Abstract
This paper provides a historic and contemporary overview of the use of focused ultrasound for treating brain disorders.
Introduction
Since the inception of ultrasound as a therapeutic tool, its potential to treat disorders throughout the brain has been explored. The objective has been to utilize ultrasound's focusing ability to target precisely within deep tissues, affecting only the interested volume while leaving all other structures unaltered. Unfortunately, ultrasound's use in the brain has been inhibited by the skull bone, as well as the inability to target and monitor treatments.
In fact, for more than 50 years it was believed that the attenuation and distortion caused by the skull was so severe that it created an impenetrable barrier, making transskull therapies impossible Citation[1]. Contemporary work, however, has shown that focusing through the intact skull is possible. Furthermore, technological advancements have made it practical, owing to the development of high-powered transducer arrays and high-performance computers to calculate the corrections necessary to restore a focus in the brain.
Similarly, the ability to target and monitor the deposition of ultrasound energy in the brain has improved dramatically over the past half-century. Radiological developments including X-ray computed tomography (CT) followed by magnetic resonance imaging (MRI) made millimeter precision registration with brain structures possible.
This progression of improvements has led to the present state of research, where non-invasive transskull focusing for the purposes of thermal ablation has reached the early stages of clinical testing. Meanwhile, advances continue in the laboratory toward expanding both the number of treatable disorders and the mechanisms of treatment.
The purpose of this paper is to review the clinical studies that used focused ultrasound beams for brain treatments and introduce the basic concepts for the future clinical use of high intensity focused ultrasound in the brain.
Early research
Early brain studies date back to the works of Lynn et al. Citation[1], Citation[2] in the 1940s which targeted areas in the brains of cats, dogs and monkeys. Although they concluded that, given their configuration, it was not possible to produce permanent changes in the brain without undesired damage, they speculated that modifications such as removal of the skull bone and use of multiple focused beams could make such treatment possible. Ensuing investigations, performed after craniotomy, indicated the ability to produce discrete deep lesions in the brain Citation[3–8] as well as an ability to open the blood–brain barrier Citation[9] in a targeted region.
Inspired by the successful clinical trials by the Fry brothers, several investigators conducted animal research to establish the biological effects of ultrasound in the brain tissue. Most notably, Lele conducted a large number of experiments, many with implanted micro-thermocouples. Lele's work established that the ultrasound-induced tissue damage was caused by temperature elevation in the focus Citation[10], Citation[11]. He continued these animal studies related to ultrasound surgery of the brain for more than a decade, with only a few publications but a wealth of information hidden in student dissertations and summarized in conference papers. His experimental results established temperature-exposure time curves for brain tissue dam Citation[12], Citation[13]. He also established the threshold (at one frequency) for inertial cavitation in brain tissue Citation[14] and observed that inertial cavitation was associated with sudden increase in the tissue temperature Citation[15].
Later with some overlap with Lele's studies, Natalia Vykhodtseva in the Soviet Union investigated the parameters for damage in the brain Citation[16], including the cavitation threshold, pulse shape and pulse duration.
Clinical treatments through a bone window
The early findings led the way to a 5-year clinical study beginning in 1957 at City Hospital in Iowa City, Iowa, led by brothers William J. and Francis J. Fry of the University of Illinois and Russell Meyers of the Department of Neurosurgery at the University of Iowa. The patients were treated for Parkinson's disease through a surgery that included opening the scalp, removing a section of skull bone, and delivering the ultrasound through the intact dura Citation[17]. The sonications were performed with a transducer head that had multiple focused beams overlapping at their focal spots. The beams were aimed with the aid of a stereotactic frame based on X-ray images of bony landmarks. The work by the Illinois group provided substantial information on the ability to treat within the brain, while also establishing quantitative thresholds for inducing permanent changes in brain tissues Citation[18], Citation[19]. The Fry method was also tested in the treatment of malignant brain tumors by Heimburger Citation[20]. These treatments were performed through the skin, which was placed over the ultrasound window created by surgically removing a piece of the skull bone. This series had a small number of patients and the results were inconclusive. A more advanced CT-guided system was developed later but was not clinically tested Citation[21].
Further insight into the focusing abilities, targeting and thermal absorption in the brain became available from focused ultrasound hyperthermia treatments performed starting i Citation[22], Citation[23]. These treatments were performed through the skin after the removal of a portion of the skull bone; the skull being viewed as a barrier to therapeutic applications in the brain since the work of Lynn ().
Figure 1. Ultrasound guided focused ultrasound treatment of brain tumors as described in Citation[22]. Top, Left: A diagram of the treatment setting showing the skull window through which the beam is propagating into the tumor. Right: A foam mold made for each of the patients to allow positioning of the head. The mold has a hole through which the ultrasound is propagating in to the brain. Bottom, Left: A CT image of a patient in a treatment position in the head mold. The image shows a thermocouple probe that was inserted to monitor and guide the treatments. In this case the prior surgery had removed most of the tumor (shown as a fluid filled cavity with tumor in the enhancing rim). Right: An ultrasound image of a patient during the treatment showing a thermocouple probe and the tumor.
![Figure 1. Ultrasound guided focused ultrasound treatment of brain tumors as described in Citation[22]. Top, Left: A diagram of the treatment setting showing the skull window through which the beam is propagating into the tumor. Right: A foam mold made for each of the patients to allow positioning of the head. The mold has a hole through which the ultrasound is propagating in to the brain. Bottom, Left: A CT image of a patient in a treatment position in the head mold. The image shows a thermocouple probe that was inserted to monitor and guide the treatments. In this case the prior surgery had removed most of the tumor (shown as a fluid filled cavity with tumor in the enhancing rim). Right: An ultrasound image of a patient during the treatment showing a thermocouple probe and the tumor.](/cms/asset/51c5f2c7-0e14-44d7-baf4-b0c4f5ea6296/ihyt_a_219931_f0001_b.gif)
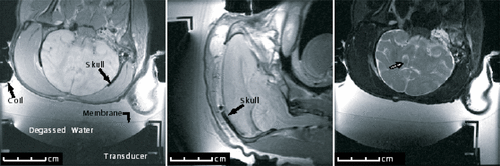
Similar through-the-skin and craniotomy sonications of brain were tested with a MRI-guided focused ultrasound system in Rhesus monkeys Citation[24] (). Locations up to 4.8 cm deep were targeted. Focal heating was observed in all cases with MRI-derived temperature imaging. Sub-threshold heating was observed at the focus when the ultrasound beam was targeted with low power sonications, and in the ultrasound beam path during high-power exposures. Lethal temperature values and histologically confirmed tissue damage were confined to the focal zone (e.g. not in the ultrasound beam path), except when the focus was close to the bone. In that case, damage to the neighboring brain tissue was observed. Focal lesions were observed on histological examination and, in some cases, in MR images acquired immediately after the ultrasound exposures.
Figure 2. MRI slices, showing (left, center) the ultrasound transducer coupled via a water interface to a Rhesus monkey that has undergone a craniotomy before replacement of the skin, and an ultrasound-induced lesion (right) is enhanced following sonication (graphic courtesy of N. McDannold).
This method of using MRI-guided focused ultrasound (Exablate 2000, InSightec, Haifa, Israel) after craniotomy was later used in the treatment of malignant brain tumors in three patients in Israel Citation[25]. The results demonstrated some tumor response and the ability of MRI to guide and monitor the ablation. It was also shown that brain damage outside of the focal zone can happen if the transducer and sonication parameters are not carefully designed for the treatments. Similar treatment of one patient with malignant brain tumor was also performed using an ultrasound imaging guided focused ultrasound system (Mode-JC HIFU System, Chongquing Hifu, China) in Korea. The follow-up imaging showed some evidence of tumor coagulation Citation[26]. Despite the clinical feasibility of performing ultrasound surgery through a craniotomy, the method has not gained clinical acceptance. This is most likely due to the need of two expensive surgeries to remove and restore the skull bone.
Development of transskull ultrasound surgery
In the mid-1970s, however, work by Fry et al. Citation[27–29] began to investigate the possibility of focusing through the skull with reduced distortion at frequencies less than 1 MHz. They Citation[28] showed that focusing is possible, but that these foci tended to be distorted and shifted.
A means to compensate for the distortion caused by the skull was demonstrated in the late 1990s. The approach made use of the development of high-power phased ultrasound arrays Citation[30–38] and driving systems suitable for thermal ablation Citation[39]. It was demonstrated that a phase conjugation approach with a small transmitter inside the brain could be used to focus through a skull fragment Citation[40]. Hynynen and Jolesz Citation[41] then demonstrated that a focus distorted by the insertion of a human skull fragment in a water bath could be restored by simply adjusting the driving phase of each element in a spherically curved transducer array with transducer elements large enough to make practical, high gain arrays feasible. The technique resembled an aberration correction method proposed for diagnostic ultrasound by Smith et al. Citation[42] a decade earlier.
While the skull distorts the ultrasound field, it also absorbs ultrasound energy, causing unwanted heating in and around the skull and attenuating the beam. To attain a focus intense enough to coagulate tissues without overheating the skull, a hemispherical transducer design was devised to maximize the surface area of the skull, and thus distribute the energy Citation[43], Citation[44]. In numeric studies it was determined that approximately 64 elements were sufficient to focus the ultrasound after phase correction while maintaining skull temperatures below the burn threshold. A 64-element array with a 30-cm diameter was prototyped Citation[45], constructed and tested Citation[46], verifying the earlier numeric work.
With the feasibility of treatment verified, practical methods for reconstructing a focus were sought. One suggested method was the use of a small—perhaps catheter-inserted—receiver in the brain that would serve as a beacon to be used in conjunction with phase conjugating electronics Citation[47], or alternatively as a receiver for phase correction Citation[48]. However, it was the development of a model-based approach to focal restoration that made the technique completely non-invasive Citation[49] (). This spectral method, as well as a related finite-difference approach Citation[50] required information from CT images in order to infer density, sound speedCitation[49], Citation[51], Citation[52], and to register the transducer with the skull ().
Figure 3. Top, Left: A diagram showing the wave distortion induced by a skull. Top, Right: The measured focused ultrasound field after it propagated through an ex vivo human skull showing the multiple foci induced. Bottom, Left: A diagram showing the adjustment of the phase of the array elements to compensate for the skull induced wave distortion. Bottom, Right: The measured ultrasound field after propagating through the ex vivo human skull when a CT correction algorithm was used to correct for the distortion induced by the skull Citation[49].
![Figure 3. Top, Left: A diagram showing the wave distortion induced by a skull. Top, Right: The measured focused ultrasound field after it propagated through an ex vivo human skull showing the multiple foci induced. Bottom, Left: A diagram showing the adjustment of the phase of the array elements to compensate for the skull induced wave distortion. Bottom, Right: The measured ultrasound field after propagating through the ex vivo human skull when a CT correction algorithm was used to correct for the distortion induced by the skull Citation[49].](/cms/asset/9d5bacf7-9ef2-4c01-93c9-b034a93920d7/ihyt_a_219931_f0003_b.gif)
From simulations of ultrasound propagation into the brain Citation[44] it was determined that a phase-corrected array on the order of 500 elements would produce a focus of about 1 mm in diameter in the brain at the array's geometric center. Thermal studies comparing the temperature rise at the focus to that on the skull surface further indicated that the optimal thermal gain between the focus in the brain and the skull surface is reached, on average, at frequencies near 0.7 MHz Citation[46], Citation[53]. Based on these studies a 500-element 1–3 piezocomposite MRI-compatible transducer was designed and constructed Citation[54]. The composite material was used to allow for flexibility in the transducer bandwidth without impeding the high-power continuous-wave operation of the array. Although this array was primarily intended to provide adequate aberration correction at the geometric focus rather than electronic steering Citation[43], the array also allowed for limited electronic beam steering.
Equally critical to brain procedures has been the ability to target and monitor the treatment region. MR-guided focused ultrasound surgery (MRgFUS) Citation[55–59] has demonstrated the ability to image both the tissue structure and the temperature rise throughout the region. Operation required that the ultrasound applicators were MR compatible, imposing unique design criteria for the ultrasound applicatorsCitation[54], Citation[55], Citation[60]. MRI studies were critical to identifying potentially dangerous thermal variation over the skull surface Citation[61], as well as methods to correct for such variation Citation[62]. These studies also indicated the need to circulate cooled, degassed water between the array and the patient to provide skin and skull surface cooling to avoid excessive temperatures and tissue damage.
The complete 500-element MRI-guided system was tested by sonicating ultrasound phantoms and in vivo rabbit muscle and brain tissue using both model-based and hydrophone-based phasing through ex vivo human skulls Citation[60]. These experiments showed that adequate energy can be delivered through the human skull to ablate an in vivo brain tissue, and that MRI can detect the focal temperature rise and tissue coagulation. It had been shown earlier with in vivo rabbit brains that the focal hot spots induced by sub-threshold sonications could be detected with MRI thermometry Citation[63] allowing accurate targeting prior to ablative sonications.
Separate experiments with an ultrasound-guided system and 300-element array Citation[64] were also performed in vivo in sheep Citation[65] demonstrating that transskull focal brain tissue coagulation is feasible. In these experiments, an invasive hydrophone was used to aid in the aberration correction. This work has recently been continued with in vivo monkey experiments that demonstrated model-based transskull brain ablation Citation[66].
Clinical transskull ultrasound surgery procedure
Based on the culmination of brain research, a clinical brain system has been produced (ExAblate 3000, InSightec, Haifa, Israel) (). This system was tested in the treatment of Rhesus monkeys to verify the system functionality and determine the level of temperature elevation at the skull bone surfaces Citation[67]. These experiments also allowed the testing of the treatment planning programs. The results clearly demonstrated the importance of having a uniform ultrasound intensity at the skull surface and showed that high enough powers can be transmitted through the monkey skull to allow focal tissue coagulation in humans.
Figure 4. A clinical prototype brain treatment system (Exablate 3000, InSightec, Inc., Haifa, Israel). (a) An illustration of the treatment planning showing how each of the ultrasound beams are propagated through a section of a skull based on the CT images obtained before the treatment and co-registered with the online MR image. (b) A photograph of the 512-element array and the mechanical positioning system. (c) A block diagram of the complete system. (d) A temperature elevation image derived from the MRI thermometry information at the end of a sonication of a monkey Citation[67]. The hotspot and the skull heating are visible in the image.
![Figure 4. A clinical prototype brain treatment system (Exablate 3000, InSightec, Inc., Haifa, Israel). (a) An illustration of the treatment planning showing how each of the ultrasound beams are propagated through a section of a skull based on the CT images obtained before the treatment and co-registered with the online MR image. (b) A photograph of the 512-element array and the mechanical positioning system. (c) A block diagram of the complete system. (d) A temperature elevation image derived from the MRI thermometry information at the end of a sonication of a monkey Citation[67]. The hotspot and the skull heating are visible in the image.](/cms/asset/0d8639d0-dfce-4dd6-9fd5-a80d2efccff4/ihyt_a_219931_f0004_b.gif)
A clinical treatment series with three patients was then performed to gather feasibility information and determine clinical patient machine interface features. Briefly, the patient treatment is executed in the following manner.
Non-invasive brain procedures begin with a high-resolution CT scan of the patient's head using a bone kernel (Typical FOV 200 mm with 0.75-mm slice thickness), which is rendered into three dimensions to provide relevant acoustic input parameters. These CT images must be registered with both the reference frame of the treatment transducer as well as the MRI scanner for treatment planning. Treatment planning is performed with the patient's head rigidly affixed to the treatment array. In the initial trial this was done using a facemask. However stereotactic frames (that have been routinely used in radiosurgery) secured to the patient's head provide the most rigid support, and offer an ability for planning to be performed hours before the actual treatment.
The planning procedure numerically simulates the ultrasound beam along its path through the skull bone and into the brain in order to determine the amplitude and phase of the ultrasound when it reaches the intended focal position. While there are many possible approaches to simulating the ultrasound field, only the spectral approach Citation[49] has been shown to repeatedly focus through the skull over a range of skull samples. The primary contribution of the planning is to determine the relative phase of the ultrasound contributed by the individual elements in the array. Once determined, the individual phases can be adjusted, so that the contributed beam from each element arrives at the focus in phase. Furthermore, the path of the ultrasound beam through the skull is identified (). If energy from a given element is severely attenuated or refracted in such a way that it cannot contribute appreciatively to the focus, the amplitude of this element can also be adjusted. In severe cases this element can be turned off, as it will only deposit energy in the skull or other undesired volumes. In cases of milder attenuation it may be more beneficial to increase the amplitude of the element in order to strengthen its pressure output Citation[62].
The patient is prepared for treatment by shaving and cleaning the scalp before inserting the head into a watertight membrane that is affixed to the treatment array. The region between the array and the patient is filled with degassed water cooled to approximately 15°C in order to cool the outer surface of the skull to prevent overheating.
The clinical trial of treating neoplastic brain tumors is currently under way at the Brigham and Women's Hospital, Boston, MA, USA. Another study expected to start also at Univeristy Children's Hospital in Zurich, Switzerland, will initially target non-invasive functional neurosurgery starting with neurogenic pain, with plans to extend into Parkinson's disease and epilepsy. The first series in Boston treated three patients, verifying transskull focusing and the quantity of skull heating. The continuation of the trial is pending modifications to be made to the patient immobilization system.
Future applications
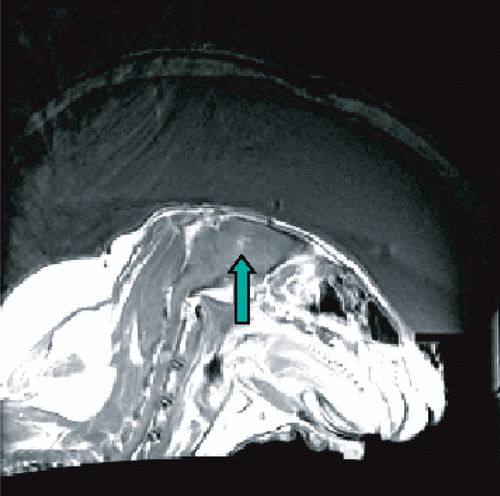
Although transskull thermal coagulation of tumors appears feasible, it may turn out that cavitation-enhanced heating Citation[68–71] or mechanical tissue destruction Citation[72] may offer benefits due to the increased focal energy absorption and thus reduced energy transmission through the skull. Owing to the reduced time, average power requirement, low-duty cycle, high-pressure amplitude sonications inducing cavitation were originally proposed as the method of choice for transskull surgery Citation[41]. By injecting an ultrasound contrast agent with preformed microbubbles into the blood stream, the thermal and mechanical tissue damage methods can be combined Citation[73]. This could results in at least an order of magnitude reduction in the required power. An example of a focal lesion that was produced with only 8 Watts of acoustic power emitted by the transducer during 20 s sonication through an ex vivo human skull in a living rabbit brain after a bolus injection of an ultrasound contrast agent is shown in .
Figure 5. Bubble-enhanced tissue destruction as seen with MRI contrast enhanced imaging after the sonication. The image shows ultrasound being delivered through an ex vivo human calvarium and brain-mimicking phantom and into a rabbit brain in vivo.
Ultrasound holds the promise of providing multiple therapeutic functions in the brain by way of coagulative necrosis, through potential reversible blocking of certain functions Citation[74], by assisting in the delivery of thrombolytic agents Citation[75–77], or through delivery of an agent to a targeted volume by opening of the blood–brain barrier. These methods have been suggested for the treatment of Parkinson's disease, epilepsy and tumors and to inhibit transmission of nerve signals in the brain Citation[74] and may be useful in targeting genetherapy Citation[78], Citation[79].
Significant attention has recently focused on the ability of ultrasound to temporarily open the blood–brain barrier (), providing a means for spatially targeted and time-windowed passage of therapeutic agents into the brain. It has long been recognized that ultrasound can disrupt the blood–brain barrier Citation[9] but the prospect of creating a controlled reversible process Citation[80], introduces significant promise for delivering agents that currently cannot be delivered into the brain. Furthermore, the prospect of targeting may protect certain areas in the brain while providing benefit to the target. Online monitoring abilities Citation[80–82] make the procedure especially exciting.
Figure 6. A T1-weighted contrast enhanced image of a rabbit brain showing two locations with disrupted blood–brain barrier Citation[80].
![Figure 6. A T1-weighted contrast enhanced image of a rabbit brain showing two locations with disrupted blood–brain barrier Citation[80].](/cms/asset/e8fce9c6-a5e6-4f94-8656-fb3108d1cff5/ihyt_a_219931_f0006_b.gif)
Much work remains to be performed for disease-specific models and drugs in order to determine clinical viability, but the body of quantitative data is increasing rapidly. A range of particle sizes has been demonstrated to pass the blood–brain barrier, including molecular weights of 961 (trypan blue), 938 (Magnevist®), 10 000 (MION) Citation[82], 40 000 (horseradish peroxidase) Citation[83], and 150 000 (antibodies) Citation[84]. Recent evidence has indicated that significant concentrations of the chemotherapy drug liposomal doxorubicin can be delivered to the normal rat brain Citation[85], and the monoclonal antibody Herceptin in mice Citation[84].
Acknowledgements
This work was supported by NIH grants EB003268, EB000705, and U41-RR 019703 and CRCP.
References
- Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942; 26: 179–193
- Lynn JG, Putnam TJ. Histology of cerebral lesions produced by focused ultrasound. Am J Path 1944; 20: 637–652
- Wall PD, Fry WJ, Stephens R, Tucker D, Lettvin JY. Changes produced in the central nervous system by ultrasound. Science 1951; 114: 686–687
- Fry WJ, Mosberg W, Barnard JW, Fry FJ. Production of focal destructive lesions in the central nervous system with ultrasound. J Neurosurg 1954; 11: 471–478
- Fry WJ, Barnard JW, Fry FJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science 1955; 122: 517–518
- Fry WJ, Barnard JW, Fry FJ. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med 1955; 34: 413–423
- Barnard JW, Fry WJ, Fry FJ, Brennan JF. Small localized ultrasonic lesions in the white and gray matter of the cat brain. AMA Arch Neurol 1956; 75: 15–35
- Barnard JW, Fry WJ, Fry FJ, Brennan JF. Small localized ultrasonic lesions in the white and gray matter of the cat brain. Arch Neurol Psychiatry 1956; 75: 15–35
- Bakay L, Hueter TF, Ballantine HT, Sosa D. Ultrasonically produced changes in the blood-brain barrier. Arch Neurol 1956; 76: 457–467
- Lele PP. A simple method for production of trackless focal lesions with focused ultrasound: Physical factors. J Physiol 1962; 160: 494–512
- Lele PP. Production of deep focal lesions by focused ultrasound—current status. Ultrasonics 1967; 5: 105–122
- Lele PP, Pierce AD. The thermal hypothesis of the mechanism of ultrasonic focal destruction in organised tissues. Interaction of ultrasound and biological tissues. Bureau of Radiological Health, Washington, DC 1973; 121–128, FDA 73-8008 BRH/DBE
- Robinson TC, Lele PP. An analysis of lesion development in the brain and in plastic by high-intensity focused ultrasound at low-megaherz frequencies. J Acoust Soc Am 1972; 51: 1333–1351
- Lele PP. Threshold and mechanisms of ultrasonic damage to organized animal tissues. Proceedings of a Symposium on Biological effects and characterization of ultrasound sources, Rockville, MDUSA, June 1–3, 1977, 224–239
- Lele PP. Effects of ultrasound on “solid” mammalian tissues and tumors in vivo. Ultrasound: Medical applications, biological effects and hazard potential, MH Repacholi, M Grondolfo, A Rindi. Plenum Pub. Corp., New York 1987; 273–306
- Vykhodtseva NI, Gavrilov LR, Mering TA, Iamshchikova NG. [Use of focused ultrasound for local destruction of different brain structures] Primenenie fokusirovannogo ul'trazvuka dlia lokal'nykh razrushenii razlichnykh struktur golovnogo mozga. Zh Nevropatol Psikhiatr 1976; 76: 1810–1816
- Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron 1960; ME-7: 166–181
- Fry FJ, Kossoff G, Eggleton RC, Dunn F. Threshold ultrasonic dosages for structural changes in the mammalian brain. J Acoust Soc Am 1970; 48: 1413–1417
- Dunn F, Lohnes JE, Fry FJ. Frequency dependence of threshold ultrasonic dosages for irreversible structural changes in mammalian brain. J Acoust Soc Am 1975; 58: 512–514
- Heimburger RF. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med 1985; 78: 469–476
- Fry FJ, Sanghvi NT, Morris RF, Smithson S, Atkinson L, Dines K, Franklin T, Hastings J, et al. A focused ultrasound system for tissue volume ablation in deep seated brain sites. IEEE 1986 Ultrasonics Symp Proc (Cat.No.86CH2375-4), 1001. 1986
- Guthkelch AN, Carter LP, Cassady JR, Hynynen K, Iacono RP, Johnson PC, Obbens EAMT, Roemer RB, Seeger JF, Shimm DS, Stea B, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J Neurooncol 1991; 10: 271–284
- Anhalt DP, Hynynen K, Roemer RB. Patterns of changes of tumour temperatures during clinical hyperthermia: Implications for treatment planning, evaluation and control. Effect of phase errors on field patterns generated by an ultrasound phased-array hyperthermia applicator. Int J Hyperthermia 1995; 11: 425–436
- McDannold N, Moss M, Killiany R, Rosene DL, King RL, Jolesz FA, Hynynen K, et al. MRI-guided focused ultrasound surgery in the brain: Tests in a primate model. Magn Reson Med 2003; 49: 1188–1191
- Ram Z, Cohen ZR, Harnof S, Tal S, Faibel M, Nass D, Maier SE, Hadoni M, Mardor Y, et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 2006; 59: 949–955
- Park JW, Jung S, Junt TY, Lee MC. Focused ultrasound surgery for the treatment of recurrent anaplastic astrocytoma: A preliminary report. Therapeutic ultrasound. 5th International Symposium on Therapeutic Ultrasound. 2006, GT Clement, NJ McDannold, K Hynynen. American Institute of Physics, New York, 238–240
- Fry FJ. Transkull transmission of an intense focused ultrasonic beam. Ultrasound Med Biol 1977; 3: 179–184
- Fry FJ, Goss SA. Further studies of the transkull transmission of an intense focused ultrasonic beam: Lesion production at 500 kHz. Ultrasound Med Biol 1980; 6: 33–38
- Fry FJ, Goss SA, Patrick JT. Transkull focal lesions in cat brain produced by ultrasound. J Neurosurg 1981; 54: 659–663
- Do-Huu JP, Hartemann P. Annular array transducer for deep acoustic hyperthermia. IEEE Ultrasonics Symp 1981; 81CH1689-9: 705–710
- Ocheltree KB, Benkeser PJ, Frizzell LA, Cain CA. An ultrasonic phased array applicator for hyperthermia. IEEE Trans Sonics Ultras 1984; SU-31: 526–531
- Benkeser PJ, Frizzell LA, Ocheltree KB, Cain CA. A tapered phased array ultrasound transducer for hyperthermia treatment. IEEE Trans Ultrason Ferroelectr Freq Control 1987; UFFC-34: 446–453
- Frizzell LA, Benkeser PJ, Ocheltree KB, Cain CA. Ultrasound phased arrays for hyperthermia treatment. IEEE Ultrasonics Symp 1985; 2: 931–935
- Cain CA, Umemura SA. Concentric-ring and sector vortex phased array applicators for ultrasound hyperthermia therapy. Effect of phase errors on field patterns generated by an ultrasound phased-array hyperthermia applicator. IEEE Trans Microwave Theory Tech 1986; MTT-34: 542–551
- Ebbini ES, Umemura SI, Ibbini M, Cain C. A cylindrical- section ultrasound phased array applicator for hyperthermia cancer therapy. Effect of phase errors on field patterns generated by an ultrasound phased-array hyperthermia applicator. IEEE Trans Ultrason Ferroelectr Freq Cont 1988; 35: 561–572
- Diederich C, Hynynen K. The feasibility of using electrically focussed ultrasound arrays to induce deep hyperthermia via body cavities. IEEE Trans Ultrason Ferroelectr Freq Cont 1991; 38: 207–219
- Fan X, Hynynen K. Control of the necrosed tissue volume during noninvasive ultrasound surgery using a 16 element phased array. Med Phys 1995; 22: 297–308
- Daum DR, Hynynen K. A 256 element ultrasonic phased array system for treatment of large volumes of deep seated tissue. IEEE Trans Ultrason Ferroelectr Freq Cont 1999; 46: 1254–1268
- Daum DR, Buchanan MT, Fjield T, Hynynen K. Design and evaluation of a feedback based phased array system for ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Cont 1998; 45: 431–438
- Thomas J-L, Fink MA. UItrasonic beam focusing through tissue inhomogeneities with a time reversal mirror: Application to transskull therapy. IEEE Trans Ultrason Ferroelectr Freq Cont 1996; 43: 1122–1129
- Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol 1998; 24: 275–283
- Smith SW, Trahey G.E, von Ramm OT. Phased array ultrasound imaging through planar tissue layers. Ultrasound Med Biol (UK) 1986; 12: 229–243
- Sun J, Hynynen K. Focusing of therapeutic ultrasound through a human skull: A numerical study. J Acoust Soc Am 1998; 104: 1705–1715
- Sun J, Hynynen K. The potential of transskull ultrasound therapy and surgery using the maximum available skull surface area. J Acoust Soc Am 1999; 105: 2519–2527
- Clement GT, White J, Hynynen K. Investigation of a large-area phased array for focused ultrasound surgery through the skull. Phys Med Biol 2000; 45: 1071–1083
- Clement GT, Sun J, Giesecke T, Hynynen K. A hemisphere array for non-invasive ultrasound brain therapy and surgery. Phys Med Biol 2000; 45: 3707–3719
- Tanter M, Thomas J-L, Fink MA. Focusing and steering through absorbing and aberrating layers: Application to ultrasonic propagation through the skull. J Acoust Soc Am 1998; 103: 2403–2410
- Clement GT, Hynynen K. Micro-receiver guided transcranial beam steering. IEEE Trans Ultrason Ferroelectr Freq Cont 2002; 49: 447–453
- Clement GT, Hynynen K. A noninvasive method for focusing ultrasound through the human skull. Phys Med Biol 2002; 47: 1219–1236
- Aubry JF, Tanter M, Pernot M, Thomas J-L, Fink MA. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am 2003; 113: 84–93
- Clement GT, Hynynen K. Correlation of ultrasound phase with physical skull properties. Ultrasound Med Biol 2002; 28: 617–624
- Connor CW, Clement GT, Hynynen K. A unified model for the speed of sound in cranial bone based on genetic algorithm optimization. Phys Med Biol 2002; 47: 3925–3944
- Clement GT, Hynynen KH. Criteria for the design and calibration of large area arrays for transskull ultrasound surgery and therapy. IEEE 2000 Ultrasonics Symp 2000; 2: 1243–1246
- Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med 2005; 24: 1117–1125
- Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI-guided noninvasive ultrasound surgery. Med Phys 1993; 20: 107–115
- Darkazanli A, Hynynen K, Unger E, Schenck JF. On-line monitoring of ultrasound surgery with MRI. J Mag Res Imag 1993; 3: 509–514
- Cline HE, Hynynen K, Hardy CJ, Watkins RD, Schenck JF, Jolesz FA. MR temperature mapping of focused ultrasound surgery. Magn Reson Med 1994; 30: 98–106
- Hynynen K, Freund WR, Cline HE, Chung AH, Watkins RD, Vetro JP, Jolesz FA, et al. A clinical noninvasive MRI monitored ultrasound surgery method. Radiographics 1996; 16: 185–195
- Hynynen K, Chung A, Fjield T, Buchanan MT, Daum D, Colucci V, Lopath, Jolesz F, et al. Feasibility of using ultrasound phased arrays for MRI monitored noninvasive surgery. IEEE Trans Ultrason Ferroelectr Freq Cont 1996; 43: 1043–1053
- Hynynen K, Clement GT, McDannold N, Vykhodtseva N, King R, White PJ, Vitek S, Jolesz FA, et al. 500-element ultrasound phased array system for noninvasive focal surgery of the brain: A preliminary rabbit study with ex vivo human skulls. Magn Reson Med 2004; 52: 100–107
- Connor CW, Hynynen K. Patterns of thermal deposition in the skull during transcranial focused ultrasound surgery. IEEE Trans Biomed Eng 2004; 51: 1693–1706
- White J, Clement GT, Hynynen K. Transcranial ultrasound focus reconstruction with phase and amplitude correction. IEEE Trans Ultrason Ferroelectr Freq Cont 2005; 52: 1518–1522
- Hynynen K, Vykhodtseva NI, Chung A, Sorrentino V, Colucci V, Jolesz FA. Thermal effects of focused ultrasound on the brain: Determination with MR Imaging. Radiology 1997; 204: 247–253
- Pernot M, Aubry JF, Tanter M, Thomas JL, Fink M. High power transcranial beam steering for ultrasonic brain therapy. Phys Med Biol 2003; 48: 2577–2589
- Pernot M, Aubry JF, Tanter M, Boch AL, Kujas M, Fink M. Adaptive focusing for ultrasonic transcranial brain therapy: First in vivo investigation on 22 sheep. AIP Conf Proc 2005; 754: 174–177
- Marquet F, Aubry JF, Tanter M, Fink M. Non invasive transcranial brain therapy guided by CT scans: An in vivo monkey study. Presented in the 6th ISTU meeting. OxfordUK, 2006
- Hynynen K, McDannold N, Clement G, Jolesz FA, Zadicario E, Killiany R, Moore T, Rosen D, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—A primate study. Eur J Radiol 2006; 59: 149–156
- Hynynen K. The threshold for thermally significant cavitation in dog's thigh muscle in vivo. Ultrasound Med Biol 1991; 17: 157–169
- Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol 2003; 27: 1399–1412
- Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced ultrasound heating in in vivo rabbit thigh. Phys Med Biol 2003; 48: 223–241
- Sokka SD, Gauthier TP, Hynynen K. Theoretical and experimental validation of a dual-frequency excitation method for spatial control of cavitation. Phys Med Biol 2005; 50: 2167–2179
- Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Cont 2003; 50: 1296–1304
- McDannold NJ, Vykhodtseva NI, Hynynen K. Microbubble contrast agent with focused ultrasound to create brain lesions at low power levels: MR imaging and histologic study in rabbits. Radiology 2006; 241: 95–106
- Vykhodtseva NI, Koroleva VI. [Changes in the steady potential in various structures of the rat brain induced by focused ultrasound] Sdvigi postoiannogo potentsiala v razlichnykh strukturakh golovnogo mozga krysy pri deistvii fokusirovannogo ul′trazvuka. Dokl Akad Nauk SSSR 1986; 287: 248–251
- Akiyama M, Ishibashi T, Yamada T, Furuhata H. Low-frequency ultrasound penetrates the cranium and enhances thrombolysis in vitro. Neurosurgery 1998; 43: 828–832
- Alexandrov AV, Demchuk AM, Felberg RA, Christou I, Barber PA, Burgin WS, Malkoff M, Wojner AW, Grotta JC, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke 2000; 31: 610–614
- Frenkel V, Oberoi J, Stone MJ, Park M, Deng C, Wood BJ, Neeman Z, Horne M, III, Li KC, et al. Pulsed high-intensity focused ultrasound enhances thrombolysis in an in vitro model. Radiology 2006; 239: 86–93
- Moonen C, Madio D, de Zwart J, Olson A, DesPres D, van Gelderen PE, Mandel M, Voisin P, Canioni P, Vekris A, et al. MRI-guided focused ultrasound as a potential tool for control of gene therapy. Eur Radiol 1997; 7: 1165
- Silcox CE, Smith RC, King R, McDannold N, Bromley P, Walsh K, et al. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol 2005; 31: 965–970
- Hynynen K, McDannold N, Vykhodtseva NI, Jolesz FA. Noninvasive MR image guided focal opening of the blood–brain barrier. Radiology 2001; 220: 640–646
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: Association with cavitation activity. Phys Med Biol 2006; 51: 793–807
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005; 24: 12–20
- Sheikov N, McDannold N, Jolesz F, Zhang YZ, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood–brain barrier. Ultrasound Med Biol 2006; 32: 1399–1409
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci USA 2006; 103: 11719–11723
- Treat LH, McDannold NJ, Vykhodtseva NI, Zhang Y, Tam K, Hynynen K (2006) Targeted drun delivery to the brain by MRI-guided focused ultrasound. Therapeutic ultrasound: 5th International Symposium on Therapeutic Ultrasound. 2005, GT Clement, NJ McDannold, K Hynynen. American Institute of Physics, 266–270