Abstract
Hyperthermia is a useful adjunct in cancer therapy as it can increase the effectiveness and decrease the toxicity of currently available cancer treatments such as chemotherapy and radiation. In the present study, we investigated whether 41°C hyperthermia (mild HT) for 20 min can enhance macrosphelide (MS5)-induced apoptosis in human lymphoma U937 cells. Our results revealed that, compared with MS5 (5 µM) and mild HT alone, the combined treatment exhibited significant enhancement in apoptosis at 6 h, which was evaluated by observing morphological changes and DNA fragmentation. Marked increase in the reactive oxygen species (ROS) generation was observed immediately after the combined treatment. Significant increase in Fas externalization, caspase-8 and caspase-3 activation, and loss of mitochondrial membrane potential (MMP) was found after the combined treatment compared with MS5 and mild HT alone. Moreover, this combination can also alter the expression of apoptosis-related proteins as evident by the cleavage of Bid and down-regulation of Bcl-2 while no change in the expression of Bax was observed. Furthermore, an immediate rise in the intracellular calcium ion ([Ca2+]i) concentration was observed after the combined treatment, which continuously increased in a time-dependent manner. In addition, mild HT treatment alone also increases [Ca2+]i concentration without inducing apoptosis. Our data indicate that early increase in ROS generation is mainly responsible for the enhancement of apoptosis after the combined treatment.
Introduction
Heat treatment of tumors, either alone or in combination with anti-cancer drugs and radiation, is a widely accepted procedure in fighting cancer. Its application enables the use of lower doses of chemotherapy or radiation; therefore, the two main obstacles of cancer therapy unwanted side effects and resistance of cancer cells to drugs and radiation are reduced.
In the enhancement of heat-induced apoptosis [Ca2+]i concentration and free radicals are involved Citation[1], Citation[2] and ROS including H2O2 play an important role as intracellular mediators of hyperthermia-induced apoptosis Citation[3]. It has been reported that mild hyperthermia shows a synergism with some cytotoxic drugs at temperatures as low as 39°C–41°C Citation[4] and combination therapy using mild hyper-thermia and anti-cancer drugs is more effective than the use of either singly Citation[5].
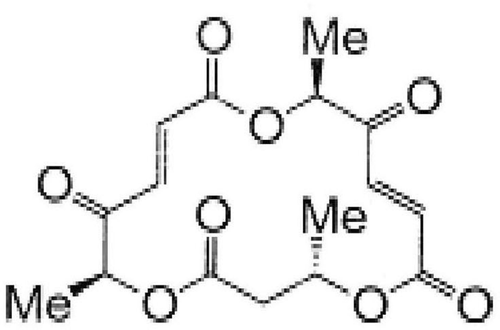
In our previous study, the synthesis and biological properties of macrosphelides, 16-membered natural macrolide compounds, were reported Citation[6], and we have noticed that one of the synthetic diketone macrosphelide (MS5) () can activate a ROS-dependent apoptotic program in U937 cells at 10 µM concentration after 12 h incubation. This preliminary result is the first observation on the apoptosis-inducing potential of macrosphelides Citation[7].
Based on the above reports that mild hyperthermia can enhance the properties of cytotoxic drugs, we aimed to examine the effects of the combination of short duration mild hyperthermia (41°C for 20 min) and MS5 on U937 cells and demonstrated its associated mechanism in detail.
Materials and methods
Cells and heat treatment
A human lymphoma cell line, U937, was obtained from the Human Sciences Research Resource Bank (Japan Human Sciences Foundation, Tokyo, Japan). The cells were grown in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) at 37°C in humidified air with 5.0% CO2. Cells were used for experiments after confirmation that they are free of any mycoplasma contamination. Cell viability before treatment was always more than 95% as evaluated by the trypan blue exclusion test Citation[8]. MS5 was synthesized as described in previous publications Citation[6], Citation[9]. The U937 cells were pretreated with 5 µM concentration of MS5 and then heat treatment was performed immediately by immersing the plastic culture tubes containing the cell suspension (3 ml) in a water bath (NTT-1200, Eyela, Tokyo, Japan) at 41°C for 20 min. The temperature of the culture medium was monitored with a digital thermometer (#7563, YOKOGAWA, Tokyo, Japan) with a 0.8-mm thermocouple. The cells were then incubated at 37°C for 6 h and then harvested for the evaluation of apoptosis.
Measurement of intracellular H2O2 production
After heat treatment with or without MS5, 5 µM of 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes, Eugene, OR) was added to the cells to measure the incidence of intracellular H2O2-positive cells by flow cytometry (Epics XL, Beckman-Coulter, Miami, FL, USA) Citation[10].
Determination of DNA fragmentation
The cellular DNA fragmentation after the heat treatment with or without MS5 was assayed using the method of Sellins and Cohen Citation[11]. Briefly, the cells were lysed in a lysis buffer (10 mM Tris, 1 mM EDTA and 0.2% Triton X-100, pH 7.5) and centrifuged at 13000 × g for 10 min. Subsequently, each DNA in the supernatant and the pellet was precipitated in 12.5% trichloroacetic acid (TCA) at 4°C and quantified using diphenylamine reagent after hydrolysis in 5% TCA at 90°C for 20 min. The percentage of fragmented DNA in each sample was calculated as the amount of DNA in the supernatant divided by total DNA for that sample (supernatant plus pellet).
Microscopic observation
To identify apoptotic cells, the cells harvested at 6 h after exposure to heat treatment with or without MS5 were washed with phosphate buffer saline (PBS) and collected by centrifugation. The cells were fixed with methanol and acetic acid (3:1) and spread on slide glasses. After drying, staining was performed with a 3% Giemsa solution (pH 6.8) for 15 min. Apoptotic cells were determined by counting a total of 500 cells per sample in randomly selected areas.
Flow cytometric analysis for apoptosis
Flow cytometry was performed with propidium iodide (PI) and fluorescein isothiocyanate (FITC)-labelled annexin V to detect the expression of phosphatidyl serine on the outside of the cell membrane as an end point of early apoptosis. The samples were washed in PBS cooled at 4°C and centrifuged at 500 × g for 5 min. The resulting pellet was adjusted to 106 cells per ml with the binding buffer of the Annexin V-FITC kit (Immunotech, Marseille, France). FITC-labelled annexin V (5 µl) and PI (5 µl) were added to the suspension (490 µl) and mixed gently. After incubation for 10 min in the dark, the cells were analyzed with a flow cytometer.
Determination of intracellular calcium ions concentration
The cells were collected by centrifugation and washed with HEPES-buffer Ringer solution (HR) containing 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.13 mM MgCl2, 1.0 mM Na2HPO4, 5.5 mM glucose and 10 mM HEPES. The buffer was supplemented with 0.2% bovine serum albumin (Sigma, St Louis, MO, USA), 2.0% Eagle essential amino acids (Flow Laboratories, Irvine, UK) and 2 mM L-glutamine. About 3 × 105 cells in 3 ml of HR were loaded with 5 µM Fura-2/AM (Dijino Laboratory, Kumamoto, Japan) for 30 min at 25°C. The cells loaded with Fura-2/AM were washed once with HR and twice with the growth medium. After centrifugation, the cells were transferred to a tube containing culture medium with or without MS5. After heat treatment, the cells were washed with HR and an aliquot of cell suspension (10 µl) was transferred onto a glass-bottom dish coated with Cell-Tak (Collaborative Research, Bedford, MA, USA). After addition of HR to the dish, digital imaging of Fura-2 fluorescence was carried out using an inverted microscope and a digital image processor (Argus 50/Ca, Hamamatsu Photonics, Hamamatsu, Japan). The fluorescence ratio (340/380 nm) at an emission wavelength of 510 nm was converted to [Ca2+]i as reported previously Citation[1], Citation[2].
Assessment of mitochondrial membrane potential
A cationic fluorophore, tetramethylrhodamine methyl ester (TMRM), was employed for examining mitochondrial membrane potential (MMP). TMRM accumulates electrophoretically in mitochondria in response to membrane potential and is released upon loss of MMP. After heat treatment with or without MS5, the cells were washed twice with PBS and exposed to 10 nM TMRM for 15 min at 37°C in PBS containing 1% FBS followed by immediate flow cytometry of red TMRM fluorescence (excitation at 488 nm; emission at 575 nm) Citation[12].
Analysis of caspase-3 and caspase-8 activity
The CaspGLOW™ Red Active caspase-3 staining kit was used to monitor the intracellular caspase-3 activity according to the manufacturer's recommendations (MBL, Nagoya, Japan). Briefly, the sample (106 cells per ml) was incubated with 1 µl of Red-DEVD-FMK at 37°C for 1 h, the samples were washed twice and diluted with 0.5 ml of ice-cold flow cytometry dilution buffer (provided in the kit). The fraction of cells showing strong caspase-3 activity was measured by flow cytometry using the FL-2 channel.
To measure caspase-8 activity, a FLICE/caspase-8 colorimetric protease assay kit (MBL, Nagoya, Japan) was used according to manufacturer's instructions. The assay is based on the spectrophotometric detection of chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate IETD-pNA. The pNA absorbance was quantified using a spectrophotometer at a wavelength of 400 nm (Beckman Instruments Inc., Fullerton, CA, USA) Citation[13].
Flow cytometric detection of Fas on the cell surface
Cells (2 × 105) were washed twice with PBS, resuspended in 20 µl of washing buffer containing 2.5 µg/ml of a FITC-labeled anti-Fas monoclonal antibody (clone: UB3, MBL, Nagoya, Japan) and incubated for 30 min at room temperature and analyzed with a flow cytometer.
Western blot analyses for apoptosis-associated proteins
Western blot analyses for Bid, Bax, Bcl-2 and cytochrome c were performed using specific polyclonal or monoclonal antibodies as described in previous papers Citation[14], Citation[15]. For the detection of these specific antibodies, the chemiluminescence ECL detection reagent (Amersham, Biosciences, UK) was used following the manufacturer's instructions.
Statistics
The results are expressed as mean ± SD. Significance was assessed with Student's t-test and was assumed for p-values <0.05. All experiments presented were performed in triplicate.
Results
Enhancement of MS5-induced apoptosis by mild HT
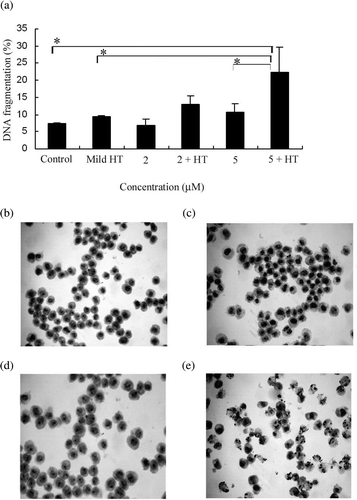
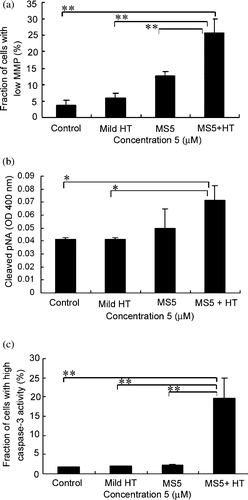
To examine whether or not mild HT potentiates MS5-induced apoptosis, U937 cells were treated with 2 µM and 5 µM concentrations of MS5, 41°C HT for 20 min and the combination of both, followed by 6 h incubation, and then DNA fragmentation, a feature characteristic of apoptosis, was measured. We found that the combination of MS5 5 µM and 41°C HT showed significant increase in the percentage of DNA fragmentation compared with the 41°C HT and MS5 alone (). With this result we selected only 5 µM concentration of MS5 for further experiments with or without mild HT. To determine the percentage of early apoptosis and secondary necrosis, cells were treated with MS5 and 41°C HT alone or in combination followed by a flow cytometry by using annexin V/FITC and PI staining. The proportion of early apoptosis after the combined treatment was higher (18.3 ± 3.0%, mean ± SD, n = 3) than the treatment with MS5 alone (11.0 ± 1.3%, mean ± SD, n = 3) and the percentage of secondary necrosis was 2.7 ± 0.2% after the combined treatment and 1.0 ± 0.7% after treatment with MS5 alone (mean ± SD, n = 3) respectively, while 41°C HT alone did not show early apoptosis or secondary necrosis. To examine the morphological changes in U937 cells, Giemsa staining was performed. Marked apoptotic changes such as chromatin condensation and nuclear fragmentation were observed under light microscope in the cells treated with the combination of MS5 and 41°C HT (. Marginal changes were found in the cells treated with MS5 alone (, while the morphology of cells treated with 41°C HT alone ( is the same as control cells (.
Figure 2. Apoptotic features induced by the combination of mild HT and MS5. (a) DNA fragmentation induced by MS5 and mild HT. U937 cells were treated with 2 µM and 5 µM concentrations of MS5 alone and in combination with mild HT (41°C, 20 min) and incubated for 6 h followed by DNA fragmentation assay. Bars indicate standard deviation (n = 3; *p < 0.05). (b) Morphological changes induced by mild HT and MS5 in U937 cells. Cells were treated with MS5 (5 µM) with or without heat shock (41°C, 20 min) and then harvested after incubation for 6 h. Signs of apoptosis were detected by Giemsa staining and examined under a microscope at a magnification of ×400. (b) Control (c) mild HT (d) MS5 (e) MS5 + HT.
Mild HT potentiates MS5-induced ROS generation
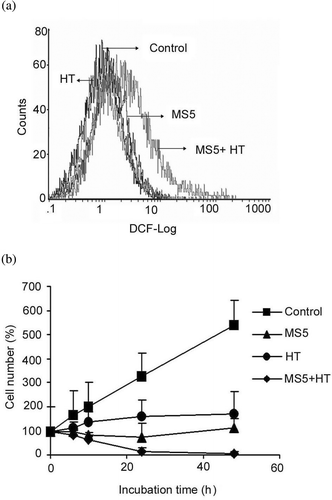
In order to examine the effects of mild HT on MS5-induced ROS generation, cells were treated with MS5, 41°C HT or both followed by immediate staining with DCFH-DA. H2O2 generation was measured by flow cytometry. Significant enhancement in the generation of H2O2 positive cells immediately after the combined treatment was observed, while the cells treated with 41°C HT and MS5 alone did not show generation of H2O2 (. These results suggest that early ROS generation induced by the combined treatment might be involved in the enhancement of MS5-induced apoptosis.
Figure 3. Effects of MS5 and mild HT on ROS generation and on cell growth. (a) U937 cells were first incubated with DCFH-DA for 30 min and then treated with MS5, mild HT alone and in combination of both. Intracellular H2O2 production was measured by flow cytometry immediately after treatment. (b) Time-dependent effect on cell growth was determined by trypan blue dye exclusion test. Bars indicate standard deviation (n = 3).
Effects of mild HT and MS5 on cell growth
Next we examined the growth rate of U937 cells after treatment with MS5 and mild HT alone and in combination of both. The results showed that the combined treatment significantly inhibits cell growth after 24 h, while the cells treated with mild HT or MS5 alone showed cytostatic effects after 24 h (.
Effects of combined treatment on the expression of Bcl-2 family proteins
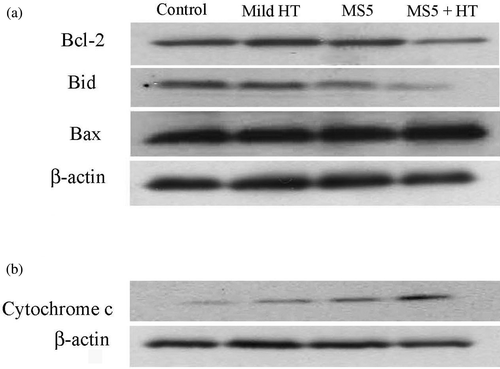
To investigate further whether mild HT enhances MS5-induced apoptosis by modulating the expression of Bcl-2 family members, which ultimately determine the cellular response to apoptotic stimuli, western blotting was done. The results revealed that an anti-apoptotic protein, Bcl-2 was significantly down-regulated and Bid, the pro-apoptotic protein and a substrate of caspase-8, was cleaved at 6 h after the combined treatment, while no change in the expression of Bax was observed (. In addition, the combined treatment also induces the release of cytochrome c from mitochondria to cytosol at 3 h (). These results indicate that mild HT enhances MS5-induced apoptosis by altering the function of Bcl-2 family proteins, which results in the release of cytochrome c from mitochondria.
Figure 4. Changes in the expression of Bcl-2 family proteins and cytochrome c release. U937 cells were treated with MS5 (5 µM) and mild HT (41°C) alone and in combination. (a) Changes in the expression of Bcl-2 family, and (b) release of cytochrome c into the cytosol were monitored by western blotting.
Mild HT enhances MS5-induced reduction in MMP
Many apoptotic stimuli including the death-inducing ligands and chemotherapy agents can activate the mitochondrial apoptosis pathway. The loss of MMP and the release of cytochrome c are the markers for the activation of mitochondrial pathways, which are regulated by the pro- and anti-apoptotic proteins of the Bcl-2 family. As the combined treatment already showed the alterations in pro- and anti-apoptotic Bcl-2 proteins, we therefore determined the decline in MMP by flow cytometry after treating the cells with MS5 and mild HT alone and in combination for 6 h. Significant loss of MMP after the combined treatment compared with MS5 and mild HT alone was observed .
Mild HT enhances MS5-induced caspase activation
Caspases are the important mediators of apoptosis caused by various apoptotic stimuli. To determine whether mild HT enhances MS5-induced apoptosis through the caspase pathway, the activation of caspase-8 and caspase-3 were examined. Treatment of U937 cells with the combination showed significant caspase-8 and caspase-3 activities compared with MS5 and mild HT alone (.
Fas externalization induced by the combination of MS5 and mild HT
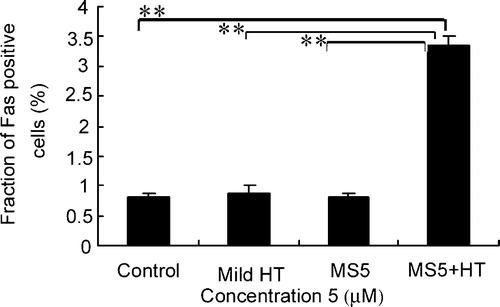
To examine the effects of mild HT on Fas externalization, U937 cells were treated with mild HT, MS5 alone and the combination of both. The externalization of Fas was markedly increased 30 min after the combined treatment as compared with mild HT and MS5 alone ().
Figure 6. Effects of mild HT and MS5 on Fas externalization. U937 cells were treated with MS5, mild HT alone and in combination of both for 30 min followed by flow cytometry to measure externalization of Fas by using anti-Fas FITC-conjugated antibody. Bars indicate standard deviation (n = 3; **p < 0.01).
Effects of mild HT on MS5-induced change in [Ca2+]i concentration
U937 cells are known to possess Ca2+-Mg2+-dependent endonucleases and a Ca2+-dependent apoptotic pathway Citation[15], Citation[16] and hyperthermia is known to enhance Ca2+-dependent apoptosis Citation[15], Citation[17]. Here, we have examined the changes in [Ca2+]i concentration by the digital imaging of Fura-2 fluorescence and we found that immediately after treatment, [Ca2+]i concentration was significantly increased only in the cells treated with the combination, while MS5 and mild HT alone showed no changes , . The cells treated with mild HT alone and in combination with MS5 shows increase in [Ca2+]i concentration in a time-dependent manner ( and ). However, the cells treated with MS5 alone showed a slight change in [Ca2+]i only at 4 h (. To examine further the role of [Ca2+]i in the enhancement of mild HT-induced apoptosis, we treated U937 cells in the presence or absence of an intracellular calcium ion chelator BAPTA-AM (5 µM) for 6 h followed by DNA fragmentation assay. Our preliminary data showed approximately 30% inhibition of DNA fragmentation in the presence of BAPTA-AM. However, mild HT treatment alone can also increase [Ca2+]i concentration but is unable to induce apoptosis. These results indicate that a rise in [Ca2+]i is partially responsible for the enhancement of MS5-induced apoptosis by mild HT.
Discussion
Hyperthermia on its own had no role to play in the curative treatment of human tumors and its clinical potential lies in its use as an adjuvant with other more conventional treatments Citation[18]. Several clinical studies have demonstrated the synergistic effects of combining mild HT with radiation or chemotherapy Citation[19].
Many studies have reported that in heat-induced apoptosis oxidative stress plays a vital role. After exposure to heat stress, increase of ROS (i.e. superoxide anion radicals, hydrogen peroxide and nitric oxide), has been confirmed in various cell lines and tumor tissues Citation[20–22]. Among these ROS, hydrogen peroxide (H2O2) is thought to be an important mediator of apoptosis due to heat shock. In the present study, significant H2O2 generation was observed immediately after treating the cells with the combination of MS5 and mild HT. Furthermore, increase in the [Ca2+]i concentration and the Fas activation was also observed after the combined treatment indicating that ROS is acting as an initial signal in the enhancement of MS5-induced apoptosis.
We have previously shown that 44°C hyperthermia induces elevation of [Ca2+]i concentration which is involved in the enhancement hyperthermia-induced apoptosis Citation[12], Citation[14]. It has been reported that ROS generation releases free calcium ions from intracellular store sites Citation[23]. In addition, oxidative stress also causes a rise in [Ca2+]i in cytoplasm, which induces calcium influx into mitochondria and nuclei to control apoptosis in chondrocytes Citation[24]. In this study, together with H2O2 generation, increase in [Ca2+]i was also observed immediately after the combined treatment, and it increases in a time-dependent manner. However, BAPTA-AM partially rescued cells from the cytotoxicity of the combined treatment, suggesting that oxidative stress is playing a key role in the enhancement of MS5-induced apoptosis by mild HT.
In the mechanism of apoptosis, caspases play an important role and are responsible for the biochemical and morphological alterations in the cell. Caspase-3 is the most prevailing executor of apoptosis in all caspases and caspase-8 is known to be located upstream in the caspase cascade as an enzyme that can induce the release of cytochrome c from the mitochondria to cytosol Citation[25]. In this study, both of these caspases were significantly activated by the combination of mild HT and MS5, suggesting the activation of caspase-dependent pathways.
Bcl-2 family members are the major regulators of apoptosis. Their function mostly depends on their ability to modulate mitochondrial function. This family comprised of both anti-apoptotic and pro-apoptotic proteins. It has been reported that the anti-apoptotic protein Bcl-2 promotes cell survival by inhibiting a variety of apoptotic pathways Citation[26] and this protective effect of Bcl-2 protein is countered by the pro-apoptotic protein, Bid Citation[27]. In the current study we observed significant down-regulation of Bcl-2 protein and marked decrease in the expression of Bid after the combined treatment, indicating that mild HT enhances MS5-induced Bid activation.
Previously, it has been demonstrated that oxidative stress due to H2O2 induced an increase in the levels of mRNA and proteins for both Fas and FasL in the intestinal epithelial cell line Citation[28]. In this study, we also found an increase in the Fas externalization 30 min after hyperthermia and it has been reported that Fas is able to trigger apoptosis via a direct activation of caspase cascade, or via mitochondria by activating caspase-8 and Bid Citation[29], Citation[30]. We also observed significant MMP collapse, activation of caspase-8, caspase-3, Bid and release of cytochrome c after the combined treatment, suggesting that mild HT enhances Fas mediated mitochondrial pathway in MS5 treated cells.
In conclusion, we demonstrated that 5 µM MS5 alone is not cytotoxic but it has cytostatic effects and the combined treatment of 41°C HT and MS5 showed significant synergistic enhancement of apoptosis in U937 cells. A further study with this combination is necessary to examine its effects on other cancer cell lines for the future use of MS5 as a heat sensitizer.
References
- Kondo T, Kano E, Habara Y, Kanno T. Enhancement of cell killing and increase in cytosolic calcium concentration by combined treatments with hyperthermia and TMB-8 in mouse mammary carcinoma FM3A cells. Cell Calcium 1993; 14: 621–629
- Kondo T, Habara Y, Kanno T, Kano E. Thermosensitization and modification of cytosolic calcium concentration by verapamil and diltiazem in mouse mammary carcinoma cells. Int J Radiat Oncol Biol Phys 1994; 29: 511–517
- Katschinski DM, Boos K, Schindler SG, Fandrey J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J Biol Chem 2000; 275: 21094–21098
- Arjen KW, Eelco B, Andres RVG, Frans ANZ. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001; 27: 365–374
- Zhu WG, Shigetoshi A, Shinobu K, Ryoji A, Katsumasa N, Hiroshi S. Enhancement of hyperthermic killing in L5178Y cells by protease inhibitors. Cancer Res 1995; 55: 739–742
- Ishihara K, Kawaguchi T, Matsuya Y, Sakurai H, Saiki I, Nemoto H. Synthesis and biological evaluation of macrosphelide cores. Eur J Org Chem 2004; 19: 3973–3978
- Unpublished results; manuscript has been submitted for publication.
- Feril LB, Jr, Tsuda Y, Kondo T, Zhao QL, Ogawa R, Cui ZG, Tsukada K, Riesz P. Ultrasound-induced killing of monocytic U937 cells enhanced by 2,2’-azobis(2-amidinopropane) dihydrochloride. Cancer Sci 2004; 95: 181–185
- Kawaguchi T, Funamori N, Matsuya Y, Nemoto H. Total synthesis of Macrosphelides A, B, and E: First application of ring-closing metathesis for macrosphelides synthesis. J Org Chem 2000; 69: 505–509
- Huang HL, Wu SL, Liao HF, Jiang CM, Huang RL, Chen YY, Yang YC, Chen YJ. Induction of apoptosis by three marine algae through generation of reactive oxygen species in human leukemic cell lines. J Agric Food Chem 2005; 53: 1776–1781
- Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol 1987; 139: 3199–3206
- Zhao QL, Fujiwara Y, Kondo T. Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 2006; 40: 1131–1143
- Datta R, Kojima H, Yoshida K, Kufe D. Caspase-3 mediated cleavage of protein kinase C theta in induction of apoptosis. J Biol Chem 1997; 272: 20317–20320
- Zhao QL, Kondo T, Noda A, Fujiwara Y. Mitochondria and intracellular free-calcium regulation of radiation-induced apoptosis in human leukemic cells. Int J Radiat Biol 1999; 75: 493–504
- Li M, Kondo T, Zhao QL, Li FJ, Tanabe K, Arai Y, Zhou ZC, Kasuya M. Apoptosis induced by cadmium in human lymphoma U937 cells through Ca2+-calpain and caspase-mitochondria-dependent pathways. J Biol Chem 2000; 275: 39702–39709
- Kimura C, Zhao QL, Kondo T, Amatsu M, Fujiwara Y. Mechanism of UV-induced apoptosis in human leukemia cells: Roles of Ca2+/Mg2+- dependent endonuclease, caspase-3, and stress activated protein kinases. Exp Cell Res 1998; 239: 411–422
- Arai Y, Kondo T, Tanabe K, Zhao QL, Li FJ, Ogawa R, Li M, Kasuya M. Enhancement of hyperthermia-induced apoptosis by local anesthetics on human histiocytic lymphoma U937 cells. J Biol Chem 2002; 277: 18986–18993
- Overgaard J. Rationale and problems in the design of clinical trials. Hyperthermic oncology, J Overgaard. Taylor & Francis, London 1985; 2: 325–338
- Harm HK. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Yoshikawa T, Kokura S, Tainaka K, Itani K, Oyamada H, Kaneko T, Naito Y, Kondo M. The role of active oxygen species and lipid peroxidation in antitumor effect of hyperthermia. Cancer Res 1993; 53: 2326–2329
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 1996; 93: 5116–5121
- Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Lett 1998; 431: 285–286
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J 1999; 18: 6349–6361
- Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 2001; 50: 19–23
- Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis 2002; 7: 313–319
- Sachs L, Lotem J. Control of programmed cell death in normal and leukemic cells. New implications for therapy. Blood 1993; 82: 15–21
- Wang K, Yin M, Chao DT, Milliman CL, Korsmeyer SJ. Bid: A novel BH3 domain-only death agonist. Genes Dev 1996; 15: 2859–2869
- Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cell. Free Radic Biol Med 2002; 33: 1641–1650
- Strasser A, Newton K. FADD/MORT1, a signal transducer that can promote cell death or cell growth. Int J Biochem Cell Biol 1999; 31: 533–537
- Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family protein, connects the death receptor and mitochondria apoptosis pathways. Cell Res 2000; 10: 161–167
![Figure 7. Assessment of [Ca2+]i. Cells were stained with Fura-2/AM and then immediately treated with heat with or without MS5. Time-dependent images of Fura-2 fluorescence: (a) immediately after the treatment, (b) after 1 h, (c) after 2 h, (d) after 4 h, and (e) after 6 h incubation.](/cms/asset/b05a93f6-3c71-42c1-96ab-568b9bf55a69/ihyt_a_229872_f0007_b.gif)
![Figure 8. Assessment of [Ca2+]i. Cells were stained with Fura-2/AM and then immediately treated with heat with or without MS5. Histograms of [Ca2+]i. (a) Immediately after treatment. (b) After 6 h incubation with MS5, mild HT alone and in combination.](/cms/asset/1863724b-7fbb-422b-a45e-3bd8e21e892e/ihyt_a_229872_f0008_b.gif)