Abstract
Background and aim: The activation of NF-κB induces production of inflammatory cytokines and up regulation of endothelial cell adhesion molecules (ECAM). ECAM (e.g., E-selectin, VCAM-1 and ICAM-1) associates to the recruitment of leukocytes into tissue exposed to inflammatory situation. In this study, we investigated the effects of hyperthermia on the activation of NF-κB and the up regulation of E-selectin and VCAM-1 in human endothelial cells stimulated by TNF-α.
Methods: Human arterial endothelial cells (HAEC) were pretreated with hyperthermia for 60 min at 42°C, followed by incubation at 37°C in a passively cooled incubator, before TNF-α stimulation. To assess the effects of hyperthermia on TNF-α-induced up regulation of ECAM and TNF-α-induced activation of NF-κB, we measured ECAM by ELISA, and evaluated the activation of NF-κB by Western blotting after TNF-α stimulation. The accumulation of HO-1, Hsp70 and IκBα in hyperthermia-treated HAEC was also assessed by Western blotting. To investigate the role of Hsp70, we treated HAEC with geranylgeranylacetone (GGA, Hsp70 inducer) 2 h before hyperthermia, and then measured ECAM in TNF-α-stimulated HAEC by ELISA.
Results: Pretreatment of hyperthermia reduced TNF-α-induced up regulation of E-selectin and VCAM-1. In addition, accumulation of Hsp70, HO-1 and IκBα protein were up-regulated after hyperthermia. Furthermore, Western blotting analysis revealed that pretreatment of hyperthermia attenuated TNF-α-induced translocation of p65 into the nuclei of HAEC. Moreover, GGA enhanced Hsp70 accumulation induced by hyperthermia. Hyperthermia pretreatment combined with GGA induced further inhibition of TNF-α-induced up regulation of ECAM when compared with hyperthermia alone.
Conclusion: Pretreatment of hyperthermia blocks TNF-α-induced NF-κB activation, resulting in the inhibition of ECAM up regulation in HAEC.
Introduction
Exposure of endothelial cells to cytokines, such as tumor necrosis factor (TNF) Citation[1] and interleukin-1 (IL-1) Citation[1], induces the up regulation of various endothelial cell adhesion molecules (ECAM). ECAM play a fundamental role in the process of leukocyte recruitment from blood for tissue infiltratio Citation[2], Citation[3]. Constant induction of ECAM leads to deregulated expression and abnormal leukocyte recruitment, as seen in chronic inflammatory diseases, such as atherosclerosis, rheumatoid arthritis and Crohn's disease. The cytokine-mediated induction of ECAM is regulated by the activity of several transcription factors. Of the many transcription factors that have been described, NF-κB seems to be particularly relevant to the regulation of ECAM Citation[4]. In particular, the promoter regions of the genes encoding for E-selectin, and VCAM-1 have binding sites for NF-κB Citation[5], Citation[6]. Therefore, the blocking of NF-κB activity may bring about anti-inflammatory responses by reducing the up regulation of ECAM.
Hyperthermia has been shown to inhibit the activation of NF-κB by several stimuli, including TNF-α Citation[7–9]. In addition, Ran et al. Citation[10] demonstrated that Hsp70 blocked NF-κB activation through the inhibition of IκB kinase. Therefore, we hypothesized that hyperthermia inhibits TNF-α-induced up regulation of E-selectin and VCAM-1 on endothelial cells via accumulation of Hsp70. The major objectives of this study were: to investigate the effects of hyperthermia on TNF-α-induced NF-κB activation and up regulation of ECAM in human arterial endothelial cells; and to determine whether Hsp70 is involved in hyperthermia-induced inhibition of the TNF-α-induced inflammatory response.
Materials and methods
Reagents
All chemicals were prepared immediately before use. Recombinant human TNF-α was purchased from R&D systems (Rockville, MD, USA). Geranylgeranylacetone (GGA) was provided by Eisai Co. (Kyoto, Japan). All other chemicals used were of reagent grade.
Cell culture
HAEC (human arterial endothelial cells) were obtained from Clonetics (San Diego, CA, USA). HAEC were cultured in 75 cm2 plastic flasks (NUNC, Roskilde, Denmark) and were maintained at 37°C under 5% CO2 and 99% humidity in endothelial cell growth medium-2 (EGM-2, Clonetics) supplemented with 2% fetal bovine serum. Cells were used after 3–4 passages.
Hyperthermia
Hyperthermia was performed by exposing cells in a temperature-controlled CO2 incubator at 42°C for 60 min. HAEC monolayers were pretreated with hyperthermia and were then exposed to passive cooling in a temperature-controlled CO2 incubator (37°C) before TNF-α stimulation.
ELISA for E-selectin and VCAM-1
HAEC monolayers were pretreated with hyperthermia and were then passively cooled in a temperature-controlled incubator at 37°C before TNF-α stimulation; they were then tested for the cellular relative contents of E-selectin and VCAM-1. To quantify the contents of ECAM, we performed endothelial cell adhesion molecule expression assay. HAEC were plated on 96-well plates. Primary antibodies for E-selectin or VCAM-1 in PBS with 5% fetal bovine serum (FBS) were added to each well, and plates were incubated for 30 min at 37°C. Cells were washed and incubated with secondary antibody, peroxidase-conjugated goat-anti-mouse IgG affinity-purified F(ab′)2 fragment (Cappel, Durham, NC, USA), for 60 min. Wells were then washed, and antibody binding was detected by the addition of 100 μl of 0.1 mg/ml 3,3-, 5,5-tetramethylbenzidine (Sigma Chemical Co) with 0.003% H2O2. The reaction was stopped by addition of 75 μl of 8 N sulfuric acid. Color development was determined on a spectrometer at 492 nm after subtracting background values from cells stained only with the second-step antibody.
Western blot analysis for NF-κB
In order to assess the effects of hyperthermia on TNF-α-induced activation of NF-κB, we evaluated the status of p65 (a subunit of NF-κB) in the nucleus by Western blotting. Briefly, HAEC monolayers were grown on 100 mm2 dishes. HAEC nuclear extraction was performed at 60 min after TNF-α stimulation using a commercial nuclear extract kit (Active Motif, Carlsbad, CA). Nuclear protein concentrations were measured by Bio-Rad protein assay (Bio-Rad, Hercules, CA). Equal amounts of extracts (15 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Millipore, Boston, MA). Membranes were blocked with 3% nonfat dried milk in phosphate-buffered saline (PBS) for 30 minutes and then washed with PBS. Subsequently, membranes were incubated with rabbit antibody against to p65 (polyclonal, Santa Cruz Biotechnology, CA), and were then washed with PBS and incubated with anti-rabbit secondary antibody (Amersham, Buckinghamshire, UK) diluted 1:2000 for 30 min at room temperature. The immunocomplexes on the membrane were visualized by treatment with a commercial kit (ECL by Amersham, Buckinghamshire, UK) according to the manufacturer's instructions. The accumulation of Hsp70, heme oxygenase-1 (HO-1) and IκBα after hyperthermia were also investigated by Western blotting.
Effects of geranylgeranylacetone (GGA)
In order to investigate the role of Hsp70, the effects of GGA (0.01–10 μM, 2 h preincubation before hyperthermia) on Hsp70 accumulation and the TNF-α-induced up regulation of E-selectin and VCAM-1 in HAEC monolayers were investigated.
Statistical analysis
Results are presented as means ± SEM. Data were analyzed by one-way analysis of variance, and differences were considered significant when P values were less than 0.05 on Scheffe multiple comparison test. Stat View 5.0 software (Abacus Concepts Inc., Berkeley, CA) on a Macintosh computer was used to conduct analyses.
Results
Effects of hyperthermia on TNF-α-induced up regulation of adhesion molecules in HAEC monolayers
HAEC was stimulated with recombinant human TNF-α (10 ng/ml) at various intervals (3, 6 and 24 h) after hyperthermia. The cellular relative contents of E-selectin and VCAM-1 in HAEC were analyzed by ELISA 4 h and 6 h after the treatment with TNF-α, respectively.
TNF-α strongly induced up regulation of E-selectin and VCAM-1 in HAEC. Pretreatment with hyperthermia alone did not alter the basal expression of E-selectin and VCAM-1. TNF-α-induced accumulation of E-selectin was significantly attenuated by the pre-treatment with hyperthermia 3 h and 6 h before the treatment with TNF-α. The accumulation of E-selectin was not inhibited when hyperthermia was performed 24 h before the treatment with TNF-α (). The accumulation of VCAM-1 was also attenuated by the pre-treatment with hyperthermia 3 h, 6 h, or 24 h before the treatment with TNF-α ().
Figure 1. Effects of hyperthermia on TNF-α-induced expression of E-selectin (a) and VCAM-1 (b) in HAEC monolayers. After hyperthermia, HAEC was stimulated with TNF-α at each time point. E-selectin or VCAM-1 expression was measured by ELISA 4 hours or 6 hours after TNF-α stimulation. Data are shown as a mean ± SEM of five samples in a representative experiment. Similar results were obtained in three independent experiments. #p < 0.001 compared with control. *p < 0.001 compared with TNF-α stimulation alone.
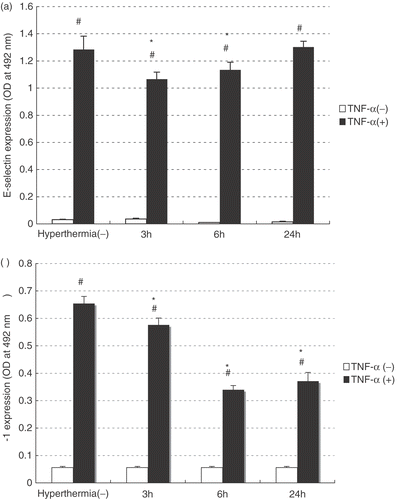
The accumulation of Hsp70, HO-1 and IκBα proteins after hyperthermia
In order to investigate accumulation of early response proteins after hyperthermia alone, the cellular contents of Hsp70, heme oxygenase-1 (HO-1, Hsp32) and IκBα in the treated cells were assessed by Western blotting. There were substantial increases in the cellular contents of both Hsp70 and HO-1 proteins 3 h after hyperthermia. The higher levels of these proteins were sustained until 24 h and declined 48 h after hyperthermia (). On the other hand, IκBα protein was up regulated from 6 to 24 h after hyperthermia.
Figure 2. The induction of HSP70, HO-1 and IkBα proteins after hyperthermia HAEC was treated with hyperthermia for 60 min. After heat treatment, total cell protein was extracted at the indicated time point. The productions of HSP70, HO-1, and IBα were assessed by Western blotting. Lane 1; control, Lane 2; 3 hours after hyperthermia, Lane 3; 6 hours after hyperthermia, Lane 4; 24 hours after hyperthermia, Lane 5; 48 hours after hyperthermia.
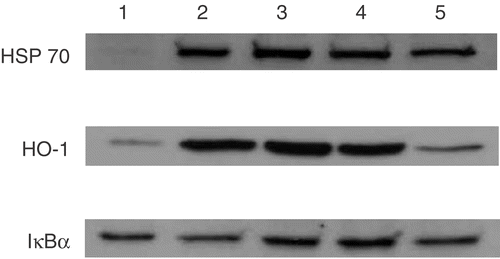
Effects of hyperthermia on TNF-α-induced NF-κB activation in HAEC
In order to assess the effect of hyperthermia on NF-κB activation by TNF-α, we evaluated the status of NF-κB by Western blotting of p65 after 1 h of the treatment with TNF-α (1 ng/ml). Hyperthermia was performed 3 h before the treatment with TNF-α.
Western blotting analysis also revealed that TNF-α stimulation induced translocation of p65 into the nucleus, and that hyperthermia inhibited TNF-α-induced translocation of p65 into the nucleus (). Hyperthermia did not affect the levels of p65 in the cytoplasm.
Figure 3. Effects of hyperthermia on TNF-α-induced activation of NF-κB in HAEC. (a) The status of NF-κB activation was evaluated by Western blotting of p65 in nuclear protein. Upper band shows the Western blotting of nuclear p65 and lower band shows the Western blotting of cytoplasmic P65. (b) Quantification of each band was performed by densitometry analysis software (image J), and results were expressed as the ratio (cytoplasm) in arbitrary units. Lane 1; control, Lane 2; hyperthermia alone, Lane 3; TNF-α alone, Lane 4; hyperthermia pretreatment with TNF-α.
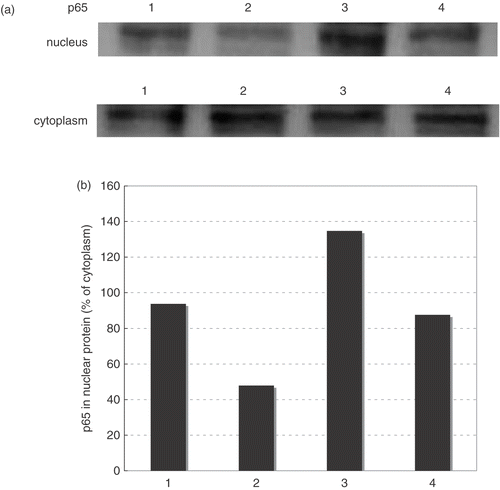
Effects of geranylgeranylacetone (GGA) on inhibition of TNF-α-induced ECAM accumulation by hyperthermia in HAEC
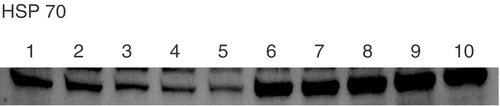
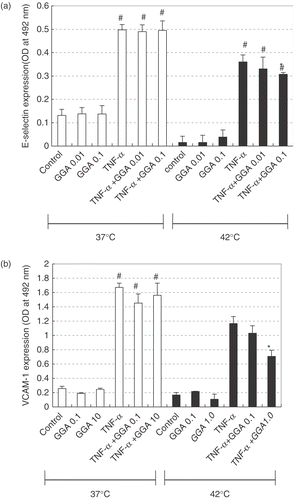
In order to investigate the role of HSP70, we used GGA as an Hsp70 inducer. We added GGA (0.01, 0.1, 1.0, 10 μM) 2 hours before hyperthermia, and Hsp70 accumulation was then assessed by Western blotting at 3 h after hyperthermia. GGA enhanced Hsp70 accumulation induced by hyperthermia (). On the other hand, when GGA was added without hyperthermia, the level of Hsp70 declined in a dose-dependent manner (). The cellular content of Hsp70 before hyperthermia is detectable in this series of experiments and this result is different from that in . () Because the property of HAEC changes after 5 passages, we used HAEC after 3–4 passages in all experiments. Therefore we used different donor's HAEC in from in . Although the cellular content of Hsp70 was sometimes different, similar results (same tendencies) were obtained for each experimental group in three independent experiments. We showed a representative result among them.
Figure 4. Effects of geranylgeranylacetone (GGA) on accumulation of HSP70 after hyperthermia in HAEC. We added GGA (0.01, 0.1, 1.0, 10 μM) 2 hours before hyperthermia, and HSP70 expression was then assessed by Western blotting at 3 h after heat treatment. Lane 1; control, Lane 2; GGA 0.01 μM alone, Lane 3; GGA 0.1 μM alone, Lane 4; GGA 1 μM alone, Lane 5; GGA 1 μM alone, Lane 6; hyperthermia alone, Lane 7; GGA 0.01 μM before hyperthermia, Lane 8; GGA 0.1 μM before hyperthermia, Lane 9; GGA 1 μM before hyperthermia, Lane 10; GGA 1 μM before hyperthermia.
We added GGA just 2 h before hyperthermia. Three hours after hyperthermia, we incubated HAEC with recombinant human TNF-α (10 ng/ml) for 4 h. We then measured the content of E-selectin in HAEC by ELISA. In the same way, we added GGA just 2 h before hyperthermia. Three hours after hyperthermia, we incubated HAEC with recombinant human TNF-α (10 ng/ml) for 6 hours. We then measured the content of VCAM-1 on HAEC by ELISA. Pre-treatment combined with GGA induced further inhibition of TNF-α-induced up regulation of adhesion molecules compared with hyperthermia alone (). The levels of E-selectin and VCAM-1 after TNF-α-stimulation are different from those in . We used different donor's HAEC in from in . When we used different donor's HAEC, the response of HAEC for TNF-α was sometimes quite different. In this series of experiments we performed three independent experiments, and similar results were obtained in these experiments. Data are shown as a mean ± SEM of five samples in a representative experiment.
Figure 5. Effects of geranylgeranylacetone (GGA) on inhibition of TNF-α-induced ECAM accumulation by hyperthermia in HAEC. We added GGA (0.01–1.0 μM) just 2 h before hyperthermia. Three hours after heat treatment, we incubated HAEC with recombinant human TNF-α (10 ng/ml). E-selectin (a) or VCAM-1 (b) expression was measured by ELISA 4 hours or 6 hours after TNF-α stimulation. Data are shown as a mean ± SEM of five samples in a representative experiment. Similar results were obtained in three independent experiments. #p < 0.001 compared with control. *p < 0.001 compared with TNF-α combined with hyperthermia.
Discussion
This study provided evidence to support the hypothesis that hyperthermia inhibits TNF-α-induced up regulation of E-selectin and VCAM-1 on endothelial cells via the blocking of NF-κB activation. During the inflammatory process, the activation of endothelium by proinflammatory cytokines is a key step, as it is directly responsible for the recruitment of the circulating leukocytes and their uptake into the inflamed tissue, a process that is mediated by the leukocyte–endothelial cell adhesion cascade Citation[11]. In this cascade, an increased surface accumulation of ECAM, notably E-selectin, and VCAM-1 play a fundamental role in the process of leukocyte recruitment from the blood for tissue infiltration. Thus, it has been proposed that controlling the ECAM accumulation may affect the degree of inflammatory injury. Previous studies have demonstrated that the promoter regions of the genes encoding for E-selectin and VCAM-1 have binding sites for NF-κB Citation[5], Citation[6]. The results of previous studies in our laboratory and by other groups also suggest that induction of heat shock protein (HSP), particularly Hsp70 and Hsp32 (HO-1), is associated with diminished proinflammatory gene expression and attenuation of inflammatory response through the inhibition of NF-κB activation Citation[12–15]. Thus, it has been postulated that hyperthermia inhibits TNF-α-induced upregulation of E-selectin and VCAM-1 on endothelial cells via induction of HSP.
The present results show that pretreatment by hyperthermia for endothelial cells increases Hsp70 and HO-1 and results in the attenuation of TNF-α-induced up regulation of E-selectin and VCAM-1. Endothelial cells can respond to extracellular stimuli such as TNF-α by the de novo synthesis of proteins. A very frequent regulatory event in such responses is the up regulation of mRNA synthesis by transcriptional activator proteins. Of the many transcription factors that have been described, NF-κB seems to be particularly relevant to the regulation of endothelial cell adhesion molecules [5, 6]. Unlike most transcription activators, NF-κB normally resides in the cytoplasm, and it must be translocated into the nucleus to elicit a response. In the cytoplasm, inactive NF-κB exists as a heterodimeric complex of subunits (p50 and p65) that bind to a cytoplasmic retention protein, IκB. Upon activation, IκB is rapidly degraded, and the p50/p65 heterodimer is translocated from the cytoplasm into the nucleus where it binds with high affinity to a κB moti Citation[16], Citation[17]. Phosphorylation and subsequent proteolysis of IκB have been reported in TNF-α-mediated inflammatory responses Citation[18], Citation[19]. It has also been reported that the heat shock response inhibits cytokine-mediated activation of NF-κB in various cells Citation[7–10], Citation[20–22]. Recently, Kohn et al. Citation[23] reported that heat shock decreased endothelial cell ICAM-1 via inhibition of IKK activity, which phosphorylates IκBα, resulting in the degradation of IκBα.
In this study, we show that hyperthermia inhibited the TNF-α-induced translocation of p65 into nucleus, thus suggesting the inhibition of NF-κB activation in HAEC. Although the heat shock response is reportedly capable of inhibiting the cytokine-mediated activation of NF-κB in a variety of cells Citation[7–10], Citation[20–22], the molecular mechanisms of the effects of hyperthermia are not fully understood. The TNF-α-induced up regulation of VCAM-1 was significantly attenuated by hyperthermic pretreatment performed between 3 h and 24 h before TNF-α treatment. At 48 h after hyperthermic pretreatment, TNF-α-induced up regulation of VCAM-1 was not inhibited (data not shown). The present results showing the kinetics of Hsp70 and HO-1 accumulation, which increased substantially from 3 h after hyperthermia with sustained accumulation for 24 h before declining at 48 h, suggest that these HSP might be involved in the inhibition of NF-κB by hyperthermic pretreatment.
In order to investigate the role of Hsp70 in the hyperthermic effects of NF-κB inhibition, evaluation using GGA was performed in our experimental system. It has been reported that GGA induces mRNA and protein expression of Hsp70 within 30 min and 90 min after GGA administration, respectively Citation[24]. Therefore, we incubated HAEC with GGA at 2 h before hyperthermia. In this model, GGA alone reduced the content of Hsp70 and GGA enhanced Hsp70 induction by hyperthermia in a dose dependent manner. In several cell lines, GGA alone didn’t induce Hsp70 and needed another stimulation such as heat stress. It is difficult for us to explain the mechanisms by which GGA reduce the content of Hsp70 in HAEC. Additional investigations are required to clarify the mechanisms by which GGA alone reduces the content of Hsp70 and GGA enhances the induction of Hsp70 by hyperthermia.
In addition, GGA further promoted the effects of hyperthermia, such as the inhibition of TNF-α-induced up regulation of E-selectin and VCAM-1. These results suggest that Hsp70 induced by hyperthermia is closely involved in the inhibition of TNF-α-induced ECAM up regulation. However, the present results do not eliminate the involvement of other HSP, such as Hsp60, because hyperthermia induces not only Hsp70 and HO-1 but also other HSP.
Another important observation in this study is the induction of IκBα by hyperthermia. The accumulation of IκBα increased substantially from 6 h after hyperthermia with sustained accumulation until 24 h, before declining at 48 h. It is known that the IκBα promoter is regulated by NF-κB Citation[25], Citation[26]. NF-κB-mediated regulation of IκBα provides an important feedback loop that prevents prolonged nuclear translocation of NF-κB. For example, TNF-α-induced NF-κB activation not only increases proinflammatory cytokine gene expression, but also increases expression of IκBα, which down-regulates TNF-α-induced NF-κB activation. However, the present study showed that hyperthermia alone does not induce nuclear translocation of p65 in HAEC. This phenomenon is supported by previous reports Citation[7], Citation[21], suggesting a novel non-NF-κB dependent pathway for the induction of IκBα by hyperthermia. It is reported that overexpression of IκBα by hyperthermia can inhibit the activation of NF-κB in human bronchial cells Citation[27]. Therefore, this phenomenon may be involved in the mechanisms by which hyperthermia inhibits the activation of NF-κB in HAEC.
Thus, an important limitation to the current study is the inability to causally link HSP induction to the inhibitory effects on NF-κB activation. We are unable to conclude that the inhibitory effects of NF-κB activation are directly related to Hsp70 or HO-1 induction because hyperthermia induces numerous stress proteins in HAEC. Current studies in our laboratory are aimed at inducing only Hsp70 or HO-1 in HAEC without hyperthermia in order to confirm which HSP are important in the inhibition of NF-κB activation.
In summary, our data demonstrate that hyperthermia inhibits p65 translocation into the nucleus, indicating NF-κB inhibition with a subsequent decrease in E-selectin and VCAM-1 in TNF-α-stimulated HAEC.
References
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell 1990; 62: 3–6
- Luscinskas F, Gimbrone M. Endothelial-dependent mechanisms in chronic inflammatory leukocytes recruitment. Annu Rev Med 1996; 47: 413–421
- Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev 1999; 10: 27–39
- Neish A, Williams A, Palmer H, Whitley M, Collins T. Functional analysis of the human vascular cell adhesion molecule 1 promoter. J Exp Med 1992; 176: 1583–1593
- Schindler U, Baichwal V. Three NF-B binding sites in the human E-selectin gene required for maximal TNF-induced expression. Mol Cell Biol 1994; 14: 5820–5831
- Ledebur H, Parks T. Transcriptional regulation of the inter-cellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-B and p65 homodimers. J Biol Chem 1995; 270: 933–943
- Kokura S, Yoshida N, Ueda M, Imamoto E, Ishikawa T, Takagi T, Naito Y, Okanoue T, Yoshikawa T. Hyperthermia enhances tumor necrosis factor alpha-induced apoptosis of a human gastric cancer cell line. Cancer Lett 2003; 201: 89–96
- Chen Y, Arrigo AP, Currie RW. Heat shock treatment suppresses angiotensin II-induced activation of NF-kappaB pathway and heart inflammation: a role for IKK depletion by heat shock?. Am J Physiol Heart Circ Physiol 2004; 287: H1104–1114
- Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2001; 280: G1157–G1162
- Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, Feng Y, Han C, Zhou G, Rigby AC, Sharp FR. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev 2004; 18: 1466–1481
- Shanley TP, Warner RL, Ward PA. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med Today 1995; 1: 40–45
- Ayad O, Stark JM, Fiedler MM, Menendez IY, Ryan MA, Wong HR. The heat shock response inhibits RANTES gene expression in cultured human lung epithelium. J Immunol 1998; 161: 2594–2599
- Rakonczay Z, Jr, Takacs T, Mandi Y, Ivanyi B, Varga S, Papai G, Boros I, Lonovics J. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: Possible role of HSP72. Int J Hyperthermia 2001; 17: 520–535
- Takacs T, Rakonczay Z, Jr, Varga IS, Ivanyi B, Mandi Y, Boros I, Lonovics J. Comparative effects of water immersion pretreatment on three different acute pancreatitis models in rats. Biochem Cell Biol 2002; 80: 241–251
- Stojadinovic A, Kiang J, Smallridge R, Galloway R, Shea-Donohue T. Induction of heat-shock protein 72 protects against ischemia/reperfusion in rat small intestine. Gastroenterology 1995; 109: 505–515
- Baeuerle PA, Baltimore D. IκB: a specific inhibitor of the NFkB transcription factor. Science 1988; 242: 540–546
- Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS, Jr. IκB interacts with the nuclear localization sequences of the subunits of NF-kB: A mechanism for cytoplasmic retension. Genes Dev 1992; 6: 1899–1913
- Beg AA, Finco TS, Nantermet PV, Baldwin AS, Jr. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBa: A mechanism for NF-kB activation. Mol Cell Biol 1993; 13: 3301–3310
- Dhawan S, Singh S, Aggarwal BB. Induction of endothelial cell surface adhesion molecules by tumor necrosis factor is blocked by protein tyrosine phosphatase inhibitors: Role of the nuclear transcription factor NF-kappa B. Eur J Immunol 1997; 27: 2172–2179
- de Vera ME, Kim YM, Wong HR, Wang Q, Billiar TR, Geller DA. Heat shock response inhibits cytokine-inducible nitric oxide synthase expression in rat hepatocytes. Hepatology 1996; 24(5)1238–1245
- Wong HR, Ryan M, Wispe JR. Stress response decreases NF-kappaB nuclear translocation and increases I-kappaB alpha expression in A549 cells. J Clin Invest 1997; 99(10)2423–2428
- Lee KH, Hwang YH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. The heat-shock-induced suppression of the IkappaB/NF-kappaB cascade is due to inactivation of upstream regulators of IkappaB alpha through insolubilization. Exp Cell Res 2004; 299(1)49–56
- Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, Morris C, Stark J, Shanley TP. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock 2002; 17(2)91–97
- Hirakawa T, Rokutan K, Nikawa T, Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 1996; 111: 345–357
- de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H, Bach FH. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J 1993; 12(7)2773–2779
- Ito CY, Kazantsev AG, Baldwin AS, Jr. Three NF-kappa B sites in the I kappa B-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res 1994; 22(18)3787–3792
- Wong HR, Ryan MA, Menendez IY, Wispe JR. Heat shock activates the I-kappaBalpha promoter and increases I-kappaBalpha mRNA expression. Cell Stress Chaperones 1999; 4(1)1–7