Abstract
Purpose: Experiments with cultured HeLa, S3 and E.A. Hy296 cells were performed to determine if exposure to acute (30 min at 45°C) or chronic (2 h at 41°C) heat shocks or to non-thermal exposures of radiofrequency radiation (RF) induce changes in HSP27 phosphorylation.
Materials and methods: The radiofrequency (RF) exposures used in this study were 847 MHz time division multiple access modulated (TDMA) at a specific absorption rate (SAR) of 5 W kg−1 for 1, 2 or 24 h or 900 MHz GSM modulated (GSM) at a SAR of 3.7 W kg−1 for 1, 2 or 5 h. HSP27 phosphorylation was evaluated by resolving the various phosphorylation forms using two-dimensional gel electrophoresis measuring the relative amount of each by densitometry. Alternatively, an antibody specific for phosphorylated HSP27 was used to detect changes in HSP27 phosphorylation levels. All heat shock and RF exposure conditions were analysed simultaneously along with a matched incubator control sample. Each experiment was repeated three times.
Results: Following heat shock, the degree of phosphorylation of HSP27 varied with the heat dose, with acute hyperthermia (45°C) having an increased proportion of higher phosphorylated forms. Exposure of HeLa S3 cells to 5 W kg−1 TDMA for 1, 2 or 24 h did not induce significant differences in the levels of HSP27 phosphorylation compared to incubator control or sham. Exposure of E.A. Hy926 cells to 3.7 W kg−1 900 MHz GSM for 1, 2 or 5 h did not induce significant differences in the levels of HSP27 phosphorylation compared to sham exposed.
Conclusions: Acute and moderate hyperthermia significantly increase HSP27 phosphorylation, but there was no significant change in the levels of HSP27 following non-thermal exposure to TDMA and GSM modulated RF radiations.
Introduction
Recent concern regarding the effects of radiofrequency (RF) radiation on biological systems is motivated by the large number of individuals (>108 worldwide) exposed to the RF signals from cellular phones Citation[1–3].Footnote1,Footnote2 The specific issue is whether there are any unknown, non-thermal, deleterious effects of RF exposure. Concerns would be magnified if RF radiation could conceivably contribute to a carcinogenic or other pathological process. Conversely, data demonstrating the lack of either non-thermal, carcinogenic or other potentially harmful effects would assure both the public and industry that non-thermal exposures to RF radiation have no or minimal risk. However, a recent report related an observed RF effect on HSP27 to a possible carcinogenic process Citation[4], thus opening the possibility that stress responses, following RF exposure, should be addressed.
Operationally, the carcinogenic process is a multi-step process involving genetic and epigenetic changes. If RF radiation contributes to a carcinogenic process, it would have to result ultimately in gene structure or gene regulation changes or both. Most known carcinogens cause changes via genotoxic effects. Currently, there is no conclusive evidence that non-thermal RF exposures have genotoxic effects. Although potentially genotoxic effects have been reported Citation[5–9] these findings have failed confirmation both in vivo and in vitro Citation[10–19]. The situation is also controversial regarding potential epigenetic effects of RF radiation on changes in gene expression. Some workers have speculated that RF induced changes in HSP27 phosphorylation could lead to carcinogenic transformation Citation[4]. If this speculation were true, the proposed carcinogenic process would have to involve an epigenic mechanism because most heat shock responses are not directly genotoxic. Many cellular stresses including heat shock induce epigenetic changes that are manifest in terms of gene expression changes. Thus, the reported effects of RF on gene expression should be considered. PC12 pheochromocytoma cells (rat neural origin) treated with nerve growth factor and exposed to time domain multiple access (TDMA) radiation under various combinations of slot average power densities (0.09, 0.9 and 9 mW cm−2) and durations (20, 40 and 60 min) showed no statistically significant RF effects on oncogene expression except for a 38% decrease in c-jun following cell exposure at 9 mW cm−2 for 20 min Citation[20]. Neither FDMA nor CDMA at a SAR of 0.6 W kg−1 had any significant effect on proto-oncogene expression in C3H10T 1/2 during transit from quiescence to exponential growth. Moreover, those exposures did not affect the binding activity of transcription factors AP-1, AP-2 and NFkB during this transition or during the transition from exponential growth to plateau phase Citation[21]. While the expression of c-jun and c-myc were not significantly affected during the transition from proliferation to plateau phases of growth, the levels of c-fos mRNA were reported to be 2-fold higher in the cells exposed FDMA and 1.45-fold higher in the cells exposed to CDMA than in the sham exposed cells Citation[21]. Follow-up studies failed to confirm these changes in c-fos expression Citation[22]. Further, there was no detectable activation of HSF-1 following exposure to 0.6 W Kg−1 of either CDMA or FDMA Citation[23]. Thus, it appeared that these RF signals did not induce a general stress response.
It has been reported by Leszcynski et al. Citation[4], Citation[24] that HSP27 phosphorylation increases following a 1 h exposure to 900 MHz GSM (3.7 W kg−1). A similar study by Miyakoshi et al. Citation[25] reported that 1950 MHz continuous wave RF radiation at 10 W kg−1 for 1 or 2 h reduced the levels of phosphorylated HSP27 in MO54 cells. It is known that phosphorylation of HSP27 is an early step in the responses of cells to numerous stresses Citation[26–33]. HSP27 plays a critical role in thermotolerance Citation[34] and heat resistance Citation[35], Citation[36]. In addition, HSP27 contributes to the regulation of apoptosis Citation[37], Citation[38]. However, it was not clear, in these reports Citation[4], Citation[24], Citation[25] that the reported RF induced changes in HSP27 phosphorylation activated any of the stress response pathways involving HSP27 phosphorylation. The possibility that HSP27 was phosphorylated without activating a known stress response pathway suggests that this phosphorylation occurred via an unknown pathway, for which there is no evidence except for the reported RF effect. Given that heat shock proteins, HSP70 and HSP27, are elevated in several human tumours, it is important that the possible role of RF exposure on HSP27 expression be addressed. Prior to searching for an unknown stress response pathway, it was decided to first confirm the observation that RF exposures induce HSP27 phosphorylation. Thus, the goal of the study presented herein is to determine if non-thermal exposures to radiofrequency radiation induce the phosphorylation of HSP27. In addition, this study compared the effects of moderate (41°C for 2 h) and acute (45°C for 30 min). Considering, for the purpose of experimental design that RF exposure could be inducing some stress response Citation[4], Citation[24], this approach would allow one to determine if there are any differences between non-thermal, moderate-chronic and acute short duration stress effects on the phosphorylation of HSP27.
Materials and methods
Cell lines maintenance and experimental procedures
HeLa S3 cells (ATCC, Manassas, VA) were maintained routinely in tissue culture flasks in α-MEM medium + 10% newborn calf serum supplemented with penicillin/streptomycin at 37°C in a 5% CO2 incubator. Forty-eight hours prior to the experiment cells were exponentially grown in T75 tissue culture flasks. The cell cultures then were exposed to TDMA (5 W kg−1; 1, 2 or 24 h) or heat shock (2 h at 41°C or 30 min at 45°C). After exposure, cells were harvested by washing the T-75 flasks three times with isotonic buffered sucrose (250 mM sucrose; 10 mM Tris pH 7.0). The cells then were lysed with 7 M Urea, 2 M thiourea, 2% CHAPS solution. The lysates were analysed for HSP27 levels and phosphorylation status as described below. HeLa cells were used because there is an extensive database on the effects of heat shock on these cells.
E.A. Hy926 endothelial cells (a gift from Dr Cora-Jean S. Edgell, University of North Carolina, Chapel Hill) Citation[39] were grown in DMEM plus 10% foetal bovine serum and penicillin/streptomycin. Exponentially growing cultures were plated in T-75 flasks 48 h prior to exposure to GSM (3.7 W kg−1; 1, 2 or 5 h) or heat shock. The cells were then harvested and samples prepared as described above for HeLa S3 cells. E.A. Hy926 cells were used to provide a direct comparison of this work with that of Leszcynski et al. Citation[4].
RF exposure system and SAR
The radial transmission line (RTL) irradiator developed at Washington University in St Louis was used to expose cells in T-75 culture flasks to TDMA or GSM modulated RF radiation. A detailed description of this device and its environmental controls has been reported in several publications Citation[40–43]. Briefly, the RTL is a waveguide formed by two horizontal parallel electrically conducting plates separated by 43 mm. The top plate is made of a lightweight composite hinged to a 6 mm thick bottom AL plate to facilitate access to the interior of the radiator. The bottom plate provides support and a highly thermally conductive surface that minimizes temperature gradients among flasks. A centrally located conical AL antenna emits transverse electromagnetic (TEM) waves, which are propagated radially outward through air and are terminated by an annulus of RF-absorbing foam material braced by a thin lamina of perforated aluminum. The lamina is located 533 mm from the antenna's centre. A total of 16 T-75 culture flasks can be distributed angularly around the antenna with their centres located at a radius of 292 mm. To boost and homogenize the SAR distribution, each flask was dielectrically loaded with a 3 mm thick shim of alumina (Al2O3) ceramic that fitted snuggly under the skirt of the flask Citation[18], Citation[43]. The nominal nearest and farthest distances of the cell layer from the antenna's centre were 247 and 338 mm, respectively. A sham RTL was identical but not connected to an RF source. The above power settings were selected to produce a nominal time-averaged cell-layer SAR of 5.0 ± 2.1 W kg−1 (mean ± SD) for both signals because of variations in power density resulting from the angular position of the flask Citation[40]. This SAR value is based on a series of comprehensive measurements and does not include the potential uncertainty in the measurement of power, which is usually estimated at ± 10%. The degree of SAR inhomogeneity reflected by the standard deviation value is typical for T-75 flasks exposed in a RTL as validated by independent finite differences time domain (FDTD) simulation studies Citation[43], Citation[44]. A computed histogram of the SAR distribution over the bottom surface of a flask exposed to FDMA in a RTL can be found in Vijayalaxmi et al. Citation[18].
In this study, the powered and sham RTLs were each loaded with T-75 culture flasks containing 40 ml of media. The larger than usual amount of culture medium (i.e. 40 ml, medium height of 5 mm) was found to reduce SAR non-uniformities. The flasks for simultaneous sham exposures were handled in the same way as the flasks exposed to RF radiation. The temperature of the culture media during the 24 h exposure to RF radiation and sham exposure was maintained at 37 ± 0.3°C with a combination of temperature control systems for the room and for the RTLs Citation[40].
Hyperthermia exposure
For acute heat shocks cell cultures were capped, then immersed in a temperature controlled waterbath (45 ± 0.5°C) for the indicated time (30 min). For chronic heat shocks cell cultures were placed in a CO2 incubator set at 41°C. This incubator has been thermally mapped using Luxtron probes as described previously Citation[45]. Cell cultures were maintained at 41 ± 0.5°C for the indicated time (2 h).
Analysis of HSP27 phosphorylation
Sample preparation; 75 µg total protein as measured by the Bradford assay (BioRad, Hercules, CA), was diluted to 125 µl with 7 M urea, 2 M thio urea, 2% CHAPS. Bio-Lyte 3/10 ampholytes (BioRad) and reducing agents were added. Resolution of proteins by 2D SDS-PAGE 7 cm, 4–7 IPG Ready Strips (BioRad) were used in the first dimension. 4–15% ready gels (BioRad) were used for the second dimension. Visualization of HSP27 by immunoblotting proteins were transferred to PVDF membranes by western blotting and then probed with anti-HSP27 antibodies, StressGen SPA-800, SPA523 for non-phosphorylated and phosphorylated HSP27 (StressGen, Ann Arbor, MI).
Quantification and statistical analysis
To quantify the HSP27 spots, immunoblots were digitized as TIFF files. The optical densities of the HSP27 spots were measured by ImageQuant software (Molecular Dynamics). The Optical density values from three experiments were averaged. Statistical significance (p ≤ 0.05) was determined using either the student's t-test or 95% confidence limits. The t-test was used to test the significance in and . The 95% confidence limits were used in due to the large frequency of zero value for several of the higher phosphorylated forms of HSP27.
Figure 1. Analysis of HSP 27 phosphorylation detected by two dimensional immunoblotting. (a) After the indicated experimental manipulations HeLa S3 cells were lysed. The proteins from whole cell extracts were resolved by 2 D polyacrylamide gel electrophoresis and probed with an antibody that recognizes all forms of HSP27. An equal protein was loaded on each gel. Results from a typical experiment are shown. (b) Western blots from three experiments like the one shown here were quantified and averaged. The average ± SD is plotted for each experimental condition. The indicated experimental conditions are: control, C; TDMA exposed for 2 or 24 h, T2 and T24, respectively; sham exposed for 2 or 24 h, S2 and S24, respectively; heated at 41°C for 2 h, 41; and heated at 45°C for 30 min, 45.
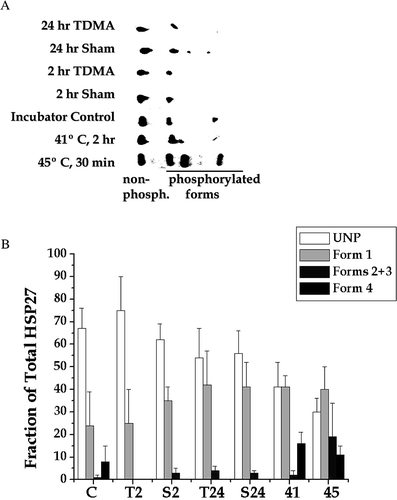
Results
Using 2D polyacrylamide gel electrophoresis, one was able to detect five isoforms of HSP27 in HeLa cells that had been heat shocked at 45°C for 30 min ( and ). One form consisted of the non-phosphorylated protein, which was present under all experimental conditions studied. The remaining four forms represented various phosphorylation states of HSP27 consistent with previous reports Citation[29], Citation[31], Citation[32], Citation[46], Citation[47]. Two of the isoforms (3 and 4, in order of increasing charge) were difficult to resolve routinely. Thus, appears to resolve only four isoforms. The analysis of RF and heat effects was performed by quantifying the non-phosphorylated and three phosphorylated forms numbered 1–4 on , with number one spot containing two unresolved forms (2 and 3). The unphosphorylated form and form 1 were detectable under all experimental conditions studied. While forms 2 and 3 appeared sporadically in some sham and control samples, form 4 was consistently present in extracts from cells heated at either 41 or 45°C. However, forms 2 and 3 were consistently present only in cells heated at 45°C. It should be noted that 2 h at 41°C is essentially a non-lethal heat shock Citation[48], while 30 min at 45°C reduces cell survival to ∼ 25% Citation[49]. Thus, heat shock clearly induced the phosphorylation of HSP27, with the acute heat shock inducing phosphorylation to a greater extent than the moderate heat shock. Exposure of HeLa cells to TDMA RF radiation for 2 or 24 h did not appear to induce any additional HSP27 phosphorylation above sham.
After both heat shocks, the fraction of unphosphorylated HSP27 decreased significantly (). Further, the fraction of forms 2 and 3 were significantly higher after 30 min at 45°C, than after 2 h at 41°C. However, the distribution of phosphorylated HSP27 among different phosphorylation states made comparing the amount of phosphorylated HSP27 to the amount of unphosphorylated difficult due to the fact that the distribution of HSP27 across phosphorylation states was changing with temperature. Therefore, to facilitate analysis of the fraction of total HSP27 that was phosphorylated, all of the phosphorylated forms were pooled into one total (). For both TDMA exposures (2 h and 24 h) the extent of HSP27 phosphorylation was less than or equal to its corresponding sham. However, both heat shocks tested showed an extent of HSP27 phosphorylation greater than the incubator control. The incubator control and the 2 h sham showed the same extent of HSP27 phosphorylation. However, the 24 h sham showed a higher extent of HSP27 phosphorylation than the incubator control. This result appears to be due to experimental variation because the 2 h sham was intermediate between the two and not significant from either.
Figure 2. The effect of heating and TDMA exposure on the fraction of phosphorylated HSP27 in HeLa cells. The data shown was analysed for the fraction of Hsp27 that was phosphorylated by summing overall phosphorylated forms. The experimental conditions are the same as indicated in . The results of t-tests show that none of the TDMA exposed samples were different from their repective shams. Both the 41 and 45°C samples were significant compared to control for both phosphorylated and unphosphorylated HSP27.
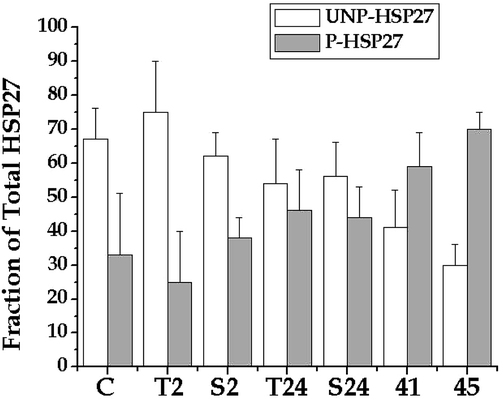
Since the above result appeared to be inconsistent with those reported by Leszczynski et al. Citation[4], Citation[24], these studies were repeated using a system and an approach that were similar to that reported by Leszczynski et al. Citation[4]. Human endothelial cells, E.A. Hy926, in culture were exposed to GSM (3.7 W kg−1) for 1, 2 or 5 h. In addition, it was modified to utilize an antibody specific to phosphorylated HSP27. The first step was to verify the specificity of the antibody. The results shown in demonstrate that the antibody for total HSP27 detected all five of the isoforms, while the antibody for phosphorylated HSP27 cross-reacted with the four most positively charged isoforms. Thus, the phosphorylated-HSP27-specific antibody did not cross-react with unphosphorylated HSP27.
Figure 3. Specificity of an antibody specific for phosphorylated HSP27. The HSP27 isoforms from HeLa cells that had been heated at 45°C for 30 min were resolved by 2D-polyacrlamide gel electrophoresis. The resolved proteins on three separate gels were probed with an antibody for total HSP27 and an antibody for phosphorylated HSP27, as indicated on the figure.
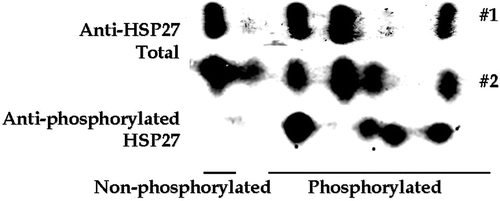
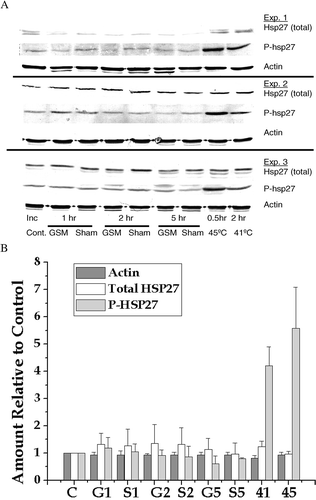
The two antibody technique was used to determine if exposure to 900 MHz GSM (3.7 W kg−1) for 1, 2 or 5 h induced HSP27 phosphorylation. Positive controls, 2 h at 41°C and 30 min at 45°C were included to ensure that the methods used will detect known changes in HSP27 phosphorylation and to provide a comparison between the two methods for detecting HSP27 phosphorylation. The western blots from three independent experiments are shown in and the quantification and averaging of these blots is shown in . Because actin, the total HSP27 and phosphorylated HSP27 are measured on separate western blots the amounts were normalized to their own control values. The actin levels did not change and the levels of total HSP27 were not significantly different for the various experimental treatments. This result shows that the loading for the various lanes on the western blots is equivalent. However, the levels of phosphorylated HSP27 were more than 4-fold elevated after the 2 h at 41°C heat shock and almost 6-fold elevated after the 30 min at 45°C heat shock. In contrast to the samples from the heat shocked cells, there were no significant changes in the levels of phosphorylated HSP27 between the control, the sham exposed and the GSM exposed for 1, 2 or 5 h. This result shows that GSM radiation at 3.7 W kg−1 did not induce measurable changes in HSP27 phosphorylation.
Figure 4. Analysis of HSP27 phosphorylation detected by Western blotting. (a) E.A. Hy296 cells were either heat shocked or exposed to GSM modulated RF radiation as described in the text. Total HSP27, phosphorylated HSP27 and actin were detected in whole cell extracts by Western blotting. Western blots from three experiments are shown. (b) Actin, total HSP27 and phosphorylated HSP27 were quantified, averaged and normalized to their control values. The normalized, average values ± SD are plotted as follows: Control = C; GSM for 1, 2 & 5 h = G1, G2 & G5, respectively; Sham for 1, 2 & 5 h = S1, S2 & S5, respectively; 41°C for 2 h = 41; and 45°C for 30 min = 45.
Discussion
The goal of the present study presented was to determine if non-thermal exposures to RF radiation induce the phosphorylation of HSP27. Clearly, the phosphorylation of HSP27 increased after either an acute, short duration heat shock or a moderate, chronic heat shock. However, exposures to TDMA for 2 or 24 h or to GSM for 1, 2, or 5 h did not alter the levels of HSP27 phosphorylation. Thus, the work presented herein did not confirm, in part, the reports of Leszcynski et al. Citation[4], Citation[24] and of Miyakoshi et al. Citation[25]. Although this study tried to duplicate the methods used in Leszcynski et al. as much as practically possible, there are differences that could account for the differences in the experimental outcomes. The main difference in methodology is that Leszcynski et al. Citation[4] used 32P-labelling to detect phosphorylated HSP27, which measures the HPS27 phosphorylated during the time that the label is available. In the Leszcynski et al. study cells were given 32P during the exposure time. Thus, the phosphorylation of HSP27 that is measured by this method will depend upon the rate of HSP27 phosphorylation and the time required for the exogenous 32P to equilibrate the intra-cellular phosphorus pools. By measuring the amount or fraction of phosphorylated HSP27 by specific immunoblotting or by resolving the phosphorylated HSP27 forms by 2D gel electrophoresis, these measurements and those of Miyakoshi et al. Citation[25] are independent of phosphorylation rates and endogenous pools. One overall interpretation of these results and those of Leszcynski et al. Citation[2], Citation[24] is that the latter group could have observed a small, transient perturbation in the rate of HSP27 phosphorylation or in the rate of 32P equilibration in the intra-cellular pools that did not affect significantly the ultimate level of HSP27 phosphorylation. The results reported in Leszcynski et al. Citation[4] are consistent with the reduction of HSP27 phosphorylation observed after a 1 h RF exposure is a transient effect, because there was no observed effect of a 2 or 5 h RF exposure. Thus, any depression of the rate of HSP27 phosphorylation during the first hour of RF exposure was swamped out by the HSP27 phosphorylation during the second hour of RF exposure, thereby having little effect on the fraction of HSP27 that was phosphorylated. This argument is supported by Miyakoshi et al. Citation[25] who reported that at 1 or 2 h after 1 or 2 W kg−1 of 1950 MHz CW there was no difference in HSP27 phosphorylation between sham and exposed samples. However, differences between the reported inhibition of HSP27 phosphorylation at 1 and 2 h during a 10 W kg−1 1950 MHz CW exposure Citation[25] was inconsistent with the lack of effect at 2 and 5 h during a 5 W kg−1 GSM exposure. This difference could be due to the difference in cell lines and in a higher SAR. However, the level of reduction in phosphorylated HSP27 in the report by Miyakoshi et al. Citation[25] was 20–25% of a low initial level suggestive of a small effect. In contrast, both acute and chronic heat shock did increase the level of HSP27 phosphorylation 4–6-fold. The magnitude of change in HSP27 phosphorylation observed after a known stress, i.e. heat shock, compared with the small changes reported after RF exposure, suggests that the small reduction in the rate of HSP27 phosphorylation, reported by Leszcynski et al. Citation[4] or in levels after a high SAR reported by Miyakoshi et al. Citation[25] are not biologically significant.
Several other studies suggest that non-thermal RF exposures do not induce a biologically significant stress response involving HSP70 Citation[23], Citation[50]. Responses of cells to environmental stresses include changes in the expression of stress responsive genes, in the levels of stress responsive proteins and/or in the phosphorylation status of signalling proteins. The results of gene expression studies of specific genes or groups of genes are mixed, with some suggesting changes Citation[20], Citation[21], Citation[27], Citation[51] while others find no effect Citation[22], Citation[23] following RF exposure. Further, the results of studies of using genome wide screening for expression changes are also mixed, with some reporting changes Citation[52] while others found no changes Citation[53] after RF exposure. Protein modifications and protein levels can not be detected directly as changes in gene expression. However, such changes are often parts of pathways that lead to changes in gene expression or occur in response to gene expression changes. Thus, one would expect that the results of proteomic studies would also find mixed results. This proved to be true for the studies of HSP27 phosphorylation by Leszcynski et al. Citation[4] that followed changes detected by a proteomics screen Citation[24], because the study by Miyakoshi et al. Citation[25] and the present study found results that differ from those reported by Leszcynski et al. Citation[4]. Thus, the results reported in this report, in the context of other studies, suggest that non-thermal RF do not induce a stress response.
In contrast to the lack of an effect of RF exposure on HSP27 phosphorylation both heat shocks studied caused a significant increase in the fraction of HSP27 that was phosphorylated. Interestingly there were differences due to heat shock in HSP27 phosphorylation, as seen in the ratio of phosphorylated to nonphosphorylated, the relative increase in phosphorylated and the relative fraction of total HSP27 represented by each of the various phosphorylated forms. The difference in the ratio of phosphorylated to non-phosphorylated was significantly different between sham and both 41 and 45°C ( and ). However, the relative increase in total phosphorylated HSP27 was only marginally different between 41 and 45°C and not significant ( and ). Since the thermal dose from the 2 h at 41°C heat shock is ∼1/8 that of the 30 min at 45°C heat shock, it is surprising that there was not more of a difference in the relative increase in phosphorylated HSP27 after these heat shocks. However, the relative fraction of total HSP27 represented by phosphorylated forms (2 and 3, ) is significantly different between the 41 and 45°C heat shocks. Thus, the pattern of HSP27 phosphorylation rather than the ratio of phosphorylated to non-phosphorylated HSP27 may be a marker for moderate vs acute heat shocks.
In summary, exposure of HeLa S3 cells to 5 W kg−1 TDMA for 1, 2 or 24 h did not induce significant differences in the levels of HSP27 phosphorylation compared to incubator control or sham. Further exposure of E.A. Hy926 cells to 3.7 W kg−1 900 MHz GSM for 1, 2 or 5 h did not induce significant differences in the levels of HSP27 phosphorylation compared to sham exposed. Thus, in contrast to chronic and acute heat shocks RF exposure did not appear to alter the phosphorylation of HSP27 at a biologically significant level. However, the possibility that subtle changes in HSP27 phosphorylation rates or levels are produced by RF exposure remains open due to the mixed results in reported studies. It is interesting to note in view of the mixed results with RF effects on HSP27 phosphorylation that after 60 Hz electromagnetic field exposure (an area of study where mixed results are often found) there was no effect on HSP27 phosphorylation, localization or expression Citation[54].
Acknowledgements
This work was supported in part by a research contract from the Mobile Manufacturers Forum (MMF) and in part by our Program Project grant (PO1 CA104457-02) from the National Cancer Institute of the US Department of Health and Human Services.
Notes
Notes
1. Health Effects from Radiofrequency Electromagnetic Fields, Documents of the NRPB, Volume 14 No. 2, National Radiological Protection Board, 2003. (http://www.nrpb.org/publications/documents_of_nrpb/abstracts/absd14-2.htm).
2. Cleveland RF, Ulcek JL. Questions and Answers about Biological Effects and Potential Hazards of Radiofrequency Electromagnetic Fields, OET Bulletin 56, 4th ed., FCC, Washington D.C., 20554, 1999. (http://www.fcc.gov/Bureaus/Engineering_Technology/Documents/bulletins/oet56/oet56e4.pdf).
References
- Habash RW, Brodsky LM, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part I: Evaluation and assessment of electric and magnetic fields. Crit Rev Biomed Eng 2003; 31: 141–195
- Habash RW, Brodsky LM, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part II: Evaluation and assessment of radio frequency radiation. Crit Rev Biomed Eng 2003; 31: 197–254
- Brodsky LM, Habash RW, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part III: Risk analysis. Crit Rev Biomed Eng 2003; 31: 333–354
- Leszcynski D, Joenvaara S, Reivinen J, Kuokka R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation 2002; 70: 120–129
- Lai H, Singh NP. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics 1995; 16: 207–210
- Lai H, Singh NP. Single- and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int J Radiat Biol 1996; 69: 513–521
- Lai H, Singh NP. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics 1997; 18: 156–165
- Phillips JL, Ivaschuk O, Ishida-Jones T, Jones RA, Campbell-Beachler M, Haggren W. DNA damage in Molt-4 T-lymphoblastoid cells exposed to cellular telephone radiofrequency fields in vitro. Bioelectrochem Bioenerget 1998; 45: 103–110
- Tice RR, Hook GG, Donner M, Mcree DI, Guy AW. Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics 2002; 23: 113–126
- Malyapa RS, Ahern EW, Straube WL, Moros EG, Pickard WF, Roti Roti JL. Measurement of DNA damage after exposure to electromagnetic radiation in the cellular phone communication frequency band (835.62 and 847.74 MHz). Radiat Res 1997; 148: 618–627
- Malyapa RS, Bi C, Ahern EW, Roti Roti JL. Detection of DNA damage by the alkaline comet assay after exposure to low-dose gamma radiation [published erratum appears in Radiat Res 1998;149:531]. Radiat Res 1998; 149: 396–400
- Lagroye I, Anane R, Wettring BA, Moros EG, Straube WL, LaRegina M, Niehoff M, Pickard WF, Baty J, Roti Roti JL. Measurement of DNA damage after acute exposure to pulsed-wave 2450 MHz microwaves in rat brain cells by two alkaline comet assay methods. Int J Radiat Biol 2004; 80: 11–20
- Lagroye I, Hook GJ, Wettring BA, Baty JD, Moros EG, Straube WL, Roti Roti JL. Measurements of alkali-labile DNA damage and protein-DNA crosslinks after 2450 MHz microwave and low-dose gamma irradiation in vitro. Radiat Res 2004; 161: 201–214
- Malyapa RS, Ahern EW, Straube WL, Moros EG, Pickard WF, Roti Roti JL. Measurement of DNA damage after exposure to 2450 MHz electromagnetic radiation. Radiat Res 1997; 148: 608–617
- Li L, Bisht KS, Lagroye I, Zhang P, Straube WL, Moros EG, Roti Roti JL. Measurement of DNA damage in mammalian cells exposed in vitro to radiofrequency fields at SARs of 3–5 W/kg. Radiat Res 2001; 156: 328–332
- Hook GJ, Zhang P, Lagroye I, Li L, Higashikubo R, Moros EG, Straube WL, Pickard WF, Baty JD, Roti Roti JL. Measurement of DNA damage and apoptosis in Molt-4 cells after in vitro exposure to radiofrequency radiation. Radiat Res 2004; 161: 193–200
- Vijayalaxmi, Bisht KS, Pickard WF, Meltz ML, Roti Roti JL, Moros EG. Chromosome damage and micronucleus formation in human blood lymphocytes exposed in vitro to radiofrequency radiation at a cellular telephone frequency (847.74 MHz, CDMA). Radiat Res 2001; 156: 430–433
- Vijayalaxmi, Leal BZ, Meltz ML, Pickard WF, Bisht KS, Roti Roti JL, Straube WL, Moros EG. Cytogenetic studies in human blood lymphocytes exposed in vitro to radiofrequency radiation at a cellular telephone frequency (835.62 MHz, FDMA). Radiat Res 2001; 155: 113–121
- Bisht KS, Moros EG, Straube WL, Baty JD, Roti Roti JL. The effect of 835.62 MHz FDMA or 847.74 MHz CDMA modulated radiofrequency radiation on the induction of micronuclei in C3H 10T(1/2) cells. Radiat Res 2002; 157: 506–515
- Ivaschuk OI, Jones RA, Ishida-Jones T, Haggren W, Adey WR, Phillips JL. Exposure of nerve growth factor-treated PC12 rat pheochromocytoma cells to a modulated radiofrequency field at 836.55 MHz: Effects on c-jun and c-fos expression. Bioelectromagnetics 1997; 18: 223–229
- Goswami PC, Albee LD, Parsian AJ, Baty JD, Moros EG, Pickard WF, Roti Roti JL, Hunt CR. Proto-oncogene mRNA levels and activities of multiple transcription factors in C3H 10T 1/2 murine embryonic fibroblasts exposed to 835.62 and 847.74 MHz cellular phone communication frequency radiation. Radiat Res 1999; 151: 300–309
- Whitehead TD, Brownstein BH, Parry JJ, Thompson N, Cha BA, Moros EG, Rogers BE, Roti Roti JL. Expression of the proto-oncogene c-fos after exposure to radiofrequency radiation relevant to wireless communications. Radiat Res 2005; 164: 420–430
- Laszlo A, Moros E, Davidson T, Bradbury M, Straube W, Roti Roti JL. The heat shock factor is not activated in mammalian cells exposed to cellular phone frequency microwaves. Radiat Res 2005; 164: 163–172
- Leszczynski D, Nylund R, Joenvarra S, Reivinen J. Applicability of discovery science approach to determine biological effects of mobile phone radiation. Proteomics 2004; 4: 426–431
- Miyakoshi J, Takemasa K, Takashima Y, Ding GR, Hirose H, Koyama S. Effects of exposure to a 1950 MHz radio frequency field on expression of Hsp70 and Hsp27 in human glioma cells. Bioelectromagnetics 2005; 26: 251–257
- Gabai VL, Sherman MY. Molecular biology of thermoregulation. Invited review: Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol 2002; 92: 1743–1748
- Valentim LM, Rodnight R, Geyer AB, Horn AP, Tavares A, Cimarosti H, Netto CA, Salbego CG. Changes in heat shock protein 27 phosphorylation and immunocontent in response to preconditioning to oxygen and glucose deprivation in organotypic hippocampal cultures. Neurosci 2003; 118: 379–386
- Arrigo A-P, Firdaus WJJ, Mellier G, Moulin M, Paul C, Diaz-latoud C, Kretz-remy C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods 2005; 35: 126–138
- Ito H, Iwamoto I, Inaguma Y, Takizawa T, Nagata K-I, Asano T, Kato K. Endoplasmic reticulum stress induces the phosphorylation of small heat shock protein, Hsp27. J Cell Biochem 2005; 95: 932–941
- Crete P, Landry J. Induction of Hsp27 phosphorylation and thermoresistance in Chinese hamster cells by arsenite, cycloheximide, A23187 and EGTA. Radiat Res 1990; 121: 320–327
- Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 1992; 267: 794–803
- Wong JW, Shi B, Farboub B, McClaren M, Shibamoto T, Cross CE, Isseroff RR. Ultraviolet B-mediated phosphorylation of the small heat shock protein HSP27 in human keratinocytes. J Invest Dermatol 2000; 115: 427–434
- Arata S, Hamaguchi S, Nose K. Effects of the overexpression of the small heat shock protein, HSP27, on the sensitivity of human fibroblast cells exposed to oxidative stress. J Cell Physiol 1995; 163: 458–465
- Landry J, Chretien P, Laszlo A, Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol 1991; 147: 93–101
- Chretien P, Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol 1998; 137: 157–166
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 1995; 15: 505–516
- Charette SJ, Landry J. The interaction of HSP27 with Daxx identifies a potential regulatory role of HSP27 in fas-induced apoptosis. Ann NY Acad Sci 2000; 926: 126–131
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2000; 20: 7602–7612
- Edgell CJS, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA 1983; 80: 3734–3737
- Moros EG, Straube WL, Pickard WF. The radial transmission line as a broad-band shielded exposure system for microwave irradiation of large numbers of culture flasks. Bioelectromagnetics 1999; 20: 65–80
- Moros EG, Straube WL, Pickard WF. A compact shielded exposure system for the simultaneous long-term UHF irradiation of forty small mammals: I. Electromagnetic and environmental design. Bioelectromagnetics 1998; 19: 459–468
- Pickard WF, Straube WL, Moros EG, Fan X. Simplified model and measurement of specific absorption rate distribution in a culture flask within a transverse electromagnetic mode exposure system. Bioelectromagnetics 1999; 20: 183–193
- Pickard WF, Straube WL, Moros EG. Experimental and numerical determination of SAR distributions within culture flasks in a dielectric loaded radial transmission line. IEEE Trans Biomed Eng 2000; 47: 202–208
- Schonborn F, Pokovic K, Burkhardt M, Kuster N. Basis for optimization of in vitro exposure apparatus for health hazard evaluations of mobile communications. Bioelectromagnetics 2001; 22: 547–559
- Horman S, Galand P, Mosselmans R, Legros N, Leclercq G, Mairesse N. Changes in the phosphorylation status of the 27 kDa heat shock protein (HSP27) associated with the modulation of growth and/or differentiation in MCF-7 cells. Cell Prolif 1997; 30: 21–35
- Xu M, Myerson RJ, Straube WL, Moros EG, LaGroye I, Wang LL, Lee JT, Roti Roti JL. Radiosensitization of heat resistant human tumor cells by 1 m @ 41°C and its effect on DNA repair. Int J Hyperthermia 2002; 18: 383–403
- Larsen JK, Yamboliev IA, Weber LA, Gerthoffer WD. Phosphorylation of the 27 kDa heat shock protein via p 38 MAP kinase and MAPKAP kinase in smooth muscle. Am J Physiol 1997; 273: 930–940
- Xu M, Wright WD. Higashikubo R, Roti Roti JL. Chronic thermotolerance with continued cell proliferation. Int J Hyperthermia 1996; 12: 645–660
- Roti Roti JL, Turkel N. Heat-shock-induced changes in nuclear protein and cell killing in thermotolerant HeLa cells. Radiat Res 1994; 138: 286–290
- Cleary SF, Cao G, Liu LM, Egle PM, Shelton KR. Stress proteins are not induced in mammalian cells exposed to radiofrequency or microwave radiation. Bioelectromagnetics 1997; 18: 499–505
- Pacini S, Ruggiero M, Sardi I, Alterini S, Gulisano F, Gulisano M. Exposure to global system for mobile communication (GSM) cellular phone radiofrequency alters gene expression, proliferation, and morphology of human skin fibroblasts. Oncol Res 2002; 13: 19–24
- Lee S, Johnson D, Dunbar K, Dong H, Ge X, Kim YC, Wing C, Jayathilaka N, Emmanuel NS, Zhou CQ, Gerber HL, Tseng CC, Wang SM. 2.45 GHz radiofrequency fields alter gene expression in cultured human cells. FEBS Lett 2005; 579: 4829–4836
- Whitehead TD, Moros EG, Brownstein BH, Roti Roti JL. Gene-expression does not significantly change in C3H 10T1/2 cells following exposure to CDMA or FDMA radiofrequency radiation. Radiat Res 2006; 165: 626–635
- Shi B, Farboud B, Nuccitelli R, Isseroff RR. Power-line frequency electromagnetic fields do not induce changes in phosphorylation, localization, or expression of the 27-kilodalton heat shock protein in human keratinocytes. Env Hlth Persp 2003; 111: 281–287