Abstract
Purpose: We investigated whether it is possible to predict the antitumour effects of thermochemotherapy from the results of anticancer agent sensitivity testing. Materials and Methods: We produced a nude mouse cancer model using 4 lung cancer cell lines. Animals were divided into 4 groups: Thermotherapy (HT group), chemotherapy (CT group), thermochemotherapy (HT+CT group), and no therapy (NT group). Comparison of in vivo and in vitro effects were performed using cisplatin (CDDP), doxorubicin (ADR) and vinorelbine (NVB). In vivo thermotherapy was performed using the Thermotron RF IV, and radiofrequency (RF) capacitative hyperthermia device that induces a localised temperature of 42.0°C for 45 min. The collagen gel embedded culture drug sensitivity test (CD-DST) was used for in vitro chemosensitivity analysis of the anticancer agents. In vitro thermochemotherapy was performed using a modified CD-DST method, with the incubator set at 42.0° for the first hour of the 24 hours drug exposure period. Results: A good correlation was seen between in vivo and in vitro treated/control ratios (T/C%) in the HT group (R = 0.91, p = 0.09). Good correlations were also seen between in vivo and in vitro T/C in all cell lines in the CT group (R = 0.759, p = 0.09) and the HT+CT group (R = 0.65, p = 0.02). True positive rate was 87.5% (7/8), and true negative rate was 100% (4/4). Sensitivity, specificity and accuracy were 100% (7/7), 80% (4/5), and 91.7% (11/12) respectively. Conclusion: A modified CD-DST using an exposure temperature of 42°C can be used to predict the antitumour effect of thermochemotherapy.
Introduction
At the time of diagnosis, more than 70% of non-small cell lung cancers are already inoperable advanced cancers, and therapeutic results remain poor. Combinations of new anti-cancer agents developed since the 1990s, such as taxol (TXL), taxotere (TXT), vinorelbine ditartrate (NVB), gemcitabine (GEM), and irinotecan (CPT-11) with platinum compounds, have yielded superior results to combinations of older agents such as vindesine (VDS) or etoposide (VP16) with platinum compounds, so the newer combinations have become the mainstay of current treatment Citation[1–6]. Outcomes are less than completely satisfactory, however, with response rates no better than 30–40%, even for initial therapy. One way of overcoming this situation is development of new anti-cancer agents that will surpass the efficacy of presently available agents; but in the meantime the goal is to improve therapeutic results as much as possible by selecting the most effective agents and adjuvant therapies for each individual patient.
Although some anti-tumour effect is seen for thermotherapy used as monotherapy, it is augmented in combination with anti-cancer agents Citation[7–11]. More effective application of thermochemotherapy would be possible if chemosensitivity analysis could predict in advance the therapeutic effect.
The collagen gel embedded culture drug sensitivity test (CD-DST) method cultures tumour cells three-dimensionally in a collagen gel, with a high level of success from resected specimens, and the cultured cells can be maintained for approximately 10 days. Denaturation of the collagen gel does not occur at 42°C, the same temperature used for thermotherapy, so we used the CD-DST method Citation[12–17] for our in vitro chemosensitivity analysis of the anti-cancer agents, and investigated whether this method can be used for the selection of the thermochemotherapy regimen, instead of in vivo testing using nude mice.
Materials and methods
Chemotherapy agents
The anti-cancer agents used were cisplatin (CDDP), doxorubicin hydrochloride (ADR) and vinorelbine ditartrate (NVB).
Materials
We used male BALB/C nunu nude mice aged 5–7 weeks (CLEA Japan, Inc.) and four cell lines of human non-small cell lung cancer transplantable to nude mice: PC-13 (large cell carcinoma), PC-14 (adenocarcinoma), LU-99 (large cell carcinoma), and A-549 (adenocarcinoma).
For the in vivo hyperthermia equipment, we used the Thermotron RF IV (Yamamoto Vinyter Co. Ltd, Osaka, Japan) 8 MHz radiofrequency (RF) capacitative hyperthermia device. Two circular electrode plates are placed on either side of the target, heating the target within the range 0–50°C with radio waves at an emission frequency of 8 MHz produced by coil oscillation Citation[18–20].
The CD-DST method, with the Primaster human cancer cell primary culture system kit (Nitta Gelatin Inc., Osaka, Japan), was used for the in vitro sensitivity testing.
Methods: In vivo study
Production of nude mouse cancer model
We injected a human non-small cell lung cancer cell line subcutaneously into the dorsal region of nude mice, and when the estimated tumour weight (1/2LW2, L: longer diameter in mm, W: shorter diameter mm) reached 200 mg, animals were sacrificed and the tumours were resected, cut into equal pieces with 3-mm sides and transplanted subcutaneously into the dorsal region of different nude mice using a transplant needle. When the transplanted tumours reached an estimated mass of 200 mg, the animals were used for the experiment.
Allocation to groups
The nude mice in the cancer model were allocated to 4 groups: thermotherapy alone (HT group), chemotherapy alone (CT group), thermochemotherapy, with hyperthermia and anti-cancer agents administered simultaneously (HT + CT group), and controls given no treatment (NT group) (n = 6 for each group). The anti-cancer agents (CDDP, ADR and NVB) were given to CT and HT + CT groups. For each anti-cancer agent (CDDP, ADR, NVB), the examination with these four groups was performed.
Thermotherapy
General anaesthesia was induced with intraperitoneal pentobarbital 40 mg/kg, and animals were placed prone on the applicator of the Thermotron RF IV. Tumour and rectal temperatures were monitored using needle thermosensor (Type IT-18, Sensortek Inc., Costamesa, CA, USA) and the tumour was heated while keeping the rectal temperature below 37°C. The tumour was subjected to hyperthermia for 45 min after a temperature of 40°C or more was reached, with the tumour temperature maintained at 40.0–42.5°C for that period with measurements taken at 5-min intervals.
Administration of anti-cancer agents
Anti-cancer agents were administered intravenously via the caudal vein at their maximum tolerance dose (MTD: CDDP 10 mg/kg, ADM 12 mg/kg, NVB 16.2 mg/kg), diluted with saline. For the HT + CT group, the anti-cancer agent was administered intravenously via the caudal vein immediately following induction of anaesthesia, at the start of hyperthermia treatment.
Assessment of therapeutic effect
Tumour diameters (L, W) were measured after treatment, and the ratio between the estimated weight on day 14 after therapy (Wt14) and the estimated weight at the commencement of therapy (Wt0) was calculated (Wt14/Wt0). Measurements commenced in the control group when the estimated tumour mass reached 200 mg. The Wt14/Wt0 ratio in each treatment group was designated T, and that for the control group as C, and the anti-tumour effect for each treatment was evaluated using the T/C (%) proliferation rate ratio. T/C (%) of a cell line was calculated as the average T/C (%) of six tumours grown from the same cell line. A T/C (%) ratio of 50% or less was regarded as being sensitive in vivo to the given anti-tumour agent.
Methods: In vitro study
Production of nude mouse cancer model
Nude mice carrying cancer were produced by the same method used for the in vivo experiment. Animals were sacrificed when the estimated tumour mass reached 200 mg, the tumours were resected and tissue specimens were allocated to the four groups: (CT, HT, HT + CT, NT: n = 6 for each group). The CT group underwent conventional CD-DST, and the HT group and the HT + CT group underwent a modified CD-DST method. To determine in vitro chemosensitivity or in vitro thermochemotherapy of a given anti-cancer agent for a cancer cell line, six tumour specimens from six nude mice were examined. Measurements were performed triplicate for each tumour specimen.
CD-DST—preparation of tumour cell suspensions
Each specimen was aseptically finely minced with a scalpel, suspended in Hanks's balanced saline solution (HBSS), and digested in a cell dispersion enzyme solution (10% EZ®, Nitta Gelatin Inc.) at 37°C for 1–3 h. The dispersed cancer cells were collected by centrifugation at 900 g for 3 min, filtered through 80-µm nylon mesh, washed in HBSS, suspended in PCM-1® medium (Nitta Gelatin Inc.), and incubated in a CG-flask® (collagen gel coated flask, Nitta Gelatin) in a CO2 incubator at 37°C for 24 h. The collagen gel in the CG-flask® was dissolved in the cell dispersion enzyme solution (10% EZ). This protocol allowed only viable cells that could adhere to the collagen gel to be collected and a polarizing microscope was used at this stage to check whether the tumour cell suspension contained sufficient number of tumour cells.
CD-DST—collagen gel droplet embedded culture
The tumour cell suspension was added to a collagen solution (Collagen Gel Culture Kit Primaster®, Nitta Gelatin Inc.) to produce a final cell density of 1 × 105 cells/mL. Three drops of collagen-cell mixture (30 µL/droplet) were placed in each well of a six-well multi-plate and allowed to form a gel at 37°C in a CO2 incubator. Testing for each anti-cancer agent was performed in triplicate. The final concentration was approximately 3 × 103 cells/collagen gel droplet. DF medium® (3 mL, Nissui Pharmaceutical Inc., Tokyo, Japan) containing 10% foetal bovine serum (FBS, Gibco, Gaitherberg, MD, USA) was overlaid on each well 1 h later, and samples were incubated in a CO2 incubator at 37°C overnight.
CD-DST—in vitro chemosensitivity test
Chemosensitivity analyses were performed for CDDP, VNR and ADR, which were added at final concentrations adjusted to give the same AUC (area under the curve) as in clinical use, and incubated for 24 h. The final concentration of each anti-cancer agent was CDDP 0.2 µg/mL, VNR 0.05 µg/mL, and ADR 0.02 µg/mL. After removal of the medium containing the anti-cancer drugs, wells were rinsed with 4 mL HBSS, overlaid with 4 mL PCM-2 medium (serum free medium, Nitta Gelatin Inc.), and incubated for 7 days. At the end of this period, neutral red was added to each well at a final concentration of 50 µg/mL, and incubated for 2 h. Each collagen droplet was fixed with 10% neutral formalin buffer, washed in water, air dried, and quantified by image analysis (image digitizer: LG-3 Image One, Tokyo, Japan; and Video-Micro Scope: VH-5910, Keyence, Osaka, Japan). The growth rates of control incubations were calculated as the total volume of living cancer cells on day 7 divided by the total volume of living cancer cells on day 1.
CD-DST—assessment of therapeutic effect
The in vitro sensitivity was expressed as the T/C (%) ratio, where T was the total volume of living cancer cells in each treatment group, and C was the total volume of living cancer cells in the control group. The T and the C values were calculated as an average of triplicate droplets. A T/C ratio of 50% or less was regarded as being sensitive in vitro, according to clinical use of CD-DST.
Heat treatment and drug exposure (modified CD-DST method)
Hyperthermia was induced by setting the incubator temperature to 42.0°C for the first hour of the 24-h drug exposure period, as described above for the CD-DST method. It takes 15 min of heating for the temperature of collagen gel droplets to rise from 37°C to greater than 40°C, so 60 min heating was set to allow for at least 45 min at 40°C or more, as in the in vivo experiment. At the completion of 1 h with the temperature setting at 42°C, it was immediately turned down to 37°C for a further 23 h of drug exposure. As for the conventional CD-DST method, following 24 h of drug exposure, cells were cultured for 7 days in serum-free medium in an incubator containing 5% CO2.
Heat treatment alone
Hyperthermia was induced by setting the incubator temperature to 42.0°C for the first hour of the 24-h drug exposure period, as described above for the modified CD-DST method. At the completion of 1 h with the temperature setting at 42°C, cells were cultured for 23 h and 7 days in serum-free medium in an incubator containing 5% CO2.
Assessment of sensitivity testing
As assessment of sensitivity testing, true positive (TP) is defined as both in vivo and in vitro being sensitive, true negative (TN) is defined as both in vivo and in vitro not being sensitive. False positive (FP) is defined as T/C ratio in vitro under 50% or less and T/C ratio in vivo above 50%. False negative (FN) is defined as which T/C ratio in vivo under 50% or less and T/C ratio in vitro above 50%. Accuracy (%) is defined as (TP + TN)/(TP + TN + FP +FN) × 100.
Statistical analyses
The anti-tumour effect was calculated for each anti-cancer agent and each cancer cell line for the CT group and the HT + CT group, and intergroup comparisons were made using the Mann–Whitney U test. In vivo and in vitro correlations for HT group, CT group, and HT + CT group were examined using simple regression. The level of significance was set at 5%, with p < 0.05 considered as statistically significant.
Results
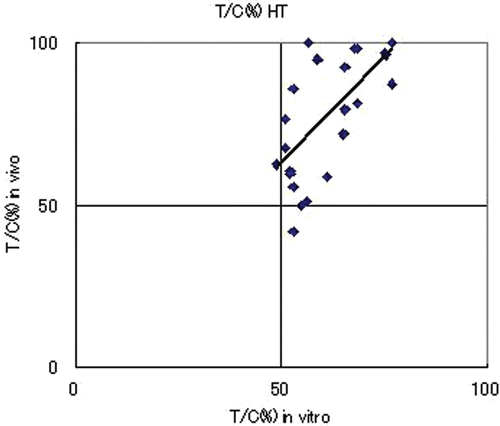
Comparison of in vivo and in vitro anti-tumour effects in the HT group
The in vivo T/C (%) for PC13, PC14, LU99 and A549 were 93 ± 7, 61 ± 21, 68 ± 12 and 90 ± 12, respectively; whereas the in vitro T/C (%) values were 74 ± 4, 55 ± 4, 52 ± 1 and 65 ± 3. There was suppression of cell proliferation by thermotherapy in all cell lines, although the T/C values remained above 50%. Comparison of the in vivo and in vitro anti-tumour effects in four lung cancer cell lines showed a good correlation (R = 0.911, p = 0.089) (). These results indicate that the in vivo therapeutic effect of heat treatment correlated with the CD-DST results.
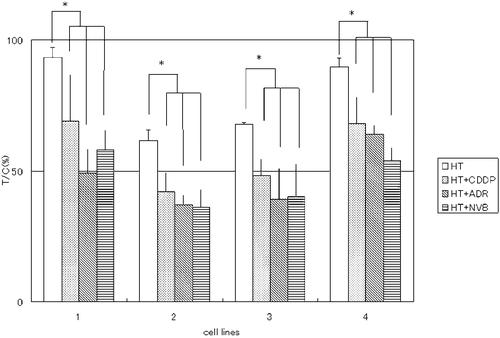
Comparison of the in vivo anti-tumour effects in the HT and HT + CT groups
Effect of hyperthermia alone showed insufficient suppression of tumour growth. A significant increase in suppression of in vivo tumour growth was seen in the HT + CT group in comparison with the HT group in all four cell lines ().
Comparison of the in vivo anti-tumour effects in the CT and HT + CT groups
A significant increase in suppression of tumour growth was seen in the HT + CT group in comparison with the CT group in all four cell lines given CDDP. A significantly suppressed tumour growth was also seen in the HT + CT group in comparison with the CT group in all four cell lines given ADR. A significant increase in the degree of suppression of tumour growth was seen in the HT + CT group in comparison with the CT group in the three cell lines given NVB, but not PC13 ().
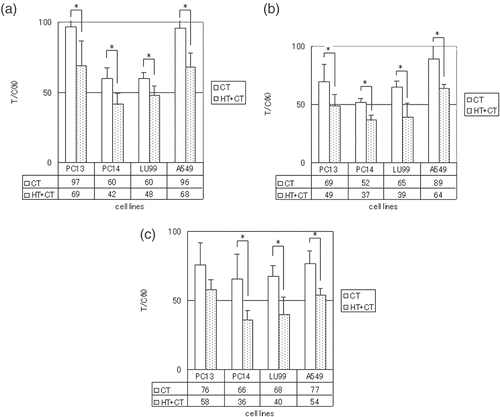
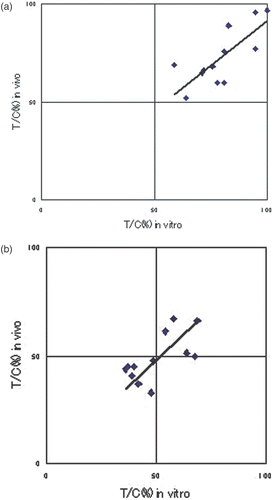
Comparison of in vivo and in vitro anti-tumour effects in the CT and HT + CT groups
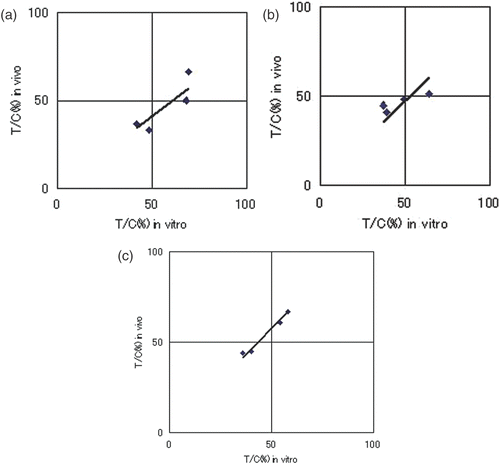
We investigated the correlation between the rate of suppression of tumour cell proliferation (in vitro), determined using the modified CD-DST method, and the actual rate of suppression of tumour growth in transplanted tumours to nude mice (in vivo). A favourable correlation was seen in the CT group (R = 0.76, p = 0.089), indicating that the CD-DST method is suitable for measuring tumour sensitivity to anti-cancer agents (). Similarly, a statistically significant positive in vitro–in vivo correlation was found in the HT + CT group overall (R = 0.65, p = 0.02), indicating that the modified CD-DST method can predict sensitivity to thermochemotherapy (). Good in vitro and in vivo correlations were also seen for each anti-cancer agent in the HT + CT group (CDDP: R = 0.87, p = 0.13 (), ADR: R = 0.88, p = 0.12 (), NVB: R = 0.99, p = 0.009 ()), thus confirming that the modified CD-DST method could predict the anti-tumour effect of each agent with hyperthermia.
Assessment of the modified CD-DST
shows in vivo/in vitro T/C of each respective anti-tumour drug and cell line. In only A549 and CDDP, the modified CD-DST revealed a false positive datum. Therefore, the true positive rate was 87.5% (7/8), and true negative rate was 100% (4/4). Sensitivity, specificity and accuracy were 100% (7/7), 80% (4/5), and 91.7% (11/12), respectively.
Table I. Accuracy, sensitivity and specificity in vivo and in vitro of HT + CT group.
Discussion
We investigated in vitro whether it is possible to use a modified CD-DST method to predict the anti-tumour effects of in vivo thermochemotherapy with CDDP, ADR and NVB. Overall results showed a statistically significant in vivo and in vitro correlation (R = 0.65, p = 0.021).
The CD-DST method of in vitro anti-cancer agent sensitivity testing obtains high cell culture success rates by culturing the tumour cells in a three-dimensional collagen gel matrix. Culture is performed in a 37°C environment, with exposure for 24 h to the anti-cancer agents at concentrations calculated to give the same AUC as in clinical use. This is followed by culture for 1 week in culture medium not containing anti-cancer agents, and the number of cells indicates the degree of suppression of cell proliferation by each agent Citation[12–15]. In the modified CD-DST method we used, the incubator temperature was set at 42°C for the first hour of the 24-h drug exposure period. If we place a cell culture in an incubator previously set at 42°C, the culture will reach 42°C after 15 min and if we return this culture to the original incubator set at 37°C 60 min later, we approximate the same conditions that occur within a tumour subjected to thermotherapy in vivo, in terms of temperature only. Under these actual conditions, we found that the modified CD-DST method detected suppressed cell proliferation in all cell lines used. In the case of thermotherapy alone (HT group), there was in vitro and in vivo correlation, with a significant difference between the two (R = 0.91, p = 0.089).
Therefore, we consider that the CD-DST method can be used as described to predict the effects of in vivo thermotherapy.
The addition of hyperthermia to chemotherapy has been reported to enhance the anti-tumour effecCitation[11], Citation[21], Citation[22].
Intracellular uptake of CDDP and ADR is known to increase with heating, and the higher the temperature the greater the increase in uptake Citation[8], Citation[9], Citation[11], Citation[21–23]. With CDDP, heating promotes DNA crosslink formation, thereby enhancing its cytoxic effect Citation[23]. Although a search of the literature failed to reveal any reports of heat enhancement of the efficacy of NVB Citation[24], the present study results demonstrate that hyperthermia does increases its anti-tumour effect. Therefore, we chose CDDP, ADR and NVB as the agents of thermochemotherapy in this study.
The modified CD-DST method showed good correlation between in vitro and in vivo thermochemotherapy. In clinical use, accuracies of CD-DST were reported to be 81.8% in unresectable non-small cell lung cancer Citation[25], 84% in recurrent non-small cell lung cancer Citation[26], and 87% in breast cancer Citation[27]. In this study the modified CD-DST for thermochemotherapy showed 91.9% of accuracy. Sensitivity, specificity, true positive rate, and true negative rate were also acceptable. Therefore, we suppose the modified CD-DST could be applicable to clinical use for thermochemotherapy.
Our results showed ADR and NVB tended to have greater in vivo than in vitro anti-tumour effects, which may be because the CD-DST method, although conducted at 42°C, does not reproduce changes in the tissue environment, such as local increases in blood flow. Another factor may be that the composition of the culture fluid is constant in the CD-DST method, even when heated to 42°C, whereas local levels of acid phosphatase increase, because of anaerobic glycolysis, and the pH falls when tumours are subjected to hyperthermia in vivoCitation[21], Citation[28], Citation[29]. Nevertheless, our findings of in vivo and in vitro correlations with satisfied accuracy, sensitivity and specificity indicate that the modified CD-DST method can predict the effects of in vivo thermochemotherapy.
When chemotherapy alone is ineffective, selection of an appropriate combination of anti-cancer agent and thermotherapy will provide the better treatment, and that the use of sensitivity testing in such cases will lead to individualized, more effective therapy.
References
- Souquet PJ, Chauvin F, Boissel JP, Cellerino R, Cormier Y, Ganz PA, Kaasa S, Pater JL, Quoix E, Rapp E. Polychemotherapy in advanced non-small cell lung cancer: A meta-analysis. Lancet 1993; 342: 19–21
- Wozniak AJ, Crowley JJ, Balcerzak SP, Weiss GR, Spiridonidis CH, Baker LH, Albain KS, Kelly K, Taylor SA, Gandara DR, Livingston RB. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small cell lung cancer: A Southwest Oncology Group study. J Clin Oncol 1998; 6: 2459–2465
- Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C, Palmer MC, Gregor A, Nguyen B, Niyikiza C, Einhorn LH. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small cell lung cancer. J Clin Oncol 2000; 18: 122–130
- Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, Jiroutek M, Johnson D. Comparison of survival and quality of life in advanced non-small cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: Results of an Eastern Cooperative Oncology Group trial. J Clin Oncol 2000; 18: 623–631
- Le Chevalier T, Brisgand D, Douillard JY, Pujol JL, Alberola V, Monnier A, Riviere A, Liones P, Chomy P, Cigolari S. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small cell lung cancer: Results of a European multicenter trial including 612 patients. J Clin Oncol 1994; 12: 360–367
- Bunn PA, Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: A review of the literature and future directions. Clin Cancer Res 1998; 4: 1087–1100
- Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 1988; 61: 232–237
- Urano M, Kahn J, Kenton LA. The effect of cis-diamminedichloroplatinum (II) treatment at elevated temperatures on murine fibrosarcoma, Fsa-II. Int J Hyperthermia 1990; 6: 563–570
- Nagaoka S, Kawasaki S, Sasaki K. Intracellular uptake, retention, and cytotoxic effect of adriamycin combined with hyperthermia in vitro. Jpn J Cancer Res 1983; 43: 517–520
- Hahn GM. Potential for therapy of drugs and hyperthermia. Cancer Res 1979; 39: 2264–2268
- Harn GM, Braun J, Har-Kedar I. Thermochemotherapy; synergism between hyperthermia (42–43KC) and adriamycin (or bleomycin) in mammalian cells inactivation. Proc Natl Acad Sci USA 1975; 72: 937–940
- Tanigawa N, Kitaoka A, Yamakawa M, Tanisaka K, Kobayashi H. In vitro chemosensitivity testing of human tumours by collagen gel droplet culture and image analysis. Anticancer Res 1996; 16: 1925–1930
- Kobayashi H, Tanisaka K, Doi O, Kodama K, Higashiyama M, Nakagawa H, Miyake M, Taki Y, Hara S, Yasutomi M, Hanatani Y, Kotake K, Kubota T. An in vitro chemosensitivity test for solid human tumors using collagen gel droplet embedded cultures. Int J Oncol 1997; 11: 449–455
- Inaba M, Tashiro T, Sato S, Ohnishi Y, Tanisaka K, Kobayashi H,. Koezuka M: In vitro-in vivo correlation in anticancer drug sensitivity test using AUC-based concentrations and collagen gel droplet-embedded culture. Oncology 1996; 53: 250–257
- Hanatani Y, Komayashi H, Kodaira S, Takami H, Asagoe T, Kaneshiro E. An in vitro chemosensitivity test for gastric cancer using collagen gel droplet-embedded culture. Oncol Rep 2000; 7: 1027–1033
- Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res 2003; 161: 48–61
- Mori T. Prediction of cell kill kinetics of anticancer agents using the collagen gel droplet embedded-culture drug sensitivity test. Oncol Rep 2002; 2: 301–305
- Kodama K, Doi O, Tatsuta M, Kuriyama K, Tateishi R. Development of postoperative intrathoracic chemo-thermotherapy for lung cancer with objective of improving local cure. Cancer 1989; 64: 1422–1428
- Fujimoto S, Kobayashi K, Takahashi M, Nemoto K, Yamamoto I, Motou T, Toyasawa T, Ashida T, Hayashi S, Igarashi N, Ohkubo H. Clinical pilot studies on pre-operative hyperthermic tumour ablation for advanced breast carcinoma using an 8 MHz radiofrequency heating device. Int J Hyperthermia 2003; 1: 13–22
- Nakatani S, Kunieda K, Seki T, Wakabayashi M, Inoue K, Nagata K, Murata T, Tanaka Y, Sougawa M, Kimura R. Fundamental study on hyperthermic chemotherapy using adriamycin-loaded hydroxyapatite. Jpn J Cancer Chemother 1992; 10: 1644–1647
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44(suppl.)4721s–4730s
- Matthew CB, DuBose DA, Sils IV, Tartartini KA. Hyperthermia-induced changes in the vascular permeability of rats: A model system to examine therapeutic interventions. J Thermal Biol 2000; 45: 219–230
- Campling BG, Pym J, Baker HM, Cole SPC, Lam YM. Chemosensitivity testing of small cell lung cancer using the MTT assay. Br J Cancer 1991; 63: 75–83
- Rietbroek RC, Katschinski DM, Reijers MHE, Robins HI, Geerdink A, Tutsch K, D'oleire F, Haveman J. Lack of thermal enhancement for taxanes in vitro. Int J Hyperthermia 1997; 13: 525–533
- Kawamura M, Gika M, Abiko T, Inoue Y, Oyama T, Izumi Y, Kobayashi H, Kobayashi K. Clinical evaluation of chemosensitivity testing for patients with unresectable non-small cell lung cancer (NSCLC) using Collagen gel Droplet embedded culture Drug Sensitivity Test (CD-DST). Cancer Chem Pharm 2007; 59: 507–513, 8(Augus) [Epub ahead of print]
- Higashiyama M, Kodama K, Yokouchi H, Takami K, Nakagawa H, Imamura F, Minamigawa K, Kobayashi H. Cisplatin-based chemotherapy for postoperative recurrence in non-small cell lung cancer patients: Relation of the in vitro chemosensitive test to clinical response. Oncol Rep 2001; 8: 279–283
- Takamura Y, Kobayashi H, Taguchi T, Motomura K, Inaji H, Noguchi S. Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancer. Int J Cancer 2002; 98: 450–455
- Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977; 2: 463–474
- Harman TS, Teicher BA, Collins LS. Effect of hypoxia and acidosis on the cytotoxicity of four platinum complex at normal and hyperthermic temperatures. Cancer Res 1988; 48: 2342–2347