Abstract
In cancer immunotherapies combined with hyperthermia, one or two cytokines have been tested to augment the anti-tumor effect. However, the therapies have not shown sufficient improvement. The aim of this study is to find a new potent tumor immunotherapy in order to augment antitumor effect of hyperthermia by the cytokine cocktails in vivo. We used a combination therapy of local hyperthermia (LH) and various cytokine cocktails composed of IFNs (IFN-α, -β, and -γ), Th1 cytokines (IL-2, -12, -15, and -18), a Th2 cytokine (IL-4), inflammatory cytokines (IL-1α and TNF-α), and dendritic cell-inducible cytokines (IL-3 and GM-CSF). These cytokines in a proper combination augmented the anti-tumor effect of LH and prolonged survival time in Lewis lung carcinoma or B16 melanoma significantly. Moreover, the 12-cytokine cocktail suppressed B16 metastasis to the lung and lymph nodes, and complete regression of the tumors without regrowth occurred in 3 of 5 mice. In the cured three B16 mice, there was hyperplasia of lymphatic organs with many CD3-positive T lymphocytes. The most effective cytokine combination should be able to augment the anti-tumor effect of other therapies besides hyperthermia that induce the necrosis of tumor cells.
Introduction
Many cancer immunotherapies, including cancer vaccines, dendritic cell (DC) therapy, and gene therapy with cytokine or apoptosis-related genes, have been tested in clinical trials Citation[1], but it is very difficult to break the barrier created by an immunosuppressed state or to overcome an abnormal cytokine network in cancer patients Citation[2]. An improvement in the anti-tumor effect of immunotherapies may require their being used in combination with other therapy that can induce tumor-cell necrosis, such as hyperthermia or radiation. After necrosis is induced in tumor cells, immunotherapies can significantly improve the impact of the treatment through the increased activation of DCs and the subsequent effective induction of killer lymphocytes such as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells Citation[1].
The anti-tumor effect of hyperthermia is due to the direct killing of tumor cells by heating and by the augmentation of the host immune response Citation[3]. Cytokines such as interleukin (IL)- Citation[4], Citation[5], IL-1α, Citation[6] tumor necrosis factor-alpha (TNF-α) Citation[6], IFN-β Citation[7] and granulocyte-macrophage colony-stimulating factor (GM-CSF) Citation[5] have been combined with hyperthermia to reinforce its anti-tumor effect by activating the host immune system. Unfortunately, these combinations have been ineffective in clinical trials.
The normal functions of cytokines, which have been elucidated in studies of cultured cells, animals, and healthy subjects, may not be exerted in the tumor-bearing immunosuppressed state Citation[2]. Activated T lymphocytes release IL-2; however, IL-2 induces CTLA-4, which suppresses lymphocyte function Citation[8]. IFN-γ released from T helper type 1 (Th1) lymphocytes suppresses differentiation and increases the number of T helper type 2 (Th2) lymphocytes Citation[9]. Conversely, IL-4 and -10 released from Th2 lymphocytes suppress differentiation of Th1 lymphocytes Citation[10]. Treatment with only one or two cytokines may not achieve a significant anti-tumor effect through suppressive and regulative mechanisms. Moreover, activation of not only Th1 lymphocytes or CTLs but also Th2 lymphocytes, DCs, or inflammation-inducible systems may be needed to overcome severe immune suppression Citation[2]. Therefore, we have tried to suppress tumor growth in mice using combination therapies with LH and cytokine cocktails composed of 3, 4, or 12 cytokines that were intended to cause a cytokine storm.
Materials and methods
Mice and Cells
Male C57BL /6J mice, 6–8 weeks old, were purchased from Crea Japan Inc., Tokyo. The mice were housed in plastic cages, 5–6 per cage, and provided with water and a standard laboratory diet under SPF conditions.
B16-BL6 melanoma cells were kindly provided by Isaiah J. Fidler, MD Anderson Cancer Center, the University of Texas, USA and Lewis lung carcinoma (LLC) cells were purchased from Riken Cell Bank, Japan. The culture medium was DEME (Cat. No. 11885-092, Gibco BRL, Rockville, MD) supplemented with 7.5% FBS, l-glutamate, and sodium bicarbonate for the B16-BL6 cells and DEME (Nissui-2, Nissui Inc., Tokyo) supplemented with 10% FBS, l-glutamate, and sodium bicarbonate for the LLC cells. Mice were inoculated with 1 × 106 viable B16-BL6 or LLC cells by s.c. injection into the dorsal surface of the right hind foot pad.
Cytokines
IL-1α(>5 × 108 units/mg), IL-3 (>2 × 107 units/mg), IL-4 (>1 × 107 units/mg), IL-12 (>1 × 107 units/mg), IL-15 (>2 × 106 units/mg), IFN-γ (>1 × 107 units/mg), TNF-α (>1 × 107 units/mg), and GM-CSF (>5 × 106 units/mg) were purchased from Pepro Tech EC, Ltd, London, UK. IL-2 (1.1 × 107 JRU/mg) and IL-18 (20 ng/ml:682.7 IU/ml, 40 ng/ml:956.2 IU/ml) were purchased from Shionogi Co., Ltd., Osaka, and MBL Co., Ltd., Nagoya, respectively. IFN-α (1.08 × 108units/mg) and -β (2.42 × 107 units/mg) were purchased from R&D Systems, Inc., Minneapolis, MN. The numerical values in the parentheses are the biological activity.
Therapy with LH and cytokine cocktails in B16-BL6-bearing mice
Mice bearing B16-BL6 cells were assigned to 4 groups and treated with PBS (the control group), LH, 12 cytokines, which included IFNs (IFN-α Citation[11], Citation[12], -β, Citation[11], Citation[12] -γ Citation[12], Citation[13]), Th1 cytokines (IL- Citation[9], Citation[12], -12 Citation[9], -15 Citation[14], -18 Citation[15]), a Th2 cytokine (IL-4) Citation[9], Citation[12], inflammatory cytokines (IL-1α, TN F-α)Citation[6], Citation[13], Citation[16], and DC-inducible cytokines (IL-3, GM-CSF) Citation[17], or LH plus the 12-cytokines.
The therapy was performed over the period from 11 to 20 days after tumor inoculation. Mice were anesthetized with an i.p. injection of 0.2 ml pentobarbital. LH was performed by warming both hind limbs up to the knee joints in circulating hot water (43.0°C ± 0.2°C), warmed by a Thermominder SX-10R, Taitec Inc., Tokyo, for 120 min on days 12 and 19. The intratumoral temperature was measured by a thermocouple probe MT-29, Physitemp, Instruments, Inc., New Jersey. It reached the same as one of water within 5 min after the tumor was sunk in the water when the tumor size was less than 10 mm. The cytokines were diluted in 0.05 ml PBS and injected s.c. into the hind limbs. The dosages of the cytokines were referred to the minimum dosage required to induce an anti-tumor effect or an immune response by individual cytokines in mice. However, we admixed 12 kinds of cytokine, their concentrations were decided ad hoc, in general, lower than the minimum dosage. The dosages were IL-1α, 5 × 104 IU/0.1 µg (0.5 ∼ 10 µg) Citation[6], Citation[18]; IL-2, 3500 IU/0.32 µg (2000 ∼ 6 × 105 IU, 0.1 ∼ 108 µg) Citation[4], Citation[14], Citation[19], Citation[20]; IL-3, 1000 IU/0.1 µg (1 × 104 ∼ 1 × 105 IU, 0.2 ∼ 1 µg) Citation[21–23]; IL-4, 1000 IU/0.1 µg (1000 IU, 0.05 ∼ 4 µg) Citation[24–26]; IL-12, 1000 IU/0.1 µg (0.08 ∼ 1 µg) Citation[27–29]; IL-15, 500 IU/0.25 µg (0.4 ∼ 108 µg) Citation[14], Citation[29]; L-18, 227 IU/0.25 µg (0.2 ∼ 1 µg) Citation[15], Citation[28]; IFN-α, 2500 IU/0.023 µg (2500 ∼ 1 × 105 IU)Citation[11], Citation[24], Citation[30]; IFN-β, 2500 IU/0.1 µg (2 × 104 ∼ 5 × 105 IU) Citation[7], Citation[24], Citation[31]; IFN-γ, 1 × 104 IU/1 µg (1 × 104 ∼ 1 × 105 IU)Citation[11], Citation[24], Citation[32]; TNF-α, 2000 IU/0.2 µg (2000 ∼ 1 × 105 IU, 1 ∼ 5 µg) Citation[6], Citation[32–34]; GM-CSF, 2500 IU/0.5 µg (3000 ∼ 1 × 104 IU, 0.1 ∼ 4 µg) Citation[5], Citation[20], Citation[24], Citation[35]. IL-1α, -3, -4, -12, -18, IFN-γ, TNF-α, and GM-CSF were administered every day on days 11-20. IL-2, -15, IFN-α, -β, and GM-CSF were administered on days 11, 14, 17, and 20. The tumor volume was calculated as V = 0.5 (ab2), where a and b were two perpendicular diameters, and b was the smaller one. The numerical values in the parentheses are the dosages used in the past experiments.
Therapy with LH and cytokine cocktails in LLC-bearing mice
To clarify the anti-tumor effect of the cytokine cocktail in B16-BL6 bearing mice, several subdivided combination of the 12 cytokines have been carried out in this experiment. And in order to decrease the adverse effect of the cytokines and LH, the interval of cytokine injection has been lengthened, and the temperature and time of hyperthermia have been changed slightly.
Mice bearing LLC cells were assigned to 6 groups, treated with (1) PBS, (2) LH, (3) the 12 cytokine cocktail, (4) LH plus the IFNs, (5) LH plus the Th1 cytokines, and (6) LH plus the 12 cytokine cocktail.
The therapy was performed over the period from 9 to 30 days after the inoculation with tumor cells. LH was performed as described above for the B16-BL6 experiments, but at 43.5°C ± 0.2°C for 90 min on days 10, 17 and 24. The method of administration and the dosage of each cytokine were the same as for the B16-BL6 experiments. IL-1α, -3, -4, -12, -18, IFN-γ, TNFF-α, and GM-CSF were administered on days 9, 11, 14, 16, 18, 21, 23, 25, 28, and 30. IL-2/15, IFN-α and -β and GM-CSF were administered on days 9, 16, 23, and 30. The tumor volume was calculated as V = 0.5 (ab2), where a and b were two perpendicular diameters, and b was the smaller one.
Immunohistological analysis
Tissues were fixed with Bouin's solution (Sigma-Aldrich Japan, Tokyo) and embedded in paraffin. Two micrometer-thick paraffin sections were cut and deparaffinized with xylene and ethanol. The sections were stained with hematoxylin and eosin for histological analysis. The lung metastasis of B16-BL6 cells could be found macroscopically as black nodules in yellowish lung fixed with Bouin's solution. The nodules with the size less than about 0.1 mm, we have confirmed the metastasis by microscopic observation.
Immunostaining was performed using the Catalyzed Signal Amplification system (CSA system; DAKO Japan Co., Kyoto, Japan). The deparaffinized sections were boiled in 10 mM citric acid buffer in a 500-W microwave oven for 10 min. They were then washed in phosphate buffered saline (PBS pH 7.6), the endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min, and they were washed again in PBS. To block nonspecific sites, the sections were incubated with Blocking solution (DAKO Japan Co., Kyoto, Japan) in PBS for 15 min. The sections were then incubated with a rat anti-human CD3 monoclonal antibody with cross-reactivity to mouse CD3 (Serotec, Oxford, UK) at 10 µg/ml for 1 h at room temperature. Negative control staining was performed with PBS or isotype-matched (IgG1) immunoglobulins. After being washed with PBS, the sections were reacted with biotinylated sheep anti-rat IgG at 10 µg/ml for 30 min at room temperature. The slides were washed in PBS and then incubated with the amplification and streptavidin solutions, according to the CSA system. The reaction cascade was visualized with 3,3′-diaminobenzidine tetrahydrochloride (DAB) Chromogen solution, resulting in brown staining. The sections were counterstained with hematoxylin and coverslipped.
Results
Anti-tumor effect in B16-BL6-bearing mice
In the B16 experiment, the average tumor size on day 25, the last day that more than half the mice were still surviving in all groups, was 1484 mm3 in the control group, 1032 mm3 in the LH group, 400 mm3 in the 12-cytokine group, and 7 mm3 in the LH plus 12-cytokine group. It was smaller in the LH plus 12-cytokine group than in the LH or control groups (P < 0.05 by two-tailed t-test) (). In the LH plus 12-cytokine group, complete regression of the tumors without regrowth occurred in 3 of 5 mice, and was maintained until they were sacrificed on day 245. In the remaining 2 mice, the tumor completely regressed temporarily in one and gradually regressed in the other.
Figure 1. Anti-tumor effects of combination therapies using LH and cytokine cocktails in B16-BL6-bearing mice. (a) Growth curves of B16 tumors on the foot-pad in each group. The 4 treatment groups are described in the text. The average tumor size on day 25 was lower in the LH plus 12 cytokines group than in the LH or PBS groups (P < 0.05 by two-tailed t-test). (b) Survival curve, P < 0.05 for LH plus 12 cytokines vs. PBS or 12 cytokines (log rank test).
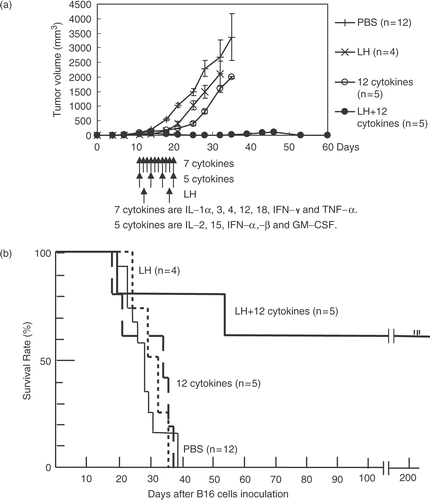
The average number of lung metastases was 23.6 in the control group, 7.5 in the LH group, 3 in the 12-cytokine group, and 0 in the LH plus 12-cytokine group. The frequency of metastasis was significantly lower in the LH plus 12-cytokine group than in the control or LH group (P < 0.05 by Fisher's exact probability test). The respective frequency of metastasis to the popliteal and inguinal lymph nodes in each group was 92% (11/12) and 92% (11/12) for the control group, 100% (4/4) and 50% (2/4) for the LH group, 60% (3/5) and 40% (2/5) for the 12-cytokine group, and 20% (1/5) and 0% (0/5) for the LH plus 12-cytokine group. This difference was significant for the LH plus 12-cytokine group compared with the control group (P < 0.01 by Fisher's exact probability test). The survival rate was highest in the LH plus 12-cytokine group (P < 0.05 by log-rank test) ().
Anti-tumor effect in LLC-bearing mice
In the LLC experiment, the average tumor size on day 21 after tumor inoculation, when all the mice still survived, was lower in the LH plus Th1 cytokines (560 mm3) and the LH plus 12-cytokine cocktail (255 mm3) groups than in the control group (2162 mm3) (P < 0.01 by two-tailed t-test) (). The average tumor size on day 31, the day after treatment ended, was lower in the LH plus 12-cytokine group (531 mm3) than in 3 groups, i.e., the control (3650 mm3), the LH (3375 mm3), and the LH plus Th1 cytokines (2330 mm3) groups (P < 0.05 by two-tailed t-test) (). In the LH plus 12-cytokine group, the tumor temporarily regressed in 2 of the 5 mice.
Figure 2. Anti-tumor effects of combination therapies using local hyperthermia (LH) and cytokine cocktails in Lewis lung carcinoma (LLC)-bearing mice. (a) Growth curves of the LLC tumors on the foot-pad in each group. The 6 treatment groups are described in the text. The average tumor size on day 21 was lower in the LH plus Th1 cytokines and LH plus 12 cytokines groups than in the control group (P < 0.01 by two-tailed t-test). (b) Survival curve. P < 0.05 in LH plus Th1 cytokines vs. PBS, and in LH plus 12 cytokines vs. 12 cytokines, P < 0.01 in LH plus 12 cytokines vs. PBS or LH (log rank test). The LH plus IFNs group was excluded for the reason described in the text.
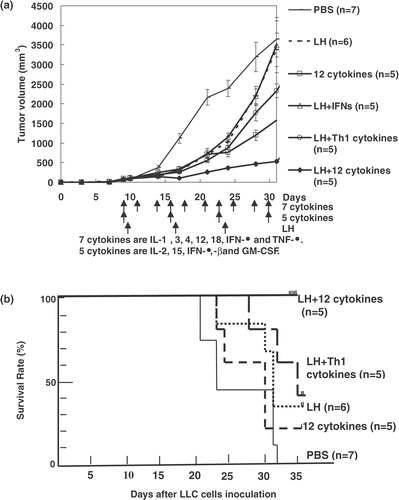
The survival rate was higher in the LH plus Th1 cytokine group than in the control group (P < 0.05 by log-rank test) (). The LH plus 12-cytokine group showed the highest survival rate and was the sole group with 100% survival on day 35 (P < 0.05 by log-rank test) (). The LH plus IFNs group was excluded from the statistical analysis after day 24, because two mice in the group died unexpectedly during the 3rd LH treatment, which was performed on that day (probably from bleeding from the tumor).
Histological analyses
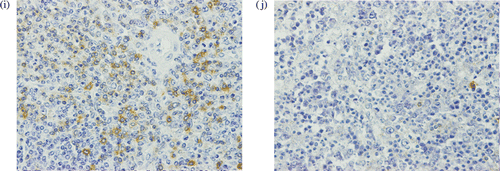
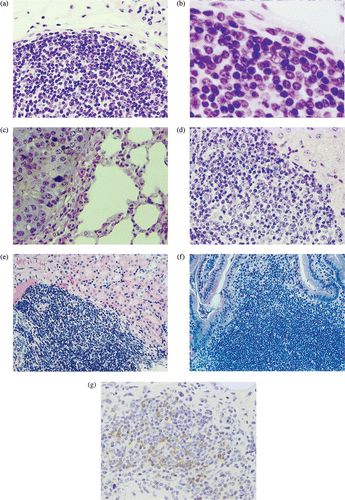
In the three B16 mice cured by the combination therapy of LH plus 12 cytokines, there was hyperplasia of lymphatic organs, including the spleen, lymph nodes, and thymus (, as compared with those of mice treated with LH alone (). The immunological staining showed many CD3-positive T lymphocytes in the hyperplastic areas of the lymphatic organs such as spleen () compared with few one in the LH group (). Several colonies composed of mononuclear cells but without a tumor nest were seen in the lung of the cured B16 mice (). On the other hand, B16 metastases with melanin pigment were shown in the lung of the LH group (). A few colonies were also seen in the liver, kidney, and small intestine in the cured B16 mice (). The colonies included many CD3-positive T lymphocytes (). In the LLC mice treated with the combination therapy of LH plus 12 cytokines, there was little hyperplasia of the lymphatic organs. Several colonies composed of mononuclear cells including CD3-positive T lymphocytes were seen in the lung only; however, the colonies were smaller than those found in the cured B16 mice.
Figure 3. (a)–(h) Hematoxylin and eosin histochemical staining. Spleen (a, b), Lymph nodes (c), and thymus (d) in a B16 mouse cured by the LH plus 12-cytokine treatment or those (e, f, g, h) from the LH group, respectively. (i, j) Immunohistological staining with anti-CD3 rat monoclonal antibody of spleen in the LH plus 12-cytokine group (i) and one in the LH group (j). (original magnification, a, c–j, 400×, b, f, 1000×).
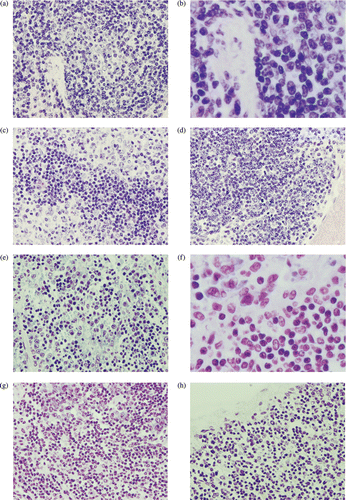
Figure 4. (a)–(f) Hematoxylin and eosin histochemical staining. (a, b) Lung of a B16 mouse cured by the LH plus 12-cytokine treatment. (c) Lung from the LH group. B16 metastasis with melanin pigment is shown. Liver (d), kidney (e) and small intestine (f) from the LH plus 12-cytokine group. (g) Immunohistological staining with anti-CD3 monoclonal antibody. Lung of a B16 mouse cured by the LH plus 12-cytokine treatment. (original magnification a, c, d, e, f, g, 400×, b, 1000×).
Discussion
The LH treatment was administered after injection of the cytokine cocktails in both B16 and LLC experiments. The rationale for this was referred to the experiment indicating that the administration of IFN-γ before hyperthermia showed synergistic interactions of the effect of the IFN-γ treatment and hyperthermia in the B16-bearing mice, although, when IFN-γ was administered in the middle or following hyperthermia, the life span were essentially the same as for IFN-γ treatment alone Citation[36]. Activation of immune cells such as DC and lymphocytes before hyperthermia might have stimulated efficient recognition of tumor-associated antigens expressed on the LH-treated necrotic tumor cells and subsequently induced strong anti-tumor immunity.
In the LLC experiments, the LH alone failed to suppress tumor growth or to raise the survival rate, compared with the control. The combination of Th1 cytokines and LH significantly suppressed tumor growth and raised the survival rate (). IL-2 Citation[9], Citation[10], Citation[12] IL- Citation[9], Citation[10], and IL-15 Citation[14] might enhance anti-tumor effect of LH due to induction of CTLs or lymphokine-activated killer cells (LAKs), and activation of NK cells. IL-12 might induce a shift in Th1/ Th2 balance to Th1 cells, which are thought to be important in tumor immunity Citation[9], Citation[12]. IL-12 and IL-18 might release IFN-γ from DCs and T lymphocytes by a different pathway and augment anti-tumor effect in the combination Citation[15]. The combination of LH plus the IFNs did not show a significant anti-tumor effect (). However, IFN-γ was administered twice a week, and IFN-α and -β were administered only every other week in this experiment. The administration of IFN-α and -β at more frequent intervals might induce a stronger anti-tumor effect. Because the signal pathway downstream of the cell membrane receptor is the same for IFN-α and -β Citation[37] one of these molecules may be sufficient to induce an anti-tumor effect in the LH plus cytokine-cocktail therapy.
Anti-tumor effect of LH plus IFNs was not effective. Anti-tumor effect of LH plus Th1 cytokines was significant compared with that of LH, but it was slightly better. Our preliminary experimental data showed that LH plus a 7 cytokine cocktail composed of Th1 cytokines plus IFNs revealed a suppressive tendency of the tumor growth (data not shown), we have tried to use a 12 cytokine cocktail, since the ad-mixed cocktail alone has shown considerably strong inhibition of LLC tumor growth (). The addition of the IFNs, Th2, inflammatory, and DC-inducible cytokines plus Th1 cytokines showed the most potent anti-tumor effect in combination with hyperthermia. Compared with LH alone, the LH plus 12-cytokine cocktail therapy significantly suppressed the tumor growth of B16 and LLC cells ( and ). Moreover, this combination also suppressed B16 metastasis to the lung and lymph nodes efficiently. Finally, the survival rate was most prolonged in mice receiving LH plus the 12-cytokine cocktail in both the B16 and LLC experiments ( and ). In particular, in the B16 experiments, three mice became tumor-free. IFNs might enhance anti-tumor effect due to activate NKs and macrophages Citation[9], Citation[12] or to augment the expression of MHC class I and II subsequent to increase the expression of tumor-related antigens Citation[11], Citation[13]. IFN-γ might be useful to the production of CTLs Citation[9], Citation[10], Citation[12]. IL-4 induces a shift in Th1/Th2 balance to Th2 cells and increases and activates B lymphocytes Citation[10]. IL-1 also increases the production of Th2 and B lymphocytes Citation[16], and showed function of a systemic adjuvant in a murine tumor model Citation[38]. In the combination of the Th1 cytokines, IL-4 and IL-1 might be useful to regulate the proper balance of Th1/Th2, which should be necessary to augment the anti-tumor effect Citation[8–10]. TNF-α might augment anti-tumor effect owing to induce NK- and lymphocytes-mediated cytotoxicity Citation[13]. IL-3 and GM-CSF might induce the differentiation of DCs Citation[17], which are important for presenting tumor-associated antigens and for inducing the subsequent tumor-specific immune response by CTLs Citation[1]. The result that the most potent anti-tumor effect was caused by the combination of the twelve cytokines may suggest that the entire immune system, including Th1, Th2, B lymphocytes, DCs, and macrophages are suppressed in the tumor-bearing state. It may be possible to overcome the various tumor-induced immunosuppressive mechanisms Citation[2] by the simultaneous administration of many cytokines with different functions. Other studies also suggest that many kinds of cytokines relate to tumor suppression and development in the LLC- or B16-BL6-bearing mic Citation[39], Citation[40]. The cytokine network is very complicated and different in various kinds of tumor and the progressive stages Citation[41], therefore the most effective cytokine combination may be different in an individual tumor.
After the treatment ended, the three cured B16 mice did not show any abnormal clinical features during the observation period, which lasted 245 days after their inoculation with the tumor cells. The findings of hyperplasia with CD3-positive T lymphocytes in lymphatic organs () and a few colonies with T lymphocytes in the liver, kidney, and small intestine () suggest that some immune response was induced by the LH plus 12-cytokine cocktail. Immunohistological analysis for the kind of the mononuclear cells other than the CD-3 positive cells in the tissues and the functional analyses of the cells for specific or non-specific anti-tumor response are needed. On the other hand, the presence in the lung of several colonies of mononuclear cells with T lymphocytes and the thickening of alveoli may suggest interstitial pneumonia (). Some autoimmune diseases have been induced by other cytokine therapies Citation[42]; therefore, careful selection of patients and a long follow-up are required for cytokine therapy.
The anti-tumor effect of the LH plus 12-cytokine cocktail therapy was stronger in the B16 than the LLC experiment. In the B16 experiment, the primary tumor completely regressed in three of the 5 mice, and there were no lung metastases in any of them. In contrast, in the LLC experiment, the primary tumor did not regress completely. The hyperplasia in the lymphatic organs and the induction of T lymphocyte colonies in the lung were stronger in the B16 than the LLC experiment. For these reasons, the LLC cells grew faster than the B16-BL6 cells in the control group, and the intervals between injections of cytokines were longer for the LLC than the B16 experiment. Taking together the results of the LLC and the B16 experiments, the 12-cytokine therapy may need to be given more than two times a week to obtain a good anti-tumor effect.
The 12 cytokine cocktail seems to be an approach from the non-realistic point of view, however it showed the potent anti-tumor effect. We have found it very hard to overcome the various immunosuppressive mechanisms in the tumor-bearing state Citation[2]. On the other hand, there are many reports that spontaneous regression of cancer have occurred following incomplete surgical removal of the tumor; next in order of frequency during some acute febrile response by bacterial or viral infection such as streptococcal pyogenes, the causative bacteria of erysipelas Citation[43]. In those cases, the cytokine storm induced by infection might lead the tumor reduction due to induction of potent anti-tumor immune response. The authors think that the cytokine cocktail used in this study can not be put into clinical application directly. However, the anti-tumor effect by the cytokine cocktail composed of various function with not only Th1 but also Th2 should be a valuable hint regarding an adjuvant therapy of LH. Th1/Th2 imbalance which are directed towards extended Th2 responses coincides with tumor progression Citation[44]. On the other hand, in some tumor-bearing mice, a shift from a Th1 to a Th2 phenotype is not clear Citation[45]. Using both Th1 and Th2 cytokines may suppress Th1- or Th2-excess activation of each other and help regulation of the Th1/Th2 balance properly in vivo Citation[8–10].
The detailed analyses including dosages, interval of administration and combination of the cytokines are needed. In regard to the cost, IFN-α or β have been administered daily for 2 weeks, subsequent to every other day for 22 ∼ 46 weeks into the Hepatitis B or C virus carrier patients. If the 12 cytokine will administer for 2 weeks into cancer patients, the cost will be possibly the same as one in the interferon therapy, since it may be possible to decrease the dosage of an individual cytokine widely less than minimum dosage with anti-tumor effect of the individual cytokine. The most effective cytokine combinations will be able to augment the anti-tumor effect of other therapies besides hyperthermia that induce the necrosis of tumor cells, such as radiation Citation[46], embolization of tumor vessels Citation[47], antiangiogenic drugs Citation[48], molecular-targeting drugs Citation[49], and chemotherapy Citation[50].
Conclusion
The cytokine cocktail composed of IFNs (IFN-α, -β, and -γ), Th1 cytokines (IL-2, -12, -15, and -18), a Th2 cytokine (IL-4), inflammatory cytokines (IL-1α and TNF-α), and dendritic cell-inducible cytokines (IL-3 and GM-CSF) augmented the anti-tumor effect of LH and prolonged survival time in Lewis lung carcinoma or B16 melanoma significantly. Moreover, the cytokine cocktail suppressed B16 metastasis to the lung and lymph nodes, and complete regression of the tumors without regrowth occurred in 3 of 5 mice. In the cured three B16 mice, there was hyperplasia of lymphatic organs with many CD3-positive T lymphocytes.
Acknowledgements
The authors wish to thank Dr. Isaiah J. Fidler for kindly providing the B16-BL6 cells, the Laboratory Animal Resource Center of the University of Tsukuba for help in bleeding the mice. This work was supported in part by the University of Tsukuba and by The Institute for Adult Diseases Asahi Life Foundation.
References
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–915
- Chouaib S, Asselin-Paturel C, Mami-Chouaib F, Caignard A, Blay JY. The host-tumor immune conflict: from immunosuppression to resistance and destruction. Immunol Today 1997; 18: 493–497
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44: 4721s–4730s
- Geehan DM, Fabian DF, Lefor AT. Combined local hyperthermia and immunotherapy treatment of an experimental subcutaneous murine melanoma. J Surg Oncol 1995; 59: 35–39
- Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, Saida T, Kobayashi T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci 2003; 94: 308–313
- Lin JC, Park HJ, Song CW. Combined treatment of IL-1α and TNF-α potentiates the antitumour effect of hyperthermia. Int J Hyperthermia 1996; 12: 335–344
- Nakayama J, Kokuba H, Kobayashi J, Yoshida Y, Hori Y. Experimental approaches for the treatment of murine B16 melanomas of various sizes. II: Injection of ethanol with combinations of β-interferon and microwaval hyperthermia for B16 melanomas with a size of greater than 10 mm in diameter. J Dermatol Sci 1997; 15: 82–88
- Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA 2002; 99: 8832–8837
- Trinchieri G. Interleukin-12: A cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 1994; 84: 4008–4027
- Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: Reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med 1994; 180: 1715–1728
- Halloran PF, Urmson J, van der Meide PH, Autenried P. Regulation of MHC expression in vivo. II. IFN-α/β inducers and recombinant IFN-α modulate MHC antigen expression in mouse tissues. J Immunol 1989; 142: 4241–4247
- Biron CA. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol 1994; 6: 530–538
- Krakauer T, Oppenheim JJ. IL-1 and tumor necrosis factor-α each up-regulate both the expression of IFN-γ receptors and enhance IFN-γ-induced HLA-DR expression on human monocytes and a human monocytic cell line (THP-1). J Immunol 1993; 150: 1205–1211
- Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA 2004; 101: 1969–1974
- Osaki T, Peron J-M, Cai Q, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. IFN-γ-inducing factor/IL-18 administration mediates IFN-γ- and IL-12-independent antitumor effects. J Immunol 1998; 160: 1742–1749
- Wood DD, Bayne EK, Goldring MB, Gowen M, Hamerman D, Humes JL, Ihrie EJ, Lipsky PE, Staruch MJ. The four biochemically distinct species of human interleukin 1 all exhibit similar biologic activities. J Immunol 1985; 134: 895–903
- Storozynsky E, Woodward JG, Frelinger JG, Lord EM. Interleukin-3 and granulocyte-macrophage colony-stimulating factor enhance the generation and function of dendritic cells. Immunology 1999; 97: 138–149
- Brunda MJ, Wright RB, Luistro L, Harbison ML, Anderson TD, Mclntyre KW. Enhanced antitumor efficacy in mice by combination treatment with interleukin-1α and interferon-α. J Immunother Emphasis Tumor Immunol 1994; 15: 233–241
- Harada M, Tamada K, Abe K, Li T, Onoe Y, Tada H, Takenoyama M, Yasumoto K, Kimura G, Nomoto K. Systemic administration of interleukin-12 can restore the anti-tumor potential of B16 melanoma-draining lymph node cells impaired at a late tumor-bearing state. Int J Cancer 1998; 75: 400–405
- Peng BG, Liu SQ, Kuang M, He Q, Totsuka S, Huang L, Huang J, Lu MD, Liang LJ, Leong KW, et al. Autologous fixed tumor vaccine: a formulation with cytokine-microparticles for protective immunity against recurrence of human hepatocellular carcinoma. Jpn J Cancer Res 2002; 93: 363–368
- Metcalf D, Begley CG, Johnson GR, Nicola NA, Lopez AF, Williamson DJ. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood 1986; 68: 46–57
- Abe T, Sugaya H, Ishida K, Khan WI, Tasdemir I, Yoshimura K. Intestinal protection against Strongyloides ratti and mastocytosis induced by administration of interleukin-3 in mice. Immunology 1993; 80: 116–121
- Lotem J, Sachs L. In vivo control of differentiation of myeloid leukemic cells by recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3. Blood 1988; 71: 375–382
- Ito A, Fujioka M, Tanaka K, Kobayashi T, Honda H. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J Biosci Bioeng 2005; 100: 36–42
- Gallagher G, Zaloom Y. Peritumoural IL-4 treatment induces systemic inhibition of tumour growth in experimental melanoma. Anticancer Res 1992; 12: 1019–1024
- Redmond HP, Schuchter L, Bartlett D, Kelly CJ, Shou J, Leon P, Daly JM. Anti-neoplastic effects of interleukin-4. J Surg Res 1992; 52: 406–411
- Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 1993; 178: 1223–1230
- Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, Lotze MT, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol 1999; 163: 583–589
- Lasek W, Golab J, MaSlinski W, Switaj T, Balkowiec EZ, Stoklosa T, Giermasz A, Malejczyk M, Jakobisiak M. Subtherapeutic doses of interleukin-15 augment the antitumor effect of interleukin-12 in a B16F10 melanoma model in mice. Eur Cytokine Netw 1999; 10: 345–356
- Prell RA, Li B, Lin JM, VanRoey M, Jooss K. Administration of IFN-α enhances the efficacy of a granulocyte macrophage colony stimulating factor-secreting tumor cell vaccine. Cancer Res 2005; 65: 2449–2456
- De Maeyer-Guignard J, Lauret E, Eusebe L, De Maeyer E. Accelerated tumor development interferon-treated B6.C-Hyal-1 a mice. Proc Natl Acad Sci USA 1993; 90: 5708–5712
- Saiki I, Sato K, Yoo YC, Murata J, Yoneda J, Kiso M, Hasegawa A, Azuma I. Inhibition of tumor-induced angiogenesis by the administration of recombinant interferon-γ followed by a synthetic lipid-A subunit analogue (GLA-60). Int J Cancer 1992; 51: 641–645
- Tsutsumi Y, Tsunoda S, Kaneda Y, Kamada H, Kihira T, Nakagawa S, Yamamoto Y, Horisawa Y, Mayumi T. In vivo anti-tumor efficacy of polyethylene glycol-modified tumor necrosis factor-alpha against tumor necrosis factor-α tumors. Jpn J Cancer Res 1996; 87: 1078–1085
- Lasek W, Feleszko W, Golab J, Stoklosa T, Marczak M, Dabrowska A, Malejczyk M, Jakobisiak M. Antitumor effects of the combination immunotherapy with interleukin-12 and tumor necrosis factor α in mice. Cancer Immunol Immunother 1997; 45: 100–108
- Charak BS, Agah R, Mazumder A. Granulocyte-macrophage colony-stimulating factor-induced antibody-dependent cellular cytotoxicity in bone marrow macrophages: application in bone marrow transplantation. Blood 1993; 81: 3474–3479
- Fleischmann WR, Jr, Fleischmann CM. Enhancement of MuIFN-γ antitumor effects by hyperthermia: sequence dependence and time dependence of hyperthermia. J Biol Regul Homeost Agents 1994; 8: 101–107
- Novick D, Cohen B, Rubinstein M. The human interferon α/β Receptor: Characterization and molecular cloning. Cell 1994; 77: 391–400
- McCune CS, Marquis DM. Interleukin 1 as an adjuvant for active specific immunotherapy in a murine tumor model. Cancer Res 1990; 50: 1212–1215
- Arca MJ, Krauss JC, Strome SE, Cameron MJ, Chang AE. Diverse manifestations of tumorigenicity and immunogenicity displayed by the poorly immunogenic B16-BL6 melanoma transduced with cytokine genes. Cancer Immunol Immunother 1996; 42: 237–245
- Rashid RM, Achille NJ, Lee JM, Lathers DM, Young MR. Decreased T-cell proliferation and skewed immune responses in LLC-bearing mice. J Environ Pathol Toxicol Oncol 2005; 24: 175–192
- Utsumi K, Takai Y, Tada T, Ohzeki S, Fujiwara H, Hamaoka T. Enhanced production of IL-6 in tumor-bearing mice and determination of cells responsible for its augmented production. J Immunol 1990; 145: 397–403
- Ito A, Matejuk A, Hopke C, Drought H, Dwyer J, Zamora A, Subramanian S, Vandenbark A, Offner H. Transfer of severe experimental autoimmune encephalomyelitis by IL-12- and IL-18-potentiated T cells is estrogen sensitive. J Immunol 2003; 170: 4802–4809
- O’Regan B, Hirshberg C. Spontaneous remission. An annotated bibliography. Infection Related Remission, GL Rohdenburg, HC Nauts, WE Swift, BL Coley, GA Fowler, FH Bogatko, L Pelner, J Fauvet, J Campagne, A Chavy, et al. Institute of Noetic Sciences, Sausalito, CA 1993; 577–646
- Lauerova L, Dusek L, Simickova M, Kocak I, Vagundova M, Zaloudik J, Kovarik J. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma 2002; 49: 159–166
- Hendel-Fernandez ME, Cheng X, Herbert LM, Lopez DM. Down-regulation of IL-12, not a shift from a T helper-1 to a T helper-2 phenotype, is responsible for impaired IFN-γ production in mammary tumor-bearing mice. J Immunol 1997; 158: 280–286
- Dillman RO, Shea WM, Tai DF, Mahdavi K, Barth NM, Kharkar BR, Poor MM, Church CK, DePriest C. Interferon-α2a and 13-cis-retinoic acid with radiation treatment for high-grade glioma. Neuro-oncol 2001; 3: 35–41
- Goker E, Sanli UA, Yuzer Y, Uslu R, Karabulut B, Memis A, Coker A, Mentes A. Bioembolisation for unresectable hepatocellular carcinoma: preliminary results of a translational research study. J Exp Clin Cancer Res 2004; 23: 403–409
- Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 1997; 390: 404–407
- Savage DG, Antman KH. Imatinib mesylate-a new oral targeted therapy. N Eng J Med 2002; 346: 683–693
- Robins HI, d’Oleire F, Kutz M, Bird A, Schmitt-Tiggelaar CL, Cohen JD, Spriggs DR. Cytotoxic interactions of tumor necrosis factor, melphalan and 41.8°C hyperthermia. Cancer Lett 1995; 89: 55–62