Abstract
Purpose: In children with locally advanced or recurrent malignant tumours, prognosis can be improved by regional deep hyperthermia (RHT) in combination with platin-based chemotherapy. However, because of the increasing number of patients that achieve long-time remission with this therapy, it is necessary to evaluate long-term sequelae of thermochemotherapy. During the years 1993–2004 one has observed avascular osteonecrosis (AON) of the femoral head after RHT in seven children with pelvic germ cell tumours or rhabdomyosarcomas.
Methods: Although AON may develop in patients with malignancies treated with chemo- or radiotherapy alone, RHT might nevertheless contribute to the occurrence of AON. In order to determine potential risk factors for AON after RHT, this study analysed the relationship of AON to the patient's age, medical history and treatment parameters such as thermal dose equivalent and power output.
Results and conclusions: In the present study AON was associated with young age as well as intensity of hyperthermia indicated by high power levels that exceed 20 W per kg body weight and/or application of eight or more heat sessions as well as additional radiotherapy. Based on this observation, it was assumed that an optimized three dimensional thermal field modelling may be helpful to avoid hazardous temperatures in the femoral heads during RHT treatment and to reduce AON of the femoral heads.
Introduction
The majority of clinical studies on the use of hyperthermia for the treatment of malignancies have been performed in superficially located malignancies, including melanoma or breast cancer Citation[1–3]. In these studies, the combination of hyperthermia and irradiation resulted in improved local tumour control. In the last two decades, the development of annular-phased array systems has allowed for the inclusion of deeply-seated tumours in the pelvis and abdomen in clinical phase II/III hyperthermia protocols Citation[4], Citation[5]. It has been shown that local heat application also enhances the local anti-cancer effects of systemically applied chemotherapy regimens Citation[6], Citation[7]. There is experimental evidence that this therapeutic effect appears to be predominantly mediated through the sensitization of tumour cells to alkylating agents under hyperthermic conditions Citation[8–10]. In refractory and unresectable germ cell tumours of children and adolescents, the addition of hyperthermia to a cisplatinum-based chemotherapy protocol (Hyper-PEI) improved the event-free survival rate by up to 65% with a median follow-up of 36 months Citation[7]. Because of the improved prognosis in this group of young patients, additional attention has to be focused on potential long-term sequelae of the combined (radio-) thermochemotherapy.
In the literature few hyperthermia related side effects have been reported. Most of the reported side effects are related to the systemic chemotherapy, e.g. myelosuppression with subsequent severe infections and nephrotoxic or ototoxic effects. Possible specific side effects of hyperthermia itself include local burn injuries, pain or complications that are related to the invasive thermometry such as infections caused by the catheters Citation[11]. One case of tumour growth along the thermometry catheter track has also been reported Citation[12]. Other than these acute side effects, peripheral neuropathy constitutes the only long-term sequela following hyperthermia described so far and is extremely rare Citation[13].
In this patient population, avascular osteonecrosis (AON) of the femoral head was found after delivery of regional hyperthermia (RHT) in combination with platinum-based chemotherapy ± radiotherapy. This complication has not been reported in the literature to date. Common reasons for AON in patients with malignancy include irradiation, application of corticosteroids Citation[14–16] and, in a few cases, chemotherapy with platinum compounds Citation[17]. Therefore, the patients were analysed for potential risk factors of the development of AON in order to evaluate whether specific measures might allow for reducing the risk of AON in future RHT treatments.
Patients
Seventy-two patients aged between 1–72 years (median 16.1 years) have been included in the present analysis. Patients suffered from locoregional pelvic malignancies that were refractory to prior standard cytostatic treatment. Twenty-four patients were male and 48 were female. The patients did not have any distant metastases. All patients received RHT in combination with systemic chemotherapy, including cisplatinum, etoposide, ifosfamide, 5-fluorouracil and adriamycin. Twenty of the paediatric patients received subsequent pelvic irradiation (19.2–50.4 Gy). Patients were treated under analgo-sedation using midazolam, promethazine and pethidine. If enduring pain of the hip or any discomfort in walking were observed, MRI of the pelvis including the hip joints was performed in order to detect possible injuries. The follow-up for the whole patient cohort after start of RHT treatment was 3–137 months (median 15 months).
Methods
RHT was performed with three annular phased array applicators that had the same design and operational characteristics (Sigma-30, -40 and -60 applicator, BSD 2000, Medical Corporation, Salt Lake City, Utah, USA). The number of heat applications per patient ranged from 2–30 sessions (median 10 sessions). Maximum radiofrequency (RF) power varied between 130–1300 W. Frequencies ranged from 87–140 MHz. Temperature distribution was monitored using non-pertubing thermometers (Bowman probes) at fixed points, including bladder, vagina, rectum and skin. Temperatures in the tumour area were measured using catheters placed in close proximity to the tumour, either under computertomographic control or surgically during tumour resection or biopsy.
In order to evaluate potential risk factors for femoral AON, a retrospective descriptional analysis of the 72 patients was performed. Patients were assigned to different sub-groups according to age at the time of treatment, previous medical history, number of heat sessions, RF power level and the achieved thermal isoeffective dose (CEM43T90):
Patients were assigned to four different age groups: ≤ 5 years (22 patients), 6–10 years (eight patients), 11–15 years (six patients), >15 years (36 patients).
The number of heat sessions was sub-divided into the following groups: <8 sessions (21 patients), 8–9 sessions (14 patients), 10–11 sessions (18 patients), 12–13 sessions (six patients), ≥14 sessions (13 patients).
RF power level per body weight was classified into four distinct groups: ≤10 W kg−1 (six patients), 10–20 W kg−1 (44 patintts), 20–30 W kg−1 (18 patients), ≥30 W kg−1 (four patients).
To estimate the thermal isoeffect dose, this study used the method of the Arrhenius relation according to Dewey Citation[18]. The measured time–temperature results were converted to an equivalent number of minutes at a standard temperature of 43°C for 90% of the tumour volume (CEM43T90 = Σ(Δt)R(43-T ), with CEM43T90 equal to cumulative equivalent minutes at a T90 converted to 43°C, Δt equal to the time increments at which the data are acquired during treatment, T equal to the average temperature over the time interval Δt and R equal to 0.25 when T < 43°C and 0.5 when it is ≥43°C). Patients were sub-divided according to the achieved thermal dose equivalent into five different CEM43T90 categories: ≤10 min (41 patients), 10–20 min (18 patients), 20–50 min (six patients), 50–100 min (two patients), >100 min (three patients), two patients with incomplete data. Results were tested for statistical significance by the two-sided Cochran-Armitage trend test in SAS (statistical analysis system). Only p-values <0.05 were considered statistically significant.
Results
Between 1993–2004, 72 patients where treated with RHT and chemotherapy ± additional radiotherapy. Seven out of these 72 patients developed AON of the femoral head.
Diagnoses and age of the patient population as well as the administered chemotherapy and the occurrence of AON are summarized in . These data show that AON occurred only in patients that suffered from germ cell tumours or rhabdomyosarcomas and were treated with PEI and/or additional EIA. As 22 additional patients with other diagnoses received the same chemotherapeutic agents (PEI, resp. EIA) without developing AON, other factors than the chemotherapy administered concommittant to hyperthermia might constitute important risk factors.
Table I. Diagnoses, age, cytostatic treatment and occurrence of AON in 72 patients treated with RHT.
To ascertain this result, this study analysed the patients with germ cell tumours or rhabdomyosarcomas for a potential impact of high cumulative doses of chemotherapy prior to RHT on the risk of AON (). However, there is no obvious correlation. Nevertheless, as five out of the patients with AON suffered from germ cell tumours, the effect of prolonged administration of PEI chemotherapy cannot be excluded.
Table II. Cytostatic treatment prior to RHT in patients with germ cell tumours (n = 25) or rhabdomyosarcoma (n = 9).
To evaluate the thermal effect in the treated area, the achieved intra-tumoural temperatures were compared in patients with and without AON. To this end, the thermal isoeffective dose was determined using the Arrhenius relation (). Only 11 patients achieved a CEM43T90 longer than 20 min and in none of them AON occurred. In contrast, six out of 41 patients with a shorter time of high isoeffective thermal dose developed AON ().
Table III. AON related to dose equivalent CEM43T90 in 70 patients. From technical reasons calculation was not possible in two patients.
The power needed to achieve temperatures in the presumed therapeutic range was highly variable when it was calculated per kg body weight (). The risk for AON increased with a higher power level ( p = 0.04), which was necessary especially in young children. However notably, none of the four patients treated with >30 W kg−1 was affected. This might be a result of the small number of patients in this group.
Table IV. AON related to power level.
As shown in , patients with germ cell tumours and rhabdomyosarcomas had the lowest median age, however the age range was grossly overlapping to patients with other diseases. Therefore, this study correlated the occurrence of AON with the defined age group at the start of thermochemotherapy (). Onset of AON was clearly correlated with young age (p = 0.004). In fact, no case of AON was detected in patients older than 15 years (n = 36).
Table V. AON related to defined age groups.
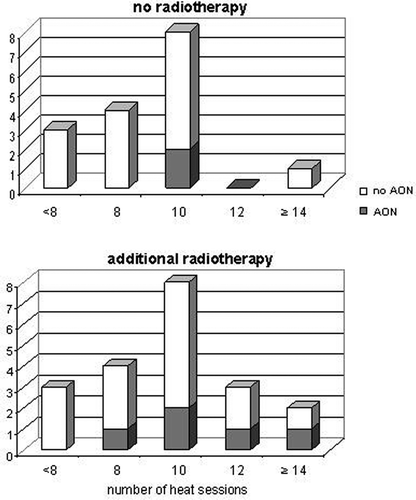
In addition, one also compared the number of heat sessions and additional radiotherapy in patients up to 15 years with and without AON (). A number of more than eight heat sessions was correlated with growing risk for AON ( p = 0.03). Only two of the 16 children treated with thermochemotherapy alone developed AON, whereas five of 20 patients with additional radiotherapy where affected. These five patients had received radiation dosages from 45–50.4 Gy. However, in the latter group there is a trend to more heat sessions. In the group of five patients treated 12 or more times, two patients developed AON.
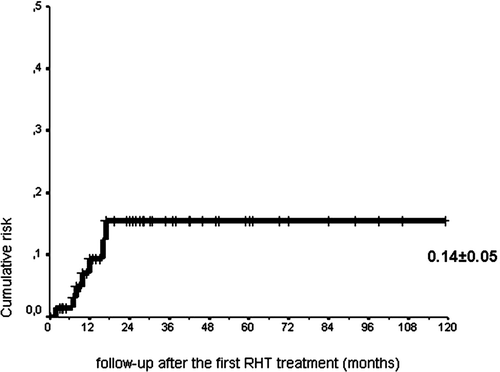
Furthermore, the patients’ charts were examined for documented pain after thermochemotherapy and the time point when AON was detected by MRI. In four out of the seven patients the initial clinical presentation of AON was characterized by acute pain that occurred within 48 h after the hyperthermia sessions but often disappeared within 1 week after the end of treatment and was therefore not considered a contraindication for the continuation of treatment. Acute hip pain was also reported in 12 of 72 patients who subsequently never developed any radiological signs of AON. shows a Kaplan-Meyer estimation of the cumulative risk of AON after the start of RHT treatment. Only in a few patients, the onset of AON was observed during thermochemotherapy, while in most of the affected patients AON was diagnosed after the end of therapy. Therefore, AON of the femoral head can be considered a real long time sequela of thermochemotherapy. The cumulative risk in the patient population (all age groups) was estimated as 14% and 28% in children up to 15 years.
Discussion
The adoption of the quality guidelines of the European Society for Hyperthermic Oncology (ESHO) Citation[19] for the application of loco-regional RF hyperthermia for superficially and deeply located tumours during the last two decades has significantly improved the safety and feasibility of hyperthermia treatment delivery in the intended temperature range from 42–44°C. The subsequent temperature monitoring and the analysis of specific apsorption rate (SAR) distribution Citation[20] in phantom models of tumour and normal tissues that is included in the ESHO quality guidelines have made severe complications of hyperthermia rather rare. In addition, a protective effect against thermal injuries is provided by the naturally occurring increased blood flow in normal tissues during hyperthermia. However, a certain risk of accidental damage to normal tissues remains, especially during analgo-sedation. To a certain extent, this risk may be related to the difficulties in accurately estimating the temperature in the surrounding normal tissues, if only data sampled through the interstitial temperature probes used for thermal mappings are available.
In the present study, AON was identified in seven out of 72 patients that were treated with RHT in combination with chemotherapy ± irradiation for tumours located in the pelvis or the abdomen. Remarkably, six of the seven cancer patients with AON were long-term survivors with a median survival time of 4 years after hyperthermia. Five of the seven patients who developed AON were children under 5 years and the two other patients were a 6-year old boy and a 12-year-old girl. The data are supported by the current literature, which includes no reports of AON in adult patients, although similar hyperthermia protocols have been utilized.
In paediatric oncology, the risk of AON correlates with the application of treatment protocols that include high-dose corticosteroids as anti-cancer agents Citation[14], Citation[16] or additional radiotherapy in order to improve local tumour control. In children with acute lymphoblastic leukaemia, the incidence of asymptomatic AON ranges from 1.1–9.3% Citation[21–25] and there is a significant increase after higher doses of corticosteroids and in girls older than 10 years. The fact that AON has also been observed as an occasional complication in adults treated with platinum-based chemotherapy suggests that this therapy may constitute a risk factor for AON that is independent from the patient's age Citation[17]. In correspondent studies of children and adolescents treated for germ cell tumours osteonecrosis has not been mentioned as a side-effect Citation[26].
Osteonecroses after anti-cancer therapy usually occur in long bones including hip and knees. Isolated osteonecroses of the femoral head after chemotherapy in children are extremely rare. In the literature only three cases of AON have been reported in children after cytostatic therapy without the use of steroids Citation[27], Citation[28]. Idiopathic AON occurs in one of 1000–5000 children Citation[29], Citation[30] and is usually associated with a certain risk profile such as extreme obesity or extreme sports. In contrast to the self-limited idiopathic juvenile osteonecrosis (Legg-Calve-Perthes disease) which is characterized by spontaneous healing, these patients with AON of the femoral head showed progressive course and poor self-healing tendencies. Therefore, this disorder seems to be a specific complication of thermochemotherapy.
Osteonecrosis of the hip joint has also been observed as a possible complication of radiotherapy of pelvic tumours Citation[31]. However, this complication is very rare and is not mentioned as a side effect in children with solid tumours such as soft tissue sarcoma who have been treated with chemoradiotherapy Citation[32], Citation[33]. In this patient cohort, an increased incidence of AON was observed in patients who underwent additional radiotherapy. Accordingly, irradiation may also be an additional risk factor for the development of AON in this patient population.
It has been previously shown that hyperthermia increases the cytotoxic effects of cisplatinum exposure during the period of increased temperatures, with increases up to 10-fold in vitro and in vivo Citation[8], Citation[10]. As the patients in this study did not receive corticosteroids, one can, therefore, postulate that cisplatinum-based chemotherapy, which is potentiated by hyperthermia, is one of the main risk factors for the development of AON in children. In addition, the clear correlation of the risk of AON with both the higher dose intensity (preferentially necessary in young children) as well as the cumulative doses (as reflected by the number of RHT sessions) indicates that thermal injuries most probably constitute the driving force for the development of AON.
Therefore, preventive measures such as optimized temperature monitoring have to be considered. In this context, it has to be noted that, while invasive thermometry can be performed by catheters placed in the tumour area, the temperature distribution in surrounding tissues, especially in the femoral heads, cannot be assessed. As has been demonstrated, the risk of AON does not correlate with high temperatures reached in the tumour area itself but with increasing power levels. Consequently, it is assumed that non-invasive thermometry during RHT would be very useful to monitor temperature distribution in the treated area surrounding the tumour in order to avoid dangerous temperatures in normal tissues. One possibility could be non-invasive temperature monitoring with nuclear magnetic imaging Citation[34]. Thus, an optimization of the energy application based on an optimized temperature monitoring during RHT rather than changes in the chemotherapeutic regimen might help to reduce the risk of AON. In small children it is more difficult to exactly adjust the field of hyperthermic treatment using the described sigma applicator with four pairs of antennae. In this context applicators that consist of more antennae like the ‘sigma eye’ applicator in combination with three dimensional temperature monitoring could be very helpful to adjust the therapeutic field more exactly and to avoid toxic temperatures in the femoral heads.
In a previous extensive comparative analysis of different factors of temperature distribution, e.g. Tmax, T90, T50, T20, Tmin and CEM43T90 in tumour and surrounding normal tissue, one did not observe significant differences between adults and children Citation[3]. Hence, the development of AON cannot be deduced from the observed temperature parameters obtained through invasive thermometry. The observation that AON after hyperthermia has not been observed in adults substantiates the assumption that anatomical or physiological features in children may promote the development of AON after the application of platinum-based chemotherapy in combination with RHT.
In a pathophysiological perspective, AON in children and adolescents commonly results from an interruption of the capital femoris epiphysis blood supply. During thermochemotherapy of pelvic tumours the femoral heads could be affected by the applied radiofrequency due to their location in the vicinity of the treated tumour. Therefore, heat-induced alterations of blood perfusion and consecutive osseous injuries of the hip might constitute a mechanism of AON development in children after RHT. The alteration of the blood supply to the area of later AON appears in some patients early after the start of thermochemotherapy, however in most patients after the end of treatment suggesting the induction of an autonomous process. In contrast to children it has been demonstrated that in adults, after obliteration of the foveolar artery (A. capitis femoris), blood perfusion of the femoral head is physiologically maintained by vessels from the metaphysis. This anatomical difference might, therefore, be a potential explanation for the higher risk of AON in children, if it is assumed that the affection of the blood flow of the foveolar artery causes the AON of the femoral head.
There are some similarities to children with ALL and AON, where also a wide time range of AON manifestation after start of therapy has been observed Citation[24]. AON in dia- and metaphysis of ALL patients show a self-healing capacity whereas epiphyseal manifestations do worse Citation[35]. Although recent data in the literature demonstrate a good therapeutic response of osteoedema to vasoactive agents such as prostacyclin analoga it remains unclear whether these treatment regimes are able to prevent a progression of an thermochemotherapy induced AON Citation[36]. The clinical manifestation of AON in the seven patients as well as treatment options and the influence on quality of life are the subject of a separate paper in preparation.
In conclusion, a cumulative risk of AON was observed of 14% of the patients receiving thermochemotherapy. These patients are characterized by young age (<5 years), high power supply (>20 W kg−1) and long duration of thermochemotherapy with more than eight RHT sessions. As a result of this experience, the number of thermochemotherapy sessions in the ongoing protocol was reduced to eight sessions in patients with germ cell tumours whereas in patients with rhabdomyosarcomas 16 sessions will be planned because of the lower AON risk and the higher risk of recurrence. Nevertheless, the risk of AON has to be accepted in some children with otherwise refractory tumours of the pelvis or abdomen, if a curative approach is intended. Notably, in this report six of seven children with AON of the femoral head are longtime survivors.
Acknowledgements
This work was supported by a grant of Deutsche Krebshilfe e.V. and Elterninitiative Kinderkrebsklinik e.V.
References
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995; 345: 540–543
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, Liu FF, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys. 1996; 35: 731–744
- Wessalowski R. Regionale Tiefenhyperthermie und Chemotherapie bei Tumoren von Kindern und Erwachsenen. Experimentelle und klinische Untersuchungen. Shaker Verlag, Aachen 2002
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000; 355: 1119–1125
- Issels RD, Prenninger SW, Nagele A, Boehm E, Sauer H, Jauch KW, Denecke H, Berger H, Peter K, Wilmanns W. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: A phase II study. J Clin Oncol 1990; 8: 1818–1829
- Wessalowski R, Schneider DT, Mils O, Hannen M, Calaminus G, Engelbrecht V, Pape H, Willers R, Engert J, Harms D, Göbel U. An approach for cure: PEI-chemotherapy and regional deep hyperthermia in children and adolescents with unresectable malignant tumors. Klin Padiatr 2003; 215: 303–309
- Issels R. Hyperthermia combined with chemotherapy—biological rationale, clinical application, and treatment results. Onkologie 1999; 22: 374–381
- Schlemmer M, Wendtner CM, Issels RD. Ifosfamide with regional hyperthermia in soft-tissue sarcomas. Oncology 2003; 65(Suppl 2)76–79
- Debes A, Rommel F, Breise M, Willers R, Göbel U, Wessalowski R. In vitro test-system for chemo- and thermosensitivity: An analysis of survival fractions and cell-cycle distributions in human Ewing's sarcomas as a modelfor tumors in pediatric oncology. Klin Padiatr 2002; 214: 223–229
- van der Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13: 1173–1184
- van der Zee J, Veeze-Kuijpers B, Wiggers T, van de Merwe SA, Treurniet-Donker AD. Risk of tumour growth along thermometry catheter trace: A case report. Int J Hyperthermia 1992; 8: 621–624
- Haveman J, Van Der Zee J, Wondergem J, Hoogeveen JF, Hulshof MC. Effects of hyperthermia on the peripheral nervous system: A review. Int J Hyperthermia 2004; 20: 371–391
- Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: A treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)—experiences from trial ALL-BFM 95. Pediatr Blood Cancer 2005; 44: 220–225
- Langebrake C, Reinhardt D, Ritter J. Minimising the long-term adverse effects of childhood leukaemia therapy. Drug Saf 2002; 25: 1057–1077
- Relling MV, Yang W, Das S, Cook EH, Rosner GL, Neel M, Koward S, Ribeiro R, Sandlund JT, Pui CH, Kaste SC. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol 2004; 22: 3930–3936
- Winquist EW, Bauman GS, Balogh J. Nontraumatic osteonecrosis after chemotherapy for testicular cancer: A systematic review. Am J Clin Oncol 2001; 24: 603–606
- Dewey WC. Interaction of heat with radiation and chemotherapy. Cancer Res 1984; 44(Suppl)4714s–4720s
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, Wust P, De Leeuw AC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998; 14: 125–133
- Edelstein-Keshet L, Dewhirst MW, Oleson JR, Samulski TV. Characterization of tumour temperature distributions in hyperthermia based on assumed mathematical forms. Int J Hyperthermia 1989; 5: 757–777
- Arico M, Boccalatte MF, Silvestri D, Barisone E, Messina C, Chiesa R, Santoro N, Tamaro P, Lippi A, Gallisai D, et al. Osteonecrosis: An emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica 2003; 88: 747–753
- Mattano Jr LA, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: A report from the Children's Cancer Group. J Clin Oncol 2000; 18: 3262–3272
- Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol 2001; 19: 3066–3072
- Bernbeck B, Christaras A, Krauth K, Lentrodt S, Strelow H, Schaper J, Janssen G, Mödder U, Göbel U. Bone marrow oedema and aseptic osteonecrosis in children and adolescents with acute lymphoblastic leukaemia or non-Hodgkin-lymphoma treated with hyperbaric-oxygen-therapy (HBO): An approach to cure?—BME/AON and hyperbaric oxygen therapy as a treatment modality. Klin Padiatr 2004; 216: 370–378
- Raab P, Kuhl J, Krauspe R. [Multifocal osteonecrosis in children and adolescents after polychemotherapy]. Z Orthop Ihre Grenzgeb 1997; 135: 444–450
- Göbel U, Schneider DT, Calaminus G, Haas RJ, Schmidt P, Harms D. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann Oncol 2000; 11: 263–271
- Geetha N, Kumary PK, Ramachandran K, Nair MK. Avascular necrosis of the femoral head in neuroblastoma: A case report. Pediatr Hematol Oncol 1998; 15: 443–446
- Ishii E, Yoshida N, Miyazaki S. Avascular necrosis of bone in neuroblastoma treated with combination chemotherapy. Eur J Pediatr 1984; 143: 152–153
- Fisher RL. An epidemiological study of Legg-Perthes disease. J Bone Joint Surg Am 1972; 54: 769–778
- Molloy MK, MacMahon B. Incidence of Legg-Perthes disease (osteochondritis deformans). N Engl J Med 1966; 275: 988–990
- Aigner C, Ehall R, Stampfel O. [Osteoradionecrosis of the hip joint]. Z Orthop Ihre Grenzgeb 1995; 133: 467–473
- Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, Graf N, Heise U, Jürgens H, Kotz R, et al. Long-term results of the co-operative German–Austrian–Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 1998; 9: 893–899
- Koscielniak E, Harms D, Henze G, Jürgens H, Gadner H, Herbst M, Klingebiel T, Schmidt BF, Morgan M, Knietig R, et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 1999; 17: 3706–3719
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Körholz D, Bruder M, Engelbrecht V, Ruther W, Göbel U. Aseptic osteonecrosis in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol 1998; 15: 307–315
- Jäger M, Werner A, Lentrodt S, Mödder U, Krauspe R. [Pain management in non-juvenile, aseptic osteonecrosis]. Schmerz 2004; 18: 481–491