Abstract
Background: Prognosis for patients with malignant pleural mesothelioma (MPM) remains poor and such patients require intensive treatment. Few studies have examined hyperthermia for MPM. The present study investigated the feasibility of hyperthermia combined with weekly chemo-radiotherapy for patients with MPM and estimated the efficacy of this regimen.
Methods: A total of 11 patients (median patient age was 67 and all had pleural effusion) with MPM were enrolled in this study. The treatment regimen comprised of weekly thermo-radiotherapy with intra-thoracic chemotherapy 2–5 times at initiation of treatment. Hyperthermia was performed once per week for ∼60 min. Hemithorax external radiotherapy was administered once weekly on the same day as hyperthermia and just before thermochemotherapy. Median total radiation dose was 6 Gy (range, 2–10 Gy). Chemotherapy was administered into the thoracic cavity through a tube. Chemotherapeutic agents administered were CDDP for seven patients, carboplatinum (CBDCA) for three patients and both CDDP and CBDCA for one patient. Dose of CDDP was 50 mg/body and dose of CBDCA was 200–300 mg m−2. Response rate and median survival time (MST) and palliative effect were investigated.
Results: Complete response was not achieved in any of the 11 patients. Partial response was achieved in three of 11 patients (27.3%), SD in six patients (54.5%) and PD in two patients (18.2%). There was no correlational relationship between thermal parameters and response. MST was 27.1 months. Pleural fluid decreased in all patients after therapy, while all patients displayed improved performance status and could be discharged from hospital. Patients with partial response had a relatively longer survival time than SD or PD. All patients underwent the complete course of treatment and only one of 11 patients developed grade 4 thrombocytopenia.
Conclusion: It was therefore concluded that hyperthermia combined with intra-thoracic chemotherapy using cisplatinum or carboplatinum may be tolerable. This approach appears effective and more acceptable for patients with MPM with pleural effusion than other multi-modality therapy.
Introduction
Malignant pleural mesothelioma (MPM) is a rare disease that is usually caused by exposure to asbestos. Although most countries have quite strict rules about asbestos now, due to the long latency of MPM and the fact that the exposure was increasing until a certain time ago, the numbers of patients with MPM has been predicted to increase over the next few decades Citation[1]. Depending on the health of the individual, time of diagnosis and other factors, survival time is ∼4–12 months from onset of symptoms Citation[2]. Treatment for mesothelioma can involve surgical resection of the tumour, chemotherapy, radiotherapy or a combination of these approaches. However, the roles of surgery, radiotherapy and chemotherapy in the treatment of mesothelioma remain undetermined Citation[3]. New chemotherapeutic agents are currently being tested in clinical trials and appear somewhat promising Citation[4], Citation[5]. Treatments using some combination of surgery, radiotherapy and chemotherapy, known as multi-modality therapy, are now being studied and may provide the most promising option for some patients. However, the majority of patients presenting with MPM are not candidates for radical surgical resection due to unresectable, locally advanced disease or comorbidity. Even with strictly selected patients, surgery is associated with relatively high risks, with mortality for pleurectomy and decortication reportedly ∼2% Citation[6] and mortality for extra-pleural pneumonectomy ranging from 6–30% Citation[7].
Ratto et al. Citation[8] demonstrated that hyperthermic intra-thoracic perfusion with cisplatinum (CDDP) offers pharmacokinetic advantages with limited systemic toxicity. DeBree et al. Citation[9] reported the feasibility and toxicity of cytoreductive surgery combined with hyperthermic intra-thoracic chemotherapy for patients with MPM. In that study, most patients underwent surgical resection. However, thermo-chemo-radiotherapy for unresectable MPM has not been reported. This study reports herein the use of thermo-chemo-radiotherapy to treat 11 patients with MPM without surgical resection.
Materials and methods
Patients
A total of 11 patients (seven men, four women) with MPM were enrolled in this study between March 1995 and March 2005. Median patient age was relatively old, ∼67 years (range, 48–85 years) at initiation of treatment. Patient characteristics are shown in .
Table I. Patient and treatment characteristics.
Hyperthermia
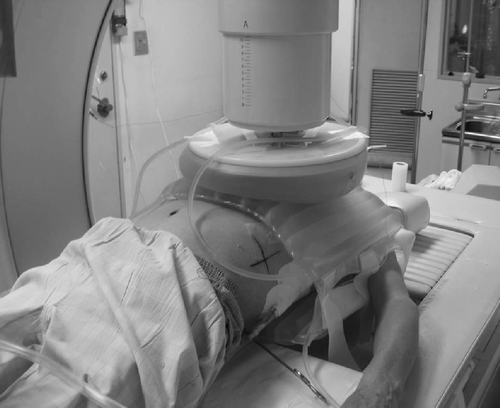
Hyperthermia was produced using a radiofrequency capacitive heating apparatus (Thermotron RF8; yamamoto Vinita Co. Ltd, Osaka, Japan). Hyperthermia was produced once per week for ∼60 min (median, 3.5 times). Electrodes were placed on the front and back of the patient and were 25–30 cm in diameter (). Overlay bolus was used to reduce the edge effect. Thermometry was performed by inserting thermocouples directly into the tumour for two patients, into the chest wall for six patients, into effusion itself for one patient or onto the skin for two patients. Since skin temperature is not an accurate indicator of tumour temperature, these two patients were excluded from thermal parameter analysis.
Radiotherapy
All patients received hemithorax external radiotherapy using a 10-MV linear accelerator (Mevatron; Toshiba medical Co. Ltd, Tokyo, Japan). In principle, MPMs are disseminated to the cavity, so the field should cover the entire hemithorax. As lung tissue is radiosensitive, radiotherapy was administered once weekly on the same day as hyperthermia and just before thermochemotherapy. Median total radiation dose was 6.5 Gy (range, 2–10 Gy) and median fraction size was 1–2 Gy.
Chemotherapy
Chemotherapy was administered into the thoracic cavity through a tube. Chemotherapeutic agents administered were CDDP for seven patients, carboplatinum (CBDCA) for three patients and both CDDP and CBDCA for one patient. Dose of CDDP was 50 mg/body and dose of CBDCA was 200–300 mg m−2.
Data analysis
Clinical tumour response was evaluated by measuring the tumour under computed tomography (CT). Complete response (CR) was defined as complete absence of disease. Partial response (PR) was defined as a ≥50% reduction from baseline of the sum of the products of perpendicular diameters for bidimensionally measurable disease or a ≥30% decrease in sum of the greatest diameters of unidimensionally measurable lesions. Progressive disease (PD) was defined as a ≥50% increase from baseline of the sum of the products of perpendicular diameters of bidimensionally measurable lesions. Stable disease (SD) represented disease that did not qualify for CR, PR or PD. Analysis of overall survival was conducted using the Kaplan–Meier method.
Results
summarizes treatment results. Complete response was not achieved in any of the 11 patients. PR was achieved in three of 11 patients (27.3%), SD in six patients (54.5%) and PD in two patients (18.2%). Survival period ranged from 4.1–68.0 months.
Table II. Treatment results.
As for thermal parameters, the average of Tmax for PR cases, SD cases and PD case were 43.2 ± 0.95°C, 42.2 ± 1.86°C and 42.0 ± 1.13°C, respectively. There was no correlational relationship between Tmax and the response.
Pleural fluid decreased in all patients after therapy, while all patients displayed improved performance status and pain relief and could be discharged from hospital. Complications comprised grade 4 thrombocytopenia in one patient. A blood transfusion was administered and no severe cardiac or pulmonary toxicity was observed.
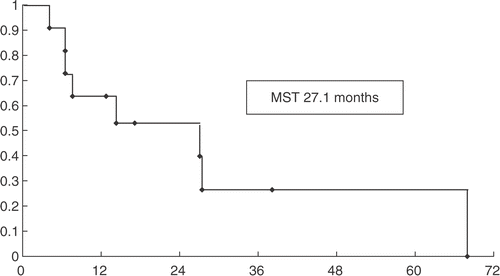
As of June 2005, a total of eight of the 11 patients were dead from respiratory failure due to intra-throracic recurrence and intractable pleural effusion. Four patients survived more than 2 years. Among them three were PR and one was SD. Two patients were PD and lived no more than 7 months. Median survival time (MST) was 27.1 months ().
Discussion
Despite many years of clinical research, no effective therapies have yet been identified for MPM. Untreated, prognosis is poor, with a median survival of <1 year Citation[10]. MPM may be treated using surgery, radiotherapy, chemotherapy or a combination of these approaches. In most patients, treatment remains palliative with symptom relief and moderate gains in survival. Some research has demonstrated that surgery can only offer symptom relief, with MST remaining poor at 8–11 months Citation[11–13].
Most monotherapies have been tested for MPM (). In general, single-agent response rates are <20% and no survival benefit for single-agent chemotherapy has been suggested by cohort studies. CDDP has demonstrated overall response rates of 14% and 36% when administrated at doses 100 mg m−2 every 21 days or 80 mg m−2 weekly, respectively Citation[17], Citation[18]. CBDCA, a better tolerated and easier-to-deliver analogue of CDDP, demonstrated response rates similar to CDDP when used with a conventional regimen Citation[19]. Some new agents, such as paclitaxel and gemcitabine, also display low response rates and therefore do not appear to represent effective monotherapies for MPM Citation[22], Citation[24]. Pemetratexed is a novel multi-targeted anti-folate that has been studied as a monotherapy in a phase II study, with a response rate ∼16% Citation[26]. Single-agent chemotherapy is thus not recommended for treatment of MPM.
Table III. Single-agent chemotherapy for MPM.
Combination chemotherapeutic regimens have been extensively evaluated in MPM () Citation[27]. The majority of these regimens have been adriamycin- or platinum-based. With few exceptions, however, most response rates have been a little higher than monotherapies and have remained <30% and MST has remained within 7–14.8 months.
Table IV. Combination chemotherapy for MPM.
While often attempted with curative intent, neither surgical management nor chemotherapy appears to offer significant improvements in survival. Efforts have therefore been focused on multi-modal approaches. Sugarbaker et al. Citation[28] conducted a large study evaluating multi-modalities against MPM. A single cohort of patients underwent EPP (extra-pleural pneumonectomy) and adjusted chemotherapy with cyclophosphamide/doxorubicin and/or CDDP and/or CBDCA/paclitaxel. That study demonstrated total MST as 19 months and a sub-set of patients with good prognostic parameters (i.e. epithelial histology, no nodal involvement and clear resection margins) achieved an MST of 51 months and 2- and 5-year survival rates of 68% and 46%, respectively. Other approaches to multi-modal therapy have been tried. Yoshino et al. Citation[29] selected 11 patients with resectable MPM who underwent hemithorax radiotherapy with gemcitabine/vinorelbine/cisaplatinum, achieving an MST of ∼22 months. Weder et al. Citation[30] investigated patients who underwent neoadjuvant chemotherapy using CDDP and gemcitabine followed by extra-pleural pneumonectomy with or without radiotherapy in patients with potentially resectable MPM. The response rate was 32% and MST was 23 months.
In the present study, hyperthermia was used for 11 patients instead of surgical management. This approach seems more practical, as the majority of patients have no opportunity to be operated on due to wide unresectable lesions when diagnosed. On the other hand, although systemic intravenous administration of the drug may be more effective in establishing diffuse dose intensity in malignant cells than local administration in patients with bulky tumours, especially with lymph-node metastases, it is not always suitable for every patient because of the toxicity of drug, especially to old patients. Also in such cases with bulky tumours local radiotherapy might be indicated. In the absence of surgical risk and with reduced invasiveness and toxicity of drug, this is more acceptable to the older patients. The regimen seemed tolerable and no severe complications were observed except for one case. All patients gained therapeutic benefits after initial therapy and were able to be discharged because of pain relief and control of pleural effusion. The stage of two patients with PD was T3N2M0, which was relatively advanced compared to other patients. It seems that local administration may be less effective in patients with bulky tumours, especially with lymph-node metastases. However, this regimen may be more tolerable to the older patients, especially without good performance status. And the patient survival was in accordance with the response rate which had a close relationship with stage of MPM. Response rate was 27.3% and MST was 27.1 months, comparable with the multi-modal approach mentioned above. This result was attributable to the fact that hyperthermia involves two biological interactions with radiation: a radiosensitizing effect Citation[31]; and a direct cytotoxic effect on tumour cells Citation[32]. When the target lesion is heated to ∼42°C, the cancer-killing effects of radiation or anti-cancer agents are enchanced Citation[33]. Intra-cavitary chemotherapy has the additional advantage of allowing high local doses with limited systemic toxicity Citation[34]. Hyperthermia also improves the efficacy and penetration depth of chemotherapy Citation[35]. This therapy is thus considered an effective approach to treat MPM with pleural effusion.
As for thermal parameters, one could not have any positive correlational relationship between Tmax and response. This is partly because of the possible heterogeneity of temperature distributions in diffuse MPM and partly because of the limited number of cases. Since one will continue to treat MPM patients in this way, any relationship would be acquired in the future.
In summary, the therapeutic effects and survival benefits described herein demonstrate the feasibility of thermo-chemo-radiotherapy for MPM with pleural effusion. The efficacy of thermo-chemo-radiotherapy remains to be confirmed in further studies involving a larger subject population.
References
- Pistloesi M, Rusthoven J. Malignant pleural mesothelioma: Update, current management, and newer therapeutic strategies. Chest 2004; 126: 1318–1329
- Curran D, Sahmoud T, Therasse P. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998; 16: 145–152
- Alberts AS, Falkson G, Goedhals L. Malignant pleural mesothelioma: A disease unaffected by current therapeutic maneuvers. J Clin Oncol 1988; 6: 527–535
- Gatzemeier U. Pemetrexed in malignant pleural mesothelioma. Oncology (Huntingt) 2004; 18(13 Suppl 8)26–31
- Tomek S, Manegold C. Chemotherapy for malignant pleural mesothelioma: Past results and recent developments. Lung Cancer 2004; 45(Suppl 1)S103–S119
- Rusch V, Saltz L, Venkatraman E, Ginsburg R, McCormack P, Burt M, Markman M, Kelsen D. A phase II trial of pleurectomy/decortication followed by intrapleural and systemic chemotherapy for malignant pleural mesothelioma. J Clin Oncol 1994; 12: 1156–1163
- Sugarbaker DJ, Mentzer SJ, DeCamp M, Lynch TJ, Jr, Strauss GM. Extrapleural pneumonectomy in the setting of a multimodality approach to malignant mesothelioma. Chest 1993; 103(Suppl 4)377–381
- Ratto GB, Civalleri D, Esposito M, Spessa E, Alloisio A, De Cian F, Vannozzi MO. Pleural space perfusion with cisplatin in the multimodality treatment of malignant mesothelioma: A feasibility and pharmacokinetic study. J Thorac Cardiovasc Surg 1999; 117: 759–765
- De Bree E, van Ruth S, Baas P, Rutgers EJ, Van Zandwijk N, Witkamp AJ, Zoetmulder FA. Cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy in patients with malignant pleural mesothelioma or pleural metastases of thymoma. Chest 2002; 121: 480–487
- Grondin SC, Sugarbaker DJ. Malignant mesothelioma of the pleural space. Oncology (Huntingt) 1999; 13: 919–926, Discussion 926, 931–932
- Brenner J, Sordillo PP, Magill GB, Golbey RB. Malignant mesothelioma of the pleura: Review of 123 patients. Cancer 1982; 49: 2431–2435
- Alberts AS, Falkson G, Goedhals L, Vorobiof DA, Van der Merwe CA. Malignant pleural mesothelioma: A disease unaffected by current therapeutic maneuvers. J Clin Oncol 1988; 6: 527–535
- Boutin C, Rey F, Gouvernet J, Viallat JR, Astoul P, Ledoray V. Thoracoscopy in pleural malignant mesothelioma: A prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 1993; 72: 394–404
- Lerner HJ, Schoenfeld DA, Matin A, Falkson G, Borden E. Malignant mesothelioma: The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1983; 52: 1981–1985
- Magri MD, Veronesi A, Folodore S, De Giovanni D, Serra C, Crismancich F, Tuveri G, Nicotra M, Tommasi M, Morassut S. Epirubicin in the treatment of malignant mesothelioma: A phase II cooperative study. Tumori 1991; 77: 49–51
- Bajorin D, Kelsen D, Mintzer DM. Phase II trial of mitomycin in malignant mesothelioma. Cancer Treat Rep 1987; 71: 857–858
- Zider BL, Green S, Pierce HL, Roach RW, Balcerzak SP, Militello L. A phase II evaluation of cisplatin in unresectable diffuse malignant mesothelioma: A Southwest Oncology Group study. Invest New Drugs 1988; 6: 223–226
- Planting AST, Schelens JHM, Goey SH, Van der Burg ME, De Boer-Dennert M, Stoter G, Verweij J. Weekly high-dose cisplatin in malignant mesothelioma. Ann Oncol 1994; 5: 373–374
- Raghavan D, Gianoutsos P, Bishop J, Lee J, Young I, Corte P, Bye P, McCaughan B. Phase II trial of carboplatin in the management of malignant mesothelioma. J Clin Oncol 1990; 8: 151–154
- Harvey VJ, Slevin ML, Ponder BA, Blackshaw AJ, Wrigley PF. Chemotherapy of diffuse malignant mesothelioma: Phase II trials of single-agent 5-fluorouracil and adriamycin. Cancer 1984; 54: 961–964
- Solheim OP, Saeter G, Finnanger AM, Stenwig AE. High-dose methotrexate in the treatment of malignant mesothelioma of the pleura: A phase II study. Br J Cancer 1992; 65: 956–960
- Van Meerbeeck J, Debruyne C, van Zandwijk N, Postmus PE, Pennucci MC, Van Breukelen F, Galdermans D, Groen H, Pinson P, Van Glabbeke M, et al. Paclitaxel for malignant pleural mesothelioma: A phase II study of the EORTC Lung Cancer Cooperative Group. Br J Cancer 1996; 74: 961–963
- Steele JPC, Shamash J, Evans MT, Gower NH, Tischkowitz MD, Rudd RM. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 2000; 18: 3912–3917
- Van Meerbeek JP, Baas P, Debruyne C, Groen HJ, Manegold C, Ardizzoni A, Gridelli C, Van Marck EA, Lentz M, Giaccone G. A phase II study of gemcitabine in patients with malignant pleural mesothelioma. Cancer 1999; 85: 2577–2582
- Anderson MK, Krarup-Hansen A, Martensson G, Winther-Nielsen H, Thylen A, Damgaard K, Olling S, Wallin J. Ifosfamide in malignant mesothelioma: A phase II study. Lung Cancer 1999; 24: 39–43
- Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, Burris H, Gatzemeier U, Blatter J, Symanowski JT, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003; 21: 1556–1561
- Bass P. Chemotherapy for malignant mesothelioma: From doxorubicin to vinorelbine. Semin Oncol 2002; 29: 62–69
- Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, DeCamp MM, Jr, Swanson SJ, Bueno R, Lukanich JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–63, Discussion 63–65
- Yoshino I, Yamaguchi M, Okamoto T, Ushijima C, Fukuyama Y, Ichinose Y, Maehara Y. Multimodal treatment for respectable epithelial type malignant pleural mesothelioma. W J Surg Oncol 2004; 2: 11–14
- Weder W, Kestenholz P, Taverna C, Bodis S, Lardinois D, Jerman M, Stahel RA. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004; 22: 3451–3457
- Arcangeli G, Casale C, Colistro F, Benassi M, Lovisolo G, Begnozzi L. One versus four heat treatments in combination with radiotherapy in metastatic mammary carcinoma. Int J Radiat Oncol Biol Phys 1991; 21: 1569–1574
- Overgaard J. Effect of hyperthermia on malignant cells in vivo. A review and hypothesis. Cancer 1977; 37: 2637–2648
- Song CW. Effect of local hyperthermia and blood flow and microenvironment: A review. Cancer Res 1984; 44(Suppl 10)4721S–4730S
- Rusch VW, Niedzwiecki D, Tao Y, Menendez-Botet C, Dnistrian A, Kelsen D, Saltz L, Markman M. Intrapleural cisplatin and mitomycin for malignant mesothelioma following pleurectomy: pharmacokinetic studies. J Clin Oncol 1992; 10: 1001–1006
- Storm FK. Clinical hyperthermia and chemotherapy. Radiol Clin North Am 1989; 27: 621–627