Abstract
Purpose: This study investigated whether hyperthermia can enhance TRAIL-induced apoptotic death.
Methods: Human prostate adenocarcinoma DU-145, human pancreatic carcinoma MIA PaCa-2 and BxPC-3, human colon fibroblast CCD-33Co and rat prostate endothelial YPEN-1 cells were treated with various concentrations of TRAIL (0–200 ng ml−1) with hyperthermia (40–42°C).
Results: It was observed in human cancer cells, but not in normal cells, that TRAIL induced apoptotic death and also that hyperthermia (40–42°C) promoted TRAIL-induced apoptotic death. Enhancement of TRAIL-mediated apoptosis by hyperthermia was detected by an increase in PARP cleavage, the hallmark feature of apoptosis, as well as by activation of caspases. There were no significant changes in the intra-cellular levels of death receptors (DRs), decoy receptors (DcRs) and anti-apoptotic proteins. Interestingly, data from in vitro enzyme kinetics assay demonstrated that hyperthermia promoted caspase enzyme activity.
Conclusions: These data suggest that cancer cells are more susceptible to TRAIL in the condition of hyperthermia (40–42°C). The promotion of caspase enzyme activity by hyperthermia may be responsible for enhancement of TRAIL-induced apoptotic death.
Abbreviations
| DcR1: | = | decoy receptor 1 |
| DcR2: | = | decoy receptor 2 |
| DR4: | = | death receptor 4 |
| DR5: | = | death receptor 5 |
| DTT: | = | dithiothreitol |
| FADD: | = | Fas-associated death domain |
| FasL: | = | Fas ligand |
| PARP: | = | Poly-ADP Ribose Polymerase |
| FLICE: | = | Fas-associated death domain-like interleukin-1β-converting enzyme |
| FLIP: | = | FLICE inhibitory protein |
| IAP: | = | inhibitor of apoptosis |
| PAGE: | = | polyacrylamide gel electrophoresis |
| PARP: | = | poly (ADP-ribose) polymerase |
| PBS: | = | phosphate-buffered saline |
| PDK-1: | = | phosphoinositide-dependent kinase-1 |
| PI3K: | = | phosphatidylinositol 3-kinase |
| PP1: | = | protein phosphatase 1 |
| PTEN: | = | phosphatase and tensin homologue deleted on chromosome 10 |
| SDS: | = | sodium dodecyl sulphate |
| TNF: | = | tumour necrosis factor |
| TRAIL: | = | tumour necrosis factor-related apoptosis-inducing ligand |
Introduction
Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo-2L), a 281-amino-acid protein, is a type II integral membrane protein belonging to the tumour necrosis factor (TNF) family, which includes Fas ligand (FasL) and TNF. The C-terminal extra-cellular region of TRAIL (amino acids 114–281) exhibits a homotrimeric sub-unit structure Citation[1] and its function is to induce apoptosis of tumour cells. The mechanism of TRAIL-induced apoptosis is initiated when TRAIL binds to death receptors such as TRAIL-R1 (DR4) and TRAIL-R2 (DR5) which induces the apoptotic signal. Both DR4 and DR5 contain a cytoplasmic death domain, which is required for TRAIL receptor-induced apoptosis. TRAIL also binds to decoy receptors (DcR1, DcR2), which results in inhibition of TRAIL signalling Citation[2–8]. Recent studies revealed that inhibition of TRAIL signalling by DcR2 critically depends on its association with DR5 via the NH2-terminal pre-ligand assembly domain overlapping the first partial cysteine-rich domain of both receptors Citation[9]. These observations suggest that DcR2 is a regulatory rather than decoy receptor. Nevertheless, the relative resistance of normal cells to the apoptotic inducing effects of TRAIL has been explained by the presence of large numbers of the decoy receptors on normal cells Citation[10], Citation[11]. Recently, this hypothesis has been challenged based on the results showing poor correlations between DR4, DR5 and DcR1 expression and sensitivity to TRAIL-induced apoptosis in normal and cancerous breast cell lines Citation[12] and melanoma cell lines Citation[13]. This discrepancy indicates that other factors such as death inhibitors including FLICE-inhibitory protein (FLIP) Citation[13], Fas-associated protein (FAP-1) Citation[14], Bcl-2 Citation[15], Bcl-XL Citation[15], Bruton's tyrosine kinase (BTK) Citation[16], silencer of death domain (SODD) Citation[17], toso Citation[18], inhibitor of apoptosis (IAP) Citation[19], X-linked inhibitor of apoptosis (XIAP) Citation[20] and survivin Citation[21] may be responsible for the differential apoptotic effect of TRAIL.
As mentioned above, TRAIL has been shown to induce apoptosis in a broad range of cancer cell types but not in normal cells and tissues, suggesting that it could be a potential therapeutic agent for the treatment of cancer Citation[22–24]. However, many tumour cells have been shown to be resistant to TRAIL Citation[25], Citation[26]. Several researchers have reported that TRAIL resistance can be overcome by various sensitizing agents such as chemotherapeutic drugs Citation[12], Citation[13], Citation[27–29], ionizing radiation Citation[30], cytokines Citation[31] and matrix metalloprotease inhibitors Citation[32]. It has also been demonstrated that tumour micro-environments such as low extra-cellular pH Citation[33], low glucos Citation[34], Citation[35] and low oxygen tensions Citation[36] augment TRAIL cytotoxicity. This study investigated whether hyperthermia also promotes TRAIL-induced cytotoxicity.
Hyperthermia, that is temperature above normal (37°C), has been successfully used to enhance the effectiveness of various forms of anti-cancer treatment, including chemotherapy and radiation therapy Citation[37–39]. Randomized clinical trials showed that complete response rate with radiotherapy plus hyperthermia was significantly better than that with radiotherapy alo Citation[40], Citation[41]. A phase II study also demonstrated that chemotherapy combined with hyperthermia improved local tumour control and overall survival in comparison with chemotherapy alo Citation[42], Citation[43]. However, several problems still remain to be solved to optimize the efficiency of hyperthermia in cancer therapy. Conventional studies have demonstrated that heating non-superficial tumours above 42°C is technically difficult and commonly leads to pai Citation[44], Citation[45]. This problem can be overcome by a multi-modality treatment strategy including the use of sub-lethal temperaturesCitation[41], Citation[43], Citation[46]. This study observed that hyperthermia (41–42°C) effectively enhances TRAIL-induced cytotoxicity by promoting caspase activity. It is believed that hyperthermia in combination with TRAIL can be used as an adjunctive therapy for cancer treatments such as radiotherapy and chemotherapy.
Materials and methods
Cell culture and survival assay
Human prostate adenocarcinoma DU-145, human pancreatic carcinoma MIA PaCa-2 and BxPC-3 and rat prostate endothelia YPEN-1 cells were cultured in DMEM medium (Gibco BRL, Gaithersburg, MD) containing 10% foetal bovine serum (HyClone, Logan, UT) and 26 mM sodium bicarbonate for monolayer cell culture. Human colorectal carcinoma CX-1 cells were cultured in RPMI-1640 medium (Gibco BRL) containing 10% foetal bovine serum. Human colon fibroblast CCD-33Co cells were cultured in Eagle's Minimal Essential medium containing 10% foetal bovine serum, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 2 mM L-glutamine and 1.5 g L−1 sodium bicarbonate. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2. One or 2 days prior to the experiment, cells were plated into 60-mm dishes. For trypan blue exclusion assay Citation[47], trypsinized cells were pelleted and resuspended in 0.2 ml of medium, 0.5 ml of 0.4% trypan blue solution and 0.3 ml of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min and examined under a light microscope. At least 300 cells were counted for each survival determination. For colony formation assay, the 60-mm Petri dishes containing monolayers of asynchronous cells were trypsinized with ice cold 0.05% trypsin and EDTA (0.1 g l−1) in PBS. After trypsinization, the cells were resuspended in 5 ml of DMEM or RPMI-1640 medium containing 10% foetal bovine serum used as a trypsin inhibitor. Cells were counted and appropriate dilutions were made. The appropriate number of cells were plated. After 10 days of incubation at 37°C, colonies were stained and counted.
Production of recombinant TRAIL
A human TRAIL cDNA fragment (amino acids 114–281) obtained by RT-PCR was cloned into a pET-23d (Novagen, Madison, WI) plasmid and His-tagged TRAIL protein was purified using the Qiagen express protein purification system (Qiagen, Valencia, CA).
Hyperthermia treatment
Cells cultured in 60-mm dishes were sealed with parafilm and were placed in a circulating water bath (Heto, Thomas Scientific, Denmark) which was maintained within ±0.02°C of the desired temperature.
Morphological evaluation
Approximately 5 × 105 cells were plated into 60-mm dishes overnight. Cells were treated with TRAIL and/or hyperthermia and then analysed by phase contrast microscopy for signs of apoptosis.
TUNEL assay
For detection of apoptosis by the TUNEL method, cells were plated in slide chambers. After treatment, cells were fixed with 4% paraformaldehyde in PBS. Cells were washed once, permeabilized by incubating with 100 µl of 0.1% Triton X-100 and 0.1% sodium citrate and then washed twice in PBS. The TUNEL reaction was carried out with 50 µl of TUNEL reaction mixture (450 µl of label solution/50 µl of enzyme solution; AP cell death detection kit, Roche, Germany). After washing three times with PBS, 50 µl of converter-AP was added on a sample. Thirty minutes after incubation in a humidified chamber at 37°C, each 100 µl of substrate (NBT/BCIP, Roche) was added. After 10 min incubation in the dark, cells were subjected to washing and were examined under a microscope.
Flow cytometry
Cells were pelleted, washed with PBS and resuspended in 200 µl of staining buffer containing fluorescein isothiocyanate (FITC)-annexin V and propidium iodide (PI) according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). After 15 min of incubation, 300 µl of sorting buffer was added and analysis was performed using the FACScan flow cytometer (Beckman Coulter, Inc., Hialeah, FL). Results were analysed with CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Antibodies
Rabbit polyclonal anti-caspase-3 antibody was purchased from Santa Cruz (Santa Cruz, CA). Anti-DR4, anti-DR-5, anti-DcR1 and anti-DcR2 antibodies were from ProSci (Poway, CA). Anti-cIAP-1 and anti-cIAP-2 antibodies were from R&D Systems (Minneapolis, MN). Anti-phospho-Akt, anti-Akt, anti-caspase-8, anti-FLIP (C-term: 447–464), anti-FLIP γ/δ and anti-FLIP-L antibodies were from Cell Signaling (Beverly, MA). Monoclonal antibodies were purchased from each of the following companies: anti-Bcl-2 and anti-Bcl-XL antibodies from Santa Cruz, anti-caspase-9 antibody from Upstate Biotechnology (Lake Placid, NY), anti-PARP antibody from Biomol Research Laboratory (Plymouth Meeting, PA) and anti-actin antibody from ICN (Costa Mesa, CA).
Protein extracts and polyacrylamide gel electrophoresis (PAGE)
Cells were lysed with 1 × Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8, 0.21 M sodium dodecyl sulphate, 0.3 mM bromophenol blue) and boiled for 10 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL). The samples were diluted with 1 × lysis buffer containing 1.28 M β-mercaptoethanol and equal amounts of protein were loaded on 8–12% sodium dodecyl sulphate (SDS)-polyacrylamide gels. SDS-PAGE analysis was performed according to Laemmli Citation[48] using a Hoefer gel apparatus.
Immunoblot analysis
Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% non-fat dry milk in PBS-Tween-20 (0.1%, v/v) at 4°C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer's instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL).
In vitro caspase activity assays
Activities of caspase-8, caspase-9 and caspase-3 were measured by spectrophotometric detection of the chromophore p-nitroalanine (pNA) at 405 nm (Chemicon, Temecula, CA). The assay was performed by the manufacturer's instructions. Briefly, CX-1 cells treated or untreated with TRAIL were harvested and lysed with 1X caspase lysis buffer. The cell lysates were chilled in ice for 10 min and the insoluble fraction was removed by centrifugation of 10 000 g, 5 min at 4°C. Supernatants were used for further caspase assay. Total protein was measured by BCA protein assay kit (Pierce). To measure caspases’ activity, 50 µg of total protein was used for each assay.
Results
Effect of hyperthermia on TRAIL-induced cytotoxicity
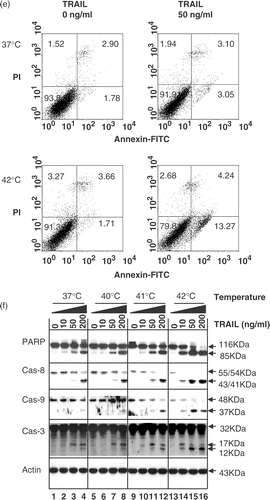
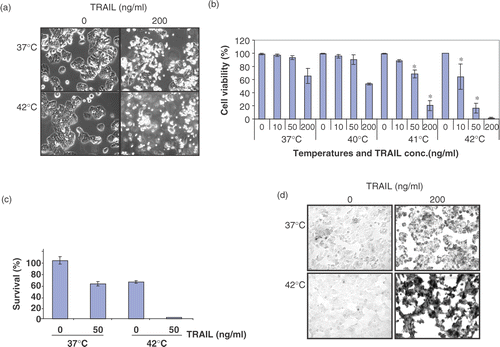
To study the effect of hyperthermia on TRAIL-induced cytotoxicity, human colorectal carcinoma CX-1 cells were treated with various concentrations of TRAIL (0–200 ng ml−1) for 4 h at various temperatures (37–42°C). Data from morphological assay and trypan blue exclusion assay show that no or minimal cytotoxicity was observed during hyperthermia (40–42°C) alone ( and ). However, unlike physiological death, colony formation assay shows that hyperthermia at 42°C for 4 h induced clonogenic cell death (). Additionally, TRAIL-induced cytotoxicity was promoted by hyperthermia (40–42°C). clearly shows that the augmentation of TRAIL-induced cytotoxicity was dependent upon heating temperatures. These observations were consistent with morphological features (). Most cells underwent apoptosis during TRAIL treatment in combination with hyperthermia as shown by cell surface blebbing and formation of apoptotic bodies (). An increase in the number of rounded cells and detached cells was observed during treatment with TRAIL in combination with hyperthermia. Similar results were observed with TUNEL assay () and flow cytometric assay (). Data from TUNEL and flow cytometric assays show that apoptotic death occurred during treatment with TRAIL and hyperthermia increased TRAIL-induced apoptotic death.
Figure 1. Effect of hyperthermia (40–42°C) on TRAIL-induced apoptosis in human colorectal carcinoma CX-1 cells. (a) Cells were treated with 200 ng ml−1 TRAIL for 4 h at 37°C or 42°C. The morphological features were analysed with a phase-contrast microscope. (b) Cells were treated with various concentrations of TRAIL (0–200 ng ml−1) for 4 h at various temperatures (37–42°C). Cell viability was determined by the trypan blue exclusion assay. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisks indicate values which are different from the respective control (t-test, p < o.05). (c) Cells were treated with 50 ng ml−1 TRAIL for 4 h at 37 or 42°C. Cell survival was determined by the colony formation assay. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisks indicate values which are different from the respective control (t-test, p < 0.05). (d) Cells were treated with TRAIL (200 ng ml−1) for 2 h at 37°C or 42°C. After treatment, apoptosis was detected by the TUNEL assay. (e) Cells were treated with TRAIL (50 ng ml−1) for 4 h at 37°C or 42°C. After treatment, apoptosis was detected by the flow cytometric assay. (f) Proteolytic cleavage of PARP and activation of caspases were assayed by Western blot analyses. Cells were treated for 4 h with various concentrations of TRAIL at various temperatures. Cell lysates were harvested and subjected to immunoblotting for caspase-8, caspase-9, caspase-3 or PARP. Antibody against caspase-8 detects inactive form (55/54 kDa) and cleaved intermediates (41, 43 kDa). Anti-caspase-9 antibody detects both inactive form (48 kDa) and cleaved intermediate (37 kDa). Anti-caspase-3 antibody detects inactive form (32 kDa) and cleaved active form (17 kDa). Immunoblots of PARP show the 116 kDa PARP and the 85 kDa apoptosis-related cleavage fragment. Actin was used to confirm the equal amount of proteins loaded in each lane.
Additional studies were designed to examine whether the combination of hyperthermia and TRAIL treatment enhances poly (ADP-ribose) polymerase (PARP) cleavage, the hallmark feature of apoptosis. PARP (116 kDa) was cleaved yielding a characteristic 85 kDa fragment in the presence of TRAIL (10–200 ng ml−1). This cleavage was enhanced by hyperthermia, in particular heating at 42°C (). It is well known that TRAIL-induced apoptosis is mediated through a caspase cascade. To examine whether hyperthermia enhances TRAIL-induced apoptosis through activation of caspases, several caspases known to be involved in TRAIL-induced apoptosis were examined. shows that hyperthermia enhanced TRAIL induced caspase-8 activation. Western blot analysis shows that procaspase-8 (55/54 kDa) cleavage to intermediate (43/41 kDa) forms was enhanced with increasing temperature in the presence of TRAIL. Hyperthermia enhanced the proteolytic processing of procaspase-9 (48 kDa) into its active form (37 kDa). Hyperthermia also increased TRAIL-induced caspase-3 activation. Western blot analysis shows that procaspase-3 (32 kDa), the pre-cursor form of caspase-3, was cleaved to active form (17 and 12 kDa) in the presence of TRAIL. The combined treatment with TRAIL and hyperthermia increased the level of active form, in particular 12 kDa.
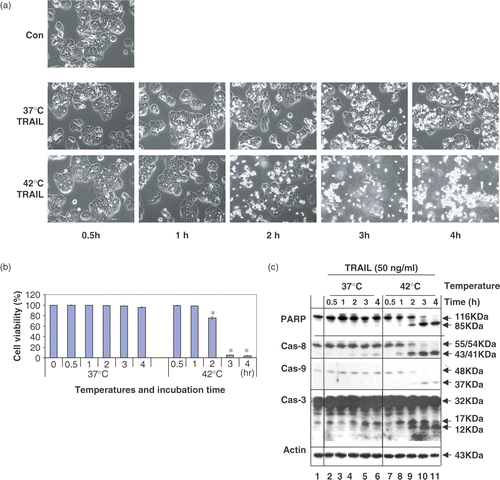
These studies were extended to investigate the time course of apoptotic death during treatment with TRAIL in combination with hyperthermia (42°C). shows that apoptotic death gradually increased when the treatment time was increased. A maximal cytotoxicity occurred within 3 h. Similar results were observed for PARP cleavage as well as activation of caspases.
Figure 2. Time course of apoptotic cell death during treatment with TRAIL in the conditions of normothermia and hyperthermia. (a) CX-1 cells were treated with 50 ng ml−1 TRAIL for various times (0.5–4 h) at 37°C or 42°C. The morphological features were analysed with a phase-contrast microscope. Con, untreated unheated control cells. (b) Cell viability was determined by the trypan blue exclusion assay. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisks indicate values which are different from the respective control (t-test, p < 0.05). (c) Time course of PARP cleavage and activation of caspases were assayed by Western blot analyses as described in .
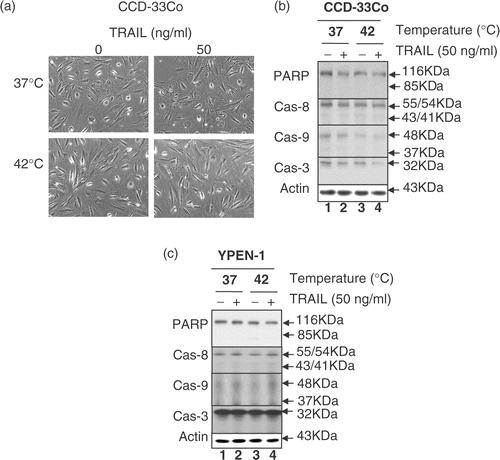
To examine whether these observations are unique to CX-1 cells, human prostate adenocarcinoma DU-145, human pancreatic carcinoma MIA PaCa-2 and BxPC-3, human colon fibroblast CCD-33Co and rat prostate endothelia YPEN-1 cells were also employed. and show that the combined treatment of TRAIL and hyperthermia resulted in an increase in cell death and PARP cleavage as well as the activation of caspases in tumour cells but not in normal CCD-33Co and YPEN-1 cells. These results suggest that the response of normal cells to TRAIL in combination with hyperthermia differs from that of tumour cells.
Figure 3. Effect of hyperthermia on TRAIL-induced apoptosis in other cell lines: MIA PaCa-2, BxPC-3 and DU-145 cells. (a) MIA PaCa-2 and BxPC-3 cells were heated at 40°C or 42°C for 4 h in the presence or absence of 50 ng ml−1 TRAIL. Cell survival was determined by the trypan blue exclusion assay. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisks indicate values which are different from the respective control (t-test, p < 0.05). (b, c) Cell lysates were subjected to immunoblotting for PARP and caspases as described in .
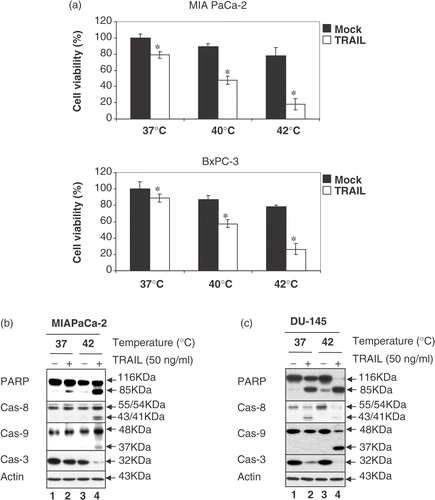
Figure 4. Effect of hyperthermia in combination with TRAIL on cell morphology, proteolytic cleavage of PARP and activation of caspases in normal cell lines CCD-33Co and YPEN-1. (a) CCD-33Co cells were heated at 42°C for 4 h in the presence or absence of 50 ng ml−1 TRAIL. The morphological features were analysed with a phase-contrast microscope. (b, c) CCD-33Co cells or YPEN-1 cells were heated at 42°C for 4 h in the presence or absence of 50 ng ml−1 TRAIL. Cell lysates were subjected to immunoblotting for PARP and caspases as described in .
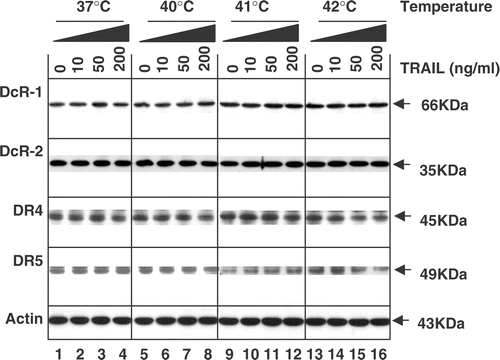
Effect of hyperthermia on the TRAIL receptor family
It is well known that TRAIL-induced apoptotic signals are triggered by interaction with two death receptors (DR4 and DR5). Such signals may be blocked by antagonistic decoy receptors (DcR1 and DcR2). Previous studies also demonstrate that increased DR5 levels induced by genotoxic agents are responsible for increasing TRAIL cytotoxicity. Thus, it was examined whether treatment with TRAIL in combination with hyperthermia affects the level of TRAIL receptors. Data from western blot analysis revealed that the combined treatment did not significantly alter the total cellular levels of death receptors DR4 and DR5 as well as decoy receptors DcR1 and DcR2 ().
Figure 5. Intra-cellular levels of TRAIL receptors during treatment with TRAIL in the conditions of normothermia and hyperthermia. CX-1 cells were treated for 4 h with various concentrations of TRAIL (0–200 ng ml−1) in the indicated temperatures. To measure the levels of death receptors and decoy receptors during treatment with TRAIL in normothermia and hyperthermia, equal amounts of protein (20 µg) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotted as described in Materials and methods. Actin was shown as an internal standard.
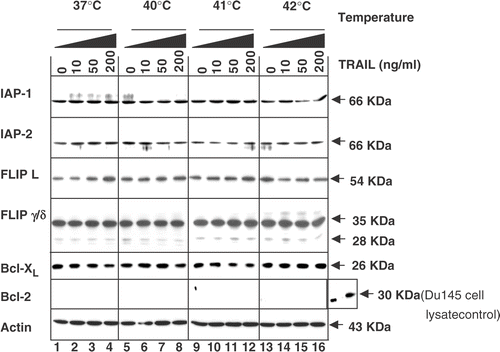
Effect of hyperthermia and TRAIL on anti-apoptotic proteins
It is well known that reduction of intra-cellular anti-apoptotic molecules such as FLIP, IAP-1, IAP-2, Bcl-2 and Bcl-XL sensitizes TRAIL-resistant cancer cells to TRAIL. Thus, this study examined whether changes in the amounts of anti-apoptotic proteins are associated with the promotion by hyperthermia of apoptosis by TRAIL. Data from western blot analysis reveal that the combined treatment did not significantly alter the levels of FLIPL, FLIPS, IAP-1, IAP-2 and Bcl-XL (). Interestingly, there is no detectable level of Bcl-2 in CX-1 cells.
Figure 6. Intra-cellular levels of anti-apoptotic proteins during treatment with TRAIL in the conditions of normothermia and hyperthermia. CX-1 cells were treated for 4 h with various concentrations of TRAIL (0–200 ng ml−1) in the indicated temperatures. To determine the intra-cellular levels of anti-apoptotic proteins during treatment with TRAIL in normothermia and hyperthermia, equal amounts of protein (20 µg) were separated and immunoblotted as described in Materials and methods. Actin was shown as an internal standard.
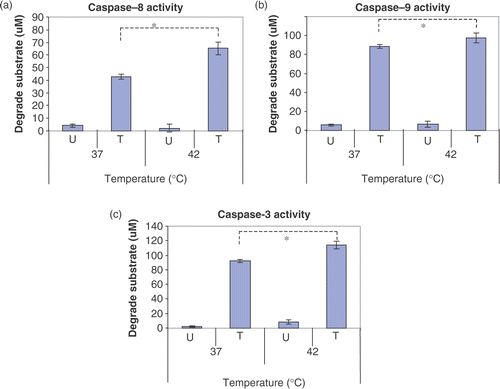
Effect of hyperthermia on caspase activity
TRAIL induced cell death is mediated through caspase cascades. So, it was hypothesized that caspase enzyme activity is thermodynamically enhanced in hyperthermic conditions and hyperthermia-enhanced caspase enzyme activity is responsible for enhancement of TRAIL-induced apoptotic death. To test this possibility, CX-1 cells were treated with TRAIL, cell lysates were harvested and then caspase activity was measured during hyperthermia (42°C). Data from in vitro enzyme kinetics assay in demonstrated that hyperthermia significantly promoted caspase enzyme activity.
Figure 7. Effects of hyperthermia on caspase activity. CX-1 cells were treated with or without 200 ng ml−1 of TRAIL for 2 h and the cell lysates were prepared for caspase activity. Cell lysates was subjected to reaction at 37°C or 42°C for 2 h. U, untreated cell lysates. T, TRAIL treated cell lysates. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisks indicate values which are different from the respective control (t-test, p < 0.05).
Discussion
TRAIL induces apoptosis in a wide variety of tumour cells, but does not cause toxicity to most normal cells. Pre-clinical studies in mice and primates have also shown that administration of TRAIL can induce apoptosis in human tumours, but no cytotoxicity to normal organs or tissue Citation[23]. Recent phase 1 and 2 clinical trials using agonistic monoclonal antibodies which bind to human TRAIL receptors DR4 and DR5 have provided encouraging results for cancer therapy Citation[49].
Previous studies have demonstrated that hyperthermia induces improvement of tumour oxygenation and increases responses of tumours to radiotherapy and chemotherapy Citation[50–53]. This study reveals that hyperthermia (40–42°C) promotes TRAIL-induced apoptotic death by facilitating caspase activity. The observations are similar to previous reports which reveal the synergistic effect of hyperthermia on TNF- and/or interferon-γ-induced cytotoxicity Citation[54–56]. However, these observations are somewhat different from a previous report that heat shock inhibits rather than promotes TRAIL-induced apoptosis Citation[57]. In the previous report, cells were heated prior to TRAIL treatment, so the resistance to TRAIL may have been due to the synthesis of anti-apoptotic molecules such as HSP70 and HSP90. It is well known that HSP70 and HSP90 interact with Apaf-1 to prevent efficient assembly of the apoptosome Citation[58–61] or antagonize the caspase-independent death effector apoptosis inducing factor (AIF) Citation[62]. Nonetheless, the data clearly demonstrated that simultaneous TRAIL treatment and hyperthermia promotes apoptotic death by enhancing caspase activation.
Several researchers have shown that an increase in DR5 gene expression or decrease in FLIP expression in response to genotoxic stress is responsible for the synergistic effect of ionizing agents or chemotherapeutic agents on TRAIL Citation[12], Citation[13], Citation[27], Citation[30], Citation[63]. This study revealed that hyperthermia-enhanced TRAIL cytotoxicity is not due to alteration of TRAIL receptor levels () or anti-apoptotic protein levels ().
Although this study has demonstrated that caspase enzyme activity is thermodynamically enhanced in hyperthermic conditions, one cannot rule out other possibilities. One possibility is that apoptotic signalling via a mitochondria-dependent pathway is also enhanced during hyperthermia. This is based on findings that over-expression of Bcl-2 gene inhibits mitochondria-dependent apoptosis pathways and that over-expression of Bcl-2 gene also inhibits the enhancing effect of hyperthermia, PARP cleavage and caspase activation (data not shown). It is also well known that cytochrome c release from mitochondria activates various caspases during apoptosis Citation[64–66]. It is possible that hyperthermia promotes cytochrome c release from mitochondria and the inclusion of the involvement of the mitochondria-dependent pathway may elucidate the enhancement of TRAIL-induced cytotoxicity by hyperthermia. It is believed that the model presented in this article provides a framework for future studies.
Acknowledgements
This work was supported by the following grants: NCI grants CA95191, CA96989 and CA121395, DOD prostate program fund (PC020530 and PC040833) and Susan G. Komen Breast Cancer Foundation (BCTR60306).
References
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12687–12690
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 1997; 7: 813–820
- Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 1997; 186: 1165–1170
- Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, et al. Identification of a ligand for the death-domain-containing receptor Apo3. Curr Biol 1997; 7: 1003–1006
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997; 277: 815–818
- Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science 1997; 276: 111–113
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997; 277: 818–821
- Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 1997; 16: 5386–5397
- Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo MJ, Chan FK. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci USA 2005; 102: 18099–18104
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999; 11: 255–260
- Gura T. How TRAIL kills cancer cells, but not normal cells. Science 1997; 277: 768
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res 1999; 59: 734–741
- Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol 1998; 161: 2833–2840
- Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science 1995; 268: 411–415
- Wen J, Ramadevi N, Nguyen D, Perkins C, Worthington E, Bhalla K. Antileukemic drugs increase death receptor 5 levels and enhance Apo-2L-induced apoptosis of human acute leukemia cells. Blood 2000; 96: 3900–3906
- Vassilev A, Ozer Z, Navara C, Mahajan S, Uckun FM. Bruton's tyrosine kinase as an inhibitor of the Fas/CD95 death-inducing signaling complex. J Biol Chem 1999; 274: 1646–1656
- Tschopp J, Martinon F, Hofmann K. Apoptosis: silencing the death receptors. Curr Biol 1999; 9: R381–R384
- Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, Francke U, Reed JC, Kinoshita S, Nolan GP. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity 1998; 8: 461–471
- Kothny-Wilkes G, Kulms D, Luger TA, Kubin M, Schwarz T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand- and CD95-induced apoptosis but not from ultraviolet radiation-induced apoptosis. J Biol Chem 1999; 274: 28916–28921
- Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev 1999; 13: 239–252
- Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 1998; 58: 5315–5320
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155–162
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Med 1999; 5: 157–163
- French LE, Tschopp J. The TRAIL to selective tumor death. Nature Med 1999; 5: 146–147
- Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br J Cancer 2003; 89: 206–214
- Tillman DM, Izeradjene K, Szucs KS, Douglas L, Houghton JA. Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res 2003; 63: 5118–5125
- Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res 2000; 60: 847–853
- Lee YJ, Lee KH, Kim HR, Jessup JM, Seol DW, Kim TH, Billiar TR, Song YK. Sodium nitroprusside enhances TRAIL-induced apoptosis via a mitochondria-dependent pathway in human colorectal carcinoma CX-1 cells. Oncogene 2001; 20: 1476–1485
- Fulda S, Jeremias I, Debatin KM. Cooperation of betulinic acid and TRAIL to induce apoptosis in tumor cells. Oncogene 2004; 23: 7611–7620
- Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA 2000; 97: 1754–1759
- Park SY, Billiar TR, Seol DW. IFN-gamma inhibition of TRAIL-induced IAP-2 upregulation, a possible mechanism of IFN-gamma-enhanced TRAIL-induced apoptosis. Biochem Biophys Res Comm 2002; 291: 233–236
- Nyormoi O, Mills L, Bar-Eli M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Diff 2003; 10: 558–569
- Lee YJ, Song JJ, Kim JH, Kim HR, Song YK. Low extracellular pH augments TRAIL-induced apoptotic death through the mitochondria-mediated caspase signal transduction pathway. Exp Cell Res 2004; 293: 129–143
- Nam SY, Amoscato AA, Lee YJ. Low glucose-enhanced TRAIL cytotoxicity is mediated through the ceramide-Akt-FLIP pathway. Oncogene 2002; 21: 337–346
- Lee YJ, Froelich CJ, Fujita N, Tsuruo T, Kim JH. Reconstitution of caspase-3 confers low glucose-enhanced tumor necrosis factor-related apoptosis-inducing ligand cytotoxicity and Akt cleavage. Clin Cancer Res 2004; 10: 1894–1900
- Lee YJ, Moon MS, Kwon SJ, Rhee JG. Hypoxia and low glucose differentially augments TRAIL-induced apoptotic death. Mol Cell Biochem 2005; 270: 89–97
- Oleson JR, Calderwood SK, Coughlin CT, Dewhirst MW, Gerweck LE, Gibbs FA, Kapp DS. Biological and clinical aspects of hyperthermia in cancer therapy. Am J Clin Oncol 1988; 11: 368–380
- Riehemann K, Schmitt O, Ehlers EM. The effects of thermochemotherapy using cyclophosphamide plus hyperthermia on the malignant pleural mesothelioma in vivo. Ann Anat 2005; 187: 215–223
- Ohguri T, Imada H, Kato F, Yahara K, Morioka T, Nakano K, Korogi Y. Radiotherapy with 8 MHz radiofrequency-capacitive regional hyperthermia for pain relief of unresectable and recurrent colorectal cancer. Int J Hyperthermia 2006; 22: 1–14
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomized trial, Dutch Deep Hyperthermia Group. Lancet 2000; 356: 771–772
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomize trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Issels RD, Prenninger SW, Nagele A, Boehm E, Sauer H, Jauch KW, Denecke H, Berger H, Peter K, Wilmanns W. Ifosfamide plus etoposide combined with regional hyperthermia in patients with locally advanced sarcomas: A phase II study. J Clin Oncol 1990; 8: 1818–1829
- Wendtner CM, Abdel-Rahman S, Krych M, Baumert J, Lindner LH, Baur A, Hiddemann W, Issels RD. Response to neoadjuvant chemotherapy combined with regional hyperthermia predicts long term survival for adult patients with retroperitoneal and visceral high-risk soft tissue sarcomas. J Clin Oncol 2002; 20: 3156–3164
- Kapp DS, Fressenden P, Samulski TV, Bagshaw MA, Cox RS, Lee ER, Lohrbach AW, Meyer JL, Prionas SD. Stanford University Institutional report. Phase I evalulation of hyperthermia equipment for hyperthermic treatment of cancer. Int J Hyperthermia 1988; 4: 75–116
- Sapozink MD, Gibbs FA, Gibbs P, Stewart JR. Phase I evaluation of hyperthermia equipment: University of Utah Institutional report. Int J Hyperthermia 1988; 4: 117–132
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Burow ME, Weldon CB, Tang Y, Navar GL, Krajewski S, Reed JC, Hammond TG, Clejan S, Beckman BS. Differences in susceptibility to tumor necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Res 1998; 58: 4940–4946
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol 2006; 84: 87–98
- Ryu S, Brown SL, Kim SH, Khil MS, Kim JH. Preferential radiosensitization of human prostatic carcinoma cells by mild hyperthermia. Int J Radiat Oncol Biol Phys 1996; 34: 133–138
- Raaphorst GP, Miao J, Ng CE. Cisplatin and mild hyperthermia in radiosensitization to low dose rate irradiation in human ovarian carcinoma cells. Anticancer Res 1997; 17: 3469–3472
- Atallah D, Marsaud V, Radanyi C, Kornprobst M, Rouzier R, Elias D, Renoir JM. Thermal enhancement of oxaliplatin-induced inhibition of cell proliferation and cell cycle progression in human carcinoma cell lines. Int J Hyperthermia 2004; 20: 405–419
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–767
- Srinivasan JM, Fajardo LF, Hahn GM. Mechanism of antitumor activity of tumor necrosis factor alpha with hyperthermia in a tumor necrosis factor alpha-resistant tumor. J Natl Cancer Inst 1990; 82: 1904–1910
- Klostergaard J, Leroux E, Siddik ZH, Khodadadian M, Tomasovic SP. Enhanced sensitivity of human colon tumor cell lines in vitro in response to thermochemoimmunotherapy. Cancer Res 1992; 52: 5271–5277
- Lee YJ, Hou Z, Curetty L, Cho JM, Corry PM. Synergistic effects of cytokine and hyperthermia on cytotoxicity in HT-29 cells are not mediated by alteration of induced protein levels. J Cell Physiol 1993; 155: 27–35
- Ozoren N, El-Deiry W. Heat shock protects HCT116 and H460 cells from TRAIL-induced apoptosis. Exp Cell Res 2002; 281: 175–181
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol 2000; 2: 469–475
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2000; 2: 476–483
- Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J 2000; 19: 4310–4322
- Ran R, Zhou G, Lu A, Zhang L, Tang Y, Rigby AC, Sharp FR. Hsp70 mutant proteins modulate additional apoptotic pathways and improve cell survival. Cell Stress Chap 2004; 9: 229–242
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3: 839–843
- Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res 1998; 58: 1593–1598
- Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 1998; 273: 7770–7775
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997; 275: 1129–1132
- Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J Biol Chem 1997; 272: 30299–30305