Abstract
Acoustic cavitation has been shown to play a key role in a wide array of novel therapeutic ultrasound applications. This paper presents a brief discussion of the physics of thermally relevant acoustic cavitation in the context of high-intensity focussed ultrasound (HIFU). Models for how different types of cavitation activity can serve to accelerate tissue heating are presented, and results suggest that the bulk of the enhanced heating effect can be attributed to the absorption of broadband acoustic emissions generated by inertial cavitation. Such emissions can be readily monitored using a passive cavitation detection (PCD) scheme and could provide a means for real-time treatment monitoring. It is also shown that the appearance of hyperechoic regions (or bright-ups) on B-mode ultrasound images constitutes neither a necessary nor a sufficient condition for inertial cavitation activity to have occurred during HIFU exposure. Once instigated at relatively large HIFU excitation amplitudes, bubble activity tends to grow unstable and to migrate toward the source transducer, causing potentially undesirable pre-focal damage. Potential means of controlling inertial cavitation activity using pulsed excitation so as to confine it to the focal region are presented, with the intention of harnessing cavitation-enhanced heating for optimal HIFU treatment delivery. The role of temperature elevation in mitigating bubble-enhanced heating effects is also discussed, along with other bubble-field effects such as multiple scattering and shielding.
Introduction
At large rarefaction pressure amplitudes, a propagating ultrasonic wave may place the surrounding tissue under sufficient tension for small cavities, which rapidly fill with gas and vapor, to form. This process is known as acoustically induced cavity nucleation, and the pressure amplitude required to induce such phenomena depends greatly on the physical properties of the medium Citation[1–3]. Once formed, subsequent ultrasonic excitation will cause these bubbles to pulsate volumetrically, a process known as acoustic cavitation Citation[4–9].
Early studies of ultrasonically induced cavitation in tissue were triggered by concerns for the safety of diagnostic ultrasound Citation[10–18]. Experimental studies (performed mostly in small animals) suggested that, at the upper end of the pressure output of diagnostic scanners, tissue damage, which occurred preferentially near gaseous interfaces, could be observed. Concerns about such bioeffects led to the formulation of the Mechanical Index, which gauges the likelihood of cavitation excitation by short-pulse, low-duty-cycle diagnostic ultrasound in the presence of pre-existing gas nuclei Citation[8], Citation[19–21]. Based on this work, operating conditions aimed at minimizing the risk of what was perceived as undesirable bubble activity in a diagnostic context have been proposed for ultrasound scanners Citation[15], Citation[16], Citation[18], Citation[22].
However, the rapid emergence of a wide array of therapeutic ultrasound applications over the last two decades has led to a re-evaluation of the desirability of acoustic cavitation in vivo. Ultrasonic waves have been reported to cause potentially beneficial bioeffects, including the sealing of blood vessels (acoustic haemostasis) Citation[23–34], dissolution of blood clots (thrombolysis) Citation[35–46], activation of drugs Citation[47–50], opening of the blood–brain barrier Citation[51–61] and the increase of cell membrane and skin permeability to molecules (sonoporation and sonophoresis) Citation[62–64]. Even though the exact biophysical mechanisms underlying these phenomena remain poorly understood, acoustic cavitation is often acknowledged as their most likely common denominator Citation[65–69].
Many therapeutic applications—particularly those involving rapid tissue heating, acoustic haemostasis, and tissue ablation—employ high-intensity beams of focused ultrasound (HIFU) Citation[70–73]. Tissue heating occurs primarily due to viscous absorption of the acoustic energy. Both the attenuation and absorption coefficients in tissue are known to increase as a power law with increasing frequency Citation[74–77], which implies that the choice of clinical HIFU frequencies constitutes a compromise between the desired treatment depth, limited by attenuation, and the maximum achievable rate of heating, determined by absorptio Citation[78], Citation[79]. In the context of HIFU ablation in particular, the objective is to induce rapid and irreversible thermal necrosis of malignant tumors, with minimal damage in the intervening path. Any mechanism which might make it possible to increase heat deposition efficiency at the focus whilst minimizing prefocal damage is therefore of considerable interest, and it is in this context that cavitation can play a potentially crucial role.
The peak negative pressures produced by HIFU fields are often sufficiently large to produce cavitation activity which is generally, but not always, initially confined to the focal region of the HIFU field. Recent studies have demonstrated that the presence of small gas bubbles at the ultrasound focus can lead to substantially higher rates of tissue heating, which can be as much as six times the rate of heating in the absence of bubbles Citation[80–84]. This enhanced rate of heating is generally attributed to two basic types of acoustic cavitation activity, described in greater detail in the next section. Stable cavitation is the prolonged linear or nonlinear oscillation of an acoustically driven bubble about its equilibrium radius, where the dynamics of the cavity motion are dominated by the compressibility of the gas. Inertial (or transient) cavitation describes the unstable expansion of a bubble followed by a rapid, violent collapse dominated by the inertia of the surrounding medium, usually occurring over a single or small number of acoustic cycles Citation[85]. During collapse, inertial cavities will radiate energy into the surrounding tissue in the form of broadband acoustic emissions Citation[86], Citation[87].
Based on the premise that the local rate of HIFU-induced heating is determined by the local, frequency-dependent absorption coefficient, any mechanism that either increases the high-frequency content of the sound field or extends the length-scales over which viscous absorption can occur will result in enhanced heating. The strong scattering of sound by cavities present within a bubble cloud at the HIFU focus will ‘trap’ acoustic energy within that region, extending the sonic propagation path and leading to greater absorption by viscous dissipation and thermal conduction. In addition, the presence of multiple cavities oscillating in the sound field increases the opportunity for viscous absorption in boundary layers at the bubble surface. Finally, in the presence of inertial cavitation, the frequency dependence of the absorption coefficient dictates that the high-frequency broadband noise emissions produced by the collapsing bubbles will be very readily and locally absorbed.
Based on these three mechanisms, acoustic cavitation has tremendous potential to enhance the heat deposition efficiency during HIFU treatment, as it can lead to preferential heating in the focal region compared to the pre-focal region. Furthermore, if this enhanced heating can be directly correlated with inertial cavitation activity, monitoring the broadband noise emissions produced by collapsing bubbles at frequencies well removed from the main HIFU frequency could make it possible to monitor treatment non-invasively. However, harnessing cavitation activity for optimal HIFU treatment delivery is not without its challenges. The pressures required to generate cavitation in vivo are generally very high and tissue-specific Citation[1], Citation[88–90]. The use of nucleating agents or particular preconditioning excitations may thus be required to instigate and promote cavity formation. The appearance of bubbles in a region of tissue will dramatically alter its acoustic impedance, causing reflection of the incident sound wave and increasing sound intensity in the region proximal to the focal volume. This in turn can cause the migration of the bubble cloud towards the HIFU transducer, resulting in damage to prefocal tissue and shielding of the original focus. These factors can contribute to the formation of HIFU lesions of unpredictable shape Citation[83], Citation[91–95], a feature that is highly undesirable during clinical treatments.
For these reasons, the cavitation nucleation and activity in tissue must be understood, monitored and controlled in order to enhance the delivery and monitoring of HIFU treatment. This paper presents a brief overview of the underlying physics of cavitation, the various mechanisms by which cavitation can enhance heating, some representative experimental results demonstrating the accelerated heating effect, and possible techniques for monitoring thermally relevant cavitation activity in real time.
Physics of thermally relevant cavitation
Linear and non-linear bubble behavior
A bubble exposed to a low-amplitude sound field will exhibit small radial oscillations that are symmetric about its equilibrium radius. In this linear regime, its behavior is analogous to that of a mass-spring-damper system, where the spring represents the compressibility of the gas, the mass the inertia of the surrounding liquid, and the damper any viscous, thermal or radiation losses Citation[96], Citation[97]. For bubbles of equilibrium diameter smaller than 10 microns, the surface tension at the liquid–gas interface will also contribute significantly to the stiffness of the system Citation[85]. As for any second-order linear system, the acoustic bubble will resonate for a particular excitation frequency, known as the resonance frequency f0 and given by Citation[97], Citation[98]where P0 is the ambient pressure, ρ the density of the liquid, σ the surface tension at the liquid–gas interface, R0 the equilibrium radius in the absence of sound and γ the polytropic exponent for the gas (an adiabatic process is frequently assumed). Conversely, the equilibrium size R0 of the bubble that will resonate at a particular excitation frequency f0 is known as the resonance size. Note that for air bubbles in water possessing radii greater than approximately 5 µm, one can ignore the surface tension term, in which case the resonance frequency and equilibrium radius are inversely proportional to each other. For such a bubble undergoing adiabatic pulsations (γ = 1.4) at atmospheric pressure, this proportionality constant is approximately 3.26 Hz-m.
In the context of the high ultrasound amplitudes encountered in HIFU, the oscillations performed by the bubble can no longer be deemed small and are generally not symmetric about its equilibrium radius. These large pulsations cause the bubble to respond non-linearly, in a manner that is ultimately determined by the initial bubble size, the spectral content of the incident sound field, the proximity of physical boundaries or other neighboring bubbles, the viscoelastic and interfacial properties of the surrounding medium, the ambient temperature, and the propensity for gas diffusion across the bubble wall. The effect that this large array of parameters has on single and multiple bubble behavior has been explored in detail elsewhere Citation[85], Citation[97], Citation[99–102] and will not be repeated here.
In order to investigate the salient features of HIFU-driven cavitation behavior, we assume here a single bubble of known equilibrium radius Ro pulsating radially in a Newtonian viscous liquid under the effect of a single-frequency acoustic plane wave of amplitude Pa, in the absence of gas transfer across the bubble wall and well away from physical boundaries. The changes in radius R of a bubble driven in this manner can be described by a force balance equation across the bubble wall, where internal pressures arise from gas compression and vapor pressure, and external pressures arise from surface tension, viscous stress, static fluid pressure and dynamic acoustic pressure fluctuations Citation[103], Citation[104]:where
Po, σ, Pv, μ, c, and ρ are respectively the ambient pressure, surface tension, vapor pressure, viscosity, sound speed, and density of the surrounding medium. It should be noted that Equation 2 reduces to the aforementioned classic second-order system in the limit of small oscillations and negligible surface tension, i.e. for acoustic pressures less than about 0.01 MPa and equilibrium radii greater than 5–10 µm.
Equation 2 is highly nonlinear and suggests a range of possible bubble behaviors that are rich in complexity, yet can be broadly classified into two categories: stable and inertial cavitation. Stable cavitation is characterized by essentially repetitive radial oscillations about an equilibrium radius that may or may not change over time owing to gradual dissolution, or to growth by a process known as rectified diffusioCitation[99], Citation[105], Citation[106]. When driven at HIFU-relevant amplitudes, a stable response is only likely to be exhibited by larger gas-filled bubbles whose radial excursions are arrested by the inertial mass of the surrounding medium. Such cavities will generally be on the order of the resonance size or larger. In an aqueous medium driven at 1 MHz and a pressure amplitude of 1 MPa, only those bubbles with radii larger than about 3–5 µm will respond stably. As the HIFU pressure amplitude is raised, this lower size limit also increases.
Probably the most important facet of stable cavitation is the formation of small-scale fluid flows known as cavitation microstreaming Citation[107–110], an effect that is greatly enhanced by the excitation of surface waves on the bubble. These flows occur over scales comparable with the bubble dimensions, and are considered to be one of the key ways by which cavitation can lyse cells, clean surfaces and promote convective drug delivery Citation[66], Citation[111–113]. Stable cavitation can also promote heat generation owing to viscous losses in the boundary layer on the surface of the pulsating bubble. In short, stable cavitation provides a means of converting acoustical energy into mechanical (streaming) and thermal (heating) energy over length scales that are comparable to the bubble size but generally much smaller than an acoustic wavelength.
If a stable gas-filled cavity is driven at sufficiently large pressure amplitudes, the rarefaction portion of the acoustic pressure cycle causes it to grow to a size for which the internal pressure drops to the vapor pressure. The progression from gas-filled microbubble to a growing vapor-filled cavity occurs much more readily if the equilibrium size of the bubble is small at the outset, or if the peak negative pressure is extremely large. During the ensuing compressive phase, the growth is arrested and the vapor-filled bubble proceeds to collapse unstably. By the time the gas pressure builds up to the point where it is dynamically significant, the bubble wall velocity approaches supersonic speeds and the collapse continues, driven by the inertia of the inrushing liquid (hence the name ‘inertial cavitation’). If this phenomenon occurs far from any boundaries (or other bubbles), the gas is profoundly compressed, resulting in intense heating and pressure. Chemical reactions ensue and light is generated from the radiative re-combination of chemical species Citation[114–116]. Microstreaming can also result, particularly if the bubble is not destroyed upon collapse and rebound. Probably the most thermally relevant aspect of intertial cavitation is the generation of broadband acoustic emissions upon collapse Citation[99], Citation[117]. Owing to the frequency dependence of the absorption coefficient, high-frequency broadband noise emissions are readily absorbed by the surrounding tissue and converted into heat. In addition, these emissions provide a diagnostic tool for monitoring both the nature and extent of cavitation activity present in the HIFU field.
For exposure conditions relevant to HIFU, only bubbles smaller than about half the resonance size at the fundamental HIFU frequency will tend to behave inertially. Indeed, there exists an optimum size for which a bubble is most likely to undergo inertial growth and collapse Citation[8]. Allen et al. showed that this size is essentially the nonlinear resonance radius of the driven bubble Citation[118] and decreases with increasing HIFU frequency. For 1 MHz HIFU in an aqueous medium, this optimum radius is of order 1 µm. At 2.5 and 5.0 MHz, the optimum size decreases to approximately 0.4 µm and 0.25 µm, respectively.
Cavitation nucleation in tissue
Our discussion thus far has assumed that bubbles of adequate sizes pre-exist in a medium prior to being excited linearly or non-linearly by the incident HIFU field. In practice, adequate inhomogeneities must be present that will enable acoustically induced cavity nucleation upon exposure to ultrasound. These cavitation nuclei are typically pre-existing gas bodies or imperfectly wetted solids Citation[101], Citation[119]. In many aqueous systems, cavitation nuclei are plentiful, but it is not clear whether this is the case everywhere in human tissue. In the absence of suitable nuclei, the pressure required to initiate cavitation can be so large (on the order of 10 MPa) that the ensuing bubble behavior rapidly grows out of control and deleterious biological effects result. Recent studies suggest that the targeted region can be populated with gas nuclei by injection of ultrasound contrast agents Citation[120–125], which are stabilized microbubbles designed to enhance imaging contrast in blood Citation[126–132]. A detailed discussion of acoustic cavitation nucleation mechanismsCitation[1], Citation[119], Citation[133] and techniques Citation[134–136] is beyond the scope of this paper. For the purposes of this discussion, we assume that some form of nucleating agent is present and cavitation can be both initiated and sustained at relatively modest HIFU pressure amplitudes on the order of 1–3 MPa.
Mechanisms of cavitation-enhanced heating
HIFU-induced heating in tissue is directly dependent on the incident intensity and the local frequency-dependent absorption coefficient. As a result, stable and inertial cavitation will increase the rate at which acoustic energy is being converted into heat because they increase the length scales over which viscous absorption can occur, or redistribute some of the energy of the incident field as higher-frequency emissions that are more readily absorbed. The three primary mechanisms by which this enhanced heat deposition will occur—multiple scattering, absorption in the viscous boundary layer at the bubble wall and absorption of secondary acoustic emissions—are described in detail hereafter.
Whether bubbles are oscillating linearly or non-linearly, they will act as strong scatterers of the incident sound field. Multiple cavities contained within a volume of tissue will therefore effectively trap the incident acoustic energy, increasing the path length over which viscous absorption can occur within that region. Multiple scattering of the incident field will therefore result in enhanced heating over the volume of tissue containing bubbles.
Furthermore, the high shear stresses present in the boundary layer between an oscillating bubble and the surrounding medium will also lead to enhanced viscous absorption. Equation 2, which describes the bubble dynamics, includes a viscous stress term that is velocity-dependent and corresponds to the dissipative work done by the bubble on the surrounding medium. The bubble acts simultaneously as a vehicle for conversion of acoustic to mechanical energy, by virtue of the bubble dynamics, and from mechanical to thermal energy, by virtue of viscous dissipation. An estimate of the cycle-averaged deposited power per bubble comes from consideration of the average rate of work done by viscous stress and is given by Citation[99], Citation[137]This energy is dissipated solely as heat and depends on the shear viscosity μ of the medium, the equilibrium bubble size and the bubble dynamics.
When a bubble undergoes a violent collapse, the acceleration associated with its rebound is considerable and results in a very short burst of broadband sound. For micron and sub-micron bubbles pulsating inertially, this so-called secondary acoustic emission exceeds the HIFU energy scattered by the original bubble by orders of magnitude. The acoustic emission from a bubble undergoing volumetric pulsations is given by Citation[99], Citation[138]where r is radial distance from the center of the bubble and α is the frequency-dependent ultrasound attenuation coefficient for the medium. Two things are immediately obvious from Equation 4. First, since sound attenuation in tissue increases with increasing frequency, broadband emissions at high frequencies will be more readily absorbed than sound scattered from the primary HIFU field at the fundamental frequency. Second, the amplitude of the emission is a strong function of both wall velocity and acceleration. The acceleration term can dominate in the case of inertial collapses, and is greatest at the point of maximum collapse.
At a distance r, the power emitted as secondary noise emissions is given bywhere I is the acoustic intensity at the distance r. If the sound power emitted as secondary emissions at the surface of the bubble (r = R) is W0, then the thermal power deposited in the volume subtended by r is given by:
It is evident from Equations 4–6 that bubbles radiating the most noise will be the bubbles that are most likely to contribute to heating through the absorption of radiated sound power.
The relative significance of cavitation-enhanced heating due to increased absorption in the viscous boundary layer near the bubble wall, described by Equation 3, and of that due to the absorption of secondary acoustic emissions, given by Equation 6, deserves further investigation. Direct simulations of the bubble response, as given by Equation 2, have been performed to compute the heat deposited by these two mechanisms Citation[99], Citation[139], Citation[140]. The exact values of the shear viscosity and range of equilibrium bubble sizes are critically important to these calculations but are not known for biologically relevant media. Therefore, these simulations are set up as a parameter study in which the equilibrium bubble size was varied from 0.1 to 50 µm, and the shear viscosity was allowed to vary between 1 and 100 times the shear viscosity of water (0.001 N-s/m2).
Results obtained for a 1-MHz HIFU exposure at a pressure amplitude of 2.8 MPa are shown in and . Both plots reveal regions in which bubble-enhanced heating is maximal: shows maximum power depositions on the order of 50 mW, but only for the highest viscosities considered and for bubble sizes equal to, or somewhat larger than, the linear resonance radius of 3.72 µm. These larger bubbles are pulsating stably, and we conclude that, for these relatively low-level HIFU exposure conditions, stable cavitation is most effective at converting acoustical energy to heat via viscous loss. shows that similar levels of heat deposition can be achieved by the absorption of secondary acoustic emissions, but this enhanced heating is associated with a broad range of subresonant bubble sizes and candidate media viscosities. Bubbles in this parameter range undergo inertial collapse, and from this we conclude that inertial cavitation is more effective at converting acoustical energy to heat via the absorption of secondary acoustic emissions.
Figure 1. Heating from a single bubble exposed to 1 MHz HIFU at 2.8 MPa pressure amplitude. Power deposited via (a) viscous boundary layer heating and (b) absorption of secondary acoustic emissions (SAE) are plotted as a function of the equilibrium bubble radius and the viscosity of the medium Citation[99], Citation[139]. The linear resonance radius at 1 MHz is 3.72 µm.
![Figure 1. Heating from a single bubble exposed to 1 MHz HIFU at 2.8 MPa pressure amplitude. Power deposited via (a) viscous boundary layer heating and (b) absorption of secondary acoustic emissions (SAE) are plotted as a function of the equilibrium bubble radius and the viscosity of the medium Citation[99], Citation[139]. The linear resonance radius at 1 MHz is 3.72 µm.](/cms/asset/08926c36-ce8a-43d3-8839-24cc1eabbb2e/ihyt_a_219335_f0001_b.gif)
Allowable bubble sizes in tissue
The results of the simulations presented in suggest that both inertial and stable cavitation can induce comparable levels of enhanced heat deposition by different mechanisms, but for completely different ranges of equilibrium bubbles sizes. However, not all bubble sizes can necessarily exist in tissue under ultrasound exposure. Bubbles exposed to HIFU undergo a gradual growth in equilibrium size owing to a process known as rectified diffusion Citation[105], Citation[106], Citation[141–143] and, once they reach a critical radius, break up into smaller bubbles due to instabilities caused by surface perturbations. The specifics of these two processes are discussed in further detail by Yang Citation[144] and Holt and Roy Citation[99]. For now, it suffices to say that there exists an upper bound to allowable bubble sizes, and bubbles smaller than this size undergo a cyclic process of growth followed by breakup, yielding an asymptotic bubble size distribution that is limited at the lower end by surface tension and the upper end by surface instabilities.
For a given frequency and pressure amplitude, the range of allowable bubble sizes depends on both viscosity and dissolved gas concentration. For water-like viscosities and exposure conditions of 1 MHz and 2.5 MPa, the allowable size range is 10 nm–1 µm Citation[140], Citation[144] irrespective of the dissolved gas concentration. For such conditions, only inertial cavities can exist, and bubble-enhanced heating is due solely to the absorption of radiated noise. For viscosities greater than about 20 times that of water, the limiting bubble size distribution expands to 10 nm–2 µm for a degassed medium (0.1% of saturation), and once again only inertial cavitation is supported. However, in a gas-saturated medium, the range becomes 10 nm–18 µm. Both inertial and stable cavities can exist and contribute to heating, but only at the highest viscosities and dissolved gas concentrations.
It must be emphasized that the viscosities and gas concentrations encountered in vivo will vary with tissue type and physiological condition. Therefore, these numbers serve only as a qualitative guide of what one might expect to occur in vivo. Nevertheless, the modeling evidence suggests that, for most media and for the relatively low-level HIFU exposures under consideration, bubble enhanced heating is most likely to result from the absorption of broadband noise emissions generated by inertial cavitation. Thus we anticipate a correlation between a physical observable (broadband noise) and a desired effect (heating). This testable hypothesis is investigated further in the context of experimental observations of cavitation-enhanced heating.
Experimental observations of cavitation- enhanced heating
Tissue-mimicking materials
There have been several investigations of the correlation between cavitation activity and the resulting rate of heating, both in vivo Citation[82–84], Citation[138], Citation[145–148] and in vitro Citation[81], Citation[83], Citation[84], Citation[139]. In order to isolate the thermal effects due to cavitation and to develop techniques on how to best monitor and control the process, it is often desirable to carry out such experimentation under precisely known acoustic and thermal conditions in gel-based tissue-mimicking materials known as ‘tissue phantoms’ Citation[139], Citation[149–151]. These media are generally designed to match at least some of the physical properties relevant to HIFU treatment in tissue, such as attenuation, density and speed of sound. However, it must be noted a priori that no phantom will provide an exact match to many other relevant tissue properties, such as the absorption coefficient, cavitation threshold, coefficient of non-linearity, or rate of tissue perfusion, and that such in vitro studies are primarily aimed at developing a qualitative rather than quantitative understanding of the cavitational and other mechanisms involved in tissue heating.
One commonly used tissue phantom consists of agar gel, graphite particles and 1-propanol Citation[139]. The 1-propanol is used to ‘tune’ the sound speed of the phantom to match that of tissue. The graphite serves to introduce acoustic scatterers and thus enhances acoustic absorption. It can also provide nucleation sites for bubble formation. Indeed, the measured cavitation nucleation threshold for this material can be as low as 1.4 MPa at 1 MHz when saturated with gas. This is significantly less than thresholds reported in tissues, many of which exceed 4–6 MPa Citation[1], Citation[88–90]. The agar/graphite phantom is an ideal medium for promoting cavitation effects and monitoring HIFU-induced bubble behavior.
Cavitation detection
A typical experimental setup used to investigate HIFU-induced cavitation and its associated heating effects is depicted in . A single-element, 1.1 MHz, focused HIFU source (Sonic Concepts H-102 S/N-6) with a transmission aperture of 6 cm and a focal length of approximately 6 cm is driven using a sine-wave generator (Agilent 33250A) and power amplifier (ENI A150), and aimed at an agar-graphite tissue phantom. A needle hydrophone (not shown) can be embedded into the phantom to monitor the pressure field in situ. The HIFU half-power beam width is approximately 1 mm. Temperature is measured using a 125 µm diameter, bare-wire, Type-E thermocouple (not shown) positioned in the focal plane of the HIFU source and offset by approximately 0.5 mm with respect to the beam axis in order to reduce the possibility of cavitation occurring on the surface of the sensor.
Figure 2. Schematic of the HIFU generation (dashed line), passive cavitation detection (continuous line) and B-mode imaging (dotted line) apparatus.
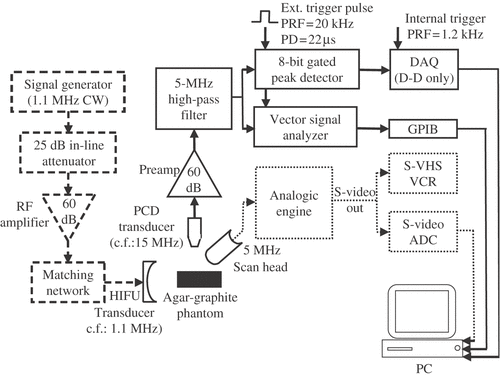
A key element in the apparatus is a tightly focused broadband acoustic sensor (Panametrics-NDT V313-SU), of centre frequency 15 MHz, positioned at 90 degrees and confocally with the HIFU beam, the objective of which is to isolate, detect and monitor broadband noise emissions resulting from inertial cavitation activity at the focus of the HIFU transducer. The signal received by the 15-MHz transducer is amplified by 60 dB (EE&G 5185) and filtered through a passive 5-MHz high-pass filter (Allen Avionics F5081-5PO-B) to remove any contributions from the HIFU fundamental, second, third and fourth harmonic frequencies arising from nonlinear sound propagation. The resulting signal is attenuated by 6 dB using a passive in-line attenuator (JFW 50F-006) so as to match the full 1-V input range of a digitizing peak detector (Panametrics CS-NDT 5607). This passive cavitation detection (PCD) scheme is therefore configured to sense broadband acoustic emissions from inertial cavitation, and not scattering from the primary HIFU beam from stable cavitation bubbles Citation[152], Citation[153]. It is a real time indicator of the extent of inertial cavitation activity present within its sensing volume, effectively defined by the focal region of the PCD transducer. Its output can be processed and displayed in real time, providing the user with a non-invasive ‘cavitation meter’. It can also be stored and later correlated with measured temperature elevations and observations of lesion formation.
Correlation of observed heating with cavitation activity
depicts the temperature elevation in an agar-graphite phantom following exposure of three different phantom locations to 1.1-MHz HIFU for 1 s at three different insonation amplitudes. Also plotted is the peak voltage from the PCD sampled in a 20 µsec window at a rate of 1000 samples/sec. At the lowest exposure pressure amplitude (1.65 MPa), the output of the PCD in indicates that no inertial cavitation occurs in the confocal region of the HIFU and PCD transducers. A modest rate of heating is observed under those conditions, resulting in a temperature elevation of less than 10°C during HIFU exposure, followed by cooling. This type of heating-cooling behavior is well-predicted by a standard heat conduction model, commonly known as the bioheat transfer equation (BHTE) Citation[99], Citation[154]. The BHTE takes into account thermal conduction, has sink terms to account for tissue perfusion and convective blood cooling (both zero for this phantom), and a source term to incorporate energy deposition from ultrasound absorption. At these low pressures, no cavitation activity is evident and linear propagation effects alone account for the observed heating.
Figure 3. (a) Measured temperature rise (labeled ‘Themocouple) and PCD output (labeled ‘PCD') as a function of time for a 1-s 1.1-MHz HIFU insonation of an agar-graphite tissue phantom at three different pressure amplitudes. No inertial cavitation occurs in (iii), whilst cavitation onsets halfway through the exposure in (ii) and at the start of exposure in (i). In (ii) and (iii), there is a dramatic increase in the observed rate of heating that is coincident with the onset of inertial cavitation activity. (b) Peak temperature rise with respect to ambient conditions vs. peak-positive acoustic pressure for the agar/graphite.
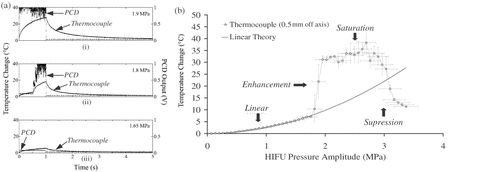
For a modest increase in HIFU pressure amplitude to 1.8 MPa, the heating rate initially observed in differs only slightly from that seen in . However, halfway through the HIFU exposure, there is a sudden and dramatic increase in heating coincident with an equally sudden and sustained burst of broadband emission. This strongly suggests that the origin of the enhanced heating phenomenon is related to the origin of acoustic emissions: inertial cavitation.
Finally, an additional small increase in the HIFU insonation amplitude to 1.9 MPa results in the immediate onset of both inertial cavitation and enhanced heating, as shown in . A temperature elevation of some 30°C is obtained over the 1-s exposure and greatly exceeds that obtained without bubbles present. The temperature rise predicted by the BHTE at 1.8 MPa in the absence of cavitation should not exceed the increase at 1.65 MPa by more than 33%, yet the measured elevation is almost triple that at 1.65 MPa. In this phantom, and for these relatively low pressures, bubbles dominate the heating process.
The temperature rise observed over a wide range of HIFU excitation amplitudes deserves further investigation. Shown in is the measured peak temperature elevation in the agar-graphite phantom exposed to 700-ms long tone bursts of high-intensity focused ultrasound. Each data point represents an average of five measurements taken at a given HIFU pressure amplitude, and the phantom was allowed to cool for 100 s between measurements. The data suggest four distinct regions, presumably corresponding to differing regimes of bubble activity: linear heating, enhancement, saturation, and suppression.
As shown in , the inertial cavitation threshold for this agar-graphite phantom is 1.8 MPa. At HIFU pressure amplitudes below that threshold, shows that the temperature rise is quadratic with pressure, as predicted by the BHTE. As the pressure is increased beyond 1.8 MPa, the level of inertial cavitation activity gradually increases and coincides with a sudden and dramatic increase in the rate of heating, with peak temperatures rising greatly in excess of those predicted by linear heating models in the absence of cavitation. However, further increases in the HIFU pressure amplitude beyond 2 MPa do not yield any further increase in the peak temperature reached. This is presumably due to gradual saturation of the HIFU focal region with cavitation activity. For excitation pressures beyond some 3 MPa, the peak temperature recorded by the thermocouple suddenly decreases. At these relatively high HIFU pressures, it is likely that cavitation is initiated in the prefocal region as well as in the focal region: this will result in shielding of the original HIFU focus, due to increased attenuation through the prefocal bubble cloud, and will lead to enhanced heat deposition ahead of the intended focus. When bubbles and tissue heating are concerned, more power is not necessarily desirable, both in terms of heat deposition efficiency at the focus and the likelihood of generating unpredictably shaped and positioned lesions that extend well into the prefocal region.
It is important to note that these results were obtained in a gassy phantom that has a lower cavitation threshold and lower absorption coefficient than most tissues. As a result, it is much more susceptible to cavitation activity. The relative contribution of cavitation-enhanced heating with respect to heating caused by absorption due to linear and non-linear sound propagation clearly depends on the relationship between the cavitation threshold pressure and that for which significant non-linear propagation occurs. This has been investigated previously Citation[83], Citation[93–95], Citation[155] and will not be addressed here. However, the effect of increasing temperature during HIFU exposure on cavitation-enhanced heating deserves further investigation and is discussed hereafter.
Role of ambient temperature
As tissue heats up during HIFU exposure, a number of significant changes take place. Proteins denature, leading to an increase in tissue stiffness and attenuation coeffici Citation[156], Citation[157]. The bubbles are stress-confined and may not be able to grow and collapse inertially Citation[102]. This potentially important effect remains poorly understood and will not be discussed here.
However, what is known is that the hot tissue becomes supersaturated and proceeds to outgas into existing bubbles. The bubble equilibrium size increases and, in the process, existing cavities are less likely to grow and collapse inertially due to the mass loading of the surrounding tissue. Finally, the increasing temperature causes a significant increase in vapor pressure. As a result, the vapor inside the bubble is less likely to condense during the compression phase and serves to inhibit inertial collaps Citation[158], Citation[159]. The combined result of all these effects is a reduction in the bubble radial expansion ratio, defined as Rmax/Rmin. A decrease in the expansion ratio leads to a decrease in the broadband noise emissions following collapse and, by extension, to a reduction in the energy available for cavitation-enhanced HIFU heati Citation[84], Citation[160].
Calculations performed using the models described earlier (Equations 2–6) confirm this observation. For an air bubble in water, the thermal power deposited by secondary acoustic emissions and viscous boundary layer heating decreases by 50% and 60% respectively when the water temperature is increased from 20°C to 90°C Citation[84]. This implies that, as the medium heats up, inertial cavitation bubbles cease to act as either thermal or broadband noise sources. At this point, a stable cavitation field in the form of acoustically driven boiling bubbles remains. However, visual observations suggest that these bubbles become very large and will therefore impact the local sound field primarily through acoustic scattering. For the reasons outlined in the second section, sound scattering from boiling cavitation at the focus enhances pre-focal heating. This effect is undesirable, as it can lead to the generation of malformed ‘tadpole-shaped’ lesions of unpredictable shape, size and position. However, boiling bubbles are sufficiently large that they become clearly visible as a bright region in diagnostic ultrasound pulse-echo images of the treatment volume. This increased echogenicity has proven beneficial for treatment monitoring and guidance using diagnostic ultrasound Citation[73], Citation[90], Citation[161–164].
Monitoring and control of cavitation during HIFU
In the previous section, we demonstrated that at relatively modest HIFU excitation amplitudes and at sub-boiling temperatures, inertial cavitation can greatly enhance the rate of heating and provides a mechanism for increased heat deposition efficiency at the focus. However, the rapidly changing conditions and increasing temperature during HIFU exposure have also been shown to impact the bubble dynamics and their resulting heating enhancement, leading to potentially undesirable pre-focal effects. This emphasizes the need for adequate monitoring and control of inertial cavitation activity during HIFU exposure, if it is to be harnessed for optimal treatment delivery.
Cavitation monitoring
HIFU therapy is often delivered under ultrasound guidance, utilizing linear phased-arrays driven in pulse-echo mode to image the HIFU focal region. Previous investigators have suggested that relatively subtle grayscale changes in such ultrasound images during HIFU exposure could provide a direct indication of inertial cavitation activity. To investigate this hypothesis, an agar-graphite tissue phantom was exposed to 1.1-MHz HIFU, whilst cavitation activity at the focus was monitored simultaneously using the previously described passive cavitation detector (PCD) and a 5-MHz ultrasound imager, as depicted in . The PCD signal was fed directly to an 8-bit peak detector with a 20-µs detection window, the output of which was transferred digitally to a 12-bit DAQ board (AT-MIO-16E-1, National Instruments) on a PC and displayed as a function of time. The peak detector also passed the raw PCD signal to a 10-MHz vector signal analyzer (89410A, Hewlett-Packard), which displays the frequency spectrum of the received PCD signal in real time. The imaging scan head (Analogic Corp. 8802) is a 192-element array with bandwidth 3.5–6 MHz, driven by an Analogic engine in pulse-echo mode. The S-video output from the engine was fed directly to an S-Video ADC on the PC, as well as recorded using an S-VHS VCR.
A B-mode image of the HIFU focal region prior to exposure is shown in , where the confocal region of the HIFU and PCD transducers is denoted by the white cross. The HIFU pressure amplitude was ramped up in steps of 0.15 MPa, as shown on the top axis of , every 5 s, as indicated on the bottom axis of . At the end of the 5-s interval for which a new HIFU excitation amplitude had been imposed, the HIFU field was switched off and the B-mode image was visually examined for the appearance of a noticeable hyperechogenic region. If none was identified, the confocal position of the HIFU, PCD and imaging transducers was moved to a different location in the phantom, whilst maintaining the propagation path length to each transducer constant. The HIFU amplitude was then ramped up again from zero in 5-s intervals until the next highest excitation amplitude increment was reached, and the procedure was repeated until a hyperechogenic region became visible on the B-mode image.
Figure 4. Comparison between peak PCD output voltage, the frequency spectra of the PCD-received traces and the pre- and post-HIFU B-mode images obtained whilst ramping up the input voltage to the HIFU transducer every 5 s (bottom x-axis), thus increasing the peak negative focal pressure in steps of 0.15 MPa (top x-axis). The confocal position of the HIFU, PCD and imaging transducers in the agar-graphite phantom is indicated by the white cross on the B-mode images, with the HFU transducer being to the left of the image, the PCD transducer pointing out of the image and the imaging transducer lying above the image. At a peak negative focal pressure of 0.9 MPa, a sudden increase in peak PCD voltage is observed, which is coincident with a clear jump in broadband noise emissions. However, a hyperechogenic region only becomes visible on the B-mode image at t = 2 s once the HIFU excitation amplitude had been ramped up to 1.35 MPa. This strongly suggests that the appearance of a bright-up on B-mode images cannot be assumed to be coincident with the onset of inertial cavitation activity.
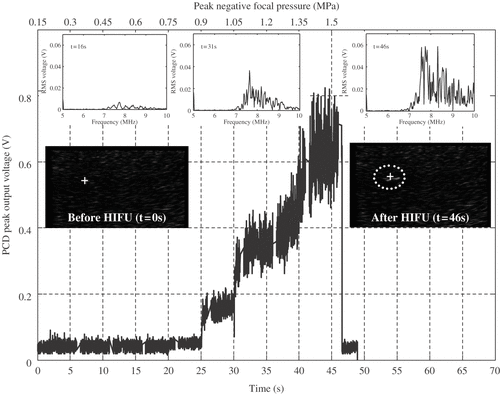
Such a region became visible for the first time following 45 s of HIFU exposure, by the end of which the HIFU amplitude had been ramped up every 5 s in 0.15-MPa increments to 1.35 MPa. The resulting B-mode image, shown in at t = 46 s, shows a clearly visible bright-up that is coincident with the confocal region of the HIFU, PCD and imaging transducers (indicated by the white cross). However, closer investigation of the peak value and spectral content of the PCD signal over the 45-s exposure, also plotted in , strongly suggests that inertial cavitation activity had been initiated as early as t = 20 s, when the HIFU pressure amplitude was 0.9 MPa. The spectral content of the PCD signal further confirms this, by exhibiting a clear jump in broadband noise emissions between t = 16 s and t = 31 s. In the time interval between t = 20 s and t = 45 s, both the PCD peak value and spectral content indicate that the level of inertial cavitation activity continues to increase and is sustained throughout the exposure, yet a hyperechogenic region does not become visible until a pressure amplitude of 1.35 MPa is reached and maintained for 5 s until t = 45 s.
From this, we conclude that the appearance of a hyperechogenic region on B-mode images constitutes neither a necessary nor a sufficient condition for inertial cavitation to have occurred during HIFU exposure Citation[165]. As suggested in the previous section, the appearance of a hyperechogenic region is most likely linked to the formation of large, boiling cavities as the temperature is increased by exposure to HIFU. Similar observations have been reported during experimentation in vivo by other investigatorsCitation[90], Citation[161], Citation[166]. In general, a PCD scheme constitutes the most reliable, cost-effective and sensitive means of real-time cavitation detection during HIFU exposure. However, when targeting deep-seated organs such as the liver or kidney with HIFU, the large ultrasound propagation path could make high-frequency secondary acoustic emissions difficult to detect. Many challenges remain in order to develop truly clinically applicable, reliable means of passive cavitation detection.
Control of inertial cavitation
As discussed in the third section, at relatively high HIFU excitation amplitudes inertial cavitation activity can migrate out of the focal region towards the HIFU transducer, leading to undesirable prefocal damage and shielding of the original focus. Potential evidence of this is shown in , where the rms voltage detected by the PCD during 2.89 MPa HIFU exposure of an agar-graphite phantom is shown to decay rapidly during continuous-wave (CW) excitation, suggesting that the bubble cloud is migrating towards the HIFU transducer and out of the field of view of the PCD. It must be noted that some of the observed decrease could also be due to the effect of increasing ambient temperature, as discussed earlier. However, shows that switching to a 20% duty cycle following 1 s of CW HIFU excitation, as previously suggested Citation[167], can help sustain the level of inertial cavitation activity throughout the HIFU exposure. Three particular implementations of a 20% duty cycle following 1 s of HIFU excitation are investigated here, using a 5-, 10- and 100-cycle on-time, and the results suggest that shorter pulses are more effective at preventing shielding of the original focus than longer pulses.
Figure 5. RMS voltage received by a passive cavitation detector (PCD) positioned confocally with the HIFU transducer during excitation of an agar-graphite tissue phantom at a 2.89 MPa peak negative focal pressure amplitude. In all cases, the phantom was exposed to 1-s continuous-wave (CW) excitation, followed by a further 60 s either CW exposure (dotted line) or 20% duty-cycle excitation (continuous dark grey, light grey and black lines). A rapid decay in cavitation activity is observed during sustained CW exposure, suggesting movement of the bubble cloud towards the HIFU transducer and out of the field of view of the PCD. Different implementations of 20% duty cycle HIFU excitations, using either 5 cycles (dark grey), 10 cycles (light grey) or 100 cycles (black continuous) on-time, make it possible to sustain cavitation activity in the focal region for extended periods of time. The 10-cycle implementation appears most effective at sustaining the initially generated level of inertial cavitation activity.
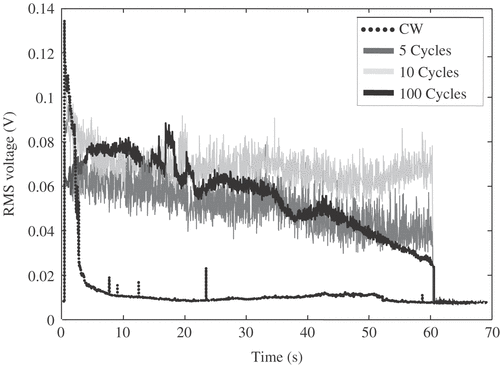
Such schemes for controlling inertial cavitation Citation[167–173] may well play a crucial role in future attempts to harness cavitation-enhanced heating for optimal treatment delivery. However, it must be noted that, during 20% duty cycle excitation, only one fifth of the power delivered during CW exposure is received at the focus. The impact that this has on the resulting rate of heating needs to be investigated further, as does the effect of varying other parameters such as HIFU frequency and excitation amplitude in real-time.
Conclusion
Cavitation has tremendous potential to enhance the rate of heating and to improve the spatial localization of heat deposition during HIFU treatment. Under exposure conditions relevant to HIFU therapy, it has been shown that most of this enhancement is due to the re-radiation of the incident sound field as broadband noise emissions by inertially cavitating bubbles, whilst scattering and viscous absorption in the bubble boundary layer play a relatively minor role. However, as the ambient temperature is increased and the properties of tissue change, inertial cavitation gradually shuts down and can give rise to larger, boiling cavities that play a significant thermal role through scattering. These boiling cavities currently play a useful role in monitoring HIFU therapy, as they are readily visible on B-mode ultrasound images, but could also be indicative of over-treatment of the target region. New means of monitoring and controlling inertial cavitation activity are being proposed, which could be used to exploit the enhanced heating, treatment localization and treatment monitoring advantages that could be provided by inertially cavitating microbubbles. The behaviour of such microbubbles in temperature-varying viscoelastic media is still poorly understood, and many challenges remain in terms of developing truly clinically relevant means of monitoring and controlling inertial cavitation activity. However, it is hoped that, in due course, these challenges can be overcome and give rise to a novel, efficacious, cavitation-based HIFU therapy system.
Acknowledgements
The authors acknowledge the efforts of students and colleagues whose work is featured in this article: Patrick Edson, R. Glynn Holt, Charles Thomas, Jamie R. T. Collin and Adam Muckle. Authors Roy and Farny acknowledge generous financial support of their primary research sponsors: the United States Army (award number DAMD17-02-2-0014, for which The US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD is the awarding and administering acquisition office), and the Bernard M. Gordon Center for Subsurface Sensing and Imaging Systems (funded under the Engineering Research Centers Program of the National Science Foundation; award number EEC-9986821). Dr Coussios's work in this area has been in part supported by the F. V. Hunt Post-Doctoral Fellowship of the Acoustical Society of America. Finally, both Dr Coussios and Dr Ter Haar would like to acknowledge the generous ongoing support of the UK's Engineering and Physical Sciences Research Council.
References
- Church CC. Spontaneous homogeneous nucleation, inertial cavitation and the safety of diagnostic ultrasound. Ultrasound Med Biol 2002; 28: 1349–1364
- Miller MW, Everbach EC, Miller WM, Battaglia LF. Biological and environmental factors affecting ultrasound-induced hemolysis in vitro: 2. Medium dissolved gas (pO(2)) content. Ultrasound Med Biol 2003; 29: 93–102
- Apfel RE. Acoustic cavitation series. 4. Acoustic cavitation inception. Ultrasonics 1984; 22: 167–173
- Neppiras EA. Acoustic cavitation. Phys Rep 1980; 61: 159–251
- Apfel RE. Acoustic cavitation. Methods of experimental physics, Edmonds. Academic. 1981; 19: 355–411
- Apfel RE. Acoustic cavitation prediction. J Acoust Soc Am 1981; 69: 1624
- Crum LA, Fowlkes JB. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature 1986; 319: 52–54
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am 1990; 88: 2059–2069
- Miller DL. Acoustic cavitation series. 6. Gas body activation. Ultrasonics 1984; 22: 261–269
- Miller DL. A review of the ultrasonic bioeffects of microsonation, gas-body activation, and related cavitation-like phenomena. Ultrasound Med Biol 1987; 13: 443–470
- Miller DL. Update on safety of diagnostic ultrasonography. J Clin Ultrasound 1991; 19: 531–540
- Cardinale A, Lagalla R, Giambanco V, Aragona F. Bioeffects of ultrasound: An experimental study on human embryos. Ultrasonics 1991; 29: 261–263
- Holland CK, Deng CX, Apfel RE, Alderman JL, Fernandez LA, Taylor KJW. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med Biol 1996; 22: 917–925
- Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol 1996; 22: 1131–1154
- Barnett SB, Ter Haar GR, Ziskin MC, Rott HD, Duck FA, Maeda K. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med Biol 2000; 26: 355–366
- Fowlkes JB, Holland CK. Mechanical bioeffects from diagnostic ultrasound: AIUM consensus statements—Introduction. J Ultrasound Med 2000; 19: 69–72
- Holland CK, Roy RA, Biddinger PW, Disimile CJ, Cawood C. Cavitation mediated rat lung bioeffects from diagnostic ultrasound. J Acoust Soc Am 2001; 109: 2433
- Church CC. A theoretical study of acoustic cavitation produced by “positive-only” and “negative-only” pressure waves in relation to in vivo studies. Ultrasound in Medicine and Biology 2003; 29: 319–330
- Holland CK, Apfel RE. An improved theory for the prediction of microcavitation thresholds. IEEE Trans Ultrason Ferroelectr Freq Control 1989; 36: 204–208
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol 1991; 17: 179–185
- Church CC. Frequency, pulse length, and the mechanical index. Acoust Res Lett Online-Arlo 2005; 6: 162–168
- Ter Haar GR. Ultrasonic contrast agents: safety considerations reviewed. European Journal of Radiology 2002; 41: 217–221
- Vaezy S, Martin R, Schmiedl U, Caps M, Taylor S, Beach K, Carter S, Kaczkowski P, Keilman G, Helton S, et al. Liver hemostasis using high-intensity focused ultrasound. Ultrasound Med Biol 1997; 23: 1413–1420
- Vaezy S, Martin R, Yaziji H, Kaczkowski P, Keilman G, Carter S, Caps M, Chi EY, Bailey M, Crum L. Hemostasis of punctured blood vessels using high-intensity focused ultrasound. Ultrasound Med Biol 1998; 24: 903–910
- Martin RW, Vaezy S, Kaczkowski P, Keilman G, Carter S, Caps M, Beach K, Plettt M, Crum L. Hemostasis of punctured vessels using Doppler-guided high-intensity ultrasound. Ultrasound Med Biol 1999; 25: 985–990
- Vaezy S, Martin R, Kaczkowski P, Keilman G, Goldman B, Yaziji H, Carter S, Caps M, Crum L. Use of high-intensity focused ultrasound to control bleeding. J Vasc Surg 1999; 29: 533–542
- Poliachik SL, Chandler WL, Mourad PD, Ollos RJ, Crum LA. Activation, aggregation and adhesion of platelets exposed to high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1567–1576
- Vaezy S, Martin R, Crum L. High intensity focused ultrasound: A method of hemostasis. Echocardiography 2001; 18: 309–315
- Hwang JH, Vaezy S, Martin RW, Cho MY, Noble ML, Crum LA, Kimmey MB. High-intensity focused US: A potential new treatment for GI bleeding. Gastrointestinal Endoscopy 2003; 58: 111–115
- Vaezy S, Noble ML, Keshavarzi A, Paun M, Prokop AF, Cornejo C, Sharar S, Chi EY, Crum LA, Martin RW. Liver hemostasis with high-intensity ultrasound—Repair and healing. J Ultrasound Med 2004; 23: 217–225
- Vaezy S, Vaezy S, Starr F, Chi E, Cornejo C, Crum L, Martin RW. Intra-operative acoustic hemostasis of liver: Production of a homogenate for effective treatment. Ultrasonics 2005; 43: 265–269
- Zderic V, Keshavarzi A, Noble ML, Paun M, Sharar SR, Crum LA, Martin RW. Hemorrhage control in arteries using high-intensity focused ultrasound: A survival study. Ultrasonics 2006; 44: 46–53
- Rivens IH, Rowland I, Denbow M, Fisk NM, Leach MO, Ter Haar GR. Focused ultrasound surgery-induced vascular occlusion in fetal medicine. Proceedings of SPIE 2003; 3249: 260
- Denbow ML, Rivens IH, Rowland IJ, Leach MO, Fisk NM, Ter Haar GR. Preclinical development of noninvasive vascular occlusion with focused ultrasonic surgery for fetal therapy. Am J Obstet Gynecol 2000; 182: 387–392
- Trubestein G, Engel C, Etzel F, Sobbe A, Cremer H, Stumpff U. Thrombolysis by ultrasound. Clin Sci Mol Med Suppl 1976; 3: 697s–698s
- Lauer CG, Burge R, Tang DB, Bass BG, Gomez ER, Alving BM. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation 1992; 86: 1257–1264
- Luo H, Steffen W, Cercek B, Arunasalam S, Maurer G, Siegel RJ. Enhancement of thrombolysis by external ultrasound. Am Heart J 1993; 125: 1564–1569
- Sehgal CM, Leveen RF, Shlansky-Goldberg RD. Ultrasound-assisted thrombolysis. Invest Radiol 1993; 28: 939–943
- Olsson SB, Johansson B, Nilsson AM, Olsson C, Roijer A. Enhancement of thrombolysis by ultrasound. Ultrasound Med Biol 1994; 20: 375–382
- Kornowski R, Meltzer RS, Chernine A, Vered Z, Battler A. Does external ultrasound accelerate thrombolysis? Results from a rabbit model. Circulation 1994; 89: 339–344
- Shlansky-Goldberg RD, Cines DB, Sehgal CM. Catheter-delivered ultrasound potentiates in vitro thrombolysis. J Vascular and Interventional Radiology 1996; 7: 313–320
- Hamm CW, Steffen W, Terres W, De Scheerder I, Reimers J, Cumberland D, Siegel RJ, Meinertz T. Intravascular therapeutic ultrasound thrombolysis in acute myocardial infarctions. Am J Cardiol 1997; 80: 200–204
- Tachibana K. Prototype therapeutic ultrasound emitting catheter for accelerating thrombolysis. Am Inst Ultrasound Med 1997; 16: 529–535
- Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med 2001; 20: 1313–1325
- Shaw GJ, Meunier JM, Cheng JY, Holland CK. Duty cycle dependence of ultrasound enhanced thrombolysis in an in-vitro human clot model. Ann Emerg Med 2005; 46: S27
- Parikh DS, Tiukinhoy-Laing S, Huang SL, Holland CK, MacDonald RC, McPherson DD, Klegerman ME. Targeting of tissue plasminogen activator-loaded echogenic liposomes for site-specific thrombolysis. Arterioscler Thromb Vasc Biol 2006; 26: E102
- Tata DB, Biglow J, Wu J, Tritton TR, Dunn F. Ultrasound-enhanced hydroxyl radical production from two clinically employed anti-cancer drugs, adriamycin and mitomycin C. Ultrason Sonochem 1996; 3: 39–45
- Umemura SI, Yumita N, Nishigaki R, Umemura K. Sonochemical activation of hematoporphyrin: A potential modality for cancer treatment. Ultrason Symp 1989 Proc, IEEE 1989 1989; 955–960
- Jeffers J, Feng RQ, Fowlkes JB, Brenner DE, Cain CA. Sonodynamic therapy: Activation of anticancer agents with ultrasound. Ultrason Symp 1991 Proc, IEEE 1991 1991; 1367–1370
- Miyoshi N, Misik V, Fukuda M, Riesz P. Effect of gallium-porphyrin analogue ATX-70 on nitroxide formation from a cyclic secondary amine by ultrasound: On the mechanism of sonodynamic activation. Radiat Res 1995; 143: 194–202
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001; 220: 640–646
- Mesiwala AH, Farrell L, Wenzel HJ, Silbergeld DL, Crum LA, Winn HR, Mourad PD. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol 2002; 28: 389–400
- Hynynen K, McDannold N, Martin H, Jolesz FA, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison (R)). Ultrasound Med Biol 2003; 29: 473–481
- McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 2004; 51: 913–923
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood–brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004; 30: 979–989
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005; 24: 12–20
- McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood–brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol 2005; 31: 1527–1537
- Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood–brain barrier due to 260-kHz ultrasound bursts: A method for molecular imaging and targeted drug delivery. J Neurosurg 2006; 105: 445–454
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci USA 2006; 103: 11719–11723
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006; 340: 1085–1090
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: Association with cavitation activity. Phys Med Biol 2006; 51: 793–807
- Bao SP, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 1997; 23: 953–959
- Miller DL, Bao SP, Gies RA, Thrall BD. Ultrasonic enhancement of gene transfection in murine melanoma tumors. Ultrasound Med Biol 1999; 25: 1425–1430
- Miller DL, Dou CY, Song JM. DNA transfer and cell killing in epidermoid cells by diagnostic ultrasound activation of contrast agent gas bodies in vitro. Ultrasound Med Biol 2003; 29: 601–607
- Everbach EC, Francis CW. Cavitational mechanisms in ultrasound-accelerated thrombolysis at 1 MHz. Ultrasound Med Biol 2000; 26: 1153–1160
- Datta S, Coussios CC, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland CK. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol 2006; 32: 1257–1267
- Miller DL, Pislaru SV, Greenleaf JF. Sonoporation: Mechanical DNA delivery by ultrasonic cavitation. Som Cell Mol Genet 2002; 27: 115–134
- Ohl CD, Arora M, Ikink R, De Jong N, Versluis M, Delius M, Lohse D. Sonoporation from jetting cavitation bubbles. Biophys J 2006; 91: 4285–4295
- Wu J, Ross JP, Chui JF. Repairable sonoporation generated by microstreaming. The Journal of the Acoustical Society of America 2002; 111: 1460–1464
- Fry WJ, Fry RB. Temperature changes produced in tissue during ultrasonic irradiation. J Acoust Soc Am 1953; 25: 6–11
- Barnard JW, Fry WJ, Fry FJ, Krumins RF. Effects of high intensity ultrasound on the central nervous system of the cat. J Comp Neurol 1955; 103: 459–484
- Fry WJ, Mosberg WH, Jr, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954; 11: 471–478
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005; 5: 321–327
- Rooney JA, Gammell PM, Hestenes JD, Chin HP, Blankenhorn DH. Velocity and attenuation of sound in arterial tissues. J Acoust Soc Am 1982; 71: 462–466
- Parker KJ. Ultrasonic attenuation and absorption in liver tissue. Ultrasound Med Biol 1983; 9: 363–369
- Hill CR, Bamber JC, Haar GR. Physical principles of medical ultrasonics. John Wiley & Sons. 2004
- Chivers RC, Hill CR. Ultrasonic attenuation in human tissue. Ultrasound Med Biol 1975; 2: 25–29
- Hynynen K, Watmough DJ, Mallard JR. Design of ultrasonic transducers for local hyperthermia. Ultrasound Med Biol 1981; 7: 397–402
- Hill CR. Optimum acoustic frequency for focused ultrasound surgery. Ultrasound Med Biol 1994; 20: 271–277
- Hynynen K. The threshold for thermally significant cavitation in dog thigh muscle in vivo. Ultrasound Med Biol 1991; 17: 157–169
- Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 2001; 27: 1399–1412
- Miller DL, Gies RA. The interaction of ultrasonic heating and cavitation in vascular bioeffects on mouse intestine. Ultrasound Med Biol 1998; 24: 123–128
- Chen WS, Lafon C, Matula TJ, Vaezy S, Crum LA. Mechanisms of lesion formation in high intensity focused ultrasound therapy. Acoust Res Lett Online 2003; 4: 41–46
- Farny CH. Identifying and monitoring the roles of cavitation in heating from high intensity focussed ultrasound. Boston University, Boston 2006
- Leighton TG. The acoustic bubble. Academic Press, London 1994
- Mellen RH. Ultrasonic spectrum of cavitation noise in water. J Acoust Soc Am 1954; 26: 356–360
- Holt RG, Crum LA. Acoustically forced-oscillations of air bubbles in water—Experimental results. J Acoust Soc Am 1992; 91: 1924–1932
- Hynynen K. The role of nonlinear ultrasound propagation during hyperthermia treatments. Med Phys 1991; 18: 1156–1163
- Yang X, Church CC. A model for the dynamics of gas bubbles in soft tissue. J Acoust Soc Am 2005; 118: 3595–3606
- Rabkin BA, Zderic V, Vaezy S. Hyperecho in ultrasound images of HIFU therapy: Involvement of cavitation. Ultrasound Med Biol 2005; 31: 947–956
- Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am 1994; 95: 1641–1649
- Damianou CA, Hynynen K, Fan XB. Evaluation of accuracy of a theoretical-model for predicting the necrosed tissue volume during focused ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control 1995; 42: 182–187
- Meaney PM, Cahill MD, Ter Haar GR. The intensity dependence of lesion position shift durig focused ultrasound surgery. Ultrasound Med Biol 2000; 26: 441–450
- Bailey MR, Couret LN, Sapozhnikov OA, Khokhlova VA, ter Haar G, Vaezy S, Shi XG, Martin R, Crum LA. Use of overpressure to assess the role of bubbles in focused ultrasound lesion shape in vitro. Ultrasound Med Biol 2001; 27: 695–708
- Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum LA. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. J Acoust Soc Am 2006; 119: 1834–1848
- Prosperetti A. Thermal effects and damping mechanisms in forced radial oscillations of gas-bubbles in liquids. J Acoust Soc Am 1977; 61: 17–27
- Prosperetti A. Acoustic cavitation series. 2. Bubble phenomena in sound fields 1. Ultrasonics 1984; 22: 69–78
- Minnaert M. On musical air bubbles and the sounds of running water. Philos Mag 1933; 16: 235–248
- Holt RG, Roy RA. Bubble dynamics in therapeutic ultrasound. Bubble and particle dynamics in acoustic fields: Modern trends and applications, A Dionikov. Transworld Research Network, Kerala 2005; 108–229
- Prosperetti A. Acoustic cavitation series 3. Bubble phenomena in sound fields 2. Ultrasonics 1984; 22: 115–124
- Apfel RE. Acoustic cavitation. Methods Exp Phys (ed PD Edmonds) 1981; 19: 355–411
- Yang XM, Church CC. A simple viscoelastic model for soft tissues in the frequency range 6–20 MHz. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: 1404–1411
- Keller JB, Miksis M. Bubble oscillations of large amplitude. J Acoust Soc Am 1980; 68: 628–633
- Prosperetti A, Lezzi A. Bubble dynamics in a compressible liquid 1. 1st-order theory. J Fluid Mech 1986; 168: 457–478
- Crum LA, Hansen GM. Generalized equations for rectified diffusion. J Acoust Soc Am 1982; 72: 1586–1592
- Church CC. Prediction of rectified diffusion during nonlinear bubble pulsations at biomedical frequencies. J Acoust Soc Am 1988; 83: 2210–2217
- Elder SA. Cavitation microstreaming. J Acoust Soc Am 1959; 31: 54–64
- Nyborg WLM. Acoustic streaming. Physical Acoustics, WP Mason. Academic Press, New York 1965; IIB: 265–331
- Miller DL. Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J Acoust Soc Am 1988; 84: 1378–1387
- Wu J, Du G. Streaming generated by a bubble in an ultrasound field. J Acoust Soc Am 1997; 101: 1899–1907
- Rooney JA. Acoustic streaming as a mechanism in treatment of suspensions. J Acoust Soc Am 1970; 48: 114–117
- Rooney JA. Hemolysis near an ultrasonically pulsating gas bubble. Science 1970; 169: 869–871
- Williams AR. Disorganization and disruption of mammalian and amoeboid cells by acoustic microstreaming. J Acoust Soc Am 1972; 52: 688–693
- Harvey EN. Sonoluminescence and sonic chemiluminescence. J Am Chem Soc 1939; 61: 2392–2398
- Suslick KS. Sonochemistry. Science 1990; 247: 1439–1445
- Roy RA. Physical aspects of sonoluminescence from acoustic cavitation. Ultrason Sonochem 1994; 1: S5–S8
- Hilgenfeldt S, Lohse D. The acoustics of diagnostic microbubbles: Dissipative effects and heat deposition. Ultrasonics 2000; 38: 99–104
- Allen JS, Roy RA, Church CC. On the role of shear viscosity in mediating inertial cavitation from short-pulse, megahertz-frequency ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control 1997; 44: 743–751
- Atchley AA, Prosperetti A. The crevice model of bubble nucleation. J Acoust Soc Am 1989; 86: 1065–1084
- Yu T, Wang G, Hu K, Ma P, Bai J, Wang Z. A microbubble agent improves the therapeutic efficiency of high intensity focused ultrasound: A rabbit kidney study. Urol Res 2004; 32: 14–19
- Umemura S, Kawabata K, Sasaki K. In vivo acceleration of ultrasonic tissue heating by microbubble agent. IEEE Trans Ultrason Ferroelectr Freq Control 2005; 52: 1690–1698
- Kaneko Y, Maruyama T, Takegami K, Watanabe T, Mitsui H, Hanajiri K, Nagawa H, Matsumoto Y. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur Radiol 2005; 15: 1415–1420
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006; 241: 95–106
- Apfel RE. Vapor nucleation at a liquid-liquid interface. J Chem Phys 1971; 54: 62–63
- Miller DL, Thomas RM. Ultrasound contrast agents nucleate inertial cavitation in-vitro. Ultrasound Med Biol 1995; 21: 1059–1065
- Ophir J, Parker KJ. Contrast agents in diagnostic ultrasound. Ultrasound Med Biol 1989; 15: 319–333
- De Jong N, Ten Cate FJ, Lancee CT, Roelandt JR, Bom N. Principles and recent developments in ultrasound contrast agents. Ultrasonics 1991; 29: 324–330
- Church CC. The effects of an elastic solid-surface layer on the radial pulsations of gas-bubbles. J Acoust Soc Am 1995; 97: 1510–1521
- Allen JS, May DJ, Ferrara KW. Dynamics of therapeutic ultrasound contrast agents. Ultrasound Med Biol 2002; 28: 805–816
- Stride E, Saffari N. Theoretical and experimental investigation of the behaviour of ultrasound contrast agent particles in whole blood. Ultrasound Med Biol 2004; 30: 1495–1509
- Poliachik SL, Chandler WL, Mourad PD, Bailey MR, Bloch S, Cleveland RO, Kaczkowski P, Keilman G, Porter T, Crum LA. Effect of high-intensity focused ultrasound on whole blood with and without microbubble contrast agent. Ultrasound Med Biol 1999; 25: 991–998
- Coussios CC, Holland CK, Jakubowska L, Huang SL, MacDonald RC, Nagaraj A, McPherson DD. In vitro characterization of liposomes and Optison (R) by acoustic scattering at 3.5 MHz. Ultrasound Med Biol 2004; 30: 181–190
- Miller DL, Williams AR. Nucleation and evolution of ultrasonic cavitation in a rotating exposure chamber. J Ultrasound Med 1992; 11: 407–412
- Farny CH, Wu TM, Holt RG, Murray TW, Roy RA. Nucleating cavitation from laser-illuminated nano-particles. Acoust Res Lett Online 2005; 6: 138–143
- Miller DL, Kripfgans OD, Fowlkes JB, Carson PL. Cavitation nucleation agents for nonthermal ultrasound therapy. J Acoust Soc Am 2000; 107: 3480–3486
- Xu Z, Fowlkes JB, Cain CA. A new strategy to enhance cavitational tissue erosion using a high-intensity, initiating sequence. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: 1412–1424
- Holt RG, Roy RA, Edson PE, Yang XM. Bubbles and HIFU: The good, the bad and the ugly. International Symposium of Therapeutic Ultrasound 2003, MA Andrew, LA Crum, S Vaezy. American Institute of Physics, Seattle 2003; 120–131
- Hilgenfeldt S, Lohse D, Zomack M. Sound scattering and localized heat deposition of pulse-driven microbubbles. J Acoust Soc Am 2000; 107: 3530–3539
- Edson PL. The role of acoustic cavitation in enhanced ultrasound-induced heating in a tissue-mimicking phantom. Boston University, Boston 2001
- Yang XM, Roy RA, Holt RG. Bubble dynamics and size distributions during focused ultrasound insonation. J Acoust Soc Am 2004; 116: 3423–3431
- Eller A, Flynn HG. Rectified diffusion during nonlinear pulsations of cavitation bubbles. J Acoust Soc Am 1965; 37: 493–503
- Eller AI. Growth of bubbles by rectified diffusion. J Acoust Soc Am 1969; 46: 1246–1250
- Crum LA, Hansen GM. Growth of air bubbles in tissue by rectified diffusion. Phys Med Biol 1982; 27: 413–417
- Yang XM. Investigation of bubble dynamics and heating during focused ultrasound insonation in tissue-mimicking materials. Boston University, Boston 2003
- Miller DL, Thomas RM. Thresholds for hemorrhages in mouse skin and intestine induced by lithotripter shock-waves. Ultrasound Med Biol 1995; 21: 249–257
- Miller DL, Creim JA, Gies RA. Heating vs. cavitation in the induction of mouse hindlimb paralysis by ultrasound. Ultrasound Med Biol 1999; 25: 1145–1150
- Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 2003; 48: 223–241
- Sokka SD, Vykhodtseva N, Hynynen K. Cavitation-enhanced ultrasound heating in vivo: Therapy protocols, mechanisms, and acoustic and MRI monitoring. J Acoust Soc Am 2006; 119: 3227
- Huang J, Holt RG, Cleveland RO, Roy RA. Experimental validation of a tractable numerical model for focused ultrasound heating in flow-through tissue phantoms. J Acoust Soc Am 2004; 116: 2451–2458
- Lafon C, Zderic V, Noble ML, Yuen JC, Kaczkowski PJ, Sapozhnikov OA, Chavrier F, Crum LA, Vaezy S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound Med Biol 2005; 31: 1383–1389
- Jiang P, Everbach EC, Apfel RE. Applications of mixture laws for predicting the compositions of tissue phantoms. Ultrasound Med Biol 1991; 17: 829–838
- ANSI technical report: Bubble detection and cavitation monitoring. Mellive, NY, 2002. Standards Secretariat, Acoustical Society of America
- Leighton TG. A strategy for the development and standardisation of measurement methods for high power/cavitating ultrasonic fields: Review of cavitation monitoring techniques. University of Southampton—Institute of Sound and Vibration Research. 1997
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Hynynen K. Demonstration of enhanced temperature elevation due to nonlinear propagation of focused ultrasound in dogs thigh in vivo. Ultrasound Med Biol 1987; 13: 85–91
- Clarke RL, Bush NL, Ter Haar GR. The changes in acoustic attenuation due to in vitro heating. Ultrasound Med Biol 2003; 29: 127–135
- Zderic V, Keshavarzi A, Andrew MA, Vaezy S, Martin RW. Attenuation of porcine tissues in vivo after high-intensity ultrasound treatment. Ultrasound Med Biol 2004; 30: 61–66
- Hao Y, Prosperetti A. The dynamics of vapor bubbles in acoustic pressure fields. Phys Fluid 1999; 11: 2008–2019
- Prosperetti A, Hao Y. Vapor bubbles in flow and acoustic fields. Microgravity transport processes in fluid, thermal, biological, and materials sciences, SS Sadhal. New York Academy of Sciences, New York 2002; 328–347
- Konofagou EE, Thierman J, Karjalainen T, Hynynen K. The temperature dependence of ultrasound-stimulated acoustic emission. Ultrasound Med Biol 2002; 28: 331–338
- Vaezy S, Shi XG, Martin RW, Chi E, Nelson PI, Bailey MR, Crum LA. Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging. Ultrasound Med Biol 2001; 27: 33–42
- Chan AH, Fujimoto VY, Moore DE, Martin RW, Vaezy S. An image-guided high intensity focused ultrasound device for uterine fibroids treatment. Med Phys 2002; 29: 2611–2620
- Seo J, Tran BC, Hall TL, Fowlkes JB, Abrams GD, O’Donnell M, Cain CA. Evaluation of ultrasound tissue damage based on changes in image echogenicity in canine kidney. IEEE Trans Ultrason Ferroelectr Freq Control 2005; 52: 1111–1120
- Held RT, Zderic V, Nguyen TN, Vaezy S. Annular phased-array high-intensity focused ultrasound device for image-guided therapy of uterine fibroids. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53: 335–348
- Coussios CC, Farny CH, Thomas CR, Cleveland RO, Holt RG, Roy RA. Cavitation detection during and following HIFU exposure in vitro. J Acoust Soc Am 2004; 115: 2448
- Rabkin BA, Zderic V, Crum LA, Vaezy S. Biological and physical mechanisms of HIFU-induced hyperecho in ultrasound images. Ultrasound Med Biol 2006; 32: 1721–1729
- Thomas CR, Farny CH, Coussios CC, Roy RA, Holt RG. Dynamics and control of cavitation during high-intensity focused ultrasound application. Acoust Res Lett Online 2005; 6: 182–187
- Chapelon JY, Dupenloup F, Cohen H, Lenz P. Reduction of cavitation using pseudorandom signals [therapeutic US]. IEEE Trans Ultrason Ferroelectr Freq Control 1996; 43: 623–625
- Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, Cain CA. Controlled ultrasound tissue erosion. IEEE Trans Ultrason Ferroelectr Freq Control 2004; 51: 726–736
- Sokka SD, Gauthier TP, Hynynen K. Theoretical and experimental validation of a dual-frequency excitation method for spatial control of cavitation. Phys Med Biol 2005; 50: 2167–2179
- Xu Z, Fowlkes JB, Rothman ED, Levin AM, Cain CA. Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am 2005; 117: 424–435
- Lo AH, Kripfgans OD, Carson PL, Fowlkes JB. Spatial control of gas bubbles and their effects on acoustic fields. Ultrasound Med Biol 2006; 32: 95–106
- Roberts WW, Hall TL, Ives K, Wolf JS, Fowlkes JB, Cain CA. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol 2006; 175: 734–738