Abstract
Augmented expression of members of the heat shock protein 70 (Hsp70) family are frequently observed in various human cancers. In this study, we examined applicability of laser scanning cytometer (LSC) to evaluate the level of Hsp72, which is the member constitutively expressed and significantly induced after heat shock, in human tumour cell lines. The relative nuclear content of Hsp72 measured by LSC correlated well with the relative intracellular content determined by Western blotting (R = 0.906). Furthermore, there was a close relationship between the relative nuclear content of Hsp72 measured by LSC and the colony-forming ability in soft agar, one of the malignant characteristics of tumour cells (R = 0.880). These results indicate that LSC measurement is useful for predicting the degree of malignancy of cancer cells, as it is reliable, faster than Western blotting and more objective and quantitative than visual measurements.
Introduction
Heat shock proteins are a group of proteins induced in cells exposed to elevated temperature and they are conserved evolutionarily from bacteria to mammalian cells Citation[1–4]. In mammalian cells, major heat shock proteins are the members of the Hsp70 family. They consist of at least eight members including Hsp72 (also known as Hsp70), which is constitutively expressed and significantly induced after heat shock, and a constitutively expressed Hsc73 protein Citation[5]. Hsp72 is induced transcriptionally not only after heat shock but also by various stimuli Citation[3], such as UV Citation[6], ionizing radiation Citation[7], and DNA-damaging agents Citation[8]. It plays a role as a molecular chaperone, which binds to unfolded or denatured proteins and facilitates their repair or degradation, and this function is also essential for preventing misfolding of newly synthesized polypeptides, thereby accomplishing protein maturation Citation[9–14]. Previously, we observed that even under basal, non-stress conditions, many cancer cell lines expressed higher Hsp72 levels than normal human diploid cells, irrespective of their origins Citation[15]. The similar results have been reported not only in human tumour cell lines Citation[16], Citation[17], but also in the primary cancer tissues Citation[18–20], indicating that augmented expression of Hsp72 is involved in the mechanism underlying cancer development. Although the mechanism has not been fully understood, we found that over-expression of human Hsp72 protein increased the UV-induced mutation frequency in immortalized Syrian hamster embryo cells Citation[17]. More recently, a couple of studies have shown that Hsp70 functions as an anti-apoptotic chaperone Citation[19–22]. At the clinical level, it has been reported that Hsp70 expression is correlated with poor prognosis in a variety of types of cancer and with lower susceptibility of cancer cells to anti-cancer treatments Citation[18]. Thus, it is becoming more and more important to determine the levels of heat shock proteins for a better diagnosis and for making a more accurate prognosis.
The laser scanning cytometer (LSC) is a microscope-based cytofluorometer that combines the advantages of the flow cytometer (FC) with those of image analysis Citation[23], Citation[24]. The fluorescence of individual cells is measured rapidly by LSC, with a sensitivity and accuracy comparable to that of FC, while the specimen is located on a microscope slide rather than in suspension Citation[25]. Because of this advantage, LSC is expected to apply for immunopathological analysis of protein levels in tissue sections. Here, we examined the Hsp72 levels in several human tumour cell lines by LSC, compared the results with those by Western blotting, and discussed the applicability of LSC to evaluate malignancy of tumour cells in histological sections.
Materials and methods
Cells and cultures
The primary normal human diploid (NHD) cells and human tumour cell lines were cultured in Eagle's MEM (Nissui Pharmaceutical Co., Ltd, Tokyo) supplemented with 10% fetal bovine serum (Biosciences PTY Ltd, Australia) and 20 mM HEPES at 37°C in a humidified atmosphere with 5% CO2. T24 (bladder carcinoma), HeLa (cervix carcinoma), KB (epidermoid carcinoma), HMV-I (skin melanoma), MCF-7 (mammary carcinoma), HT1080 (fibrosarcoma), A431 (epidermoid carcinoma), G361 (malignant melanoma) were obtained from Japanese Cancer Research Resources Bank (JCRB). RKO (colon carcinoma) and NCI-H1299 (non-small cell lung carcinoma) were purchased from the American type culture collection (ATCC).
Heat treatment
Exponentially growing cells in the culture flasks or the dishes were heated at 43°C for 2 h in a water bath controlled to ±0.1°C. For the time-course study, the cells after heat shock were incubated at 37°C in a CO2 incubator.
Determination of Hsp72 by Western blotting analysis
Cells were lysed in RIPA buffer (50 mM Tris, pH 7.2, 150 mM NaCl, 1% NP–40, 1% sodium deoxycholate, 0.05% SDS). After freezing at −20°C, the cell lysate was centrifuged for 10 min at 15 000 rpm. The supernatant was harvested and the protein content was measured by the bicinchoninic acid method (Pierce, Rockford, IL, USA). Four to eight micrograms of proteins were subjected to electrophoresis through SDS-polyacrylamide gel as described by Laemmli Citation[26]. The proteins were transferred to polyvinyl difluoride (PVDF) filters electrophoretically in transfer buffer (0.1 M Tris, 0.192 M glycine, 20% methanol). Then, the filters were blocked with 0.5% dry milk overnight and incubated with monoclonal antibodies recognizing the member of Hsp70 family, which include anti-Hsp72/73 antibody (clone W27, Oncogene Research Products, San Diego, CA, USA), anti-Hsp70 antibody (clone C92F3A-5, StressGen Biotech Co., Victoria, BC, Canada), anti-Hsp70/Hsc70 antibody (clone N27F3-4, StressGen Biotech Co., Victoria, BC, Canada), and anti-Hsc70 antibody (clone 1B5, StressGen Biotech Co., Victoria, BC, Canada). For visualization of the primary antibodies, the filters were incubated with biotinylated anti-mouse Ig monoclonal antibody (Amersham Japan Co., Ltd, Tokyo, code RPN. 1001), and streptavidin/biotinylated alkaline phosphatase conjugate (Amersham Japan Co., Ltd, Tokyo, code RPN. 1234), followed by incubation with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (BCIP) and nitroblue tetra-zolium chloride (NBT) (Bethesda Research Laboratories, Gaithersburg, MD, USA) for colour development. The amount of Hsp72 was determined by densitometrical analysis using a 2-dimensional densitometer (Scanning Imager, Molecular Dynamics, Sunnyvale, CA, USA). Three independent experiments were performed, and the relative amount of Hsp72 was expressed as means ± SD.
Immunocytochemistry/immunohistochemistry
The cells grown on 22 mm × 22 mm cover-slips were fixed with cold absolute methanol (−20°C) for 5 min. After washing with PBS(−), cells were incubated with a primary antibody, anti-Hsp72/73 monoclonal antibody (clone W27, and diluted 1:100), for 1 h in a CO2 incubator. The cells were then washed with PBS(−) and incubated with a secondary antibody, an anti-mouse Ig antibody directly linked to FITC (diluted 1:100, Amersham Japan Co., Ltd, Tokyo, code N 1031), for 30 min in a CO2 incubator. Finally, the nuclei were counterstained with 25 µg/ml propidium iodide (PI) (Sigma Chemical Co., St Louis, MO, USA) and 0.02% RNase (Sigma) for 15 min in a CO2 incubator. Then, the cover-slips were mounted on microscope slides with the mounting reagent EUKITT® (O. Kindler, Germany).
For tissue sections, surgical specimens from 13 cases of primary invasive breast carcinoma were used. Specimens were fixed with 20% neutral buffered formalin and embedded in paraffin. Serial sections of 5 µm thickness were mounted onto MAS-coated (SUPERFROST®, Matsunami Glass Ind., Ltd, Osaka, Japan) slides. After deparaffination and rehydration, one slide from a tissue block was processed for histological study and the other for immunohistochemical study. For the histological study, slides were stained with hematoxylin and eosin (HE). Histological types were classified according to the General Rules for Clinical and Pathological Recording of Breast Cancer (Japanese Breast Cancer Society). Immunohistochemistry was performed according to the same procedure as immunocytochemistry, except for the concentration (diluted 1:20) of the primary and the secondary antibodies. Nuclear fluorescence of tumour tissue and adjacent normal tissue on the same slide was individually measured by LSC.
Determination of the nuclear Hsp72 level by LSC
After immunofluorescence staining, nuclear fluorescence was measured by LSC (LSC101, Olympus Optical Co., Tokyo). Fluorescence emission is separated optically into green fluorescence of FITC and red fluorescence of PI-stained nuclei. After adjusting the contouring threshold based on scan data display, the FITC fluorescence of each cell was measured using the PI fluorescence signal for contouring. The level of Hsp72 was determined by measuring the mean FITC fluorescence value of the cells based on the PI fluorescence value vs. the FITC fluorescence value scattergram. Laser power and photomultiplier gain and offset were held stable throughout the study. At least 106 cells were measured per measurement, and three independent measurements were performed. The relative amount of Hsp72 was expressed as means ± SD.
Measurement of colony-forming ability in soft agar
To determine the growth of cells in soft agar, cells suspended in 0.3% soft agar medium were plated onto 2% basal agar and incubated in a CO2 incubator for 28 days. Colony-forming ability was expressed as a percentage calculated from the number of colonies formed divided by the number of cells plated. At least three dishes were used, and the data were expressed as means ± SD.
Results
Determination of the member of Hsp70 family detected by anti-Hsp72/73 antibody
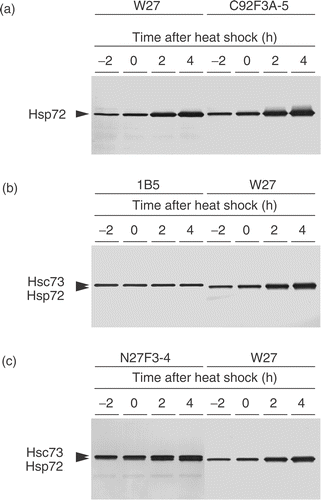
The human Hsp70 family consists of at least eight genes, whose products encode highly homologous proteins. The antibody, clone W27, used in this study was originally established by immunizing mouse with Hsp72/73 proteins purified from HeLa cells, and it was mentioned to react with both the constitutive and inducible forms of Hsp70. However, several studies used this antibody detected a single protein band in Western blotting analysis Citation[15], Citation[27–29]. Here, we determined whether the anti-Hsp72/73 antibody, clone W27, recognizes both Hsp72 and Hsc73. According to the report by Welch and Feramisco Citation[30], we used the name, Hsp72, for the member of Hsp70 family whose molecular weight is 72 kDa. It is expressed constitutively and is highly inducible after heat shock. Hsp72 is also known as Hsp70, and Hsp70 includes those induced only after heat shock, and those constitutively expressed and induced after heat shock Citation[5]. By contrast, Hsc73 is a 73-kDa protein constitutively expressed under normal conditions Citation[5]. As shown in , clone W27 detected a single protein band in NHD cells. Molecular weight of this protein is the same as that detected by clone C92F3A-5, which specifically recognizes Hsp72 Citation[31]. In , the bands visualized by clone W27 were compared with those visualized by clone 1B5, which specifically recognizes Hsc73 Citation[32], and the bands revealed the difference in their mobility. Furthermore, clone N27F3-4 specific for both Hsp72 and Hsc73 manifested two different bands, and the lower bands corresponded to those visualized by clone W27. From these results, we concluded that clone W27 is specific for Hsp72, but not for Hsc73.
Figure 1. Determination of the member of Hsp70 family detected by clone W27. Exponentially growing NHD cells were heat shocked at 43°C for 2 h, and the proteins were extracted after incubation at 37°C for indicated times. (a) The same blot was probed with clone W27 and clone C92F3A-5. (b) The upper blot was probed with clone 1B5, while the lower blot was probed with clone W27. (c) The same blot was separated into two parts, and both bands were probed with clone N27F3-4, while only the lower band was probed with clone W27.
Induction of Hsp72 by heat treatment
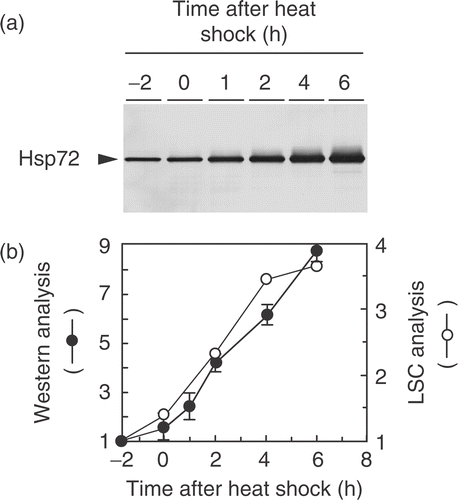
The time-course experiment demonstrates the induction of Hsp72 in NHD cells treated at 43°C for 2 h (). The relative levels of Hsp72, which are expressed as the ratio of the amount at each time to that of unheated NHD cells, increased almost linearly as a function of time up to 6 h after the heat shock (). We found that the induction kinetics of Hsp72 determined by Western blotting were similar to those obtained by LSC (). Because LSC measures FITC value on PI-stained nucleus, the results indicated that the change in the Hsp72 level in the nuclei well correlated with the change in the total amount of Hsp72 protein.
Figure 2. Time-course experiment of Hsp72 induction in NHD cells after heat shock. Exponentially growing NHD cells were treated at 43°C for 2 h. (a) Typical Western blotting pattern of Hsp72 induction detected by clone W27. (b) The relative amount of Hsp72 measured by Western blotting (•) and LSC (○); the relative amount was expressed as the ratio of the amount of Hsp72 to that of unheated NHD cells. The band intensity of the blots was determined by densitometrical analysis.
Hsp72 expression under unheated condition
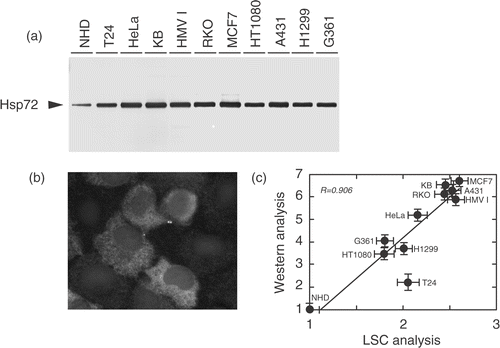
The level of constitutively expressed Hsp72 is compared between NHD cells and cancer cells in . The Hsp72 levels were significantly higher in cancer-derived cell lines, and the levels in those cell lines were comparable to that detected in normal human cells 2 h after heat shock (, 3a). Augmented expression of Hsp72 in human tumour cell lines was detected not only in the cytoplasm but also in the nucleus (); therefore, the level of Hsp72 in the nuclear area was determined by LSC, and the relative levels of Hsp72 were plotted vs. those obtained by Western blotting analysis. As shown in , there is a close relationship between the relative intracellular content of Hsp72 determined by Western blotting and the relative nuclear content measured by LSC (R = 0.906).
Figure 3. Augmented expression of Hsp72 protein in unheated human tumour cell lines. (a) Total proteins were extracted from exponentially growing cells, and the Hsp72 level was examined by Western blotting using clone W27. (b) HT1080 cells grown on cover-slips were fixed with methanol and stained with clone W27, followed by the incubation with FITC-conjugated anti-mouse Ig. Hsp72 is depicted in white. (c) Relationship between the relative intracellular amount of Hsp72 determined by Western blotting and the relative nuclear expression of Hsp72 measured by LSC. A close correlation was observed between them (R = 0.906).
Anchorage-independency and Hsp72 expression
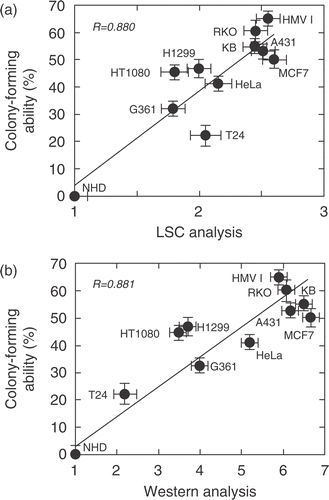
We applied the colony-forming ability in soft agar as an index of malignant characteristics of tumour cells. HMV1 cells showed the highest colony-forming ability (65.7%), and the lowest ability was observed in T24 cells (21.2%), whereas NHD cells did not show any colony-forming ability. There is a close relationship between the colony-forming ability in soft agar and the relative nuclear content of Hsp72 measured by LSC (R = 0.880) (), as well as the relative content determined by Western blotting (R = 0.881) ().
Figure 4. Relationship between the colony-forming ability in soft agar and relative amount of Hsp72 determined by Western blotting and LSC. (a) The nuclear expression of Hsp72 was measured by LSC. The colony-forming ability was determined in 0.3% soft agar as described in the Materials and methods. A close correlation was observed between them (R = 0.880). (b) The total amount of Hsp72 was measured by Western blotting. The colony-forming ability was determined as described above. A close correlation was observed between them (R = 0.881).
Hsp72 expression in pathologic section of breast cancer tissues
To confirm that LSC measurement of Hsp72 expression is applicable to tissue sections, we measured nuclear Hsp72 expression in pathologic section of breast cancer tissues. For this experiment, sections containing tumour tissue and a sufficient amount of adjacent normal tissue on the same slide were chosen. The field for LSC scanning was chosen by comparison with an HE-stained parallel section to identify an area composed of tumour cells and that composed of normal cells. Areas with mixed populations of cells were omitted from the analysis. Cells counted for each area analysed ranged from 4600 to 25 000 for tumour tissue, and from 2800 to 16 000 for normal tissue. As shown in , FITC fluorescence in tumour tissue was higher than that in adjacent normal tissue in 6 of 13 cases, and the higher FITC fluorescence was observed in more malignant histological type of breast cancer tissues; FITC fluorescence in tumour tissue of scirrhous was higher than that in adjacent normal tissue, but FITC fluorescence in tumour tissue of papillotubular and solid-tubular was smaller than that in adjacent normal tissue.
Table I. Augmented expression of Hsp72 in breast cancer tissues.
Discussion
Since the Hsp70 family consists of multiple homologous proteins, the antibodies raised against Hsp70 protein may have different specificity to the different Hsp70 family members. One of the antibody, clone W27, used in this study was originally established by immunizing mouse with Hsp72/73 proteins purified from HeLa cells. It has been indicated in the literature provided by the manufacturer that the antibody can react with both the constitutive and inducible forms of Hsp70. Therefore, we determined whether the antibody, clone W27, recognizes both Hsp72 and Hsc73 or not. As shown in and , clone W27 detected the molecule in normal human cells, whose molecular weight is 72 kDa. It also recognized 72 kDa proteins in human tumour cell lines (). The molecular weight of the protein recognized by clone W27 is the same as that detected by clone C92F3A-5, which recognizes Hsp72 Citation[31], and, as shown in , clone W27 did not show any reactivity to Hsc73. Thus, it can be concluded that clone W27 detected only Hsp72, and this was supported by the previous studies that showed a single protein band visualized by clone W27 Citation[27–29].
As shown in , augmented expression of Hsp72 is commonly observed in human cancer-derived cells by Western blotting. The total amount of Hsp72, as well as the nuclear Hsp72 level, showed a good correlation with colony-forming ability in soft agar, which is an in vitro representation of the malignancy. Thus, the augmented expression of Hsp72 could be a hallmark of malignant human cancer cells in culture. Similar mutual correlation between the Hsp72 level and poor prognosis was demonstrated in a certain type of cancer tissu Citation[18], Citation[19]. For example, Hsp70 expression in primary breast tumours from patients with negative axillary lymph nodes was correlated with shorter disease-free survival Citation[33]. A higher nuclear expression of Hsp70 in breast tumour cells was correlated with shorter disease-free survival as well as drug resistance Citation[34]. Recently, a couple of studies have shown that Hsp70 functions as an anti-apoptotic chaperone. Hsp70 binds to apoptosis protease activating factor-1 (Apaf-1) Citation[35], Citation[36] and antagonizes apoptosis-inducing factor (AIF) Citation[37]. Furthermore, down-regulation of Hsp70 has been reported to induce apoptotic cell death in cultured human tumour cell lines Citation[38–40]. Thus, augmented expression of Hsp72 is likely to function as a survival signal, which protects cancer cells from the lethal effects of physiological stresses Citation[21].
The LSC has been developed in order to combine the advantages of the flow cytometer (FC) with those of image analysis Citation[23], Citation[24]. Because the specimen is located on a microscope slide rather than in suspension, LSC is expected to apply for immunopathological analysis of protein levels in tissue sections. In the present study, we examined the Hsp72 levels in several human tumour cell lines by LSC. So far, several studies have reported augmented expression of Hsp70 family in human cancer. Immunopathological analysis using antibodies recognizing the Hsp70 family members demonstrated that they were over-expressed not only in the cytoplasm but also in the nucleus. For example, clone W27 detected an increased level of nuclear Hsp72 in gastric and colon cancers Citation[41], Citation[42]. Since it is not applicable to measure the total amount of Hsp72 in individual cells in tissues, we evaluated the level of Hsp72 using the nuclear PI signal for contouring. As shown in , the nuclear Hsp72 level after heat shock is well correlated with the total amount of Hsp72 detected by Western blotting. Furthermore, the LSC analysis showed a significant correlation (R = 0.906) between the nuclear level of Hsp72 and the total amount of Hsp72 in most of the human tumour cell lines, except T24 (). This is because the nuclear to cytoplasmic ratio of Hsp72 levels was slightly changed in T24. Without T24, we obtained significantly higher correlation (R = 0.979), indicating that the nuclear fraction of Hsp72 could be a representation of the total amount of Hsp72. Therefore, we have concluded that the nuclear levels of Hsp72 can be used for comparison of the levels of Hsp72 in tissue sections.
In the present study, we have shown that LSC measurement of nuclear Hsp72 expression is applicable to tissue sections, and augmented expression of nuclear Hsp72 was observed in some cases of the breast cancer tissues analysed. Until now, LSC has also been applied to determine the protein levels in the histological sections of paraffin-embedded tissues of human cancers. For example, the nuclear expression of estrogen receptor (ER) Citation[43] or Ki-67 antigen Citation[25] was examined in the histological sections of human breast cancers. In the former study, the authors further demonstrated that percentage ER-positive tumour cells by LSC of immunofluorescence-stained sections correlated well with manual light microscopic counts of immunoperoxidase-stained sections. These studies together with the present study demonstrate the usefulness of LSC for measuring the amount of proteins in tissue sections. Particularly, we propose that LSC could be the valuable option for the measurement of the members of Hsp70 family in cancer tissues, because LSC measurement is both faster than Western blotting and more objective and quantitative than visual counts of the signals.
As discussed previously Citation[18], augmented expression of Hsp72 is not a useful marker in diagnostic immunopathology, because it is observed generally in human cancer cells. However, an increased level of Hsp72 is associated with poor differentiation and enhanced tumour cell proliferation, which determine malignancy of cancer cells. We have also shown, in this study, that augmented expression of Hsp72 compared with adjacent normal tissue was observed in the scirrhous type of breast cancer but not in papillotubular and solid-tubular types, which coincides with the clinical impression that scirrhous types are more malignant than papillotubular and solid-tubular types in breast cancer. Moreover, HSP expression decreases the efficacy of cancer therapy, as it functions as a survival factor. Thus, at this point, the determination of the nuclear Hsp72 levels by LSC could be useful in predicting the prognosis of cancer cells. Future studies will facilitate the better anti-cancer treatment by targeting the Hsp70 family, and application of LSC is expected to aid successful cancer treatment.
References
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet 1988; 22: 631–637
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia 1995; 11: 459–488
- Morimoto RI. Heat shock: The role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cell 1991; 3: 295–301
- Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol 1986; 103: 2035–2052
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones 1996; 1: 23–28
- Suzuki K, Watanabe M. Augmented expression of Hsp72 protein in normal human fibroblasts irradiated with ultraviolet light. Biochem Biophys Res Commun 1992; 186: 1257–1264
- Sierra-Rivera E, Voorhees GJ, Freeman ML. Gamma irradiation increases Hsp-70 in Chinese hamster ovary cells. Radiat Res 1993; 135: 40–45
- Fornace AJ, Jr, Alamo I, Jr, Hollander MC, Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res 1989; 182: 61–74
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem 1994; 219: 11–23
- Ellis RJ, Hemmingsen SM. Molecular chaperones: Proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci 1989; 14: 339–342
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol 1993; 9: 601–634
- Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40-A review. Int J Hyperthermia 2000; 16: 231–245
- Hartl EU. Molecular chaperones in cellular protein folding. Nature 1996; 381: 571–580
- Welch WJ. Mammalian stress response: Cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev 1992; 72: 1063–1081
- Watanabe M, Suzuki K, Kodama S, Sugahara T. Normal human cells at confluence get heat resistance by efficient accumulation of Hsp72 in nucleus. Carcinogenesis 1995; 16: 2373–2380
- Hermission M, Strik H, Rieger J, Dichgans J, Meyermann R, Weller M. Expression and functional activity of heat shock proteins in human glioblastoma multiforme. Neurology 2000; 54: 1357–1364
- Suzuki K, Watanabe M. Modulation of cell growth and mutation induction by introduction of the expression vector of human Hsp70 gene. Exp Cell Res 1994; 215: 75–81
- Ciocca D, Calderwood SK. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005; 10: 86–103
- Jäättelä M. Heat shock proteins as cellular lifeguards. Ann Med 1999; 31: 261–271
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trend Biochem Sci 2006; 31: 164–172
- Jäättelä M. Escaping cell death: Survival proteins in cancer. Exp Cell Res 1999; 248: 30–43
- Xanthoudakis S, Nicholson DW. Heat-shock proteins as death determinants. Nat Cell Biol 2000; 2: E163–E165
- Kamentsky LA, Burger DE, Gershman RJ, Kamentsky LD, Luther E. Slide-based laser scanning cytometry. Acta Cytol 1997; 41: 123–143
- Kamentsky LA, Kamentsky LD. Microscope-based multiparameter laser scanning cytometer yielding data comparable to flow cytometry data. Cytometry 1991; 12: 381–387
- Gorczyca W, Deptala A, Bedner E, Li X, Melamed MR, Darzynkiewicz Z. Analysis of human tumors by laser scanning cytometry. Methods Cell Biol 2001; 64: 421–443
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Fujita M, Nagai M, Murata M, Kawakami K, Irino S, Takahara J. Synergistic cytotoxic effect of quercetin and heat treatment in a lymphoid cell line (OZ) with low Hsp70 expression. Leukemia Res 1997; 21: 139–145
- Shinder GA, Lacourse M-C, Minotti S, Durham H. Mutant Cu/Zu-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J Biol Chem 2001; 276: 12791–12796
- Timblin CR, Janssen YM, Goldberg JL, Mossman BT. GRP78, Hsp72/73, and cJun stress protein levels in lung epithelial cells exposed to asbestos, cadmium, or H2O2. Free Radic Biol Med 1998; 24: 632–642
- Welch WJ, Feramisco JR. Rapid purification of mammalin 70,000-dalton stress proteins: Affinity of the proteins for nucleotides. Mol Cell Biol 1985; 5: 1229–1237
- Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol 1986; 103: 2035–2052
- Hattori H, Kaneda T, Lokeshwar B, Laszlo A. A stress-inducible 40 kDa protein (hsp40): Purification by modified two-demensional gel electrophoresis and co-localization with hsc (p73) in heat-shocked HeLa cells. J Cell Sci 1993; 104: 629–638
- Ciocca DR, Clark GM, Tandon AK, Fuqua SAW, Welch WJ, McGuire WL. Heat shock protein Hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J Natl Cancer Inst 1993; 85: 570–574
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer (Pred Oncol) 1998; 79: 468–475
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000; 2: 469–475
- Leh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2000; 2: 476–483
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3: 839–843
- Nylandsted J, Rohde M, Brand K, Batholm L, Elling F, Jäättelä M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA 2000; 97: 7871–7876
- Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jäättelä M. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res 2002; 62: 7139–7142
- Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jäättelä M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechnisms. Genes Dev 2005; 19: 570–582
- Sun X-F, Zhang H, Carstensen J, Jansson A, Nordenskjöld. Heat shock protein 72/73 in relation to cytoplasmic p53 expression and prognosis in colorectal adenocarcinomas. Int J Cancer 1997; 74: 600–604
- Villaseca MA, Roa I, Araya JC, Roa JC, Flores P. Double immunostaining for p53 and molecular chaperone hsp72/73 in gastric carcinoma. J Clin Pathol Mol Pathol 1997; 50: 317–321
- Gorczyca W, Davidian M, Gherson J, Ashikari R, Darzynkiewicz Z, Melamed MR. Laser scanning cytometry quantification of estrogen receptors in breast cancer. Analyt Quant Cytol Histol 1998; 20: 470–476