Abstract
Intraoperative chemotherapy with heat has been identified as a treatment option for patients with cancer spread to peritoneal surfaces. This treatment modality is viewed as a supplement to several other treatments for this group of patients including cytoreductive surgery, systemic chemotherapy, early postoperative intraperitoneal chemotherapy, and long-term bidirectional chemotherapy. The pharmacologic basis for using heat to supplement chemotherapy effects are related to the increased penetration of chemotherapy into tumor with hyperthermia, the delayed clearance of chemotherapy from the peritoneal cavity after direct instillation, and an increased cytotoxicity that has been documented with selected chemotherapy agents. Data to support the use of perioperative hyperthermic intraperitoneal chemotherapy with mucinous appendiceal carcinomatosis comes from a large number of single institution phase II studies. Also, peritoneal and pleural mesothelioma are benefited. In colon cancer carcinomatosis, large phase II multi-institutional trials and a single phase III trial documented an increased median survival of these patients from approximately 1 year to over 2 years. Prophylaxis against peritoneal carcinomatosis in gastric cancer has been demonstrated in phase III trials. In ovarian cancer the rationale for this treatment remains large but its current application is limited. Much work needs to be done to identify a proper clinical perspective on hyperthermia used with chemotherapy in patients with peritoneal surface malignancy.
Rationale for heated intraoperative intraperitoneal chemotherapy (HIIC)
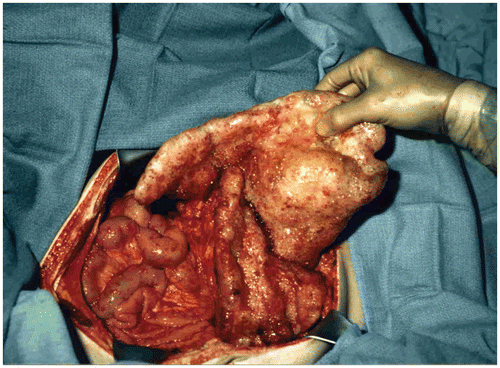
Carcinomatosis from gastrointestinal or gynecologic malignancy was in the past a lethal condition associated with a short survival and an increasingly poor quality of life Citation[1–3]. A terminal event of intestinal obstruction, intra-abdominal abscess or fistula was the expected outcome. Recently, management strategies have emerged that suggest that prevention of carcinomatosis is possible, that cure of selected patients is a reality, and that strategies for improved palliation should be accepted as standard of practice Citation[4–7]. These changes in the management of this lethal condition come about from three separate observations. First, peritoneal dissemination is not a uniform process on the abdominal and pelvic surfaces. As the cancer disseminates and progresses within the coelomic space, it does so in a characteristic fashion governed, for the most part, by physical principles (). The original observation that led surgeons to attempt debulking procedures is the profound sparing of small bowel surfaces by mucinous cancers even though a large volume of disease is present within the abdomen and pelvis Citation[8], Citation[9]. Visceral resection and peritonectomy procedures can remove all visible evidence of disease Citation[10].
Figure 1. Small bowel sparing in a patient with mucinous adenocarcinoma arising in the appendix. Large volumes of cancer have accumulated in the omentum (omental cake), in the crevice between the undersurface of the right hemidiaphragm and liver, and by gravity within the pelvis. Cytoreductive surgical procedures have evolved to the extent that all visible evidence of disease can be surgically removed.
Second, Weiss and colleagues documented the phenomenon of ‘metastatic inefficiency’. In patients with gastrointestinal cancer, even though the portal blood is ‘teeming with cancer cells’, usually the liver and other systemic sites will not develop any metastatic disease Citation[11]. By contrast, Schott and colleagues showed that even a small number of gastric or colorectal cancer cells free in the peritoneal cavity had a profound effect in reducing survival. A similar number of cancer cells identified within the bone marrow produced no reduction in the survival Citation[12]. Apparently, cancer cells in the coelomic cavity have a profound capability to implant, progress and disrupt function. Cancer cells disseminated haematogenously or lymphatically are much less efficient in resulting in implantation, progression and disruption of function. In summary, cancer cells contained within the vascular system and bone marrow are not likely to progress. However, cancer cells detected within the peritoneal cavity almost always progress and have a profound negative impact on survival. A logical conclusion from these observations is that carcinomatosis often occurs in the absence of systemic metastases and its effective treatment will improve survival.
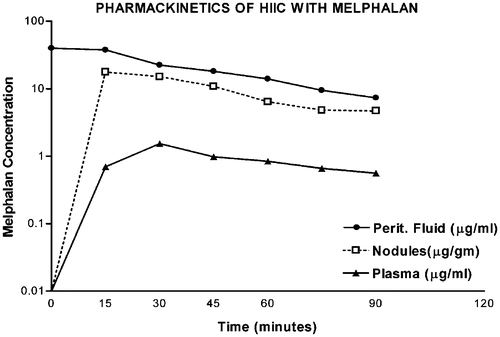
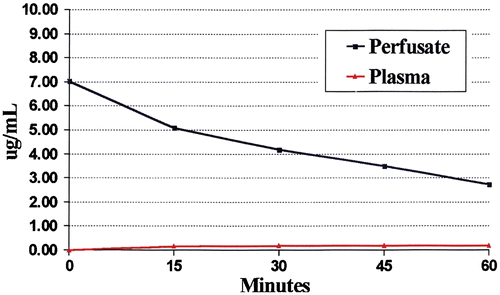
The third essential conceptual advance was the pharmacologic description of the ‘peritoneal space-to-plasma barrier’. Flessner and colleagues clearly showed that large molecules placed into the peritoneal cavity would remain sequestered there for many hours Citation[13]. The slow clearance of the chemotherapy agent from the peritoneal cavity combined with its rapid elimination from the plasma resulted in a profound local-regional treatment with minimal systemic toxicity. As shown in , the area under the curve (AUC) of concentration time for an intraperitoneal large molecule can be many times greater than the AUC of concentration time for the exposure within the plasma compartment. The crucial change in chemotherapy delivery for carcinomatosis is the intraperitoneal route of administration as compared with an intravenous one.
Figure 2. Pharmacokinetics of heated intraoperative intraperitoneal melphalan 120 mg in 3 litres of 1.5% dextrose peritoneal dialysis solution. Melphalan concentrations in peritoneal fluid, tumor nodules and plasma were determined by high-pressure liquid chromatography. The AUC ratio of peritoneal fluid to plasma was 25. The ratio of tumor nodules to plasma was 12.
Intraperitoneal chemotherapy with hyperthermia
As these new concepts were increasingly accepted, combined treatment strategies that would use intraperitoneal chemotherapy as a planned part of a multidisciplinary approach to carcinomatosis were developed. Depending on the pharmacology of the chemotherapy agent, intraperitoneal use of cancer chemotherapy evolved into three different but not mutually exclusive treatment plans. In one, intraperitoneal chemotherapy agents that were cytotoxic within 1–2 h and were augmented in their effects by heat were used in the operating room as ‘heated intraoperative intraperitoneal chemotherapy’. In the oncology literature this is often referred to as HIIC (heated intraoperative intraperitoneal chemotherapy), IPHC (intraperitoneal chemohyperthermia), or HIPEC (hyperthermic intraperitoneal chemotherapy). In this review we will use HIIC.
Intraperitoneal chemotherapy in the absence of hyperthermia
The second surgical use of intraperitoneal chemotherapy utilizes the drugs throughout the first 4–7 postoperative days. This timing is crucial so that the chemotherapy distribution is uniform and occurs prior to the inevitable adhesive process that results from an extensive cancer resection. This chemotherapy administration is referred to as early postoperative intraperitoneal chemotherapy. Drugs selected for early postoperative intraperitoneal chemotherapy should be maintained for a long time period within the peritoneal cavity because each instillation is usually 24 h. Cell cycle-specific drugs that require cell division to be effective are appropriate in that the treatments are continued over several days.
Long-term bidirectional chemotherapy
The third use of intraperitoneal chemotherapy is referred to as long-term bidirectional chemotherapy. This has been shown to be of benefit in three large randomized trials in ovarian cancer patients Citation[14–16]. Drugs pharmacologically appropriate for intraperitoneal administration are given by this route; chemotherapy agents pharmacologically appropriate for intravenous administration are given intravenously. Long-term bidirectional chemotherapy is usually continued for approximately 6 months. Some chemotherapy agents may be given by both routes of administration.
It is important for the oncologist to remember that multi-agent chemotherapy has nearly always been associated with greater benefits than a single agent. The new concept is that not only multiple agents are required but also multiple routes of administration are needed in the treatment of peritoneal surface malignancy. To do this in the operating room the oncologist uses high-molecular-weight chemotherapy agents that exert their full effect in 60–90 min and are augmented by heat. The drugs used for early postoperative intraperitoneal chemotherapy should have high molecular weight to prolong exposure to peritoneal surfaces and have maximal effect over 5–7 days. The same rationale is used for the intraperitoneal agents in long-term bidirectional chemotherapy. In this review we will focus on the use of multi-agent intracavitary chemotherapy with hyperthermia in the operating theatre as a planned part of a surgical procedure for cancer.
Data supporting hyperthermia as a compliment to intracavitary chemotherapy
In the past, hyperthermia was suggested to improve the effects of intraperitoneal chemotherapy by three mechanisms. First, heat alone can bring about the destruction of cancer cells. Second, heat will augment the cytotoxicity directed toward cancer cells of selected chemotherapy agents. Third, heat increases the penetration of chemotherapy into cancerous tissues and thereby increases the local-regional effect by increasing the depth to which the chemotherapy would kill cancer cells. Currently, because intracavitary chemotherapy is generally ‘moderate hyperthermia’ less than 42.5°C, hyperthermia alone for 60–90 min in the operating room will not cause anticancer effects. However, the other two mechanisms are thought to be important from both preclinical and clinical studies.
Hyperthermia augmenting chemotherapy cytotoxicity
By the close of the 1980s, sufficient data had accumulated to allow the publication of several complete and ordered reviews concerning the interaction of heat with chemotherapy. A review of data documenting in vitro and in vivo augmentation of cancer chemotherapy cytotoxicity occurred as well as a study of modifying factors of the hyperthermia–drug interaction. Engelhardt compiled a large number of data on the heat–drug interaction focusing on one drug at a time Citation[17]. His goal was to provide insight for the clinical oncologist to facilitate possible clinical application of combined treatment. He also reviewed the different routes for heat application; systemic heating, local or regional heating or regional heating by isolated perfusion. His data confirmed the basic phenomenon of thermally improved ability of selected drugs to kill cancer cells both in vitro and in vivo. He emphasized the heterogeneous reaction of different tumours to similar heat–drug exposure. Also, heterogeneity within the same tumour to thermal augmentation of drug toxicity was documented. Engelhardt concluded that hyperthermia with isolated limb perfusion provides the most convincing clinical data in terms of response. However, benefits of this technology in terms of survival had been published.
Dahl reviewed the interaction of hyperthermia and cancer chemotherapy to facilitate rational clinical application of the combined modality approach Citation[18]. In his review of alkylating agents, only melphalan showed greater anti-tumour effects in the absence of increased normal tissue side effects in whole body hyperthermia in a rat model. He emphasized that some negative experiments that failed to show augmentation of cytotoxicity by heat, may result from the shut down of blood flow due to stasis within tumours. If blood flow is stopped by hyperthermia, systemically administered chemotherapy cannot reach cancer cells.
Meyer and colleagues reviewed the hyperthermic oncology literature concerning combined radiation-hyperthermia effects and cancer chemotherapy effects Citation[19]. They emphasized that combined treatments in a clinical setting may result in augmentation of cell kill or induce cells into a refractory state. The role of thermotolerance induced by heat shock proteins needs to be considered when the use of heat is contemplated in cancer treatment.
More recently, Urano and colleagues quantitated the enhanced cytotoxicity that hyperthermia induced with chemotherapy agents using in vivo studies in a mouse model Citation[20]. Thermal enhancement of many chemotherapeutic agents was maximized at temperatures between 40.5°C and 43°C and referred to as moderate hyperthermia. In studies of nine different chemotherapy agents, they documented that the drug of choice at physiological temperatures may not be the drug of choice under hyperthermic conditions. Also, the chemotherapy concentration in the target tissue must be sufficiently high for thermal enhancement to occur. Urano's data showed that some chemotherapy agents are markedly augmented in cytotoxicity by hyperthermia. Others show a moderate increase and still other agents are not enhanced by moderate hyperthermia at all. In their tumour growth delay assay, the chemotherapy agents that showed the greatest augmentation by heat were melphalan, cyclophosphamide, ifosfamide and cisplatin. Moderate augmentation was seen with doxorubicin, mitomycin C and bleomycin.
Of course the timing of hyperthermia in relation to the administration of chemotherapy is of utmost importance in planning clinical studies. Studies by Marmor in mice with intradermally implanted tumours were performed with both bleomycin and doxorubicin Citation[21]. They reported that the greatest potentiation occurred when the two modalities were administered close together. This suggested to them a true interaction. Engelhardt, in his lengthy review concludes that the greatest enhancement was found when both modalities were used simultaneously or within 30 min of each other Citation[17]. This was true for all the drugs commonly combined with heat for intraperitoneal administration including mitomycin C, doxorubicin, cisplatin and melphalan. Mella and Dahl specifically addressed the issue of timing in a rat model using cisplatin Citation[22]. The chemotherapy was given intraperitoneally. The greatest enhancement of cancer control by heat occurred when the total body hyperthermia to 41°C was administered just after the drug. Considering that the intraperitoneal chemotherapy would require several minutes to reach maximal systemic concentration, the experiment could be interpreted to suggest that simultaneous administration would result in the greatest benefit. These authors reported the same results in a similar model with cyclophosphamide Citation[23].
Mohamed and colleagues studied docetaxel in an in vivo model similar to that used by Urano and co-workers in an attempt to document augmentation by hyperthermia in an in vivo model Citation[24]. They found that docetaxel at 41.5°C for 30 min did not significantly increase mean tumour growth time compared with tumours treated with docetaxel at room temperature. However, treatment for 90 min was significantly increased (p = 0.04). Application of hyperthermia immediately and at 3 h was effective, whereas treatment at 3 h had no effect. These investigators concluded that thermal enhancement of docetaxel was moderate, it required prolonged heat exposure after intraperitoneal administration and that heat and chemotherapy should be given together to show an effect.
Mohamed and colleagues determined factors that modify thermal enhancement of melphalan Citation[25]. These studies were performed in vivo with a spontaneous mouse sarcoma. Hyperthermia in the absence of melphalan had a small but significant effect on tumour growth at 43.5°C but not at 41.5°C. Hyperthermia at 41.5°C after melphalan administration doubled mean tumour growth delay at 30 min and caused a 3-fold increase when hyperthermia was used for 90 min compared with melphalan at room temperature. Application of hyperthermia for 30 min immediately following drug administration was the most effective in delaying tumour growth. This tumour growth delay was as effective at 41.5°C as at 43.5°C. The effects were most obvious in treating large tumours. These studies showed that important parameters were necessary to combine melphalan with heat optimally. Heat profoundly increased the cytotoxicity of melphalan in this mouse model.
Increased penetration of chemotherapy into tumours with hyperthermia
Los and colleagues studied the penetration of chemotherapy into cancerous tissues with and without heat Citation[26]. Their model was an experimental tumour in the peritoneal cavity of rats. They found an increased drug exposure to tumour via the circulation by adding hyperthermia. Platinum concentrations in tumours increased with hyperthermia if the carboplatin was administered intraperitoneally and also increased if the chemotherapy was administered intravenously.
Jacquet and colleagues studied the effects of heat on intraperitoneal doxorubicin, a chemotherapy agent of large molecular weight and a slow clearance from the peritoneal cavity Citation[27]. Hyperthermia significantly augmented the uptake of intraperitoneal doxorubicin in all of the tissues directly exposed to the intraperitoneal heat such as small intestine, bladder and peritoneal surfaces. It did not increase drug concentrations in heart or liver. These investigators concluded that intraperitoneal doxorubicin penetration into normal tissues was increased by hyperthermia.
Dahl reviewed the mechanisms of interaction of hyperthermia and chemotherapy agents Citation[18]. He concluded that membrane damage by hyperthermia resulting in increased drug uptake at elevated temperatures was a main cause of augmented killing. The uptake of doxorubicin, melphalan and cisplatin was increased at increased temperatures when intracellular concentrations were measured. Hahn and Li studied substances such as alcohol and plant lectins that intensify cancer cell destruction by chemotherapy and heat Citation[28]. They reasoned that alcohol localized in lipid-rich cell membranes. Also, plant lectins modify the cell surface. They suggested that their data showed that the first step in hyperthermic killing involved damage to specific cell-membrane-associated proteins.
As discussed above, many convincing in vivo studies show that heat increases cancer chemotherapy penetration into cancerous and normal tissue. In the absence of heat, Ozols and colleagues in a murine ovarian cancer model showed limited tissue penetration of doxorubicin; it was limited to a few cell layers Citation[29]. Studies that document a change in drug penetration into cancerous nodules on peritoneal surfaces have yet to be reported.
These laboratory experiments can be used as a rationale to pursue bidirectional (combined intravenous and intraperitoneal) chemotherapy treatments with hyperthermia in humans. Intraperitoneal heat would be expected to increase access of chemotherapy administered systemically to carcinomatosis nodules and result in a greater response. Also, intraperitoneal heat would increase the direct penetration of chemotherapy administered intraperitoneally into carcinomatosis nodules. Chemotherapy would augment cytotoxicity of both intravenous and intraperitoneal drug in the absence of systemic toxicity. From a theoretical perspective multi-agent chemotherapy used by multiple routes of administration and targeted to the peritoneal surface by hyperthermia may result in an optimal combination of cancer chemotherapy and hyperthermia in patients with peritoneal carcinomatosis.
Using the intraperitoneal chemotherapy as a planned part of the surgical procedure not only optimizes drug distribution, it also maximizes drug penetration into the cancer nodules in that a cytoreductive surgery precedes the HIIC. If eradication of cancer on peritoneal surfaces is to occur, the cancer nodules to be treated must be of a minute size. The cancer chemotherapy enters normal tissues and cancer nodules by simple diffusion Citation[30]. Penetration can be expected to occur only over a few cell layers of the malignancy. Also, as the chemotherapy solution leaves the cancer nodule and enters the interstitial fluids or the blood in the first capillary bed, it will be rapidly removed.
Pharmacology of HIIC
lists the chemotherapy agents including their molecular weight and the AUC ratio that are now routinely used either as single agents or in combination for HIIC Citation[31]. The drug that has been most frequently used is mitomycin C. It is an anti-tumour antibiotic whose effects are fully developed within approximately 90 min at high concentration. Approximately 90% of the chemotherapy is absorbed with a 90-min intraperitoneal treatment. The AUC ratio is approximately 30. The renal toxicities of this drug can be prevented with a forced diuresis that can be routinely accomplished with a patient under general anaesthesia and with a Foley catheter in place. Mitomycin C is a drug rarely administered intravenously because of its high toxicity profile with prolonged treatment. It is possible that this chemotherapy rarely used intravenously may show benefit after systemic agents have failed. Currently, it is the standard of practice for HIIC to which all other drugs should be compared.
Table I. Chemotherapy agents used for HIIC.
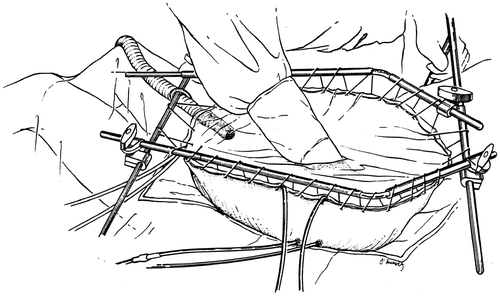
The technology for HIIC has followed two major directions. The group at the Washington Hospital Center has used a covered abdomen with manual distribution of the heated chemotherapy solution throughout the treatment interval Citation[32]. Others have used a closed technique whereby the skin of the abdomen is closed with a running suture during the HIIC treatment. Distribution is facilitated by continuous and firm intermittent pressure on the abdominal sidewall Citation[33]. To date, no major differences in outcome, complication rates or environmental contamination have been apparent. illustrates the covered technique for HIIC.
Figure 3. Line diagram of the covered technique in HIIC. Using a heavy gauge monofilament suture the skin edges are elevated on a self-retaining retractor. A small cruciate incision is made in the clear plastic to allow the surgeon's double-gloved hand to reach all portions of the peritoneal cavity and optimize the distribution of the heated chemotherapy solution. Temperature probes are placed over the skin edge. An infusion catheter and three drainage catheters are used. The inflow catheter is usually placed beneath the right hemidiaphragm and above the right lobe of the liver so that the hottest solution does not directly flow onto the small bowel. One and often two smoke evacuators are placed beneath the clear plastic sheet in order to remove any possible chemotherapy aerosols and trap them in a charcoal filtration system.
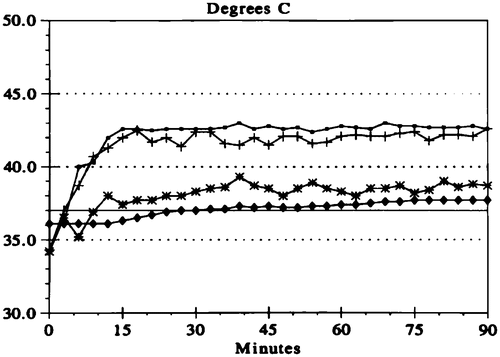
Early in the work with hyperthermia and intraperitoneal chemotherapy, temperatures within the peritoneal cavity were generated that would result in hyperthermic killing of cancer cells as a result of the heat effects alone. This meant a target temperature of 43–44°C throughout the entire abdomen. Intraoperative problems with blood pressure and urine output, combined with postoperative adult respiratory distress syndrome discouraged the use of these high temperatures. Now, most groups use ‘moderate hyperthermia’ with a target temperature for the whole abdomen of 41.5°C. At this temperature, hyperthermia significantly augments the cytotoxicity of chemotherapy agents and is much better tolerated with the preservation of the normal cardiopulmonary function Citation[20]. shows the typical temperature profile in a patient treated for 90 min by the open technique with manual distribution of the heated chemotherapy solution. The inflow is maintained at 45°C. A distant catheter usually within the pelvis is at approximately 40°C. The whole abdomen remains at approximately 42.5°C.
Figure 4. Temperature profile for HIIC. The line with open circles indicates the temperature maintained along the inflow catheter. The crossed lines show the temperature within the peritoneal cavity at the tip of the inflow catheter. The lines with stars show the temperature at a remote site, usually the base of the pelvis. The lines with diamonds show the core temperature usually within the oesophagus.
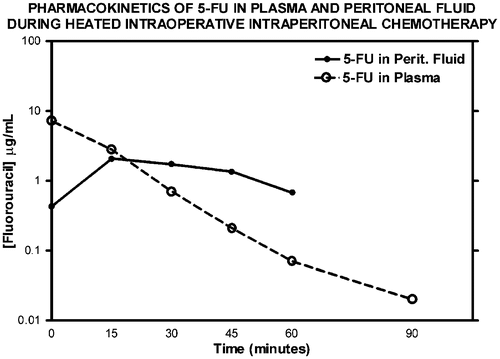
shows the pharmacology of intraperitoneal mitomycin C when using the covered technique, manual distribution and hyperthermia. High levels of drug are maintained for approximately 60 min and approximately 75% of the chemotherapy has been removed from the peritoneal cavity by 90 min. In this treatment plan, a single dose of chemotherapy is given at the initiation of the HIIC. Some groups use a double or triple dose of mitomycin C given over the 90 min of treatment Citation[34].
Figure 5. Pharmacokinetic study of intraperitoneal mitomycin C at 10 mg/m2 in 3 litres of 1.5% peritoneal dialysis solution. Mitomycin C levels were determined by high-pressure liquid chromatography in plasma and peritoneal fluid in a single patient. The data are representative of multiple similar studies performed in the operating room.
Pharmacodynamics of heated intraoperative intraperitoneal doxorubicin
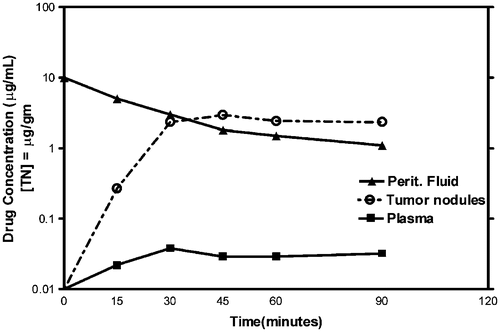
Only recently has it been possible to determine the actual levels of chemotherapy within the tumour nodules during HIIC. Some drugs appear to enter the cancer cells and remain for a prolonged time. shows a pharmacodynamic study of intraperitoneal doxorubicin used at 15 mg/m2 in 3 litres of 1.5% dextrose peritoneal dialysis solution. The levels of doxorubicin within small (<1 cm) tumour nodules were slightly elevated compared with chemotherapy concentration in the intraperitoneal fluid. By determination of the AUC ratio there was 110 times the exposure of peritoneal surfaces to doxorubicin compared with tissues in the plasma compartment. The exposure of the actual tumour nodule to doxorubicin is even greater.
Figure 6. Pharmacodynamic study of intraperitoneal doxorubicin in a single patient. Fifteen milligrams per m2 of doxorubicin was used in 3 litres of 1.5% Dianeal. Doxorubicin levels were determined by high-pressure liquid chromatography in the peritoneal fluid, tumor nodules, and plasma at 15-min intervals for 90 min. The area under the curve ratio of peritoneal fluid to plasma doxorubicin was 110. The data are representative of multiple similar studies performed in the operating room.
A new concept recently put into practice involves the simultaneous use in the operating room of intravenous chemotherapy and intraperitoneal chemotherapy. Conceptually, one uses the heat present in and around the tiny cancer nodule on peritoneal surfaces to augment the cytotoxicity of drugs given intraperitoneally as well as those given intravenously. shows the tendency of 5-fluorouracil given intravenously to concentrate in an artificial ascites. The treatment regimen, piloted by Elias and colleagues, was intravenous 5-fluorouracil and intraperitoneal oxaliplatin Citation[35]. Other drug combinations for other diseases could also fit into this theoretically improved model for combined intravenous and intraperitoneal drug delivery. Cyclophosphamide or ifosfamide given systemically would be heat-targeted to small ovarian cancer nodules. This is a promising regimen for patients who have persistent peritoneal surface malignancy from ovarian cancer Citation[36]. Protocols such as these may allow the eradication of larger tumour nodules and promote disease stabilization in those patients in whom complete cytoreduction is not possible. Much has been done to develop treatments for peritoneal surface malignancy; certainly much further laboratory and clinical investigation is needed.
Figure 7. Pharmacologic study of systemic heated intraoperative 5-fluorouracil chemotherapy in a single patient. Systemic 5-fluorouracil at 400 mg/m2 was given simultaneously with intraperitoneal oxaliplatin at 140 mg/m2. The heat should increase the cytotoxicity of chemotherapy agents entering the tumour nodule from the plasma as well as entering by diffusion from the peritoneal fluid. The data are representative of multiple similar studies performed in the operating room.
Data currently available to support HIIC as a treatment option for gastrointestinal and gynecologic malignancy
Appendiceal mucinous neoplasms
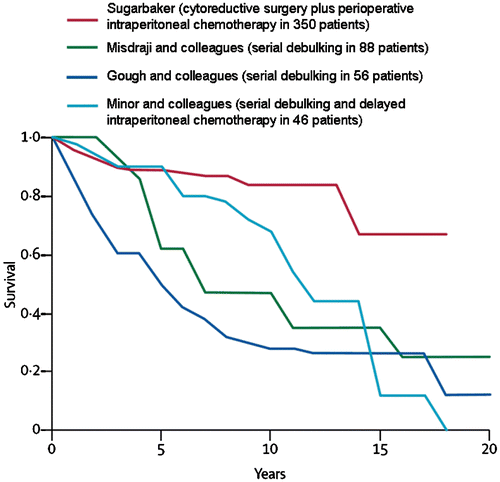
Appendiceal mucinous neoplasms have been treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for more than 20 years Citation[37]. As the cytoreductive surgical procedures improved and the HIIC became more refined, this new treatment option for appendiceal mucinous neoplasms was accepted as a new standard of care for this disease Citation[38]. It replaces serial debulking and multiple cycles of systemic chemotherapy for management; however, to see the survival differences in these patients, a long-term (20-year) follow-up is necessary. compares the results of cytoreduction plus HIIC to serial debulking procedures plus systemic chemotherapy or delayed intraperitoneal chemotherapy at four prominent cancer centres Citation[39–41]. With long-term follow-up, cytoreductive surgery plus HIIC is the only treatment associated with cure of this disease process.
Recently, a systematic review presented the results from the world's literature in the treatment of appendiceal mucinous neoplasms. Yan and colleagues concluded that cytoreductive surgery plus HIIC showed promising long-term results compared with historical controls Citation[42]. Because of the many difficulties in performing a phase-III study in this rare disease requiring long-term follow-up to show survival benefits, these are likely to be the best data available.
Peritoneal mesothelioma
Peritoneal mesothelioma is a second disease process in which cytoreductive surgery combined with perioperative intraperitoneal chemotherapy has become a new standard of care. Four groups have now reported on approximately 300 malignant peritoneal mesothelioma patients: the National Cancer Institute in Bethesda, MD Citation[43], the Washington Cancer Institute in Washington, DC Citation[44], the Columbia Mesothelioma Center in New York Citation[45] and the National Cancer Institute in Milan, Italy Citation[46]. All groups presented an experience with treatment results markedly improved over those reported in the past with conservative management by palliative surgery and systemic chemotherapy. Each group presented their experience with between 50 and 100 patients. With current treatment all the groups report a median survival of 5 years or better. The median survival in the past was approximately 1 year. As a result of this apparent major improvement in survival with a new treatment strategy in a rare disease, a new standard of care to which other options should be compared has emerged.
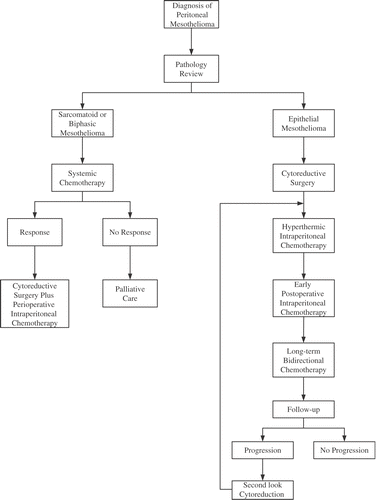
For HIIC, all groups advocate a cisplatin-based hyperthermic intraperitoneal chemotherapy. The doses were different at all four institutions. The heat, approximately 42.5°C, was the same at all institutions. The drugs combined with cisplatin were either doxorubicin or mitomycin C. At the National Cancer Institute, USA, high-dose cisplatin with systemic thiosulfate has been used. The clinical pathway used to treat peritoneal mesothelioma patients at the Washington Cancer Institute is shown in .
Pleural mesothelioma
Recently, promising results that combine thoracic surgery with intraoperative intrapleural hyperthermic cisplatin have been reported Citation[47]. In this study, 44 patients with pleural mesothelioma underwent pleurectomy followed by a 1-h lavage of the resection cavity with dose-escalated cisplatin at 42°C. Immediately after the chemotherapy lavage, intravenous sodium thiosulfate (16 g/m2 over 6 h) was administered. Survival estimates differed significantly for patients treated with low-dose compared with high-dose cisplatin (p = 0.0019). In these patients with minimal treatment options, the use of high doses of intracavitary hyperthermic cisplatin added to a complete mesothelioma resection resulted in a dose-dependent survival benefit.
Colorectal cancer
Colorectal cancer peritoneal carcinomatosis is a result of transcoelomic invasion by the primary cancer or intraperitoneal seeding during surgical manipulation. In contrast to lymphatic and haematogenous dissemination, colorectal cancer carcinomatosis should be regarded as a local-regional extension of disease rather than another manifestation of systemic metastasis. Approximately 8% of patients have isolated peritoneal seeding at the time of primary surgery, and 25% of patients with recurrence have disease confined to the peritoneal cavity. With better insight into the natural history and the incidence of carcinomatosis, new interest in its management has occurred over the last decade. The combined treatment modality of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy has gained increased attention. In the last 5 years, phase-II studies have suggested that cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy is associated with an improved survival compared with historical controls using systemic chemotherapy. Verwaal et al. recently conducted a randomized controlled trial comparing standard systemic chemotherapy with cytoreductive surgery combined with intraperitoneal hyperthermic chemotherapy and demonstrated the superiority in survival of the combined treatment group Citation[6]. In 2004, results of a registry study from 28 international institutions for colorectal cancer peritoneal carcinomatosis were reported Citation[48]. A median survival of 32.4 months was reported in patients who had a complete cytoreduction; it was only 8.4 months in patients having a suboptimal cytoreduction.
Yan and colleagues performed a systematic review to estimate the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for patients with peritoneal carcinomatosis from colorectal carcinoma Citation[49]. Two randomized controlled trials, one comparative study, one multi-institutional registry study, and the 10 most recent case-series studies were evaluated. The level of evidence was low in 13 of the 14 eligible studies. The median survival varied from 13 to 29 months, and 5-year survival rates ranged from 11% to 19%. Patients who received complete cytoreduction benefited most, with median survival varying from 28 to 60 months and 5-year survival ranging from 22% to 49%. The overall morbidity rate varied from 23% to 44%, and the mortality rate ranged from 0% to 12%.
The current evidence suggests that cytoreductive surgery combined with perioperative intraperitoneal chemotherapy is associated with an improved survival, compared with systemic chemotherapy for peritoneal carcinomatosis from colorectal carcinoma.
If hyperthermic intraperitoneal chemotherapy is effective in treating selected patients with carcinomatosis from colorectal cancer, one would suspect that prevention of local-regional recurrence on peritoneal surfaces or the resection site may be possible. This causes a dilemma that cancer surgeons must currently answer for themselves on numerous patients. Until clinical trials information becomes available, it is my opinion that patients with primary cancer that demonstrate a high risk of dissemination in the peritoneal cavity should be treated. This includes patients with T4 lesions, positive peritoneal cytology, involvement of the ovaries, and patients whose cancer is disrupted with resection Citation[50]. A phase-III trial in this group of patients may be difficult, even impossible, to complete.
Gastric cancer
Combined peritoneal carcinomatosis and resection site disease recurrence of gastric cancer occurs in as high as 50% of patients who are treated with gastrectomy. This pattern of recurrence is most prominent in patients who have stage-III or resectable stage-IV disease. Xu and colleagues performed a meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for advanced gastric cancer Citation[51]. Eleven trials involving 1161 patients were included for data extraction. A significant improvement in survival was associated with HIIC. The meta-analysis indicated that hyperthermic intraperitoneal chemotherapy after resection of advanced primary gastric cancer is associated with an improved overall survival. An adequately powered trial in Western gastric cancer patients is needed.
Ovarian cancer
Ovarian cancer is the fifth leading cause of cancer-related deaths among females in the USA with 15 310 deaths projected in 2006. The majority of cases are diagnosed with carcinomatosis and progressive malignant ascites as the presenting sign. Despite a 60–80% response rate to platinum-based systemic chemotherapy, the prognosis remains poor owing to a high rate of recurrence. Because epithelial ovarian cancer has a marked propensity for peritoneal spread, it is suitable for aggressive local-regional therapies. Consequently, the combination of intravenous and intraperitoneal route of administration for chemotherapeutic agents in ovarian cancer has been extensively investigated Citation[14–16]. Despite convincing data, intraperitoneal chemotherapy treatment is still not universally accepted owing to the increased rate of complications associated with intraperitoneal drug delivery. Cytoreductive surgery combined with HIIC is a comprehensive treatment modality directed at the whole abdomen and pelvis that has shown efficacy. Its use of intraperitoneal chemotherapy at the time of surgery and/or in the immediate postoperative period facilitates uniform drug delivery and may avoid some of the complications of prolonged peritoneal access.
Bijelic and colleagues performed a systematic review to critically evaluate cytoreductive surgery combined with HIIC in the treatment of ovarian cancer Citation[52]. This was a systematic review of all manuscripts published in the English literature that met predetermined inclusion criteria. Fourteen studies were analysed. A wide variety of drug doses, methods of intraperitoneal chemotherapy administration and volume of chemotherapy solution was used. Seven studies showed that patients with complete cytoreduction had the greatest benefit. The median overall survival for primary and recurrent disease ranged from 22 to 54 months and the median disease-free survival from 10 to 26 months. The rates of significant morbidity associated with the combined treatment were low, ranging from 5% to 36%. The mortality did not exceed 10%. These authors concluded that cytoreductive surgery combined with HIIC is a treatment option for patients with ovarian cancer that is worthy of further investigation. Selection criteria for patients most likely to benefit need to be defined.
Projections regarding number of patients requiring intraperitoneal chemotherapy with hyperthermia
Very often, the first step in finding new treatments for cancer involves studies in patients with advanced disease. A response that results in improved or prolonged life is then taken to patients with less advanced disease. Usually, this second step in development is in a randomized trial. Surprisingly, intraperitoneal chemotherapy and the appropriate use of hyperthermia has resulted in a survival benefit in numerous phase-II trials and in a single phase-III trial in colorectal cancer with carcinomatosis. Benefit is strongly suggested in ovarian cancer. In appendiceal cancer and peritoneal mesothelioma it is a new standard of care and, for ethical and statistical reasons, phase-III studies will probably never be performed.
Currently, limited application of this treatment is occurring worldwide. An inadequate number of treatment centres are operational. The practising oncologist is usually content to give intravenous chemotherapy compared with the more technologically demanding intraperitoneal chemotherapy. Also, the practising surgeon is not eager to have his time in the operating room extended approximately 2 h. Nevertheless, the number of patients who could profit from this new treatment option can be estimated. Nearly all of the 1500 new appendiceal mucinous neoplasms that occur annually in the USA should be treated. Also, a majority of the 300 new peritoneal mesothelioma patients should be treated. Approximately half of the 20 000 patients with peritoneal dissemination of colorectal cancer should be treated. Most of the 30 000 new ovarian cancer patients are eligible for this treatment option.
Of course, if this application of hyperthermia and intraperitoneal chemotherapy is extended to the adjuvant treatment of primary disease, there will be a much larger number of patients eligible. Of the 150 000 new colorectal cancer patients each year, approximately 30 000 will be at high risk for subsequent local recurrence or peritoneal carcinomatosis. These patients are excellent candidates for this treatment option. Also, at least half of the 30 000 new gastric cancer patients in the USA are stage III or resectable stage IV and are candidates for this treatment option. Many other diseases such as small bowel adenocarcinoma, endometrial cancer and gallbladder cancer may be considered for treatment because of the high risk for resection site recurrence and peritoneal carcinomatosis.
Of course, such dramatic changes in practice patterns are not going to occur quickly. Numerous additional prospective clinical trials will be necessary. Perhaps the most direct way to bridge the gap between current practice of intravenous chemotherapy only to bidirectional chemotherapy using both intraperitoneal and intravenous chemotherapy is through the initiation of studies with hyperthermic intraoperative intraperitoneal chemotherapy. In this treatment modality the effects of the intraperitoneal treatment are likely to be most profound. Also, the technical problems of intraperitoneal drug delivery are absent. The technological requirements for this treatment option are low and its morbidity and mortality is very limited Citation[53]. It seems to be a good place in which to initiate the multiple changes that may be necessary in the practice of oncology.
References
- Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy: A prospective study of prognostic factors. Cancer 1989; 63: 364–367
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies. Result of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88: 358–363
- Jayne DG, Fook S, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002; 89: 1545–1550
- Yu W, Whang I, Chung HY, Averbach A, Sugarbaker PH. Indications for early postoperative intraperitoneal chemotherapy for advanced gastric cancer: Results of a prospective randomized trial. World J Surg 2001; 25: 985–990
- Glehen O, Gilly F, Sugarbaker PH. New perspectives in the management of colorectal cancer: What about peritoneal carcinomatosis?. Scand J Surg 2003; 92: 178–179
- Verwaal VJ, Van Ruth S, de Bree E, Van Sloothen GW, Van Tinteren H, Boot H, Zoetmulder FAN. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743
- Link KH, Roitman M, Holtappels M, Runnebaum I, Urbanzyk H, Leder G, Staib L. Intraperitoneal chemotherapy with mitoxantrone in malignant ascites. Surg Oncol Clin N Am 2003; 12: 865–872
- Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Peritoneal Carcinomatosis: Principles of Management, PH Sugarbaker. Kluwer, Boston 1996; 79–100
- Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: Mechanisms of the patterns of spread. Cancer Metastasis Rev 2003; 22: 465–472
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221: 29–42
- Weiss L. Metastatic inefficiency: Causes and consequences. Cancer Rev 1986; 3: 1–24
- Schott A, Vogel I, Krueger U, Kalthoff H, Schreiber HW, Schmiegel W, Henne-Bruns D, Kremer B, Juhl H. Isolated tumor cells are frequently detectable in the peritoneal cavity of gastric and colorectal cancer patients and serve as a new prognostic marker. Ann Surg 1998; 227: 372–379
- Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: Analysis of experimental data in the rat. Am J Physiol 1985; 248: 413–424
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Eng J Med 1996; 335: 1950–1955
- Markman M, Bundy BN, Alberts DS. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001; 19: 1001–1007
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34–43
- Engelhardt R. Hyperthermia and drugs. Recent Results Cancer Res 1987; 104: 136–203
- Dahl O. Interaction of hyperthermia and chemotherapy. Recent Results Cancer Res 1988; 107: 157–169
- Meyer JL. Kapp DS, Fessenden P, Hahn GH. Hyperthermic oncology: Current biology, physics and clinical results. Pharmac Ther 1989; 42: 251–288
- Urano M, Kuroda M, Nishimura Y. Invited review for the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 1999; 15: 79–107
- Marmor JB, Kozak D, Hahn GM. Effects of systemically administered bleomycin or adriamycin with local hyperthermia on primary tumor and lung metastases. Cancer Treat Rep 1979; 63: 1279–1290
- Mella O, Dahl O. Timing of combined hyperthermia and 1,3-bis(2-chloroethyl)-1-nitrosurea or cis-diamminedichloroplatinum in BD IX rats with BT4A tumours. Anticancer Res 1985; 5: 259–264
- Dahl O, Mella O. Effect of timing and sequence of hyperthermia and cyclophosphamide on a neurogenic rat tumor (BT4A) in vivo. Cancer 1983; 52: 983–987
- Mohamed F, Stuart OA, Glehen O, Urano M, Sugarbaker PH. Docetaxel and hyperthermia: Factors that modify thermal enhancement. J Surg Oncol 2004; 88: 14–20
- Mohamed F, Stuart OA, Glehen O, Urano M, Sugarbaker PH. Optimizing factors which modify thermal enhancement of melphalan in a spontaneous murine tumor. Cancer Chemother Pharmacol 2006; 58: 719–724
- Los G, Smals OAG, Van Vugt MJH, Van der Vlist M, den Engelse L, McVie JG, Pinedo HM. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res 1992; 52: 1252–1258
- Jacquet P, Averbach A, Stuart OA, Chang D, Sugarbaker PH. Hyperthermic intraperitoneal doxorubicin: Pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother Pharmacol 1998; 41: 147–154
- Hahn GM, Li GC. Interactions of hyperthermia and drugs: Treatments and probes. Nat Cancer Inst Monogr 1982; 61: 317–323
- Ozols RF, Locker GY, Doroshow JH, Grotzinger KR, Myers CE, Young RC. Pharmacokinetics of adriamycin and tissue penetration in murine ovarian cancer. Cancer Res 1979; 39: 3209–3214
- Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal-plasma transport: Analysis of experimental data in the rat. Am J Physiol 1985; 248: 413–424
- Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 2005; 10: 112–122
- Sugarbaker PH. An instrument to provide containment of intraoperative intraperitoneal chemotherapy with optimized distribution. J Surg Oncol 2005; 92: 142–146
- Jacquet P, Averbach A, Stephens AD, Stuart OA, Chang D, Sugarbaker PH. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: Pharmacokinetic studies. Oncology 1998; 55: 130–138
- Verwaal VJ, Van Tinteren H, Van Ruth S, Zoetmulder FAN. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg 2004; 91: 739–746
- Elias D, Pocard M, Sideris L, Ede C, Ducreux M, Boige V, Lasser P. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg 2004; 91: 455–456
- Zylberberg B, Dormont D, Madelenat P, Darai E. First-line intraperitoneal cisplatin-paclitaxel and intravenous ifosfamide in stage IIIc ovarian epithelial cancer. Eur J Gynaecol Oncol 2004; 25: 327–332
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999; 6: 727–731
- Sugarbaker PH. New standard of care for appendiceal epithelial malignancies and pseudomyxoma peritonei syndrome. Lancet Oncol 2006; 7: 69–76
- Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO, Naessens JM, O’Brien PC, Van Heerden JA. Pseudomyxoma peritonei: Long-term patient survival with an aggressive regional approach. Ann Surg 1994; 2: 112–119
- Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: A clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003; 27: 1089–1103
- Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: An analysis of surgical therapy. Ann Surg 2005; 241: 300–308
- Yan TD, Zappa L, Edwards G, Alderman R, Marquardt CE, Sugarbaker PH. Perioperative outcomes of cytoreductive surgery perioperative intraperitoneal chemotherapy for non-appendiceal peritoneal carcinomatosis from a prospective database. J Surg Oncol 2007, 12 Jan [Epub ahead of print]
- Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003; 21: 4560–4567
- Sugarbaker PH, Yan TD, Stuart OA, Yoo D. Comprehensive management of diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol 2006; 32: 644–647
- Taub RN, Hesdorffer ME, Keohan ML. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for malignant peritoneal mesothelioma (MPM). J Clin Oncol 2005, (Abstract)
- Deraco M, Nonaka D, Baratti D, Casali P, Rosai J, Younan R, Salvatore A, Cabras AD, Kusamura S. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2006; 13: 229–237
- Richards WG, Zellos L, Bueno R, Jaklitsch MT, Janne PA, Chirieac LR, Yeap BY, Dekkers RJ, Hartigan PM, Capalbo L, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006; 10: 1561–1567
- Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol 2004; 22: 3284–3292
- Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006; 24: 4011–4019
- Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999; 43(Suppl): S15–S25
- Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004; 10: 2727–2730
- Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol 2007, May 11 [Ep06]
- Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, Chang D. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol 2006; 13: 635–644