs 2, 3, 5 and 6), there were fluctuations in tumour oxygenation following hyperthermia but there was not a persistent change in either direction. The significance of such fluctuations and reasons why less vacillation in post-treatment oxygenation were not observed are not known.
One must recognize that limited sampling of oxygenation may be characterized by a sampling error meaning that limited sampling is not reflective of the more global environment. This could be overcome by using imaging methods such as positron emission tomography with a hypoxia marker but this was not available for this project. On the other hand, based upon a simulation using Eppendorf oxygen probe data in cervical cancer patients, it was determined that the chance of incorrectly categorizing a tumour as oxic or hypoxic based on limited sampling was only 10% if 20 measurements were acquired Citation[20]. Therefore, although one cannot completely eliminate a possible influence of sampling error, there is no reason to believe that useful information was not be obtained from measurements as described herein.
In summary, the data indicate that hyperthermia can result in improved tumour oxygenation that persists throughout a course of fractionated radiation therapy. However, this is not a universal phenomenon as other tumours either experience a fluctuation in oxygenation or a permanent decrease. Identification of the genesis if these changes in oxygenation, their clinical signfiicance and how they are affected by hyperthermia fractionation is critical to the optimization of hyperthermia in combination with radiation for treatment of human cancer.
References
- Rosner G, Clegg ST, Prescott DM, Dewhirst MW. Estimation of cell survival in tumours heated to nonuniform temperature distributions. Int J Hyperthermia 1996; 12: 303–304
- Sapareto SA, Hopwood LE, Dewey WC. Combined effects of x-irradiation and hyperthemia on CHO cells for various temperatures and orders of application. Radiat Res 1978; 43: 221–233
- Schulte J, Schramm A, Pressel T, Klein-Hitpass L, Ells J, Havers W, Eggert A. Microarray-analysis: A new approach to study the molecular mechanisms of thermo-chemotherapy. Klin Padiatr 2003; 215: 298–302
- Narita N, Noda I, Ohtsubo T, Fujieda S, Tokuriki M, Saito T, Saito H. Analysis of heat-shock related gene expression in head-and-neck cancer using cDNA arrays. Int J Radiat Oncol Biol Phys 2002; 53: 190–196
- Oleson JR. Hyperthermia from the clinic to the laboratory: A hypothesis. Int J Hyperthermia 1995; 11: 315–322
- Vaupel PW, Otte J, Manz R. Oxygenation of malignant tumors after localized microwave hyperthermia. Radiat Environ Biophys 1982; 20: 289–300
- Song CW, Shakil A, Osborn JL, Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 1996; 12: 367–373
- Thews O, Li Y, Kelleher DK, Chance B, Vaupel PW. Microcirculatory function, tissue oxygenation, microregional redox status and ATP distribution in tumors upon localized infrared-A-Hyperthermia at 42 degrees C. Adv Exp Med Biol 2003; 530: 237–247
- Song CW, Park H, Griffin RJ. Improvement in tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Jones E, Prosnitz L, Marcom K, Hargenbergh P, Marks L, Brizel D, Vujaskovic Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 2004; 10: 4287–4293
- Brizel D, Scully S, Harrelson J, Layfield L, Dodge R, Charles H, Samulski T, Prosnitz L, Dewhirst M. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996; 56: 5347–5350
- Vujaskovic Z, Poulson J, Gaskin A, Thrall D, Page R, Meyer R, Prescott D, Samulski T, Dewhirst M. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000; 46: 179–185
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44: 4721s–4730s
- Waterman FM, Komarnicky L, Leeper DB. The response of human tumour blood flow to a fractionated course of thermoradiotherapy. Int J Hyperthermia 1998; 14: 421–434
- Hand J, Machin D, Vernon C, Whaley J. Analysis of thermal parameters obtained during Phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia 1997; 13: 343–364
- Sapozink M, Corry P, Kapp D, Myerson R, Dewhirst M, Emami B, Herman T, Prionas S, Ryan T, Samulski T, Sapareto S, Shrivastawa P, Stauffer P, Waterman F. RTOG quality assurance guidelines for clinical trials using hyperthermia for deep-seated malignancy. Int J Radiat Oncol Biol Phys 1991; 20: 1109–1115
- Dewhirst M, Phillips T, Samulski T, Strauffer P, Shrivastava P, Paliwal P, Pajak T, Gillim Y, Sapozink M, Myerson R, Waterman F, Sapareto S, Corry P, Cetas TC, Leeper DB, Fessenden P, Kapp D, Oleson JR, Emami B. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys 1990; 18: 1249–1259
- Thrall DE, Rosner GL, Azuma C, LaRue SM, Case BC, Samulski T, Dewhirst MW. Using units of CEM 43°C T90, local hyperthermia thermal dose can be delivered as prescribed. Int J Hyperthermia 2000; 16: 415–428
- Leopold KA, Dewhirst MW, Samulski TV, Harrelson J, Tucker JA, George SL, Dodge RK, Grant W, Clegg S, Prosnitz LR, Oleson UR. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1992; 22: 989–998
- Doll C, Milosevic M, Pintille M, Hill R, Fyles A. Estimating hypoxic status in human tumors: A simulation using Eppendorf oxygen probe data in cervical cancer patients. Int J Radiat Oncol Biol Phys 2003; 55: 1239–1246
- Achermann RE, Ohlerth SM, Rohrer Bley C, Gassmann M, Inteeworn N, Roos M, Scharz M, Wergin MC, Kaser-Hotz B, Roger E. Oxygenation of spontaneous canine tumors during fractionated radiation therapy. Strahlenther Onkol 2004; 180: 297–305
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L, Case B, Rosner G, Azuma C, Poulson J, Pruitt AF, Stanley W, Hauck ML, Williams L, Hess P, Dewhirst MW. Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res 2005; 11: 5206–5214