Abstract
Purpose: Hyperthermia is known to protect against cellular injury through the expression of heat shock proteins. In this study, the therapeutic effects of hyperthermia on experimental colitis in the rat were evaluated.
Materials and methods: Male Wistar rats were given a single intracolonic injection of 2,4,6-trinitrobenzene sulphonic acid (TNBS). Hyperthermia was induced in anesthetized rats by placing them in a temperature-controlled water bath. We started the hyperthermic treatment on the day after the enema. The severity of colitis was evaluated pathologically, and the activities of tissue myeloperoxidase were measured 6 days after the induction of colitis. Furthermore, cytokines, and hyperthermia-induced heat shock proteins in colonic mucosa were detected by enzyme-linked immunosorbent assay and Western blotting. We also investigated the effects of geranylgeranylacetone and zinc protoporphyrin IX on the therapeutic effect of hyperthermia.
Results: Hyperthermia significantly improved the macroscopic scores of colitis. The TNBS-induced increases in the activities of myeloperoxidase in the colonic tissue were blunted significantly in hyperthermia-treated animals. Furthermore, hyperthermia attenuated increases in cytokine-induced neutrophil chemoattractants-1 and tumor necrosis factor-α in the colon. Furthermore, hyperthermia induced the production of heat shock proteins in rat colonic mucosa, and the combination of geranylgeranylacetone with hyperthermia further induced the heat shock protein HSP70, which resulted in further improvement of TNBS-induced colitis. On the other hand, the combination of zinc protoporphyrin IX with hyperthermia attenuated the therapeutic effect of hyperthermia.
Conclusions: Hyperthermia ameliorates TNBS-induced colitis in rats through the expression of HSP70 and HO-1. It is postulated that hyperthermia may be useful for the treatment of inflammatory bowel diseases.
Introduction
Inflammatory bowel diseases (IBDs), which include Crohn's disease and ulcerative colitis, are chronic recurrent inflammatory disorders characterized by marked infiltration of neutrophils into colonic lesions accompanied by epithelial cell necrosis and ulceration. The exact pathogenesis of these diseases remains unclear and no satisfactory therapy has been established.
Recently, hyperthermia has been found to attenuate several types of inflammation, such as ischemia-reperfusion injury Citation[1–3] and pancreatitis Citation[4], Citation[5]. Hyperthermia causes eukaryotic and prokaryotic cells to decrease production of some polypeptides and to up-regulate a class of proteins known as stress proteins Citation[6]. When thermal stress starts this response, the induced proteins are known as heat shock proteins (HSPs). The heat shock response can limit inflammatory responses, protect proteins from damage, restore function to proteins that are damaged, and prevent cellular destruction. Most HSPs chaperone other proteins during synthesis, folding, unfolding, assembly and translocation Citation[7]. As molecular chaperones, HSPs transiently stabilize and refold damaged intracellular proteins and prevent intracellular protein aggregation during times of stress Citation[8]. Another key feature of the heat shock response is the inhibition of proinflammatory gene expression, which may in part explain the mechanism by which HSPs protect against acute inflammatory injury Citation[1–5]. A representative example of this response is the observation that over-expression of HSP-70 inhibits NF-kB translocation from the cytoplasm to the nucleus Citation[9], Citation[10]. Recently, hyperthermia-induced HSPs have been shown to inhibit IKK-β, resulting in the inhibition of NF-kB activation Citation[11]. The transcriptional regulation of cytokines/chemokines such as TNF-α or IL-8 has been shown to be, in part, dependent on NF-kB activatio Citation[12], Citation[13]. The composite results suggest that hyperthermia-induced HSPs may inhibit the production of cytokines/chemokines such as TNF-α or IL-8. These profound phenomena lead to the hypothesis that hyperthermia may ameliorate IBDs; however, the effect of hyperthermia on inflammation in the gut has not been elucidated yet. The objective of this study, therefore, was to evaluate the effect of hyperthermia in models of experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid (TNBS) in rats.
Materials and methods
Chemicals
All chemicals were prepared immediately before use. 3,3′,5,5′-tetramethylbenzipine was obtained from Wako Pure Chemical (Osaka, Japan). Zinc protoporphyrin IX (ZnPP) was obtained from the Sigma Chemical Co. (St Louis, MO, USA). Geranylgeranylacetone (GGA) was a gift from Eisai Co., Ltd (Tokyo, Japan). All other chemicals used were of reagent grade.
Experimental animals
Male Wistar rats weighing 190–210 g were obtained from Keari Co., Ltd (Osaka, Japan). They were housed in stainless steel cages with wire bottoms and maintained on a 12-h light/12-h dark cycle under a controlled temperature of 21–23°C. They were not fed for 48 h prior to the experiments but were allowed free access to water.
All experimental procedures described below were approved by the Animal Care Committee of the Kyoto Prefectural University of Medicine (Kyoto, Japan) and maintenance of the animals was carried out in accordance with the guidelines of the Japan Council on Animal Care.
Induction of colitis
Colitis was induced by TNBS using the method of Moriss et al. Citation[14]. In brief, rats were lightly anesthetized with pentobarbital following a 48-h fast, and then a rubber catheter (OD 2 mm) was inserted via the anus so that the tip was inserted 8 cm into the anus. Through this catheter, 0.25 ml of TNBS dissolved in 50% ethanol (120 mg/ml) was instilled into the lumen of the colon. Following the instillation of TNBS, the anus was occluded with a clip for 60 min.
Hyperthermia
Animals were lightly anesthetized with pentobarbital (10 mg/kg injected intraperitoneally). Hyperthermia was induced in anesthetized rats by placing the lower half of the body of rats in a temperature-controlled water bath. During hyperthermia, the rat was supported by a wire apparatus to prevent its aspiration of water and to facilitate the measurement of its rectal temperature. Hyperthermia was maintained at 42.0°C for 20 min. Rats in the control group were placed in a water bath maintained at 36.5°C for 20 min. Rats underwent passive cooling after hyperthermia.
Experimental protocols
All rats were divided into two groups at random on the day after the induction of colitis. The sham group was treated with an enema of physiological saline instead of TNBS solution. The TNBS group was treated in a water bath maintained at 36.5°C for 20 min after TNBS administration. The TNBS-plus-hyperthermia group was treated in a water bath maintained at 42°C for 20 min after TNBS administration. Hyperthermia was performed once a day for 6 days beginning 24 h after TNBS instillation ().
Figure 1. Experimental schedule: (a) first series of experiments; (b) second series of experiments; (c) third series of experiments.
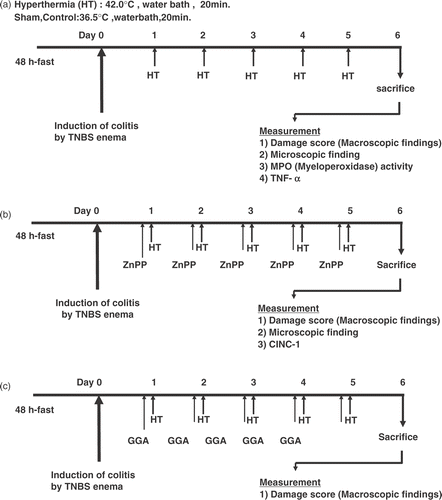
In another series of experiments, TNBS-plus-hyperthermia-plus-ZnPP group was given ZnPP (an inhibitor of heme oxygenase Citation[15]) dissolved in 50 mM Na2CO3 solution (20 mg/kg, injected intraperitoneally) 60 min before hyperthermia. The TNBS-plus-hyperthermia-plus-vehicle group was given an equal volume of Na2CO3 60 min before hyperthermia. Hyperthermia with/without ZnPP was performed once a day for 6 days beginning 24 h after TNBS instillation ().
In another series of experiments, all rats were divided into three groups at random on the day after the induction of colitis. The sham group was treated with an enema of physiological saline instead of TNBS. The TNBS group was treated in a water bath maintained at 36.5°C for 20 min after TNBS administration. The TNBS-plus-hyperthermia-plus-GGA group was given GGA (200 mg/kg as an emulsion with 5% gum arabic and 0.008% α-tocopherol) 60 min before hyperthermia. The TNBS-plus-hyperthermia-plus-vehicle group was given an equal volume of vehicle (5% gum arabic emulsion containing 0.008% α-tocopherol) 60 min before hyperthermia. Hyperthermia with/without GGA was performed once a day for 6 days beginning 24 h after TNBS instillation ().
Assessment of colitis
One week following the induction of colitis, all rats were killed by exsanguination via the abdominal aorta. The distal colon was removed and opened by a longitudinal incision. The wet weight of a segment of the colon 8 cm in length was measured. For microscopic study, specimens of the distal colon were fixed in 10% formalin and embedded in paraffin. They were then cut into serial 4 µm thick sections and stained with hematoxylin-eosin. As indices of macroscopic injury, colonic damage was estimated macroscopically as the sum of the mucosal score and the serosal score. The mucosal score was rated on a six-point scale (0–5) according to the criteria established by Moriss et al. Citation[14]. The serosal score was rated on a four-point scale (0–3) according to the criteria established by Yoshida et al. Citation[16] (). All scoring was performed by the same individual under blind conditions to prevent observer bias.
Table I. Criteria for scoring gross morphologic damage of the colo Citation[14], Citation[16].
Measurement of myeloperoxidase activity
The tissue-associated myeloperoxidase activity was determined using a modification of the method of Grisham et al. Citation[17] as an index of neutrophil accumulation. Samples (2 mL) of mucosal homogenates were centrifuged at 20 000 Xg for 15 min at 4°C to pellet the insoluble cellular debris. The pellet was then rehomogenized in an equivalent volume of 0.05 M potassium phosphate buffer (pH 5.4), containing 0.5% hexadecyltrimethylammonium bromide. The samples were centrifuged at 20 000 × g for 15 min at 4°C and the supernatants were collected. The myeloperoxidase activity was assessed by measuring the hydrogen peroxide-dependent oxidation of 3,3′,5,5′-tetramethylbenzidine. One unit of enzyme activity was defined as the amount of myeloperoxidase present that caused a change in absorbance of 1.0/min at 655 nm and 25°C. The total protein in the tissue homogenates was measured using the method of Lowry Citation[18].
Determination of cytokines
The contents of tumor necrosis factor (TNF)-α and cytokine-induced neutrophil chemoattractants-1 (CINC-1) in the colonic mucosa were determined using a rat TNF-α ELISA kit (BioSource International, Inc., Camarilla, CA, USA) and a rat GRO/CINC-1 ELISA kit (Immuno Biological Laboratories, Gunma, Japan) according to the manufacturers’ instructions.
Western blot analysis
The colonic mucosa was scraped off using two glass slides, thawed on ice, and homogenized at 4°C in a solution of 50 mmol/L Tris-HCL, pH 7.6, 300 mmol/L NaCl, 0.5% Triton X-100, 10 µg/mL aprotinin, 10 µg/mL leuptin, 1 mmol/L phenylmethylsulfonyl fluoride, 1.8 mg/mL iodoacetamide, 50 mmol/L sodium fluoride, and 1 mM DTT for extracting total cell protein. Proteins obtained from colonic mucosa and whole-cell lysate or nuclear protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with specific antibodies against HO-1 (Stressgen, Victoria, Canada), and Hsp70 (Stressgen, Victoria, Canada) separately. The immune complexes on the membranes were visualized with a commercial kit (ECL, Amersham, Buckinghamshire, England) according to the manufacturer's recommendations.
Statistical analysis
Colon damage scores are presented as scatter plots. Differences between groups were compared by analysis of variance (Kruskal–Wallis test) followed by the Mann–Whitney U test, a nonparametric test. Data on wet weight of the colon, TBA-reactive substances, myeloperoxidase activity, and the concentrations of TNF-α, and CINC-1 are presented as the means ± SEM, and were compared using an analysis of variance (ANOVA) followed by Fischer's protected least significant difference test (Fischer's PLSD). A level of p < 0.05 was considered statistically significant.
Results
Kinetics of the rectal temperature
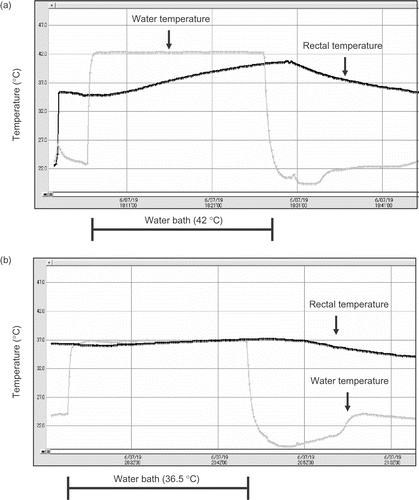
Rectal temperature was measured by a thermo-sensor probe (T & D, Nagano, Japan) inserted to the depth of 4 cm past the anal sphincter during hyperthermia. Water temperature in the water bath was also monitored. During hyperthermia, the rectal temperature of rats rose 40.6°C from 36.2°C. On the other hand, the rectal temperature of rats in the control group rose to 37.0°C from 36.2°C ().
Effects of hyperthermia on the gross appearance of TNBS colitis
The severity of TNBS-induced colitis was examined macroscopically and compared among groups. In the group that received TNBS alone (control group), the macroscopic findings of the colon demonstrated severe colitis with hyperemia, edema, wall thickening, ulceration and necrosis, as expected. Treatment with hyperthermia, however, ameliorated ulceration and inflammatory changes in the colon (). The weights of 8-cm portions of distal colon were increased remarkably in control groups compared with the sham group. However, the values were significantly lower in the hyperthermia-treated group, even though they received the same amount of TNBS (data not shown). To evaluate the macroscopic observation in a quantitative way, the severity of colonic inflammation and ulceration was graded using criteria for macroscopic damage scoring as described in detail in ‘Materials and methods’. The colonic damage score was significantly increased by TNBS administration. Hyperthermia significantly improved damage scores 6 days after TNBS treatment ().
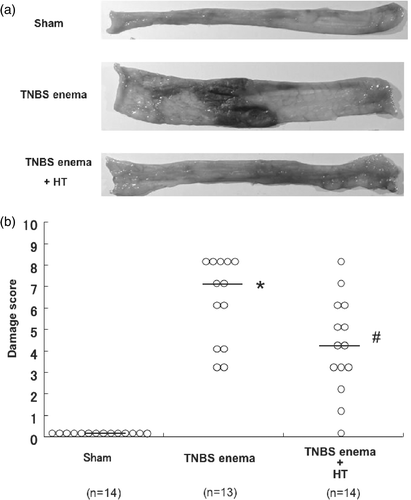
Figure 3. Macroscopic findings of colonic mucosa in TNBS-induced rat colitis. Rats were given a single enema of TNBS (30 mg in 50% ethanol/rat) and were treated in a water bath maintained at 42°C for 20 min (Hyperthermia; HT) after TNBS administration as described in detail in ‘Materials and methods’. (a) The macroscopic changes of the luminal side of the distal colon were observed 6 days after the enema of TNBS. (b) Effect of HT on the damage score 6 days after the enema of TNBS. *Values are the means ±SEM of 6–8 mice. p < 0.01 vs. sham, #p < 0.05 vs. TNBS enema.
Effect of hyperthermia on histopathologic change and myeloperoxidase activity in the colon after TNBS treatment
The severity of colonic inflammation and ulceration was evaluated further by histopathologic observations. shows typical histological features in the sham group, the group that received TNBS alone (control group), and the hyperthermia-treated group (TNBS-plus-hyperthermia). Light microscopic study of the hemoxylin/eosin-stained colonic specimens of rats that received TNBS alone showed marked thickening of the colonic wall with ulcer formation and massive transluminal infiltration of inflammatory cells, predominantly neutrophils, 6 days after a single TNBS enema. However, treatment with hyperthermia resulted in only a moderate degree of infiltration of inflammatory cells and slight thickening of the colonic wall.
Figure 4. Histological changes, tissue MPO activities in the colon after TNBS treatment. (a) Microscopic findings of colonic mucosa in TNBS-induced rat colitis. (b) Effect of HT on the increase of neutrophil accumulation in colonic mucosa 6 days after the enema of TNBS. Values are the means ±SEM of 6–8 mice. *p < 0.001 vs. sham, #p < 0.05 vs. TNBS enema.
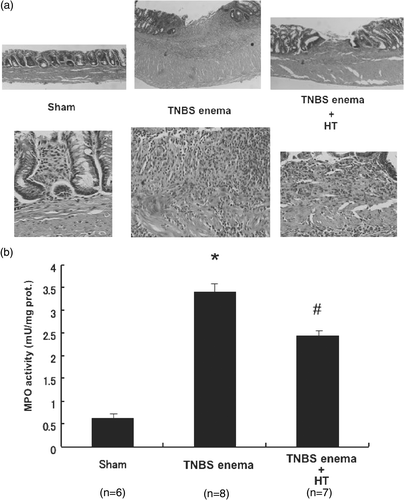
To evaluate the inflammatory changes in the colon in a quantitative manner, the activities of myeloperoxidase in the distal colon were measured 6 days after a single TNBS enema. The activities of myeloperoxidase were markedly increased in the control group. These increases were significantly attenuated by the treatment with hyperthermia ().
Effect of hyperthermia on colonic expression of tumor necrosis factor-α
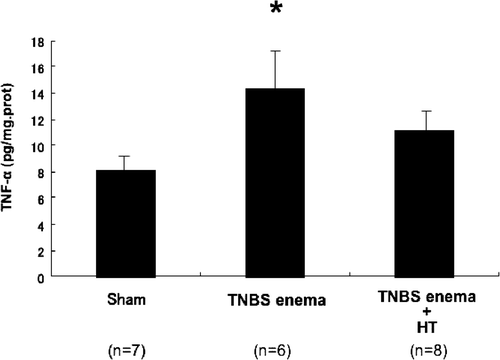
Because TNF-α has been well characterized as a key inflammatory cytokine, we measured the tissue protein level of TNF-α by ELISA. As shown in , the protein level of TNF-α was increased significantly in the control group. This increase was attenuated by hyperthermia.
Induction of heat shock proteins in rat colon
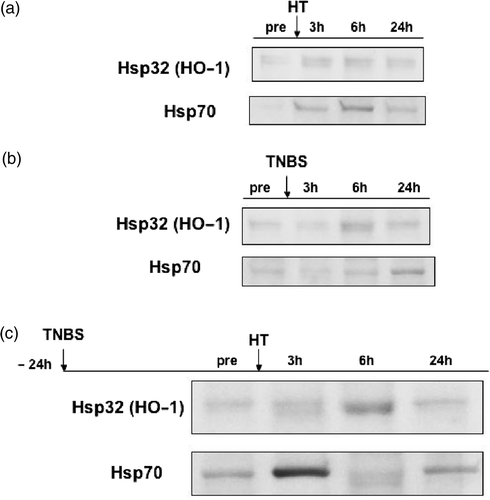
The induction of HSPs was determined by Western blotting with antibody against HO-1 or Hsp70. The levels of HO-1 and Hsp70 proteins were very low in the sham group. However, HO-1 and Hsp70 proteins were extremely accumulated in the colon between 3 h and 6 h after hyperthermia at 42°C (). On the other hand, the administration of TNBS weakly induced HO-1 and Hsp70 between 6 h and 24 h after TNBS administration (). However, when hyperthermia was performed 24 h after TNBS administration, there was substantial increase in HO-1 protein at 3 h after hyperthermia with peak expression at 6 h before levels declined at 24 h. Hsp70 was strongly induced 3 h after hyperthermia. We showed the representative band, which indicated the strong induction of Hsp70 at 3 h (). In this figure, the induction of Hsp70 at 6 h seemed to decrease and to be a double chain. It was difficult to explain why the band of Hsp70 showed a double chain at 6 h after hyperthermia in this experiment. Furthermore, while the degree of Hsp70 was different in each experiment, the peak of Hsp70 induction was always at 3 h after hyperthermia and the level of Hsp70 gradually decreased after 6 h and returned to the pre-hyperthermic level after 24 h (data not shown).
Effect of zinc protoporphyrin IX or geranylgeranylacetone on hyperthermia in TNBS-induced colitis
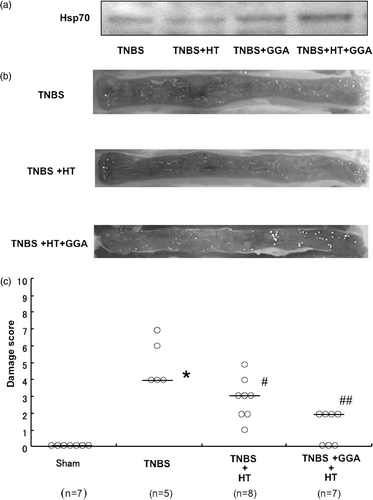
The following experiments were designed to elucidate the mechanism of the therapeutic effect of hyperthermia. The administration of TNBS caused severe colitis with hyperemia, edema, wall thickening, ulceration and necrosis. The treatment with hyperthermia ameliorated the TNBS-induced colonic damage. By contrast, the combination of ZnPP with hyperthermia extinguished the therapeutic effects of hyperthermia. As indices of macroscopic injury, colonic damage was estimated macroscopically as the sum of the mucosal score and the serosal score, and the protein level of CINC-1, rat homologues of IL-8, in colonic tissue was measured by ELISA. The colonic damage score was significantly increased by TNBS administration. The increase in damage score was significantly decreased by treatment with hyperthermia. Importantly, the damage score was again increased by treatment with ZnPP (). Mucosal CINC-1 induced by TNBS was attenuated by the treatment of hyperthermia. Similarly, ZnPP reduced the therapeutic effect of hyperthermia (). These results suggested that hyperthermia-induced HO-1 might mediate the therapeutic effect of hyperthermia against TNBS colitis. The mechanisms of the therapeutic effect of hyperthermia were further examined in the presence of GGA, an inducer of Hsp70. GGA administration induced Hsp70 in TNBS-treated colonic mucosa. The combination of GGA with hyperthermia further increased the induction of Hsp70 in colonic mucosa () and the therapeutic effects of hyperthermia against TNBS-induced colitis were significantly enhanced by GGA administration (). The colonic damage score was significantly increased by TNBS administration. The increase in damage score was significantly decreased by treatment with hyperthermia. Importantly, the damage score was further decreased by treatment with GGA (). These results suggested that the enhanced expression of Hsp70 might further improve TNBS colitis.
Figure 7. Effect of zinc protoporphyrin η on hyperthermia in TNBS-induced colitis. (a) Effects of zinc protoporphyrin IX on the damage score in TNBS colitis treated with HT. *p < 0.01 vs. sham, #p < 0.05 vs. TNBS. (b) Effects of zinc protoporphyrin IX on colonic CINC-1 levels in TNBS colitis treated with HT. Values are the means ± SEM of 6–8 mice.
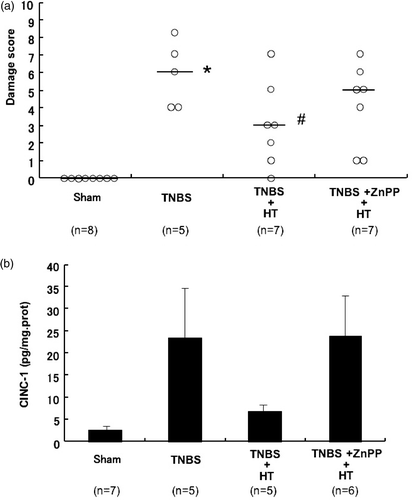
Figure 8. Effect of geranylgeranylacetone on hyperthermia in TNBS-induced colitis. (a) Effects of GGA on HT-induced Hsp70 in colonic mucosa. (b) The macroscopic changes of the luminal side of the distal colon were observed 6 days after the enema of TNBS. (c) Effects of GGA on the damage score in TNBS colitis treated with HT. *p < 0.01 vs. sham, #p < 0.05 vs. TNBS, ##p < 0.01 vs. TNBS.
Discussion
Hyperthermia has reversed TNBS-induced colitis once the colitis was established, suggesting a potential clinical value in terms of therapeutic application for IBDs. It is notable that induction of pro-inflammatory cytokines/chemokines in the colonic tissue was decreased by hyperthermia.
Colitis induced by TNBS exhibits clinical, histologic and microscopic similarities to Crohn's disease Citation[14] and the course of colonic injury has been well characterized Citation[19]. Previous studies have shown that pro-inflammatory cytokines, especially TNF-α, play a central role in the inflammatory cascade Citation[20]. This is evidenced by the fact that a neutralizing antibody for TNF-α abrogates Crohn's disease in humans Citation[21–23] and TNBS-induced colitis in mice Citation[24]. In the present study, a TNBS enema induced the production of TNF-α in colonic mucosa and hyperthermia showed attenuation of TNF-α production. It is postulated that the mechanisms by which hyperthermia ameliorate TNBS colitis partially involve suppression of TNF-α production from the colonic mucosa. It has been reported that hyperthermia blunts increases in serum TNF-α levels after lipopolysaccharide injection in rats Citation[25] and mice Citation[26] and that hyperthermia inhibits TNF-α production from isolated macroph Citation[27], Citation[28] in a direct manner.
In another aspect, TNBS-induced colitis is characterized as inflammation of colonic tissue owing to severe infiltration of inflammatory cells, predominantly neutrophils Citation[19]. Recently, it has been shown that chemokines play an important role in the accumulation of circulating leukocytes into inflammatory foci in TNBS-induced colitis Citation[29]. Administration of TNBS caused increases in CINC-1 levels in colonic mucosa, resulting in neutrophil infiltration into colonic mucosa and increases in the activities of myeloperoxidase. Interestingly, hyperthermia attenuated TNBS-induced increases in CINC-1 in the colon.
Among the mechanisms involved in the curative effects conferred by hyperthermia, the induction of HSPs might be important because HSPs are generally considered to function as a molecular chaperone and to contribute to the folding, assembly and stabilization of intracellular proteins Citation[7]. Indeed, Hsp70 and HO-1 were rapidly induced in colonic mucosa after hyperthermia. Previous studies have demonstrated that activation of NF-kB plays an important role in the generation of TNBS-induced colitis Citation[30]. Recent studies further suggested that heat shock or elevated Hsp70 level suppressed NF-kB activity in several cell lines and animal models Citation[9–11]. With these previous reports, this study suggests that hyperthermia may inhibit TNBS-induced activation of NF-kB via Hsp70, resulting in the protection of the aggravation of TNBS-induced colitis. In addition, several recent studies have shown that the beneficial effect of heat stress is not related to the Hsp70 family Citation[31], Citation[32]. Numerous other small proteins have also been reported to be HSPs, as determined by their enhanced rate of synthesis in response to heat stress. Included in this group is Hsp32, which is also known as heme oxygenase-1 (HO-1), an isoform of heme oxygenase that catalyzes the rate-limiting step in heme degradation, yielding biliverdin, iron and carbon monoxide Citation[33]. Recent studies have shown that HO-1 inhibits the induction of endothelial adhesion molecules via blocking NF-kB activatio Citation[34], Citation[35]. Therefore, it is possible that HO-1 also plays an important role in the protection of the aggravation of TNBS-induced colitis. For more detailed evaluation of the association between HSPs and the promotion of the healing of TNBS-induced colitis by hyperthermia, we used two chemicals: ZnPP as an HO-1 inhibitor Citation[15] and GGA as an Hsp70 inducer Citation[36]. Zinc protoporphyrin IX decreased the therapeutic effects of hyperthermia. This result suggests that HO-1 induced by hyperthermia is closely involved in the promotion of the healing of TNBS-induced colitis. Previous studies have also suggested the close involvement of HO-1 in the prevention of the development of experimental enteritis and the inhibition of its aggravatio Citation[37], Citation[38], which supports the results of our study. Evaluation using GGA was performed in our experimental system (TNBS-induced colitis model). It was reported that GGA induces mRNA expression and the protein level of Hsp70 within 30 min and 90 min after GGA administration respectively Citation[36]. Therefore, we administered GGA to rats 60 min before hyperthermia. In this model, GGA induced Hsp70 when used alone and further increased the Hsp70 induction by hyperthermia. In addition, GGA further promoted the effects of hyperthermia such as the promotion of the healing of TNBS-induced colitis. These results suggest that Hsp70 induced by hyperthermia is closely involved in the promotion of the healing of TNBS-induced colitis. The possible mechanisms of this promotion are the inhibition of CINC-1 production in the mucosa of the large intestine. These results together with the inhibition of NF-kB activation by HO-1 or Hsp70 reported by many previous studies Citation[9–11], Citation[34], Citation[35] suggest that HO-1 and Hsp70 induced by hyperthermia induced the healing of TNBS-induced colitis via the inhibition of NF-kB activation.
In summary, this study showed the effectiveness of hyperthermia for the treatment of TNBS-induced colitis as a model of IBD. In this mechanism, HSP induced by hyperthermia may be involved and the inhibition of NF-kB activation by HSP, in addition to its function as a molecular chaperone, may be also important. Thus, hyperthermia may provide a new therapeutic modality against IBDs such as Crohn's disease and ulcerative colitis.
References
- Stojadinovic A, Kiang J, Smallridge R, Galloway R, Shea-Donohue T. Induction of heat-shock protein 72 protects against ischemia/reperfusion in rat small intestine. Gastroenterology 1995; 109: 505–515
- Fleming SD, Starnes BW, Kiang JG, Stojadinovic A, Tsokos GC, Shea-Donohue T. Heat stress protection against mesenteric I/R-induced alterations in intestinal mucosa in rats. J Appl Physiol 2002; 92: 2600–2607
- Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. Whole-body hyperthermia provides biphasic cardioprotection against ischemia/reperfusion injury in the rat. Circulation 1998; 98: 1414–1421
- Rakonczay Z, Jr, Takacs T, Mandi Y, Ivanyi B, Varga S, Papai G, Boros I, Lonovics J. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: Possible role of HSP72. Int J Hyperthermia 2001; 17: 520–535
- Takacs T, Rakonczay Jr Z, Varga IS, Ivanyi B, Mandi Y, Boros I, Lonovics J. Comparative effects of water immersion pretreatment on three different acute pancreatitis models in rats. Biochem Cell Biol 2002; 80: 241–251
- Naylor DJ, Hartl FU. Contribution of molecular chaperones to protein folding in the cytoplasm of prokaryotic and eukaryotic cells. Biochem Soc Symp 2001; 68: 45–68
- Hendrick JP, Hartl FU. The role of molecular chaperones in protein folding. FASEB J 1995; 9: 1559–1569
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem 1997; 32: 17–29
- Kokura S, Yoshida N, Ueda M, Imamoto E, Ishikawa T, Takagi T, Naito Y, Okanoue T, Yoshikawa T. Hyperthermia enhances tumor necrosis factor alpha-induced apoptosis of a human gastric cancer cell line. Cancer Lett 2003; 201: 89–96
- Chen Y, Arrigo AP, Currie RW. Heat shock treatment suppresses angiotensin II-induced activation of NF-kappa B pathway and heart inflammation: A role for IKK depletion by heat shock?. Am J Physiol Heart Circ Physiol 2004; 287: H1104–1114
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev 2004; 18: 1466–1481
- Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem 1992; 267: 22506–22511
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: Involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol 1990; 10: 1498–1506
- Moriss GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989; 96: 795–803
- Maines MD, Trakshel GM. Differential regulation of heme oxygenase isozymes by Sn-and Zn-protoporphyrins: Possible relevance to suppression of hyperbilirubinemia. Biochem Biophys Acta 1992; 1131: 166–174
- Yoshida N, Yoshikawa T, Yamaguchi T, Naito Y, Tanigawa T, Murase H, Kondo M. A novel water-soluble vitamin E derived protects against experimental colitis in rats. Antioxid Redox Signal 1999; 1: 555–562
- Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol 1986; 252: G567–574
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology 1995; 109: 1344–1367
- Garside P. Cytokines in experimental colitis. Clin Exp Immunol 1999; 118: 337–339
- van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995; 109: 129–135
- Feldman M, Taylor P, Paleolog E, Brennan FM, Maini RN. Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn's disease: Analysis of the mechanism of action predicts utility in other diseases. Transplant Proc 1998; 30: 4126–4127
- D’haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology 1999; 116: 1029–1034
- Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Buschenfelde KH, Strober W, Kollias G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur J Immunol 1997; 27: 1743–1750
- Mikami K, Otaka M, Goto T, Miura K, Ohshima S, Yoneyama K, Lin JG, Watanabe D, Segawa D, Kataoka E, et al. Induction of a 72-kDa heat shock protein and protection against lipopolysaccharide-induced liver injury in cirrhotic rats. J Gastroenterol Hepatol 2004; 19: 884–890
- Suganuma T, Irie K, Fujii E, Yoshioka T, Muraki T. Effect of heat stress on lipopolysaccharide-induced vascular permeability change in mice. J Pharmacol Exp Ther 2002; 303: 656–663
- Ensor JE, Wiener SM, McCrea KA, Viscardi RM, Crawford EK, Hasday JD. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-alpha expression. Am J Physiol 1994; 266: C967–974
- Ensor JE, Crawford EK, Hasday JD. Warming macrophages to febrile range destabilizes tumor necrosis factor-alpha mRNA without inducing heat shock. Am J Physiol 1995; 269: C1140–1146
- Harada K, Toyonaga A, Mitsuyama K, Sasaki E, Tanikawa K. Role of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in rat experimental colitis. Digestion 1994; 55: 179–184
- Dikopoulos N, Nussler AK, Liptay S, Bachem M, Reinshagen M, Stiegler M, Schmid RM, Adler G, Weidenbach H. Inhibition of nitric oxide synthesis by aminoguanidine increases intestinal damage in the acute phase of rat TNB-colitis. Eur J Clin Invest 2001; 31: 234–239
- Qian YZ, Shipley JB, Levasseur JE, Kukreja RC. Dissociation of heat shock proteins expression with ischemic tolerance by whole body hyperthermia in rat heart. J Mol Cell Cardiol 1998; 30: 1163–1172
- Kukreja RC, Qian YZ, Okubo S, Flaherty EE. Role of protein kinase C and 72 kDa heat shock protein in ischemic tolerance following heat stress in the rat heart. Mol Cell Biochem 1999; 195: 123–131
- Ewing JF, Maines MD. Rapid induction of heme oxygenase 1 mRNA and protein by hyperthermia in rat brain: Heme oxygenase 2 is not a heat shock protein. Proc Natl Acad Sci USA 1991; 88: 5364–5368
- Nakao A, Otterbein LE, Overhaus M, Sarady JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F, et al. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology 2004; 127: 595–606
- Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 2004; 172: 3553–3563
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 1996; 111: 345–357
- Paul G, Bataille F, Obermeier F, Bock J, Klebl F, Strauch U, Lochbaum D, Rummele P, Farkas S, Scholmerich J, et al. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin Exp Immunol 2005; 140: 547–555
- Naito Y, Takagi T, Yoshikawa T. Heme oxygenase-1: A new therapeutic target for inflammatory bowel disease. Aliment Pharmacol Ther 2004; 20: 177–184