Abstract
Purpose: Interleukin-12 (IL-12) is a pro-inflammatory cytokine possessing anti-cancer and anti-angiogenic properties. This study quantitatively assessed the anti-angiogenic effect of IL-12 delivered using an adenoviral vector with murine IL-12 placed under control of a heat shock promoter. This approach limits systemic toxicity by restricting IL-12 delivery locally to the tumour. The kinetics of the downstream cytokines interferon-γ (IFN-γ) and interferon inducible protein-10 (IP-10) and other molecules affecting angiogenesis, vascular endothelial growth factor (VEGF) and plasminogen activator inhibitor-1 (PAI-1) were also studied.
Materials and methods: 4T1 tumours were grown in Balb/C mice and the AdhspmIL-12 construct was injected intra-tumourally. The tumours were heated after 24 h using a water bath. At various time points post-heating the tumours were collected and quantitatively assessed for cytokine production and vascularity.
Results: A significant reduction was seen in the tumour vasculature of the treated group vs. the control group mice. Systemic effects of IL-12 were limited to generalized immunostimulation. No hepatoxicity was noted.
Conclusions: This study suggests that IL-12 can be effectively delivered using a gene-based approach with a heat shock promoter. This results in quantitatively measurable anti-angiogenesis and general immunostimulation. The complex inter-play of other pro- and anti-angiogenic factors (IFN-γ, IP-10, VEGF and PAI-1) was also studied.
Introduction
Interleukin-12 (IL-12) is a potent pro-inflammatory cytokine possessing anti-cancer properties. A review on IL-12 by Colombo and Trinchieri Citation[1] outlines its anti-tumour, anti-metastatic, anti-angiogenic properties and the ability to elicit long-term anti-tumour immunity. Voest et al. Citation[2] first studied the role and possible mechanisms of action of IL-12 in anti-angiogenesis. The effect of IL-12 against basic fibroblast growth factor (bFGF) induced corneal neovascularization in mice was evaluated. IL-12 (1 µg per day) was given intra-peritoneally for two cycles of 5 days each with a 2-day interval. They found significant reduction in bFGF induced corneal neovascularization in the IL-12 treated mice. This effect was lost when the mice received a neutralizing IFN-γ antibody, suggesting that IFN-γ was an important mediator in the anti-angiogenic effects of IL-12. They proposed that IFN-γ has two major effects; (a) it induces production of interferon inducible protein-10 (IP-10), a member of the –C–X–C– chemokine family, which is directly anti-angiogenic and (b) IFN-γ inhibits metalloproteinase production. Metalloproteinases break down the extra-cellular matrix allowing new capillary sprouts to grow. IFN-γ induces production of soluble anti-angiogenic factor interferon-inducible protein 10 (IP-10) Citation[3]. IP-10 is expressed in activated mononuclear cells, keratinocytes, fibroblasts, endothelial cells and T-cells. It was found to inhibit bFGF-induced neovascularization of Matrigel injected subcutaneously into athymic mice. IP-10 also suppressed endothelial cell differentiation into tubular capillary structures in vitro Citation[4]. It has been suggested that the balance between the local concentrations of pro-angiogenic and anti-angiogenic factors within a tumour dictates whether angiogenesis occurs. Since angiogenesis is required for tumours and metastases to grow, angiogenesis inhibition is thought to be an important mechanism by which IL-12 controls tumour growth. Hence, IL-12 is proposed to act through the activation of IFN-γ/IP-10 axis. Other proposed mechanisms implicate direct natural killer (NK) cell mediated endothelial cytotoxicity Citation[5] and downregulation of the VEGFR-3 receptor Citation[6].
Local and systemic administration of IL-12 protein has been studied in various murine models Citation[7–10] and in Phase I/II human trials Citation[11], Citation[12]. IL-12 protein therapy has largely been limited by dose-dependent toxicity Citation[13]. Human recombinant IL-12 is associated with severe toxicity, even at doses as low as 1 µg kg−1 per day Citation[14]. Common toxicities included fever/chills, fatigue, nausea, vomiting and headache. Routine laboratory changes included anaemia, neutropenia, lymphopenia, hyperglycemia, thrombocytopenia and hypoalbuminemia. Dose limiting toxicities included oral stomatitis and liver function test abnormalities, predominantly elevated transaminases. Also, poor accumulation of IL-12 in solid tumours after intravenous injections (tumour:organ ratio <1) may partly explain its minimal anti-tumour effects Citation[15].
Local expression of IL-12 may avoid systemic toxicity of recombinant interleukin-12 administration Citation[16–18]. Intra-tumoural injections of adenoviral vectors or IL-12 plasmid DNA, naked or in complex with cationic lipid, have been attempted to deliver therapeutic IL-12 with the rationale that IL-12 will be produced only in the tumour, thereby reducing systemic side effects Citation[19]. A few reports have indicated the efficacy of this approach Citation[20–22], however, elevated systemic transgene levels were observed as the adenovirus can reach the circulation infecting other organs Citation[23–25]. The promoters in most previous reports are constitutively active, such as cytomegalovirus (CMV) based promoters. This combination makes it likely that intra-tumoural injection of this constitutively active adenovirus approach will still result in toxicity Citation[26], Citation[27].
To achieve localized gene expression, an adenoviral vector was developed with murine IL-12 placed under control of a heat inducible promoter, hsp 70B. This promoter is highly inducible at temperatures between 39–42°C and large fold-inductions in downstream transgene expression can be achieved Citation[28–30]. Heating the tumour after local injection of the adenoviral gene-therapy vector leads to activation of the hsp promoter and subsequent local IL-12 production, thus providing spatial and temporal control over transgene expression. The feasibility of combining radiotherapy, hyperthermia and heat inducible murine IL-12 gene therapy in a non-immunogenic B16.F10 melanoma line was studied Citation[31]. It was concluded that hyperthermia-regulated gene therapy in combination with radiation is feasible and therapeutically effective in murine tumours with no apparent systemic toxicity Citation[31], Citation[32].
This study assessed the dynamics and inter-play of five cytokines known to affect tumour angiogenesis (IL-12, IFN-γ, IP-10, VEGF and PAI-1) and quantitatively measured anti-angiogenesis using lectin intra-vital perfusion (FITC-conjugated Lycopersicon esculentum lectin) and vascular antigen staining (CD31). A significant reduction in vasculature was noted in the Ad hsp murine IL-12 + hyperthermia treated group as compared to the controls. Indications of the anti-metastatic and generalized immunostimulatory properties of IL-12 were also noted with little or no evidence of systemic toxicity.
Materials and methods
Animals
BALB/c mice, 9–12 weeks of age, weighing 20–25 g, were obtained from the Laboratory Animal Resources at Colorado State University.
Design of vector
The AdEasy system (Stratagene, La Jolla, CA) was used to construct the AdhspmIL12. The two sub-units (p35 and p40) of the murine IL-12 were amplified and sequence-verified. They were then connected into one gene expression unit by use of a flexible linker sequence (Gly4Ser). A 400-bp hsp70B promoter was then used to control the expression of the modified murine IL-12 gene. The whole hsp-mIL12 gene expression cassette was then transferred into an adenovirus shuttle plasmid. The shuttle plasmid was cotransfected into 293 cells to derive AdhspmIL12. Amplification and isolation of the virus was achieved following standard protocols Citation[33], Citation[34].
Tumour cell injection
4T1 cells were obtained from American Type Culture Collection (ATCC); 5 × 105 cells in 50 µl of PBS were injected into the left hind legs of the mice. Eight-to-ten days later the tumours reached a volume of 200 mm3. Daily tumour measurements were done using calipers. The tumour volume was calculated using the formula (short axis)2 × long axis × π/6 mm3. Mice were euthanized at a tumour volume of ∼1000–1200 mm3. This study was approved by the Colorado State University Animal Care and Use Committee.
Treatment groups
Tumour bearing mice were divided into the following treatment groups: (1) Control group receiving intra-tumoural normal saline injection 50 µl; (2) Intra-tumoural injection of 108 pfu of Ad LacZ (empty vector) suspended in 50 µl of normal saline + HT; (3) Intra-tumoural injection of 108 pfu of AdhspmIL-12 suspended in 50 µl of normal saline (No HT); (4) Intra-tumoural normal saline injection 50 µl + hyperthermia (HT); and (5) Intra-tumoural injection of 108 pfu of AdhspmIL-12 suspended in 50 µl of normal saline + HT.
All intra-tumoural injections were delivered 24 h prior to delivering HT through a single entry point. Multiple tracks were made in the tumour to achieve a more homogeneous distribution.
Hyperthermia
The mice were anaesthetized using 2% isoflurane and the tumours heated with the tumour bearing leg immersed in a water bath. The tumours were heated at 41°C for 60 min.
In vivo hypoxia and vascular markers
Hypoxyprobe-1 (Chemicon International, Temecula, CA) was injected intra-peritoneally at a dose of 75 mg kg−1 dissolved in 100 µl of normal saline 90 mi before euthanasia. Lycopersicon (tomato plant) lectin (Vector Laboratories, Burlingame, CA) conjugated with FITC was used as a marker for functional vasculature. It was injected via the tail vein in a dose of 100 µg in 100 µl of normal saline 3 min prior to euthanasia.
Tissue harvesting
At 6 h, 24 h and 5 days post-hyperthermia mice were euthanized by cervical dislocation under anaesthesia. Within 1 min of death, part of the tumour was collected in a cryotube (for RT-PCR) and part embedded in Tissue-Tek optimum cutting temperature compound (OCT, for imaging). Both parts were immediately frozen in liquid nitrogen. Lungs, liver and spleen were collected over the next few minutes and preserved in formalin. Haematoxylin and eosin (H&E) stains were done on these for histological examination.
RNA isolation and cDNA synthesis
Tumour samples were lysed and RNA isolated using TRIzol Reagent (GibcoBRL) as per manufacturer's protocol. RNA purity was assessed spectrophotometrically. Total isolated RNA was treated with DNase I (Invitrogen) to remove any genomic DNA. cDNA synthesis was then carried out using Superscript II RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA). Each reaction mixture contained 10 µl of RNA solution to which was added 4 µl First strand buffer (5×), 1 µl dNTP (10 mM), 1 µl DTT (0.1 M), 0.25 µl RNase out (40 U µl−1), 0.25 µl SuperScript II (200 U µl−1), 2 µl of random hexamers (300 ng µl−1) and 1.5 µl DNase, RNase free water giving a total of 20 µl per reaction mixture. This was incubated at 42°C for 50 min following which 30 µl of DNase, RNase free water was added to the mix and the enzymes inactivated by placing the reaction tubes on a 95°C heat- block for 5 min. The cDNA was then stored at −20°C until the time of RT- PCR.
Real time PCR
Real-time reverse transcriptase PCR was employed to detect and quantitatively express the production of murine genes of interest and GAPDH mRNA. Sequences for the primers and probes were obtained from the literature Citation[35–38] and purchased from MWG Biotech (High Point, NC). The reporter dye attached covalently at the 5’ end was FAM (6-carboxyfluorescin) and the quencher bound to the 3’ end was TAMRA (6-carboxytetramethylrhodamine). Real time PCR was performed using the Applied Biosystems ABI Prism 7000 (Foster City, CA). The amplification protocol was: 2 min at 50°C, 10 min at 95°C, 45 cycles of 15 s at 95°C and 60 s at 60°C.
Cycle threshold values were obtained from the ABI software and were exported to Microsoft Excel and the 2−ΔΔCt method was used to determine the relative expression of the genes of interest Citation[39]. Briefly, a standard housekeeping gene (e.g. GAPDH, β-actin, β2 microglobulin) is chosen as an internal control gene. This serves to normalize the amount of cDNA loaded for each reaction. An untreated control was selected as the ‘calibrator’ and the relative expression data is obtained as the fold change in gene expression normalized to the chosen endogenous reference gene and relative to the untreated control.
Prior to using the 2−ΔΔCt method for relative quantification, its use was validated for the primer and probe sequences and PCR conditions being employed in the experimental conditions. The methods and acceptable results are described in the literatu Citation[39], Citation[40]. Briefly, the target cDNA was serially diluted and real-time PCR performed. Cycle threshold values for the serially diluted samples are plotted on a graph with the log dilution on the x-axis and cycle threshold values on the y-axis. The slope of the line is used to obtain the efficiency of PCR amplification using the formula: Efficiency = 10(−1/slope) − 1.
Imaging for functional vasculature
Five micron thick frozen sections of the tumours were stored in the dark at −80°C. Tumour sections were imaged for FITC fluorescence using a Zeiss Axioplan 2 microscope (Carl Zeiss) with the KS 400 image analysis software. An area of the tumour away from the periphery was chosen for image capture. Images were captured at 100× magnification. Five-to-six separate images from four-to-five mice per group were captured for further image analysis. The x- and y-coordinates of each image were recorded to enable the same area to be imaged after CD31 and hypoxyprobe staining.
Immunohistochemistry
Immunohistochemical staining was performed using standard manual techniques on a vertical slide staining system (Credenza, Thermo Electron Corporation, Philadelphia, PA). Cryosections were fixed in −20°C acetone for 10 min and then allowed to dry at room temperature for 30 min. All reagents and kits were purchased from Vector Laboratories, Burlingam, CA unless otherwise noted. In all cases a control section was processed in which the primary antibody was replaced with antibody diluent. For monoclonal antibodies another negative control was run with an isotype similar non-specific antibody replacing the primary antibody at a similar concentration.
Hypoxyprobe/CD 31 staining
Identification of hypoxic regions were in fixed sections stained with the horseradish peroxidase based M.O.M. immunodetection kit with a hypoxyprobe 1-Mab1 (Chemicon International, Temecula, CA) as the primary Ab at 1:50 dilution in antibody diluent (DakoCytomation, Carpinteria, CA). DAB substrate was used to visualize the immunoreactive complexes. Tumour vessels were identified in sections incubated overnight in a rat monoclonal anti-mouse CD31 primary antibody (Clone MEC 13.3, BD Pharmingen, San Diego, CA) at 1:100 dilution in antibody diluent. A secondary biotinylated rabbit anti-rat IgG antibody at 1:100 dilution was applied and followed with a horseradish peroxidase streptavidin at 1:250 dilution in PBS. A VIP peroxidase substrate kit was used for visualization. Sections were dehydrated through ascending serially concentrated alcohol baths and mounted in a xylene-based permanent mounting media.
IL-12/IP-10 staining
Fixed sections were incubated overnight in primary antibody, rat monoclonal anti-mouse IL-12 (Clone C15.6, Biosource, Camarillo, CA) at 1:100 dilution in antibody diluent or were incubated in rabbit anti-mouse IP-10 (Peprotech, Rocky Hill, NJ) at 1:200 dilution in antibody diluent. A biotinylated secondary rabbit anti-rat IgG or goat anti-rabbit IgG at 1:100 dilution was applied and followed with a horseradish peroxidase streptavidin at 1:250 dilution. DAB substrate was used to visualize the immunoreactive complexes. Sections were dehydrated through ascending serially concentrated alcohol baths and mounted in a xylene-based permanent mounting media.
Image analysis
The saved images were quantitatively analysed for the parameters of interest using Scion Image (NIH). The ‘density slice’ was fixed for a colour of interest (FITC–green, CD31–blue, hypoxyprobe–orange/red) and the area occupied by the stain was obtained from the software expressed as pixels. All images for a particular colour were analysed using the same ‘density slice’.
Statistics
Pixel areas for FITC, CD31 and hypoxyprobe staining were compared between the treatment groups using the ANOVA test with pairwise comparison between groups.
Examination of lungs, liver and spleen
The lungs harvested from the control mice and the AdhspmIL-12 + HT treated mice were paraffin embedded, sectioned 10 µm thick and examined for the presence of metastases. Random sections of both lungs from the five mice in each group were stained using haematoxylin and eosin. The total number of metastases counted in 10 lungs each (right and left lungs) from the control and treated groups were compared.
The livers of the mice in all groups were paraffin embedded, sectioned 10 µm thick and examined for evidence of any hepatocellular changes, specifically hepatocellular necrosis. The spleens of all mice were similarly sectioned and examined for any evidence of generalized immunostimulation.
Results
Cytokine RT-PCR
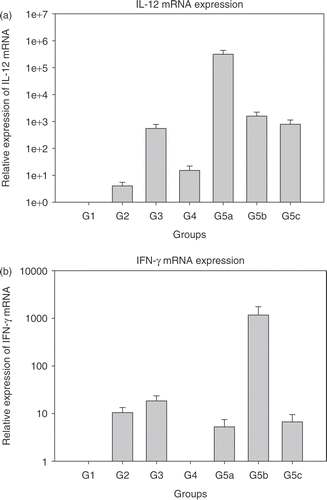
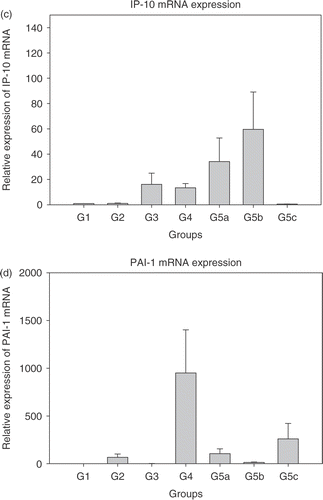
Real time PCR was performed to quantitatively detect IL-12, IFN-γ, IP-10, PAI-1 and VEGF mRNAs with GAPDH as the housekeeping gene. Initially, experiments were performed to detect efficiency of amplification for the genes of interest. After ascertaining that the PCR amplification efficiency was in the 90–95% range using the primer and probe sequences, the real time PCR for various treatment groups was performed. Results are shown in . Each bar represents the average ± standard error of mean of cytokine expression in tumours from five mice with each sample run in triplicate. The expression levels in the treatment groups are normalized to control group (group 1).
Figure 1. Relative expression of mRNAs in the treated groups 2–5c normalized to group 1 (each bar represents samples from five mice each in triplicate ± SEM). (a) Interleukin-12 (IL-12); (b) Interferon-γ (IFN-γ); (c) Interferon inducible protein-10 (IP-10); (d) Plasminogen activator inhibitor (PAI-1); and (e) Vascular endothelial growth factor (VEGF). Group 1: Normal saline injection, Control. Tumour and tissue samples collected at the same time as treatment group (5 days post-HT); Group 2: Ad LacZ + HT. Tumour and tissue samples collected 5 days post-HT; Group 3: AdhspmIL-12, no HT. Tumour and tissue samples collected at the same time as treatment group (5 days post- HT); Group 4: Normal saline injection, HT only. Tumour and tissue samples collected 5 days post-HT; Group 5: AdhspmIL-12 + HT (Group 5a: tumour sampled 6 h post-HT; Group 5b: tumour sampled 24 h post-HT; Group 5c: tumour sampled 5 days post-HT).
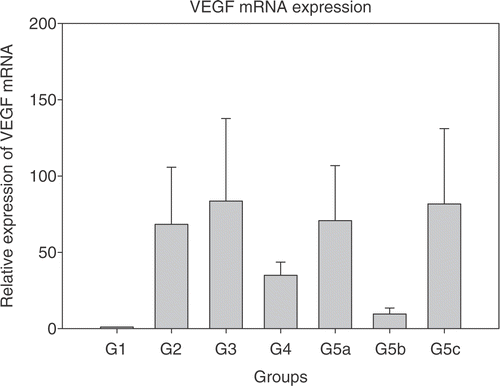
Maximum IL-12 mRNA levels were seen in the tumour samples collected 6 h post- HT (group 5a). By 24 h post-HT (group 5b) the level had reduced by ∼2.5 logs (). IL-12 mRNA was detectable up to 5 days post-HT (group 5c). IL-12 mRNA was also elevated in group 3, where the AdhspmIL-12 had been injected intra-tumourally but no hyperthermia had been delivered. This suggested a baseline level of hsp induction by factors other than hyperthermia. Peak IFN-γ mRNA levels were noted 24 h post- HT () corresponding to the time-point at which the IL-12 protein levels (16–20 h after IL-12 mRNA peak) would likely be highest. IP-10 mRNA also followed the same trend. However, as opposed to IL-12 and IFN-γ mRNA levels, which were still high in group 5c, the IP-10 levels had returned to baseline by 5 days post-treatment (). PAI-1 mRNA levels were higher in group 4 (HT only) than in other groups where hyperthermia was combined with adenoviral infection (groups 2 and 5) (). VEGF levels were elevated in all treated groups (). There were highly varying fold-increases in mRNA within the groups, as represented by the large error-bars. In groups 5a–c, VEGF levels appeared to be inversely related to the IFN-γ mRNA levels PAI-1 too.
Tumour growth curves
After the tumours reached sizes of ∼3–4 mm in diameter, daily tumour measurements were done using calipers and volumes calculated. Relative tumour volumes are shown in . A statistical analysis using the Kruskal-Wallis test revealed a statistically significant difference between the control and AdhspmIL-12 + HT group (p = 0.017) and between AdhspmIL-12 alone and AdhspmIL-12 + HT (p = 0.02). There was no statistically significant difference between any other groups compared pairwise.
Figure 2. Tumour growth curves. The relative tumour volumes for the treatment groups are shown. A statistically significant difference was noted between the control and virus + hyperthermia groups (p = 0.017) and between AdhspmIL-12 alone vs. AdhspmIL-12 + HT groups (p = 0.02). However, on pairwise comparisons using the Kruskal-Wallis test no significant difference was found between any other group.
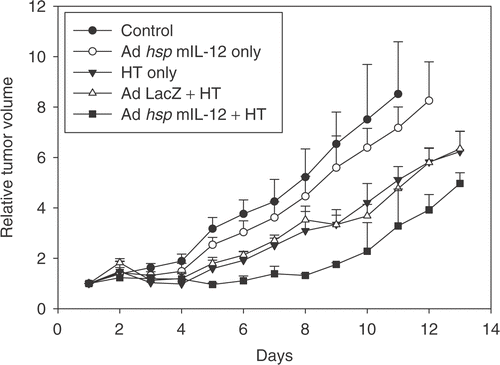
Image analysis for vascular area
Five-to-six tumour sections from four-to-five mice per group were examined at 100× for Tomato lectin-FITC fluorescence and CD31 staining (). Image analysis was done and the results are shown in .
Figure 3. Tomato lectin-FITC and CD31 & hypoxyprobe staining. (a) Tomato lectin-FITC. (b) Same coordinates on the slide imaged for CD31 and hypoxyprobe staining. Area stained by CD31 is larger than FITC indicative of greater presence of the antigen than is actually patent functional vasculature. (c) Image from a control tumour section showing the patent vasculature in green. (d) IL-12 treated tumour 5 days post-hyperthermia showing reduced vasculature. (e) Section from a control group tumour showing large areas staining positive for CD31 antigen with small areas of hypoxia. (f) Section from a treated group tumour showing reduced CD31 staining and larger areas of hypoxia.
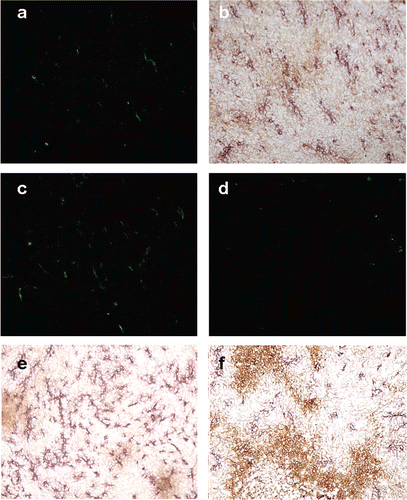
Figure 4. Area occupied by FITC and CD31 staining. (a) Percentage of field positive for Tomato lectin-FITC fluorescence, suggestive of functional vasculature. (b) Percentage of field staining positive for CD31 vasculature. The groups mentioned in the graphs are as follows: (1) Normal saline injection, Control. Tumours collected at the same time as treatment group (5 days post-HT); (2) Ad LacZ + HT. 5 days post-HT; (3) AdhspmIL-12, no HT. Same time as treatment group (5 days post-HT); (4) Normal saline injection, HT only. 5 days post-HT; (5) AdhspmIL-12 + HT. 5 days post-HT. Reduction in tumour vasculature was statistically significant (p < 0.05) in group 5 as compared to all other groups and for group 3 when compared to Control group (group 1).
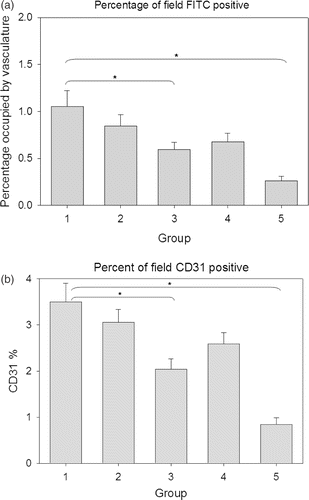
The area occupied by functional patent vasculature (FITC staining) is less than the area staining for total vascular antigens (CD31 staining). In the control group it was 1.05 ± 0.85% and 3.5 ± 1.75% for FITC and CD31, respectively, while in the treatment group (group 5c) it was 0.26 ± 0.22% and 0.84 ± 0.62% for FITC and CD31.
A statistically significant (p < 0.05) decrease in tumour vasculature was seen in group 5 as compared to all other groups for functional patent vasculature (FITC) and for total vascular antigen (CD31). In group 3, where the vector had been injected but the tumour was not heated, the difference was also significant as compared to controls.
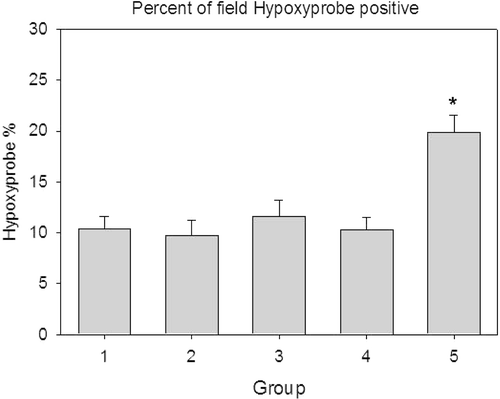
Image analysis for hypoxia
Hypoxyprobe staining was done to assess areas of tumour hypoxia in the different groups. shows the results. In the control group and groups 2–4 the tumour hypoxic area was 9.5–11.5% with standard deviations of 6–7%, however in the AdhspmIL-12 + HT group, the hypoxic area was 19.8 ± 7.5%. This increase was statistically significant (p < 0.05).
Figure 5. Area occupied by hypoxyprobe staining. The hypoxic area was higher for the AdhspmIL-12 + HT treated group as compared to all other groups. The groups mentioned in the graphs are as follows: (1) Normal saline injection, Control. Tumours collected at the same time as treatment group (5 days post- HT); (2) Ad LacZ + HT. 5 days post-HT; (3) AdhspmIL-12, no HT. Same time as treatment group (5 days post-HT); (4) Normal saline injection, HT only. 5 days post-HT; (5) AdhspmIL-12 + HT. 5 days post-HT.
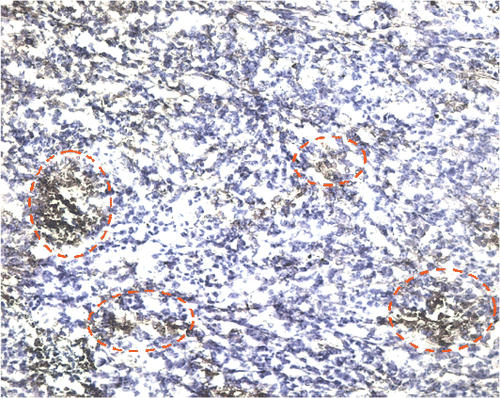
Cytokine staining
The IL-12 stain is cytoplasmic and multi-focally distributed throughout the tumour section () in the AdhspmIL-12 + HT group. In the other groups (Control, Ad LacZ + HT, AdhspmIL-12 alone and HT alone) no IL-12 was detected in the immunohistochemically stained tumour sections. An estimate for the area staining positive for IL-12 was 5–10% for the tumours collected 24 h post-HT and 10–20% for the tumours collected 5 days post-HT.
Figure 6. Interleukin-12 staining. The red circles outline some of the areas staining positive for IL-12 in a haematoxylin background in the AdhspmIL-12 + HT group. This image is from one of the tumours collected 5 days post HT (100×). In the control, Ad LacZ + HT, AdhspmIL-12 alone and HT alone groups no IL-12 was detected in the immunohistochemically stained tumour sections.
Lung metastasis
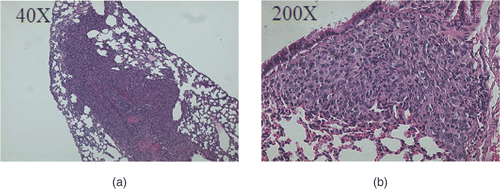
To assess the anti-metastatic properties of IL-12, random histopathologic sections of both lungs from all five mice in the AdhspmIL-12 + HT group were compared to the five mice from the control group. No lung metastasis was identified in the five mice in the AdhspmIL-12 + HT group compared to a total of nine metastases noted in 10 individual lungs of the control group. Most lung metastases were aggregates of a few tumour cells, however, some of them appeared as large sheets of tumour cells replacing the normal lung parenchyma, as shown in .
Liver and spleen histology
Mice livers were examined for pathological changes reflective of IL-12 toxicity with H&E stained sections. Liver tissue was normal with no hepatic necrosis noted in the control or the treated groups.
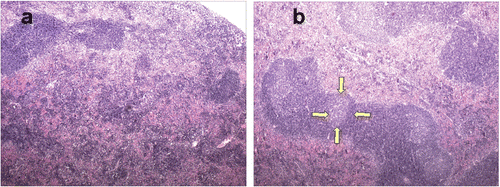
The spleen in the IL-12 treated mice had multiple coalescing large secondary follicles with large expanded germinal centres. Lymphoid follicles in the spleens of control mice were less prominent, smaller and had no or smaller germinal centres (). This suggested generalized immunostimulation in the mice where the IL-12 was expressed.
Discussion
Tumour anti-angiogenesis was studied using a hyperthermia-inducible gene construct to deliver IL-12 intra-tumourally. This construct has been designed to achieve a better spatial and temporal control over IL-12 expression. The aim was to have high tumour levels of the cytokines with limited systemic circulation to prevent generalized haematologic and hepatic toxicity. The vector was injected into the tumour and 24 h allowed for adenoviral infection in order to achieve maximum possible efficiency of infectio Citation[32], Citation[41]. Hyperthermia was then delivered to the tumour bearing leg using a water bath as previously reportedCitation[31], Citation[32], Citation[42].
Interleukin-12 mRNA levels have been reported to be maximally induced at 4–12 h after stimulation or induction Citation[43–45]. In in vitro studies the time point of maximal IL-12 mRNA levels had been found to be 6 h post- hyperthermia Citation[30]; thus this time point was selected for tumour sample collection in the AdhspmIL-12 + HT group to measure cytokine levels. Based on the in vitro experiments and from data in the literatuCitation[30], Citation[41], Citation[42], the protein levels peak ∼16–20 h post-induction and a few hours following the mRNA peak. Hence, it was reasoned that the downstream cytokines IFN-γ and IP-10 would show their peak mRNA levels in the tumour sample collected 24 h post-HT. This temporal relationship was confirmed in the tumour tissue samples of this study. The 5 days post-HT tumour sample was collected for IHC for analysis of anti-angiogenesis. It was expected that the cytokine levels would be returning toward baseline by this time. However, the IL-12 and IFN-γ mRNA levels were still ∼1000-fold and 8-fold higher than baseline. In contrast the IP-10 mRNA levels had reached baseline at day 5.
PAI-1 levels were maximally induced in the group receiving HT only (group 4). In a prior report, Roca et al. Citation[46] studied the anti-angiogenic properties of hyperthermia. They heated cultured human endothelial cells at 39°C, 41°C, 43°C and 45°C for 60 min and determined cell viability immediately and 16 h after heating. cDNA expression array was used to study the gene expression profile of endothelial cells after heat shock. It was determined that hyperthermia activates a gene response involving the transcription of plasminogen activator inhibitor (PAI-1), a regulator of the plasminogen activation pathway involved in vascular thrombosis, metastasis diffusion, inflammation and angiogenesis. A dose-dependent inverse relationship between IFN-γ and PAI-1 has been reported Citation[47–49]. A similar inverse relationship was present in this study and would explain why the hyperthermia alone group, in which no IFN-γ was induced, had higher levels of PAI-1 expression than the AdhspmIL-12 + HT group.
Elevated levels of VEGF mRNA were seen in all the treatment groups. Any anti-angiogenic measure such as hyperthermia Citation[50] or interleukin-12 could possibly result in increased VEGF to counter the anti-angiogenesis Citation[51]. An inverse relationship has been noted between IL-12 and VEGF Citation[52–54] and also IFN-γ and V Citation[55], Citation[56]. Maximum IL-12 protein levels, which would be expected ∼18–24 h post-HT, coincide with the lowest VEGF mRNA levels in groups 5a–c.
For tumour growth studies the 4T1 tumours growing in Balb/C mice were treated with HT alone or AdhspmIL-12 + HT and compared with the control group. A statistically significant reduction in tumour growth was observed in the AdhspmIL-12 + HT group as compared to the control group. The tumour growth curve for the Ad LacZ + HT was similar to that for HT alone. Intra-tumoural injection of AdhspmIL-12 alone resulted in a decreased rate of tumour growth as compared to the controls. However, a statistically significant difference was seen only when high levels of IL-12 were produced by hyperthermia in similarly injected tumours. The relative volumes in the AdhspmIL-12 + HT treated mice were lower than those in the HT only group, however this difference was not statistically significant. Similarly, the difference between HT only and control was not statistically significant. A similar study Citation[32] using this construct in B16F10 melanoma cell line tumours had also shown a delayed tumour growth in the IL-12 treated group. The reduction in tumour vasculature in group 4 may be responsible for the delayed tumour growth. In vitro studies done on 4T1 cells did not show evidence of appreciable reduction in percentage of viable cells using the trypan blue dye exclusion test when heated at 39–42°C. Only when heated at 43°C, the cell viability was reduced to ∼75% (data not shown).
For studying patent functional vasculature FITC-conjugated Lycopersicon esculentum (Tomato plant) lectin was injected 3 min prior to euthanasia. Lectins are rapidly distributed through all patent vessels within a circulation period of ∼60 s in mice Citation[57]. They bind to the glycoproteins expressed on the endothelial cells Citation[58]. CD31 staining, routinely used to study angiogenesis and anti-angiogenesis in tissue sections, over-estimates vascularity of a tumour Citation[59–62] as it stains endothelial cells in the tumour sections even in the absence of a patent vascular lumen. In this study, the area of the tumour section occupied by functional vasculature (∼1%) was one-third of the area staining positive for total vasculature (∼3%). CD31 positive area in the tumour have been reported as ∼3.5% for A431 human squamous cell carcinomas and B16F10 melanomas Citation[63] to ∼6.5–7% for neuroblastomas Citation[64] and LLC tumours Citation[63].
The vascular percentage of the treated group was 24% and 25% of the control group by CD31 and FITC studies, respectively. These values are close to those reported in literature for IL-12 induced anti-angiogenesis in Balb/C and C57CL/6 mice using Matrigel assay Citation[5] and in Balb/C mice in a breast carcinoma model Citation[52]. A significant reduction in vascularity was also noted in group 3, where the construct had been injected but hyperthermia had not been delivered. Approximately a 500-fold increase in IL-12 mRNA levels and a 20-fold and 16-fold increase in IFN-γ and IP-10 levels, respectively, were also seen. This suggested that some factor, other than HT, was leading to hsp induction in the tumour micro-environment although in a less efficient manner.
Various methods to measure tumour hypoxia have been described in the literature and the percentage of hypoxic cells, as assessed by hypoxia marker drugs, have been found to range from 0–50% with most tumours having hypoxic areas between 10–15% Citation[65]. The level of hypoxia in 4T1 tumours, using Hypoxyprobe-1, was found to be 16 ± 7% Citation[66]. In the tumour sections, using the same compound, the tumour hypoxic area was 9.7 ± 7.6% to 11.6 ± 7.3% for groups 1–4. However, in treatment group 5 (AdhspmIL-12 + HT) the hypoxic area had increased to 19.8 ± 7.5%. This difference was statistically significant and reflective of the reduction in tumour vascularity.
Adenoviruses are efficient vectors for use in gene therapy protocols. In vitro they have been found to have a high percentage infection of cells exposed Citation[67], Citation[68]. Infection efficiencies of 90–95% were found in the in vitro experiments using a GFP expressing adenoviral vector. However, the in vivo efficiencies, by intra-tumoural injections, are much lower. In malignant glioma cells, Puumalainen et al. Citation[69] reported adenoviral infection as low as <0.01–11%. Adenoviral infections are highly dependent on the coxsackievirus and adenovirus receptor (CAR) status Citation[70], Citation[71]. Gu et al. Citation[72] found variations in CAR status among human musculoendothelial tumours. They found that CAR mRNA was expressed at highest levels in osteosarcoma, Ewing's sarcoma, neurofibroma and schwannoma; at intermediate levels in bony exostosis, giant cell tumour, liposarcoma, synovial sarcoma, malignant peripheral nerve sheath tumour and haemangioma; and at low levels in alveolar soft part sarcoma and desmoid. In this study, immunohistochemical staining for IL-12 showed very sparse and patchy distribution suggestive of very low transfection efficiency in vivo. The distribution was more widespread on the 6th day post-HT as compared to 24 h post-HT. Still, only ∼10–15% of the cells appeared to be producing IL-12. IP-10, the downstream cytokine, showed a comparatively larger distribution in the tumour sections. This could be from the paracrine effects of IL-12 and IFN-γ.
It was surprising to find IL-12 protein in the tumours present and better distributed on the 6th day post-induction. The plasma half-life of IL-12 has been reported to be ∼10 h Citation[73]. This is much longer than the half-lives of other cytokines that generally have t1/2 of less than 30 min. Its persistence in the tumour for up to and possibly beyond 6 days may be due to a reduced rate of clearance from the tumour, resulting in a longer effective half-life and/or initiation of a positive feedback loop for cytokine production.
The anti-metastatic properties of interleukin-12 have been extensively studied in the literature Citation[1] and are attributed to generalized immunostimulation and anti-angiogenic properties of IL- Citation[2], Citation[5]. Rakhmilevich et al. Citation[74] delivered IL-12 using a gene-gun mediated approach and found a significant reduction in the number of lung metastases from 4T1 tumours with an increase in the survival time of the mice. In the treated group no metastases were detected in the lungs of the five mice examined as compared to a total of nine individual metastases in five control mice. The number of metastases is much lower than those reported in other studies Citation[75], Citation[76] where a total of 30–40 individual metastases were counted 3–4 weeks after 4T1 cell line inoculation in the control group. In this study only one random H&E section of both lungs from each mouse was examined for metastases ∼3 weeks post-inoculation and, thus, the number of metastases in both groups (control and treated) would be low.
Administration of recombinant IL-12 can cause liver toxicity in the form of hepatic necrosis Citation[77] or steatosis Citation[78]. No liver pathology was noted using the localized method of delivery in this study. The spleens from the IL-12 treated group showed evidence for generalized immunostimulation in the form of coalescing follicles with enlarged germinal centres. Splenic lymphoid hyperplasia has been reported in squirrel monkeys systemically treated with recombinant human IL-12 Citation[79]. This feature was not observed in the spleens of the control mice.
In conclusion, using the AdhspmIL-12 construct, one was able to deliver IL-12 intra-tumourally and demonstrate its potent anti-angiogenic effects and delayed tumour growth with limited systemic toxicity. This study also investigated the role of other pro- and anti-angiogenic factors and their complex inter-play in influencing tumour angiogenesis.
References
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13: 155–168
- Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst 1995; 87: 581–586
- Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985; 315: 672–676
- Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 1995; 182: 155–162
- Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood 1999; 93: 1612–1621
- Gerber SA, Moran JP, Frelinger JG, Frelinger JA, Fenton BM, Lord EM. Mechanism of IL-12 mediated alterations in tumour blood vessel morphology: Analysis using whole-tissue mounts. Br J Cancer 2003; 88: 1453–1461
- Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol 1994; 153: 1697–1706
- Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, Sondel PM, Grota K, Yang NS. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci USA 1996; 93: 6291–6296
- Watanabe M, Fenton RG, Wigginton JM, McCormick KL, Volker KM, Fogler WE, Roessler PG, Wiltrout RH. Intradermal delivery of IL-12 naked DNA induces systemic NK cell activation and Th1 response in vivo that is independent of endogenous IL-12 production. J Immunol 1999; 163: 1943–1950
- Tahara H, Zitvogel L, Storkus WJ, Robbins PD, Lotze MT. Murine models of cancer cytokine gene therapy using interleukin-12. Ann NY Acad Sci 1996; 795: 275–283
- Golab J, Zagozdzon R. Antitumor effects of interleukin-12 in pre-clinical and early clinical studies (Review). Int J Mol Med 1999; 3: 537–544
- Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, Witmer WK, Rockwell KA, Shane RB, Lessin SR, Vonderheid EC. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 1999; 94: 902–908
- Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: A review. Toxicol Pathol 1999; 27: 58–63
- Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res 1997; 3: 409–417
- Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, Neri D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol 2002; 20: 264–269
- Saffran DC, Horton HM, Yankauckas MA, Anderson D, Barnhart KM, Abai AM, Hobart P, Manthorpe M, Norman JA, Parker SE. Immunotherapy of established tumors in mice by intratumoral injection of interleukin-2 plasmid DNA: Induction of CD8 + T-cell immunity. Cancer Gene Ther 1998; 5: 321–330
- Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res 1996; 56: 2531–2534
- Rakhmilevich AL, Timmins JG, Janssen K, Pohlmann EL, Sheehy MJ, Yang NS. Gene gun-mediated IL-12 gene therapy induces antitumor effects in the absence of toxicity: A direct comparison with systemic IL-12 protein therapy. J Immunother 1999; 22: 135–144
- Shi F, Rakhmilevich AL, Heise CP, Oshikawa K, Sondel PM, Yang NS, Mahvi DM. Intratumoral injection of interleukin-12 plasmid DNA, either naked or in complex with cationic lipid, results in similar tumor regression in a murine model. Mol Cancer Ther 2002; 1: 949–957
- Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, Favrot M. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther 1998; 9: 2481–2492
- Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, Weichselbaum RR. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol 1999; 15: 769–773
- Putzer BM, Hitt M, Muller WJ, Emtage P, Gauldie J, Graham FL. Interleukin 12 and B7-1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proc Natl Acad Sci USA 1997; 94: 10889–10894
- Lohr F, Huang Q, Hu K, Dewhirst MW, Li CY. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin Cancer Res 2001; 7: 3625–3628
- Bramson JL, Hitt M, Gauldie J, Graham FL. Pre-existing immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther 1997; 4: 1069–1076
- Zhang R, Straus FH, DeGroot LJ. Effective genetic therapy of established medullary thyroid carcinomas with murine interleukin-2: Dissemination and cytotoxicity studies in a rat tumor model. Endocrinology 1999; 140: 2152–2158
- Nasu Y, Bangma CH, Hull GW, Lee HM, Hu J, Wang J, McCurdy MA, Shimura S, Yang G, Timme TL, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: Suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther 1999; 6: 338–349
- Emtage PC, Wan Y, Hitt M, Graham FL, Muller WJ, Zlotnik A, Gauldie J. Adenoviral vectors expressing lymphotactin and interleukin 2 or lymphotactin and interleukin 12 synergize to facilitate tumor regression in murine breast cancer models. Hum Gene Ther 1999; 10: 697–709
- Gerner EW, Hersh EM, Pennington M, Tsang TC, Harris D, Vasanwala F, Brailey J. Heat-inducible vectors for use in gene therapy. Int J Hyperthermia 2000; 16: 171–181
- Li CY, Dewhirst MW. Hyperthermia-regulated immunogene therapy. Int J Hyperthermia 2002; 18: 586–596
- Siddiqui F, Li CY, Zhang X, Larue SM, Dewhirst MW, Ullrich RL, Avery PR. Characterization of a recombinant adenovirus vector encoding heat-inducible feline interleukin-12 for use in hyperthermia-induced gene-therapy. Int J Hyperthermia 2006; 22: 117–134
- Lohr F, Hu K, Huang Q, Zhang L, Samulski TV, Dewhirst MW, Li CY. Enhancement of radiotherapy by hyperthermia-regulated gene therapy. Int J Radiat Oncol Biol Phys 2000; 48: 1513–1518
- Huang Q, Hu JK, Lohr F, Zhang L, Braun R, Lanzen J, Little JB, Dewhirst MW, Li CY. Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res 2000; 60: 3435–3439
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977; 36: 59–74
- Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 1999; 11: 305–312
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech 2003; 14: 33–43
- Huang MC, Lee HY, Yeh CC, Kong Y, Zaloudek CJ, Goetzl EJ. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2). Oncogene 2004; 23: 122–129
- Gerber HP, Kowalski J, Sherman D, Eberhard DA, Ferrara N. Complete inhibition of rhabdomyosarcoma xenograft growth and neovascularization requires blockade of both tumor and host vascular endothelial growth factor. Cancer Res 2000; 60: 6253–6258
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45
- Borrelli MJ, Schoenherr DM, Wong A, Bernock LJ, Corry PM. Heat-activated transgene expression from adenovirus vectors infected into human prostate cancer cells. Cancer Res 2001; 61: 1113–1121
- Li GC, He F, Shao X, Urano M, Shen L, Kim D, Borrelli M, Leibel SA, Gutin PH, Ling CC. Adenovirus-mediated heat-activated antisense Ku70 expression radiosensitizes tumor cells in vitro and in vivo. Cancer Res 2003; 63: 3268–3274
- Taoufik Y, Lantz O, Wallon C, Charles A, Dussaix E, Delfraissy JF. Human immunodeficiency virus gp120 inhibits interleukin-12 secretion by human monocytes: An indirect interleukin-10-mediated effect. Blood 1997; 89: 2842–2848
- Matsumoto H, Suzuki K, Tsuyuguchi K, Tanaka E, Amitani R, Maeda A, Yamamoto K, Sasada M, Kuze F. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG: Cytokine regulation and effect of NK cells. Infect Immun 1997; 65: 4405–4410
- Wittmann M, Larsson VA, Schmidt P, Begemann G, Kapp A, Werfel T. Suppression of interleukin-12 production by human monocytes after preincubation with lipopolysaccharide. Blood 1999; 94: 1717–1726
- Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, Gabriele P, Bussolino F. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res 2003; 63: 1500–1507
- Siren V, Immonen I, Cantell K, Vaheri A. Alpha- and gamma-interferon inhibit plasminogen activator inhibitor-1 gene expression in human retinal pigment epithelial cells. Ophthalmic Res 1994; 26: 1–7
- Gallicchio M, Hufnagl P, Wojta J, Tipping P. IFN-gamma inhibits thrombin- and endotoxin-induced plasminogen activator inhibitor type 1 in human endothelial cells. J Immunol 1996; 157: 2610–2617
- Giannopoulou M, Iszkula SC, Dai C, Tan X, Yang J, Michalopoulos GK, Liu Y. Distinctive role of Stat3 and Erk-1/2 activation in mediating interferon-gamma inhibition of TGF-beta1 action. Am J Physiol Renal Physiol 2006; 290: F1234–F1240
- Kanamori S, Nishimura Y, Okuno Y, Horii N, Saga T, Hiraoka M. Induction of vascular endothelial growth factor (VEGF) by hyperthermia and/or an angiogenesis inhibitor. Int J Hyperthermia 1999; 15: 267–278
- Bhujwalla ZM, Artemov D, Natarajan K, Solaiyappan M, Kollars P, Kristjansen PE. Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470. Clin Cancer Res 2003; 9: 355–362
- Dias S, Boyd R, Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int J Cancer 1998; 78: 361–365
- Duda DG, Sunamura M, Lozonschi L, Kodama T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K, Matsuno S. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res 2000; 60: 1111–1116
- Nakayama Y, Sako T, Shibao K, Onitsuka K, Nagashima N, Hirata K, Nagata N, Itoh H. Relationship between plasma levels of vascular endothelial growth factor and serum levels of interleukin-12 in patients with colorectal cancer. Anticancer Res 2000; 20: 4097–4102
- Wen FQ, Liu X, Manda W, Terasaki Y, Kobayashi T, Abe S, Fang Q, Ertl R, Manouilova L, Rennard SI. TH2 Cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol 2003; 111: 1307–1318
- Mitra-Kaushik S, Harding J, Hess J, Schreiber R, Ratner L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood 2004; 104: 3305–3311
- Debbage PL, Griebel J, Ried M, Gneiting T, DeVries A, Hutzler P. Lectin intravital perfusion studies in tumor-bearing mice: Micrometer-resolution, wide-area mapping of microvascular labeling, distinguishing efficiently and inefficiently perfused microregions in the tumor. J Histochem Cytochem 1998; 46: 627–639
- Roth J, Binder M, Gerhard UJ. Conjugation of lectins with fluorochromes: An approach to histochemical double labeling of carbohydrate components. Histochemistry 1978; 56: 265–273
- Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, New T, O’Toole K, Zabski S, Rudge JS, et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci USA 2003; 100: 7785–7790
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156: 1363–1380
- Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol 2003; 162: 183–193
- McDonald DM, Choyke PL. Imaging of angiogenesis: From microscope to clinic. Nat Med 2003; 9: 713–725
- Streit M, Stephen AE, Hawighorst T, Matsuda K, Lange-Asschenfeldt B, Brown LF, Vacanti JP, Detmar M. Systemic inhibition of tumor growth and angiogenesis by thrombospondin-2 using cell-based antiangiogenic gene therapy. Cancer Res 2002; 62: 2004–2012
- Chantrain CF, DeClerck YA, Groshen S, McNamara G. Computerized quantification of tissue vascularization using high-resolution slide scanning of whole tumor sections. J Histochem Cytochem 2003; 51: 151–158
- Hall EJ. Radiobiology for the radiologist. 5th ed. Lippincott Williams & Wilkins, Philadelphia 2000
- Samoszuk M, Corwin MA. Mast cell inhibitor cromolyn increases blood clotting and hypoxia in murine breast cancer. Int J Cancer 2003; 107: 159–163
- Meeker TC, Lay LT, Wroblewski JM, Turturro F, Li Z, Seth P. Adenoviral vectors efficiently target cell lines derived from selected lymphocytic malignancies, including anaplastic large cell lymphoma and Hodgkin's disease. Clin Cancer Res 1997; 3: 357–364
- Communal C, Huq F, Lebeche D, Mestel C, Gwathmey JK, Hajjar RJ. Decreased efficiency of adenovirus-mediated gene transfer in aging cardiomyocytes. Circulation 2003; 107: 1170–1175
- Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, Viita H, Paljarvi L, Vanninen R, Yla-Herttuala S. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther 1998; 9: 1769–1774
- Li D, Duan L, Freimuth P, O’Malley BW, Jr. Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin Cancer Res 1999; 5: 4175–4181
- Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res 2001; 61: 813–817
- Gu W, Ogose A, Kawashima H, Ito M, Ito T, Matsuba A, Kitahara H, Hotta T, Tokunaga K, Hatano H, et al. High-level expression of the coxsackievirus and adenovirus receptor messenger RNA in osteosarcoma, Ewing's sarcoma, and benign neurogenic tumors among musculoskeletal tumors. Clin Cancer Res 2004; 10: 3831–3838
- Lauw FN, Dekkers PE, te Velde AA, Speelman P, Levi M, Kurimoto M, Hack CE, van Deventer SJ, van der Poll T. Interleukin-12 induces sustained activation of multiple host inflammatory mediator systems in chimpanzees. J Infect Dis 1999; 179: 646–652
- Rakhmilevich AL, Janssen K, Hao Z, Sondel PM, Yang NS. Interleukin-12 gene therapy of a weakly immunogenic mouse mammary carcinoma results in reduction of spontaneous lung metastases via a T-cell-independent mechanism. Cancer Gene Ther 2000; 7: 826–838
- Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res 2004; 10: 4559–4567
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res 2005; 11: 728–734
- Dickerson EB, Akhtar N, Steinberg H, Wang ZY, Lindstrom MJ, Padilla ML, Auerbach R, Helfand SC. Enhancement of the antiangiogenic activity of interleukin-12 by peptide targeted delivery of the cytokine to alphavbeta3 integrin. Mol Cancer Res 2004; 2: 663–673
- Kaneda M, Kashiwamura S, Ueda H, Sawada K, Sugihara A, Terada N, Kimura-Shimmyo A, Fukuda Y, Shimoyama T, Okamura H. Inflammatory liver steatosis caused by IL-12 and IL-18. J Interferon Cytokine Res 2003; 23: 155–162
- Sarmiento UM, Riley JH, Knaack PA, Lipman JM, Becker JM, Gately MK, Chizzonite R, Anderson TD. Biologic effects of recombinant human interleukin-12 in squirrel monkeys (Sciureus saimiri). Lab Invest 1994; 71: 862–873